Abstract
The purpose of this study was to assess health status (HS) in patients with clinical suspicion of obstructive sleep apnea (OSA) in order to estimate the dose response relationship between HS and OSA severity, and to compare HS in this clinical cohort with a general population sample (N = 5000). Patients referred to an overnight sleep study due to suspected OSA, whom also responded to the SF-36 questionnaire, were included (N = 418). Of these, 194 showed normal findings, while 111, 60 and 53 demonstrated mild, moderate and severe OSA, respectively. Mean age was 47.5 (SD 11.9) and 69% were males. Only the mental health scale (p = 0.015) and mental component summary score (p = 0.023) were associated with OSA severity. This association, however, disappeared in multivariable analysis. All SF-36 scores in the sleep study group were lower than that of the general population sample, in both unadjusted and multivariable linear regression analysis. In this study, there was a lack of association between OSA severity and general HS. However, as a whole, patients in this clinical population referred to an overnight sleep study due to suspected OSA had impaired HS on all scales compared to a general population, with greatest differences in the vitality domain.
Introduction
Obstructive sleep apnea (OSA) is a common disorder characterized by recurrent episodes of complete and partial airway obstruction, which may result in an increased risk of cardiovascular diseases and metabolic disorders and subsequently have an adverse effect on quality of life1. Recent studies show increasing prevalence of OSA in the general adult population with findings of moderate OSA from 6 to 23% for adult women and from 13 to 50% for adult men in the general population2,3.
During the last decades, quality of life assessments in OSA patients have become a main focus in statements on sleep apnea published by The American Thoracic Society4 and the American Sleep Disorders Association5, leading to increased use of health status measures in both the clinical and research setting. Health status (HS) is a multidimensional concept that usually includes self-report of the way in which physical, mental, social, or other domains of well-being are affected by a disease or its treatment6. There is no absolute consensus for the definition of HS. This results in interchangeable use of the terms health-related quality of life (HRQoL) and HS, as both describe self-assessment of the aforementioned domains7. One of the most widely used questionnaires for the assessment of general HS is the SF-36, which is used to compare general and specific populations, assess the relative burden of disease, and differentiate health benefits according to treatments8.
Several studies have found that HS, as assessed by the SF-36, in untreated OSA patients is significantly impaired in numerous domains compared to general population samples9–11. Higher apnea-hypopnea index (AHI) has been in a dose-response fashion related to lower scores on six of eight SF-36 domains (mental health, social functioning, role physical, physical functioning, vitality and general health) in a large population based sample12. Males with mild OSA have shown poorer scores for the domains of role emotional, role physical, vitality and mental health when compared to patients with normal findings13. In another clinical population sample, patients with mild and moderate OSA scored lower in the domains of physical functioning and role physical, and patients with moderate to severe OSA scored lower on vitality compared to patients without respiratory disturbances14. In contrast, others have found no significant associations between AHI and HS in clinical populations15–17. Sleep quality has been shown to broadly influence general HS as assessed by the SF-3618, and the vitality domain has also shown associations with sleep disruption in OSA patients19.
The estimated prevalence of OSA in middle-aged adults in the general Norwegian population is approximately 25%20, but there is limited knowledge of the HS of persons with OSA in Norway21. The objective of this study was to use the SF-36 to: (1) estimate the dose response relationship between HS and OSA severity, grouped as mild, moderate or severe OSA, and (2) to compare HS in this clinical cohort with a general population sample.
Methods
Participants
Sleep study group
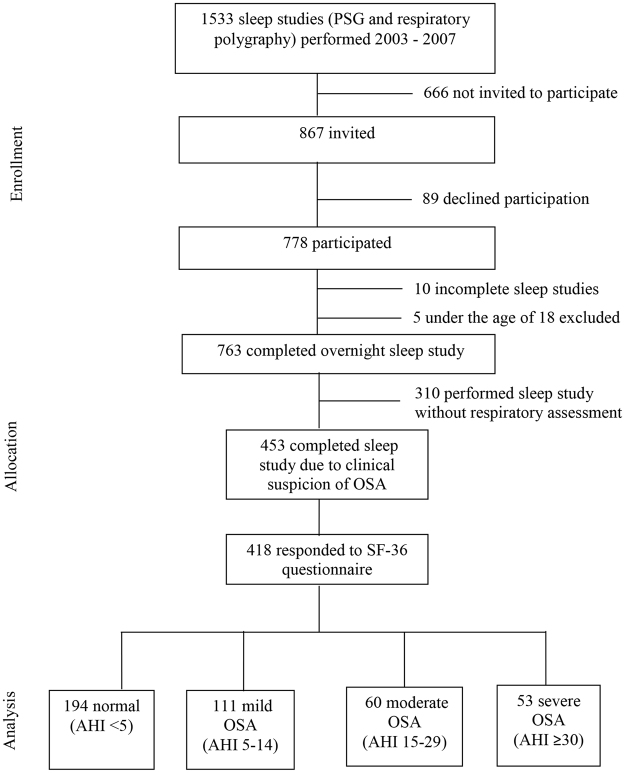
This cross-sectional study wanted to include all subjects referred to the Department of Clinical Neurophysiology, Akershus University Hospital between October 2003 and December 2007 for a sleep study due to clinical suspicion of OSA. Referrals for the majority of patients came from general practitioners, neurologists or ear-nose-throat specialists. The inclusion process was delegated to neurophysiology technicians at the Department of Clinical Neurophysiology. During this period 1533 sleep studies were performed, 89 subjects declined to participate and 666 were not invited for unknown reasons, resulting in a total of 755 non-participants, and an inclusion rate of 51%. During this inclusion period, PSGs without respiratory assessments were also performed for patients who did not have clinical findings consistent with sleep apnea. These patients were not included in this study. In total, 418 subjects with clinically suspect OSA complaints who also fully completed the SF-36 questionnaire were included for further analysis (Figure 1).
Figure 1.
Study population flow chart.
The Regional Committee for Medical Research Ethics, Helse-Sør-Øst approved the project’s ethical aspects (210-03096). Informed, written consent was obtained from all participants included in the study.
General population
General population values were collected from a survey conducted as part of the Norwegian Lifestyle Survey by Statistics Norway in 200222. The survey comprised 9698 members of the general population ≥16 years of age that were representative for Norway. The study included home and telephone interviews prior to a postal survey. The postal questionnaire included the Norwegian SF-36 version 1.223 mailed between 15 November 2002 and 15 May 2003. We had access to data collected from persons that responded to both postal survey and interview. Of these, 5000 also responded to all SF-36 questionnaire domains, and were used in further analysis.
Questionnaire
Participants in the sleep study group filled out a questionnaire that included items about demographics, smoking status, presence of comorbid conditions (high blood pressure, diabetes mellitus and anxiety or depression), a Norwegian version of the Epworth sleepiness scale (ESS)24, and a Norwegian version of the standard SF-36 questionnaire, version 1.223.
The SF-36 is a general HS questionnaire. It assesses eight dimensions of health including physical functioning, role limitations related to physical problems, bodily pain, general health perception, vitality, social functioning, role limitations due to emotional problems and mental health. Raw scores are converted to a scale from 0 to 100. Higher transformed scores indicate better HS according to the SF-36 scoring manual8. The scales are also aggregated to provide two summary scales, the physical component summary (PCS) and mental component summary (MCS), which are scored on a standardized scale designed for comparison with a US general population with mean 50 (SD 10)25.
Sleep studies
Participants in the sleep study group who were evaluated only for sleep apnea underwent an ambulant PG (n = 343) using the Embla system (EMBLA; Medcare-Flaga; Reykjavik, Iceland), recording airflow with thermistors, oxygen saturation by finger-pulse oximeter, heart rate, abdominal and thoracic respiratory movements, body position and snoring. Participants with symptoms suspect for both OSA and other sleep disorders were referred to a polysomnography (PSG) (n = 75) using the same ambulant Embla system, but with additional surface electrode recordings of the electroencephalogram (C3-A2, C4-A1, O1-A2, O2-A1), electrooculograms (EOG1 and EOG2), electrocardiogram, as well as m. submentalis and bilateral m. tibialis anterior electromyograms. The sleep studies were scored according to the criteria of the American Academy of Sleep Medicine Task Force by physicians specializing in clinical neurophysiology26.
Statistical analysis
Descriptive statistics were presented using means with SDs or number (%). The characteristics of participants in the sleep study group and the general population sample were compared using the t-test for continuous and chi-square test for discrete variables.
SF-36 scale scores were computed according to standardized criteria8,25. We compared SF-36 scale scores when participants were grouped according to OSA severity (normal, mild, moderate, severe) using the Kruskal-Wallis test, and the Wilcoxon-Mann-Whitney test when comparing participants in the sleep study group with the general population sample.
In further analysis, we focused on the SF-36 domains of physical functioning and vitality, which in previous studies have been associated with sleep disorders and therefore expected to be most sensitive to symptoms of OSA10,12,27, as well as the two component summary scores, PCS and MCS. We chose to adjust the comparisons of HS for the variables age, gender, education, body mass index (BMI), ESS > 10 and comorbid conditions, which in previous studies have shown associations between OSA and quality of life10,12,14,27,28, where available.
In the sleep study group we performed multivariable linear regression analysis for the SF-36 domains physical functioning, vitality, PCS and MCS adjusting for age per 10 years, gender, education level, BMI as well as ESS ≤ 10 or >10, presence of high blood pressure, diabetes mellitus and anxiety or depression for participants grouped according to OSA severity compared to those with AHI < 5 (normal). All independent variables were forced into the models.
When comparing participants in the sleep study group to the general population sample using multivariable linear regression analysis and the same dependent variables as above, we adjusted for age per 10 years, gender, education level, and BMI, again forcing all independent variables into the models.
Some of the residuals in the multivariable linear regression models did not comply with a normal distribution, but log-transformation or square-root transformation of the affected dependent variables did not resolve this situation. We therefore used the untransformed values for the dependent variables, but used bootstrapped 95% confidence intervals with 500 replications in all models. Missing values were not imputed.
We chose a 5% significance level, using two-sided tests. The Stata version 14.1 (StataCorp, College Station, TX, USA) was used for analyses.
Data availability statement
The patient data analysed during the current study are not publicly available due to ethical and legal constraints, but anonymized data are available from the corresponding author on reasonable request.
The general population data that support the findings of this study are available from Norwegian Centre for Research Data (NSD) but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of NSD.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Results
Descriptive statistics for the sleep study group
Participants in the sleep study group comprised more males (p < 0.001), had higher BMI (p < 0.001), a lower percentage of smokers (p = 0.044) and a greater percentage of participants with university or college degree than the general population sample (p < 0.001), but there was no difference in age (p = 0.37) between the groups. There was increasing BMI with increasing OSA severity, and participants with severe OSA had the highest percentage of ESS > 10 (Table 1).
Table 1.
Descriptive statistics for patients referred to an overnight sleep study due to a clinical suspicion of obstructive sleep apnea (sleep study group) and general population sample who responded to all SF-36 questionnaire domains.
| Normal | Obstructive sleep apnea | Total | General population | p** | |||
|---|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | 418 | 5000 | |||
| AHI* | <5 | 5–14 | 15–29 | ≥30 | |||
| N | 194 | 111 | 60 | 53 | |||
| Mean (SD) or N (%)** | Mean (SD) or N (%)** | Mean (SD) or N (%)** | Mean (SD) or N (%)** | Mean (SD) or N (%)** | Mean (SD) or N (%)** | ||
| Age, years | 43.7 (12.1) | 49.7 (11.2) | 52.2 (9.9) | 50.8 (10.1) | 47.4 (11.9) | 46.7 (16.5) | 0.37 |
| Male gender | 105 (54) | 82 (74) | 50 (83) | 49 (92) | 286 (68) | 2435 (49) | <0.001 |
| Body mass index, kg/m² | 27.4 (5.1)1 | 28.7 (4.8) | 29.8 (4.5) | 32.7 (5.9) | 28.8 (5.3) | 24.8 (5.1)2 | <0.001 |
| Education | <0.001 | ||||||
| Schooling up to 10 years | 34 (18) | 28 (25) | 14 (24) | 13 (25) | 89 (22) | 659 (13) | |
| Schooling 11–13 years | 76 (40) | 42 (38) | 20 (34) | 20 (39) | 158 (38) | 2816 (57) | |
| University or college degree | 81 (42) | 41 (37) | 25 (42) | 18 (35) | 165 (40) | 1471 (30) | |
| Current smoker | 63 (33)1 | 31 (28) | 17 (28) | 20 (38)3 | 131 (32) | 1825 (37)4 | 0.044 |
| Epworth sleepiness scale >10 | 87 (47)5 | 42 (42)6 | 24 (44)7 | 28 (55)8 | 181 (46) | ||
| High blood pressure | 17 (11)9 | 14 (15)10 | 12 (25)11 | 12 (30)12 | 55 (17) | ||
| Diabetes mellitus | 8 (5)9 | 8 (9)10 | 4 (8)11 | 5 (13)12 | 25 (8) | ||
| Anxiety or depression | 34 (23)9 | 10 (11)10 | 13 (27)11 | 7 (18)12 | 64 (19) | ||
*Apnea-hypopnea index.
**Continuous variables are presented using mean (SD), and categorical variables using number (percent).
***Comparison of total sleep group and general population using the t-test for continuous and Chi-square test for discrete variables.
1N = 192.
2N = 4926.
3N = 52.
4N = 4998.
5N = 187.
6N = 101.
7N = 54.
8N = 51.
9N = 151.
10N = 91.
11N = 48.
12N = 40.
There was no difference in age when comparing participants (N = 778) to non-participants (N = 755), with mean (SD) age of 45.1 (12.9) and 45.8 (16.0), respectively (p = 0.38). There was a similar distribution of gender among participants and non-participants, 62% and 59% males respectively (p = 0.37).
Dose-response between health status and OSA severity
Comparison of unadjusted SF-36 scale scores between participants in the sleep study group categorized according to OSA severity showed higher values for participants with mild, moderate and severe OSA on the mental health and MCS scales than those with normal findings (AHI < 5) (Table 2). Higher transformed SF-36 scale scores indicate better HS.
Table 2.
SF-36 scale scores in patients referred to an overnight sleep study due to a clinical suspicion of obstructive sleep apnea (sleep study group; N = 418) categorized according to OSA findings.
| Normal | Obstructive sleep apnea | P *** | |||
|---|---|---|---|---|---|
| Mild | Moderate | Severe | |||
| AHI * | <5 | 5–14 | 15–29 | ≥30 | |
| N | 194 | 111 | 60 | 53 | |
| SF-36 scales** | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Physical functioning | 81.4 (19.6) | 79.9 (20.0) | 79.5 (21.1) | 76.7 (16.9) | 0.052 |
| Role physical | 54.5 (41.0) | 53.8 (41.0) | 51.7 (41.4) | 51.9 (39.8) | 0.92 |
| Bodily pain | 60.8 (28.2) | 62.6 (26.8) | 63.1 (29.9) | 59.7 (30.2) | 0.92 |
| General health | 59.5 (25.0) | 61.4 (23.4) | 59.1 (24.2) | 59.2 (21.7) | 0.88 |
| Vitality | 34.8 (23.5) | 40.4 (20.4) | 39.7 (23.2) | 37.6 (20.4) | 0.118 |
| Social functioning | 69.1 (27.6) | 76.0 (25.2) | 71.0 (28.1) | 75.9 (22.2) | 0.143 |
| Role emotional | 67.4 (38.7) | 67.9 (39.2) | 70.0 (36.7) | 73.6 (38.9) | 0.68 |
| Mental health | 68.8 (18.8) | 75.4 (15.9) | 73.3 (17.9) | 73.1 (19.2) | 0.020 |
| Physical component summary**** | 44.3 (10.8) | 43.7 (10.6) | 43.3 (10.6) | 42.1 (9.7) | 0.49 |
| Mental component summary**** | 43.8 (11.0) | 47.2 (10.4) | 46.4 (10.6) | 47.6 (11.0) | 0.023 |
*Apnea-hypopnea index.
**Scored on a 0–100 scale.
***Kruskal-Wallis test.
****Standardized for comparison with a U.S. general population with mean 50 (SD 10).
There was no difference in overall HS between participants in the sleep study group categorized according to OSA severity and those in the normal group (AHI < 5) after adjustment for gender, education, age per 10 years, BMI, ESS > 10, presence of high blood pressure, diabetes mellitus and anxiety or depression. In this multivariable linear regression model, vitality and MCS scales were positively associated with age, but negatively with the presence of anxiety or depression. ESS score > 10 was negatively associated with the vitality scale, and BMI and the presence of diabetes mellitus were negatively associated with the physical functioning scale. The PCS scale was negatively associated with high blood pressure (Table 3).
Table 3.
Determinants of health status in sleep study participants (sleep study group). Coefficients and 95% confidence intervals (95% CI) for SF-36 scales according to categories of obstructive sleep apnea severity. Multivariable linear regression adjusted for gender, education, age, body mass index, Epworth sleepiness scale score > 10 as well as presence of high blood pressure, diabetes and anxiety or depression.
| Physical functioning (N = 304) | Vitality (N = 304) | Physical component summary (N = 304) | Mental component summary (N = 304) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coef.* | 95% CI | P | Coef.* | 95% CI | P | Coef.* | 95% CI | P | Coef.* | 95% CI | P | |
| Obstructive sleep apnea | ||||||||||||
| Normal (AHI** < 5) | 0 | |||||||||||
| Mild (AHI** 5–14) | −0.7 | −5.1–3.7 | 0.76 | −0.6 | −6.1–4.8 | 0.82 | −0.8 | −3.6–2.1 | 0.60 | 0.1 | −2.5–2.7 | 0.95 |
| Moderate (AHI** 15–29) | 4.1 | −1.2–9.4 | 0.126 | 3.4 | −4.6–11.2 | 0.41 | 0.4 | −3.5–4.4 | 0.83 | 1.4 | −2.0–4.8 | 0.43 |
| Severe (AHI** > 30) | −0.1 | −7.1–6.8 | 0.98 | 1.9 | −8.1–9.0 | 0.92 | −1.1 | −5.1–2.9 | 0.59 | 1.4 | −1.7–4.5 | 0.36 |
| Female gender | −3.6 | −8.3–1.0 | 0.128 | −2.0 | −7.3–3.2 | 0.45 | −0.9 | −3.8–1.9 | 0.53 | −1.9 | −4.5–0.67 | 0.147 |
| Education | ||||||||||||
| Schooling up to 10 years | 0 | |||||||||||
| Schooling 11–13 years | 2.2 | −3.7–8.1 | 0.47 | 1.9 | −5.5–9.3 | 0.61 | 0.6 | −3.0–4.2 | 0.74 | 0.3 | −2.7–3.3 | 0.84 |
| University or college degree | 3.2 | −2.8–9.2 | 0.29 | 1.9 | −5.4–9.1 | 0.62 | 0.1 | −3.3–3.5 | 0.94 | −1.0 | −3.8–1.8 | 0.48 |
| Age per 10 years | −1.1 | −3.0–0.7 | 0.24 | 4.4 | 2.1–6.6 | <0.001 | −0.3 | −1.3–0.8 | 0.64 | 1.85 | 0.8–2.9 | <0.001 |
| Body mass index, kg/m2 | −0.8 | −1.3–−0.3 | 0.001 | 0.4 | −0.1–1.0 | 0.122 | −0.1 | −0.4–0.1 | 0.31 | 0.2 | −0.06–0.4 | 0.143 |
| Epworth Sleepiness Scale > 10 | −1.0 | −5.0–3.0 | 0.63 | −9.7 | −14.3–−5.1 | <0.001 | −1.2 | −3.7–1.3 | 0.34 | −1.2 | −3.1–0.8 | 0.25 |
| High blood pressure | −5.2 | −11.4–1.0 | 0.101 | −2.0 | −9.1–5.2 | 0.59 | −3.8 | −6.8–−0.7 | 0.016 | 0.3 | −2.4–3.1 | 0.81 |
| Diabetes mellitus | −9.9 | −19.6–−0.3 | 0.043 | −7.8 | −16.9–1.4 | 0.098 | −3.8 | −8.9–1.2 | 0.135 | −1.6 | −5.6–2.5 | 0.45 |
| Anxiety or depression | −4.1 | −9.9–1.7 | 0.163 | −12.1 | −18.3–−5.8 | <0.001 | −1.3 | −4.2–1.6 | 0.38 | −12.7 | −15.7–−9.6 | <0.001 |
*Unstandardized beta coefficient.
**Apnea-hypopnea index.
Comparison of health status between sleep study group and general population
Unadjusted SF-36 scores in the sleep study group were lower on all scales than in the general population sample (Table 4).
Table 4.
Distribution of SF-36 scale scores in patients referred to an overnight sleep study due to a clinical suspicion of obstructive sleep apnea (sleep study group) compared to the general population sample.
| N | Sleep study group | General population | P ** |
|---|---|---|---|
| 418 | 5000 | ||
| SF-36 scale* | Mean (SD) | Mean (SD) | |
| Physical functioning (PF) | 80.1 (19.6) | 87.1 (19.5) | <0.001 |
| Role-physical (RP) | 53.6 (40.8) | 77.4 (36.9) | <0.001 |
| Bodily pain (BP) | 61.5 (28.3) | 74.0 (25.6) | <0.001 |
| General health (GH) | 59.9 (24.0) | 75.4 (21.7) | <0.001 |
| Vitality (VT) | 37.3 (22.4) | 61.1 (20.4) | <0.001 |
| Social functioning (SF) | 72.1 (26.5) | 86.8 (20.7) | <0.001 |
| Role emotional (RE) | 68.7 (38.5) | 84.9 (31.0) | <0.001 |
| Mental health (MH) | 71.8 (18.1) | 80.4 (15.4) | <0.001 |
| Physical component summary*** | 43.7 (10.6) | 49.4 (10.2) | <0.001 |
| Mental component summary*** | 45.6 (10.9) | 52.3 (9.0) | <0.001 |
*Scored on a 0–100 scale.
**Wilcoxon-Mann-Whitney test.
***Standardized for comparison with a U.S. general population with mean 50 (SD 10).
After adjustment for gender, education, age per 10 years and BMI, participants in the sleep study group scored lower than the general population on the physical functioning, vitality, PCS and MCS scales, with the largest difference found on the vitality scale (unstandardized beta coefficient of −23.1 (CI 95% −25.4 to −20.9) p < 0.001) (Table 5). All variables used for adjustment, except BMI for the MCS scale, showed associations with the assessed domains.
Table 5.
Coefficients and 95% confidence intervals (95% CI) for SF-36 scales in participants with suspected obstructive sleep apnea (sleep study group; N = 410) compared to the general population sample (N = 4875).
| Physical functioning (N = 5285) | Vitality (N = 5285) | Physical component summary (N = 5285) | Mental component summary (N = 5285) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coef.* | 95% CI | P | Coef.* | 95% CI | P | Coef.* | 95% CI | P | Coef.* | 95% CI | P | |
| Cohort | ||||||||||||
| General population | 0 | |||||||||||
| Clinical suspicion of OSA | −4.7 | −6.7–−2.7 | <0.001 | −23.1 | −25.4–−20.9 | <0.001 | −4.5 | −5.6–−3.5 | <0.001 | −7.0 | −8.1–−5.8 | <0.001 |
| Gender | ||||||||||||
| Female | −4.8 | −5.7–−3.9 | <0.001 | −5.6 | −6.7–−4.4 | <0.001 | −2.1 | −2.6–−1.6 | <0.001 | −1.3 | −1.8–−0.8 | <0.001 |
| Education | ||||||||||||
| Schooling up to 10 years | 0 | |||||||||||
| Schooling 11–13 years | 6.3 | 4.4–8.1 | <0.001 | 3.6 | 1.7–5.5 | <0.001 | 2.5 | 1.6–3.3 | <0.001 | 1.8 | 0.9–2.6 | <0.001 |
| University or college degree | 10.7 | 8.9–12.5 | <0.001 | 5.7 | 3.7–7.7 | <0.001 | 4.7 | 3.8–5.6 | <0.001 | 2.3 | 1.4–3.2 | <0.001 |
| Age per 10 years | −4.4 | −4.7–−4.0 | <0.001 | 0.7 | 0.3–1.1 | <0.001 | −2.1 | −2.2–−1.9 | <0.001 | 0.6 | 0.5–0.8 | <0.001 |
| Body mass index, kg/m2 | −0.7 | −0.8–−0.5 | <0.001 | −0.4 | −0.5–−0.2 | <0.001 | −0.3 | −0.4–−0.3 | <0.001 | 0.02 | −0.05–−0.1 | 0.53 |
Multivariable linear regression adjusted for gender, education, age and body mass index.
*Unstandardized beta coefficient.
Discussion
Principal findings
In this study of patients with suspected OSA referred to an overnight sleep study, we could not show an overall difference in SF-36 scale scores according to OSA severity after adjusting for confounders. However, when comparing participants with OSA to those with normal findings, increasing age was associated with higher SF-36 scores for the domains of vitality and MCS, while the presence of anxiety or depression was associated with lower scores in these domains. ESS > 10 was also associated with lower vitality scale scores.
The lack of a dose response relationship between HS and OSA severity found in this study is in line with previous studies assessing OSA severity and HS29,30, although associations between OSA severity and certain SF-36 domains have also been found in both clinical and non-clinical populations12–14. In a population-based study, mild OSA was related to lower scores on the vitality scale, while more severe OSA was more broadly associated with reduced HS10.
Unadjusted analysis of the sleep study group showed that patients with OSA had higher SF-36 scores for both the mental health scale and MCS scores compared to patients with normal findings. This supports findings from other studies where patients with severe OSA reported higher mental health scale scores than normative SF-36 data10, and less depressive symptoms than patients with normal findings31. However, this finding is not universal across studies, as other studies have reported lower mental health scale scores for patients with OSA than normal controls28,32. These inconsistencies may be related to differences in sample composition, investigation methods, and varying adjustments of potential confounding variables such as age, BMI, and comorbid medical conditions.
All SF-36 scale scores in the sleep study group were lower compared to the general population sample, both unadjusted and when controlled for age, gender, education and BMI. The largest difference was found on the vitality scale, which may be explained by this domain’s responsiveness to sleep disruption19. Lower HS in OSA patients compared to general population samples in several SF-36 domains are previously documented33,34, with some of them also showing significant improvements in HS after treatment with continuous positive airway pressure (CPAP) therapy, with most obvious improvements in those with severe OSA34,35. However, the present study was a cross-sectional study, and none had CPAP therapy.
Strengths of the study
The present study included a relatively large number of patients referred to an overnight sleep study with suspected OSA and could be compared to the SF-36 scores of a general population sample directly. Some previous studies assessing HS in OSA patients have similarly included consecutive patients referred to a sleep study15,27, while others have used a selection of OSA patients9,18 or investigated general population samples10,12. To our knowledge, there are no prior studies assessing general HS in a Norwegian clinical OSA population in relation to general population norms. Data from the general population sample used in this study was collected at about the same time and had similar age and smoking status as the sleep study group. Increasing age and smoking are both associated with OSA36,37.
Limitations of the study
Participant selection
One main limitation of the present study was the recruitment process of participants. The intention of the study was to include all consecutive patients referred to an overnight sleep study. Recruitment to the study was difficult to accomplish during periods of the year and varied over time, possibly leading to a recruitment bias. This non-consecutive sampling may have resulted in a sampling bias, and consequently question the representativeness of the sample and generalizability of the findings. In spite of adjustment for important demographic characteristics, findings from this study, based on a selected clinical population referred to sleep study due to clinical symptoms of OSA, cannot fairly be compared with a general population sample. For example, the selected clinical population will naturally have a higher prevalence of OSA than a random general population sample.
Sleep study recordings and scoring
Another limitation is the difference in measurement methods of OSA. More than 80% of the sleep studies were performed using PG, which may underestimate the AHI compared to a PSG with both respiratory and EEG parameters. In addition, the recordings were performed using only a thermistor, which may have affected correct identification of respiratory events. According to latest scoring guidelines, a thermistor is recommended for identifying apneas and a nasal pressure transducer for identifying hypopneas38. The 1999 American Academy of Sleep Medicine (AASM) scoring rules used in this study are more liberal than current criteria, as they do not necessarily distinguish obstructive apneas from hypopneas, and do not require a minimum fall in oxygen desaturation or arousal in order to score respiratory events26. Thus, the chosen recording and scoring methods make comparison of results with studies using current AASM guidelines difficult.
Excessive daytime sleepiness and comorbidities
Comparison of ESS scores and presence of comorbidities between the sleep study group and general population would have been advantageous, as EDS and comorbid illnesses such as hypertension and depression are associated with lower HS in OSA patients18,27, but neither ESS scores nor comparable information regarding comorbidities were available for the general population sample.
Clinical implications
Although OSA severity was not associated with impaired HS, patients in the sleep study group had lower HS than the general population sample. This indicates that the impairment in HS experienced by OSA patients may be caused by factors not necessarily related to OSA severity as currently defined by the AHI. For example, this may be related to sleep problems or reduced sleep quality in general, as sleep quality broadly influences general HS18, and the vitality domain is associated with sleep disruption in OSA patients19. Similarly, persons reporting poor sleep quality due to other sleep disorders, e.g. RLS and central disorders of hypersomnolence, also have lower general HS compared to general population samples39,40. For this reason, clinicians should also consider other sleep disorders as well as physical and mental conditions when assessing these patients.
The findings also serve as a reminder that the patients being referred for a sleep study are heterogeneous and present with a number of complaints or unspecific symptoms, which may lead to reduced HS independent of OSA or not. The unspecific nature of the symptoms makes screening for OSA difficult, and there is currently uncertainty about the accuracy or clinical utility of all potential screening tools41.
Conclusion
The present study has affirmed the lack of association between OSA severity and general HS, as measured by the SF-36 instrument. In contrast, compared to a general population sample, the general HS of Norwegian patients referred to an overnight sleep study due to clinical suspicion of OSA was impaired, with greatest differences in the vitality domain. These findings suggest that this difference may not necessarily be due to the presence of OSA, but may reflect the unspecific nature of symptoms in OSA compared to other sleep disorders or other confounding factors.
Acknowledgements
The data used in this publication is from the survey “Level of living 2002 - Cross sectional study - Health”. Statistics Norway (SSB) was responsible for the data collection and the survey was financed by SSB, Norwegian Institute of Public Health, and Department of Community Medicine, University of Oslo. The data has been prepared and delivered in anonymized form by Norwegian Centre for Research Data AS (NSD). Neither SSB, Norwegian Institute of Public Health, Department of Community Medicine nor NSD are responsible for the data analysis or the interpretations of the data in this study.
Author Contributions
K.K.B. and K.S. conceived and designed the study as well as performed statistical analyses. K.K.B. wrote the manuscript and K.S. critically revised the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.American Academy of Sleep Medicine. International classification of sleep disorders (American Academy of Sleep Medicine, 2014).
- 2.Heinzer R, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. The Lancet. Respiratory medicine. 2015;3:310–318. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peppard PE, et al. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013;177:1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mukherjee S, et al. An Official American Thoracic Society Statement: The Importance of Healthy Sleep. Recommendations and Future Priorities. Am. J. Respir. Crit. Care Med. 2015;191:1450–1458. doi: 10.1164/rccm.201504-0767ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Thoracic Society/AmericanSleep Disorders Association Statement on health outcomes research in sleep apnea, Am. J. Respir. Crit. Care Med. 1998;157:335–341. doi: 10.1164/ajrccm.157.1.ats1-9. [DOI] [PubMed] [Google Scholar]
- 6.Bergner M. Measurement of health status. Med. Care. 1985;23:696–704. doi: 10.1097/00005650-198505000-00028. [DOI] [PubMed] [Google Scholar]
- 7.Elberg Dengso K., Hillingso J., Marcussen A. M. & Thomsen T. Health-related quality of life and anxiety and depression in patients diagnosed with cholangiocarcinoma: a prospective cohort study, Acta Oncol. 1–7 (2016). [DOI] [PubMed]
- 8.Ware J. E., Snow K. K. & Kosinski M. SF-36 Health Survey Manual and Interpretation Guide (New England Medical Center, The Health Institute, 1993).
- 9.D’Ambrosio C, Bowman T, Mohsenin V. Quality of life in patients with obstructive sleep apnea: effect of nasal continuous positive airway pressure–a prospective study. Chest. 1999;115:123–129. doi: 10.1378/chest.115.1.123. [DOI] [PubMed] [Google Scholar]
- 10.Baldwin CM, et al. The association of sleep-disordered breathing and sleep symptoms with quality of life in the Sleep Heart Health Study. Sleep. 2001;24:96–105. doi: 10.1093/sleep/24.1.96. [DOI] [PubMed] [Google Scholar]
- 11.Bennett LS, Barbour C, Langford B, Stradling JR, Davies RJ. Health status in obstructive sleep apnea: relationship with sleep fragmentation and daytine sleepiness, and effects of continuous positive airway pressure treatment. Am. J. Respir. Crit. Care Med. 1999;159:1884–1890. doi: 10.1164/ajrccm.159.6.9808107. [DOI] [PubMed] [Google Scholar]
- 12.Finn L, Young T, Palta M, Fryback DG. Sleep-disordered breathing and self-reported general health status in the Wisconsin Sleep Cohort Study. Sleep. 1998;21:701–706. [PubMed] [Google Scholar]
- 13.Gall R, Isaac L, Kryger M. Quality of life in mild obstructive sleep apnea. Sleep. 1993;16:S59–61. doi: 10.1093/sleep/16.suppl_8.S59. [DOI] [PubMed] [Google Scholar]
- 14.Yang EH, et al. Sleep apnea and quality of life. Sleep. 2000;23:535–541. doi: 10.1093/sleep/23.4.1g. [DOI] [PubMed] [Google Scholar]
- 15.Iacono Isidoro S, et al. Quality of life in patients at first time visit for sleep disorders of breathing at a sleep centre. Health and quality of life outcomes. 2013;11:207. doi: 10.1186/1477-7525-11-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee W, Lee SA, Ryu HU, Chung YS, Kim WS. Quality of life in patients with obstructive sleep apnea: Relationship with daytime sleepiness, sleep quality, depression, and apnea severity, Chron. Respir. Dis. 2016;13:33–39. doi: 10.1177/1479972315606312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weaver EM, Woodson BT, Steward DL. Polysomnography indexes are discordant with quality of life, symptoms, and reaction times in sleep apnea patients. Otolaryngol. Head Neck Surg. 2005;132:255–262. doi: 10.1016/j.otohns.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Briones B, et al. Relationship between sleepiness and general health status. Sleep. 1996;19:583–588. doi: 10.1093/sleep/19.7.583. [DOI] [PubMed] [Google Scholar]
- 19.Smith IE, Shneerson JM. Is the SF 36 sensitive to sleep disruption? A study in subjects with sleep apnoea. J. Sleep Res. 1995;4:183–188. doi: 10.1111/j.1365-2869.1995.tb00167.x. [DOI] [PubMed] [Google Scholar]
- 20.Hrubos-Strom H, et al. A Norwegian population-based study on the risk and prevalence of obstructive sleep apnea. The Akershus Sleep Apnea Project (ASAP) J. Sleep Res. 2011;20:162–170. doi: 10.1111/j.1365-2869.2010.00861.x. [DOI] [PubMed] [Google Scholar]
- 21.Stavem K, Kjelsberg FN, Ruud EA. Reliability and validity of the Norwegian version of the Functional Outcomes of Sleep Questionnaire. Qual. Life Res. 2004;13:541–549. doi: 10.1023/B:QURE.0000018490.43009.bf. [DOI] [PubMed] [Google Scholar]
- 22.Hougen H. & Gløboden M. Samordnet levekårsundersøkelse 2002 - tverrsnittsundersøkelsen. https://www.ssb.no/sosiale-forhold-og-kriminalitet/artikler-og-publikasjoner/samordnet-levekaarsundersokelse-2002-tverrsnittsundersokelsen (2004).
- 23.Loge JH, Kaasa S, Hjermstad MJ, Kvien TK. Translation and performance of the Norwegian SF-36 Health Survey in patients with rheumatoid arthritis. I. Data quality, scaling assumptions, reliability, and construct validity. J. Clin. Epidemiol. 1998;51:1069–1076. doi: 10.1016/S0895-4356(98)00098-5. [DOI] [PubMed] [Google Scholar]
- 24.Beiske KK, Kjelsberg FN, Ruud EA, Stavem K. Reliability and validity of a Norwegian version of the Epworth sleepiness scale. Sleep & Breath. 2009;13:65–72. doi: 10.1007/s11325-008-0202-x. [DOI] [PubMed] [Google Scholar]
- 25.Ware J. E., Kosinski M, Keller SD. SF-36 Physical and Mental Health Summary Scales: A User’s Manual (The Health Institute, 1994).
- 26.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force, Sleep. 22, 667–689 (1999). [PubMed]
- 27.Bulcun E, Ekici A, Ekici M. Quality of life and metabolic disorders in patients with obstructive sleep apnea. Clin. Invest. Med. 2012;35:E105–113. doi: 10.25011/cim.v35i2.16294. [DOI] [PubMed] [Google Scholar]
- 28.Akashiba T, et al. Relationship between quality of life and mood or depression in patients with severe obstructive sleep apnea syndrome. Chest. 2002;122:861–865. doi: 10.1378/chest.122.3.861. [DOI] [PubMed] [Google Scholar]
- 29.Kim H., et al. Association of Mild Obstructive Sleep Apnea With Cognitive Performance, Excessive Daytime Sleepiness, and Quality of Life in the General Population: The Korean Genome and Epidemiology Study (KoGES), Sleep. 40 (2017). [DOI] [PubMed]
- 30.Gulbay BE, et al. Health-related quality of life in patients with sleep-related breathing disorders: relationship with nocturnal parameters, daytime symptoms and comorbid diseases. Respiration. 2008;75:393–401. doi: 10.1159/000104865. [DOI] [PubMed] [Google Scholar]
- 31.Bjorvatn B., Rajakulendren N., Lehmann S., Pallesen S. Increased severity of obstructive sleep apnea is associated with less anxiety and depression, J. Sleep Res. (2017). [DOI] [PubMed]
- 32.Sforza E, Janssens JP, Rochat T, Ibanez V. Determinants of altered quality of life in patients with sleep-related breathing disorders. Eur. Respir. J. 2003;21:682–687. doi: 10.1183/09031936.03.00087303. [DOI] [PubMed] [Google Scholar]
- 33.Bjornsdottir E, et al. Quality of life among untreated sleep apnea patients compared with the general population and changes after treatment with positive airway pressure. J. Sleep Res. 2015;24:328–338. doi: 10.1111/jsr.12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsara V, Kaimakamis E, Serasli E, Katsarou Z, Christaki P. Health related quality of life in Greek patients with sleep apnea-hypopnea syndrome treated with continuous positive airway pressure. Sleep Med. 2009;10:217–225. doi: 10.1016/j.sleep.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Avlonitou E, Kapsimalis F, Varouchakis G, Vardavas CI, Behrakis P. Adherence to CPAP therapy improves quality of life and reduces symptoms among obstructive sleep apnea syndrome patients. Sleep & Breath. 2012;16:563–569. doi: 10.1007/s11325-011-0543-8. [DOI] [PubMed] [Google Scholar]
- 36.Varol Y, Anar C, Tuzel OE, Guclu SZ, Ucar ZZ. The impact of active and former smoking on the severity of obstructive sleep apnea. Sleep & Breath. 2015;19:1279–1284. doi: 10.1007/s11325-015-1159-1. [DOI] [PubMed] [Google Scholar]
- 37.Young T, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N. Engl. J. Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 38.Berry R. B. et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications (American Academy of Sleep Medicine, 2017).
- 39.Kushida C, et al. Burden of restless legs syndrome on health-related quality of life. Qual. Life Res. 2007;16:617–624. doi: 10.1007/s11136-006-9142-8. [DOI] [PubMed] [Google Scholar]
- 40.Ozaki A, et al. Quality of life in patients with narcolepsy with cataplexy, narcolepsy without cataplexy, and idiopathic hypersomnia without long sleep time: comparison between patients on psychostimulants, drug-naive patients and the general Japanese population. Sleep Med. 2012;13:200–206. doi: 10.1016/j.sleep.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 41.Jonas DE, et al. Screening for Obstructive Sleep Apnea in Adults: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2017;317:415–433. doi: 10.1001/jama.2016.19635. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The patient data analysed during the current study are not publicly available due to ethical and legal constraints, but anonymized data are available from the corresponding author on reasonable request.
The general population data that support the findings of this study are available from Norwegian Centre for Research Data (NSD) but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of NSD.