Abstract
Night shift work is associated with cardiovascular disease and central nervous system disorders in female nurses. Brain-derived neurotrophic factor (BDNF) exerts protective effects on neural and endothelial functions. This study examined the association between serum BDNF levels and pulse pressure after rest in female nurses working night shifts. In this study, blood samples were collected for BDNF measurement after a night shift when nurses had been working night shifts for three continuous weeks. Blood pressure was assessed before and after a one-hour morning rest within a week of resuming the night shift after one month without any night shift work. The pulse pressure of nurses (n = 48, age 29 ± 5 years) was significantly reduced (from 43 ± 7 to 41 ± 6 mmHg, P = 0.003) after rest, and serum BDNF were significantly and inversely correlated with pulse pressure changes (r = −0.435, P = 0.002). Higher serum BDNF was an independent factor for greater reduction in pulse pressure (95%CI = −0.609 ‒ −0.174, P = 0.001). Using a receiver operating characteristic curve analysis, serum BDNF >20.6 ng/mL predicted a pulse pressure reduction after a one-hour rest (sensitivity 66.7%, specificity 77.8%). In conclusion, higher serum BDNF predicted greater recovery of pulse pressure after a one-hour rest in female nurses after night shift work.
Introduction
Night shift work is inevitable for nurses caring for inpatients. Pooled data from the Nurses’ Health Study (NHS) I and NHS II show that approximately 60% of female nurses had experienced night shift work1. According to the data from 39 hospitals in Taiwan, approximately 40% of female nurses had performed night shifts for more than 10% of their total work time2. In other reports from the NHS I, night shift work was significantly associated with coronary heart disease and ischemic stroke in female nurses3,4. Night shift work was recently reported to be significantly associated with all-cause mortality and cardiovascular mortality in female nurses who were followed for 22 years5.
Rotating shift work is also associated with the central nervous system disorders6,7. In one mouse study, the disturbance of circadian rhythms induced by exposure to light at night caused a depressive response8. Brain-derived neurotrophic factor (BDNF), a member of the neurotrophin family, plays important roles in protecting neurons and synaptic activity9. A reduction in circulating BDNF level is associated with depressive symptoms10–12, and serum BDNF levels can reflect an improvement in depressive symptoms after lifestyle interventions13,14 or pharmacological treatments15,16.
Notably, a low serum BDNF level is associated with forearm vascular resistance17. In one recent cross-sectional study, lower circulating BDNF concentrations were reported to be associated with coronary artery disease18; in a prospective study, a lower BDNF concentration was reported to predict coronary heart disease and higher all-cause mortality19. Therefore, insufficient BDNF levels may play an important role in linking central nervous system disorders and arterial stiffness20.
Pulse pressure, the difference between systolic and diastolic blood pressures, can reflect the severity of arterial stiffening21, and a higher pulse pressure predicts a higher cardiovascular mortality rate22,23. According to studies that stratified by age, pulse pressure is a more powerful predictor of cardiovascular mortality in young subjects than in older subjects24,25. Furthermore, long-term reduction in pulse pressure is significantly associated with survival26. A short-term reduction in pulse pressure from peak exercise to rest is predictive of survival in subjects with normal myocardial perfusion imaging27, or after hemodialysis in patients with end-stage renal disease28.
Based on psychological stress, delayed recovery of systolic blood pressure after 45 minutes might predict the carotid intima-media thickness and hypertension29,30. According to Mathiassen et al.31 a delayed recovery of blood pressure increased by physical work was observed after a one-hour rest period. In the present study, we examined the relationship between serum BDNF levels and changes in pulse pressure after a one-hour rest period in female nurses working night shifts.
Methods
Subjects
This prospective observational study was conducted at Taichung Veterans General Hospital, Taiwan. The included subjects were female nursing staff members aged ≥20 years who worked in wards with a monthly rotating shift pattern. The exclusion criteria were (1) working night shifts at the time of the screening, (2) known diabetes mellitus, (3) established cardiovascular diseases, (4) current severe infection or inflammation, (5) a history of malignancy, (6) a history of psychological or mood disorder, (7) a history of substance or alcohol abuse, and (8) post-menopause or pregnancy. The study complied with the Declaration of Helsinki. All experimental protocols were approved by the Institutional Review Board of Taichung Veterans General Hospital and the written informed consent was obtained from all subjects. The methods were carried out in accordance with the approved guidelines.
Study procedures
The aim of this study was to determine whether serum BDNF levels in female nurses who had worked a stable night shift predicted pulse pressure after a one-hour rest period when they rotated to the next night shift. The study subjects were screened when they were just before they rotated to night shift work for the following month. During the first night shift month, a three-week observation period confirmed that the subjects were performing their night shifts in a stable pattern. In the fourth week, the subjects were scheduled for a morning appointment after overnight fasting. Blood samples were collected to measure fasting glucose, insulin, lipid profile, serum creatinine, C-reactive protein (CRP) and BDNF levels after anthropometric measurements were obtained.
After the first night shift month, the subjects underwent a wash-out period during which they did not perform night shift work for a month. The participants were scheduled for another morning visit during the first week of their rotation to a second month of night shift work after the one-month wash-out period. During this visit, blood pressure was measured at the right brachial artery, and the mean of two separate measurements with a interval of 1 min was recorded after the subjects had rested in a lying position for 10 minutes in the morning following the end of the night shift. Blood samples were not collected during this visit to avoid potential impacts of any invasive procedures on the blood pressure measurements. Then, the subjects were asked to take a nap on the same bed for one hour. After the nap, blood pressure was measured again.
Biochemical analyses
Glucose levels were determined using an oxidase‒peroxidase method (Wako Diagnostics, Tokyo, Japan), and insulin levels were determined using a commercial immunoassay (Roche Diagnostics, Indianapolis, USA). Serum levels of creatinine and lipids were measured using commercial kits (Beckman Coulter, Fullerton, USA), and CRP levels were measured using an immunochemical assay with purified Duck IgY (∆Fc) antibodies (Good Biotech Corp., Taichung, Taiwan). Serum human BDNF levels was measured using an enzyme immunoassay method (R&D Systems, Minneapolis, USA). The intra‒ and inter‒ assay coefficients of variation for BDNF measurements were 4.1% and 9.0%, respectively. The sensitivity of the BDNF measurement was 0.02 ng/mL. The formula [fasting insulin (μU/mL) × fasting glucose (mmol/L)]/22.5 was used to calculate the homeostasis model assessment for insulin resistance (HOMA-IR) index32. The estimated glomerular filtration rate (eGFR) was calculated as 186 × [serum creatinine (mg/dL)]−1.154 × [age (years)]−0.203 × 0.742 based on the Modification of Diet in Renal Disease (MDRD) equation33.
Statistical analysis
Continuous variables are presented as the means ± standard deviations (SD). An independent samples t-test was used to detect significant differences between two groups. A paired t-test was used to detect significant differences in blood pressure before and after the one-hour rest. The correlation between serum BDNF levels and changes in the pulse pressure was determined using Pearson’s correlation coefficient. A multivariate linear regression analysis was used to assess the association between the changes in pulse pressure and serum BDNF levels. Statistical analyses were performed using SPSS version 22.0 software (International Business Machines Corp, New York, USA).
Results
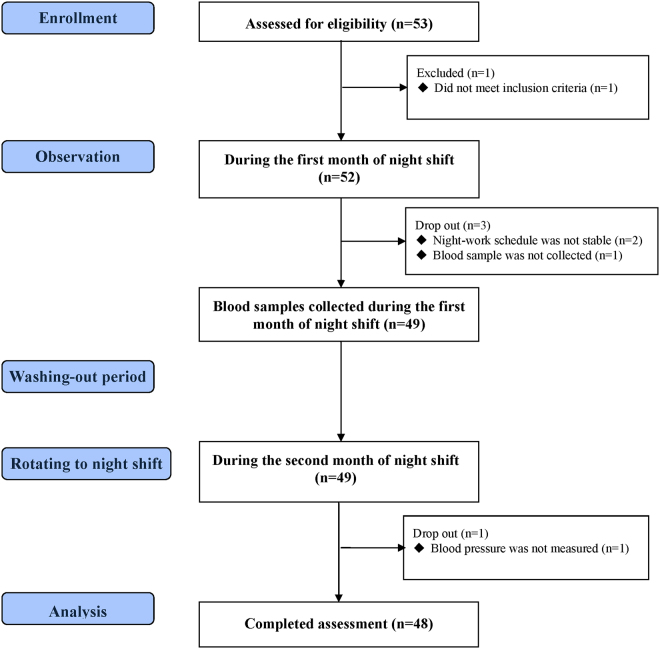
A total of 53 female nurses were screened, of whom 48 completed the study assessments (Fig. 1). Table 1 shows the baseline clinical characteristics of the study subjects during the first night shift month, measured after they had performed night shift work for three weeks. The mean age of the subjects was 29 ± 5 years, and the mean serum BDNF was 21.6 ± 6.2 ng/mL. After rotating back to night shifts following a wash-out month, the subjects showed a significant reduction in the mean systolic pressure (112 ± 9 mmHg vs. 109 ± 9 mmHg, P = 0.002) after a one-hour rest period but not a significant change in the mean diastolic pressure (69 ± 7 mmHg vs. 68 ± 7 mmHg, P = 0.766). Pulse pressure (43 ± 7 mmHg vs. 41 ± 6 mmHg, P = 0.003) was also significantly reduced after rest (Fig. 2). Serum BDNF levels during the first night shift month were significantly and inversely correlated with the change in pulse pressure (correlation coefficient of −0.435, P = 0.002; Fig. 3).
Figure 1.
Flow diagram of the enrollment of study subjects.
Table 1.
Clinical data of subjects (n = 48) at baseline.
| Mean ± SD | |
|---|---|
| Age (year) | 29 ± 5 |
| BMI (kg/m2) | 22.5 ± 3.6 |
| Waist circumference (cm) | 76.2 ± 11.1 |
| Fasting glucose (mmol/L) | 4.5 ± 0.5 |
| Fasting insulin (µIU/mL) | 7.2 ± 4.9 |
| HOMA-IR | 1.5 ± 1.1 |
| Total cholesterol (mmol/L) | 4.4 ± 0.6 |
| HDL cholesterol (mmol/L) | 1.7 ± 0.4 |
| Triglyceride (mmol/L) | 0.7 ± 0.4 |
| AST (U/L) | 18 ± 6 |
| ALT (U/L) | 14 ± 8 |
| eGFR (mL/min/1.73 m2) | 118 ± 18 |
| TSH (μU/mL) | 1.5 ± 0.8 |
| CRP (mg/L) | 1.1 ± 1.8 |
| BDNF (ng/mL) | 21.6 ± 6.2 |
| Work experience (month) | 71 ± 62 |
| Night-shift work experience (month)* | 63 ± 62 |
| Frequency of alcohol consumption | |
| <1 time/month, N (%) | 26 (54.2%) |
| ≥1 time/month and <1 time/week, N (%) | 17 (35.4%) |
| ≥1 time/week, N (%) | 5 (10.4%) |
| Frequency of coffee consumption | |
| <1 time/day, N (%) | 28 (58.3%) |
| ≥1 time/day, N (%) | 20 (41.7%) |
| Smoking history, N (%) | 0 (0%) |
| Use of sedatives in the past 3 months, N (%) | 2 (4.2%) |
| Use of anti-hypertensive drugs in the past 3 months, N (%) | 0 (0%) |
| Exercise | |
| <150 min/week, N (%) | 46 (95.8%) |
| ≥150 min/week, N (%) | 2 (4.2%) |
AST = aspartate aminotransferase, ATL = alanine aminotransferase, BDNF = brain-derived neurotrophic factor, BMI = body mass index, CRP = C-reactive protein, eGFR = estimated glomerular filtration rate, HDL = high-density lipoprotein, HOMA-IR = homeostasis model assessment of insulin resistance, TSH = thyroid stimulating hormone.
*Night-shift work experience refers to the total work experience after having worked the night shift.
Figure 2.
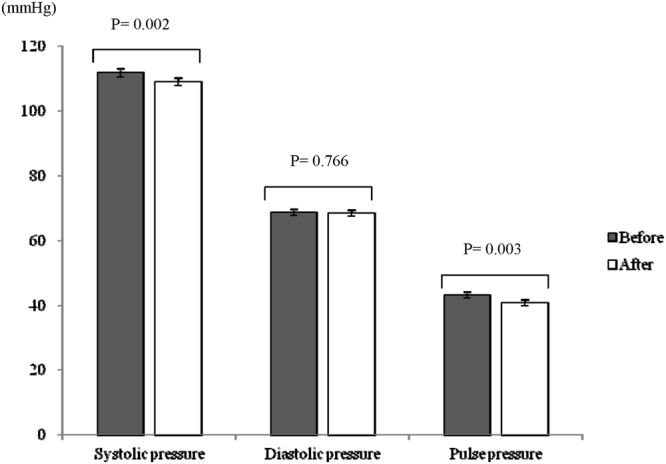
Blood pressure measured before and after a one-hour rest in subjects within a week of starting a new period of night-shift work.
Figure 3.
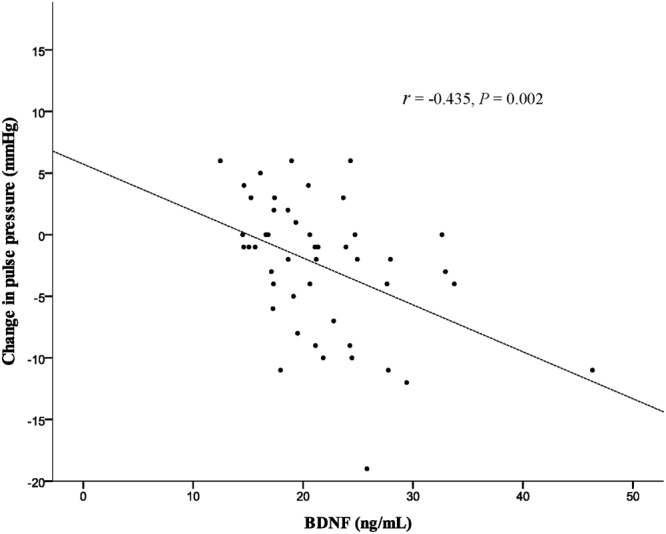
A significant inverse correlation is demonstrated between serum BDNF levels and changes in pulse pressure (r = correlation coefficient).
According to the changes in pulse pressure after a one-hour rest, we divided the subjects into a pulse-pressure (P-P) reduction group (n = 30) and a no P-P reduction group (n = 18). Table 2 shows that serum BDNF was significantly higher in the P-P reduction group than in the no P-P reduction group (23.1 ± 6.6 ng/mL vs. 19.1 ± 4.8 ng/mL, P = 0.032). Pre-rest diastolic pressure in the P-P reduction group was significantly lower than in the no P-P reduction group (67 ± 5 mmHg vs. 71 ± 8 mmHg, P = 0.017). Multivariate linear regression analyses performed after adjustment for age, body mass index and pre-rest diastolic blood pressure showed that higher serum BDNF was significantly associated with a greater reduction in pulse pressure after a one-hour rest (linear regression coefficient of −0.391, 95% confidence interval (CI) between −0.609 and −0.174; P = 0.001; Table 3).
Table 2.
The characteristics of subjects grouped according to pulse-pressure (P‒P) change.
| P‒P reduction (n = 30) | no P‒P reduction (n = 18) | P | |
|---|---|---|---|
| At baseline | |||
| Age (year) | 29 ± 5 | 29 ± 6 | 0.935 |
| BMI (kg/m2) | 22.1 ± 3.6 | 23.1 ± 3.8 | 0.383 |
| Waist circumference (cm) | 76.0 ± 11.3 | 76.6 ± 11.1 | 0.862 |
| Fasting glucose (mmol/L) | 4.5 ± 0.6 | 4.5 ± 0.4 | 0.666 |
| HOMA-IR | 1.6 ± 1.2 | 1.3 ± 0.6 | 0.368 |
| Total cholesterol (mmol/L) | 4.4 ± 0.6 | 4.2 ± 0.6 | 0.277 |
| HDL cholesterol (mmol/L) | 1.7 ± 0.4 | 1.6 ± 0.3 | 0.605 |
| Triglyceride (mmol/L) | 0.7 ± 0.4 | 0.6 ± 0.4 | 0.606 |
| AST (U/L) | 19 ± 8 | 17 ± 4 | 0.466 |
| ALT (U/L) | 14 ± 9 | 15 ± 7 | 0.862 |
| eGFR (mL/min/1.73 m2) | 119 ± 19 | 115 ± 17 | 0.465 |
| TSH (μU/mL) | 1.5 ± 0.8 | 1.5 ± 0.7 | 0.926 |
| CRP (mg/L)* | 1.1 ± 1.6 | 1.3 ± 2.0 | 0.482 |
| BDNF (ng/mL) | 23.1 ± 6.6 | 19.1 ± 4.8 | 0.032 |
| Work experience | |||
| Total (month) | 65 ± 54 | 81 ± 75 | 0.402 |
| Night shift (month)# | 58 ± 55 | 70 ± 74 | 0.527 |
| Frequency of alcohol consumption | 0.454 | ||
| <1 time/month, N (%) | 18(60.0%) | 8(44.4%) | |
| ≥1 time/month, N (%) | 12(40.0%) | 10(55.6%) | |
| Frequency of coffee consumption | 0.545 | ||
| <1 time/day, N (%) | 19(63.0%) | 9(50.0%) | |
| ≥1 time/day, N (%) | 11(36.7%) | 9(50.0%) | |
| Rotating to night shift after wash-out period | |||
| Blood pressure before rest | |||
| Systolic pressure (mmHg) | 111 ± 8 | 113 ± 11 | 0.666 |
| Diastolic pressure (mmHg) | 67 ± 5 | 71 ± 8 | 0.017 |
| Pulse pressure (mmHg) | 45 ± 7 | 41 ± 6 | 0.082 |
| Blood pressure after rest | |||
| Systolic pressure (mmHg) | 108 ± 9 | 111 ± 9 | 0.178 |
| Diastolic pressure (mmHg) | 69 ± 6 | 68 ± 7 | 0.635 |
| Pulse pressure (mmHg) | 39 ± 6 | 44 ± 6 | 0.013 |
| Heart rate | |||
| Before rest (min−1) | 70 ± 10 | 71 ± 11 | 0.775 |
| After rest (min−1) | 69 ± 11 | 68 ± 10 | 0.611 |
ATL = alanine aminotransferase, AST = aspartate aminotransferase, BDNF = brain-derived neurotrophic factor, BMI = body mass index, eGFR = estimated glomerular filtration rate, HDL = high-density lipoprotein, HOMA-IR = homeostasis model assessment of insulin resistance, P‒P reduction = pulse‒pressure reduction, TSH = thyroid stimulating hormone.
*CRP was logarithm-transformed (log) in analyses due to skewed distribution.
#Night-shift work experience refers to the total work experience after having worked the night shift.
Table 3.
Multivariate regression analysis showing an independent association between serum BDNF and pulse-pressure change.
| Crude | Model 1 | Model 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B | 95%CI | P | B | 95%CI | P | B | 95%CI | P | |
| BDNF (ng/mL) | −0.381 | (−0.615, −0.147) | 0.002 | −0.375 | (−0.611, −0.140) | 0.002 | −0.391 | (−0.609, −0.174) | 0.001 |
| Age (years) | 0.197 | (−0.102, 0.496) | 0.190 | 0.219 | (−0.057, 0.495) | 0.118 | |||
| BMI (kg/m2) | 0.012 | (−0.393, 0.417) | 0.953 | −0.285 | (−0.710, 0.140) | 0.183 | |||
| Diastolic blood pressure (mmHg) | 0.343 | (0.109, 0.576) | 0.005 | ||||||
B = linear regression coefficient, BDNF = brain-derived neurotrophic factor, BMI = body mass index, CI = confidence interval.
Model 1: adjusted for age and BMI; model 2: adjusted for age, BMI and diastolic blood pressure before taking a nap.
Based on the receiver operating characteristic (ROC) curve analysis, a cut-off value of 20.6 ng/mL for serum BDNF levels provided a sensitivity of 66.7% and specificity of 77.8% for differentiating a reduction in pulse pressure after a one-hour rest in nurses performing night shift work (Fig. 4).
Figure 4.
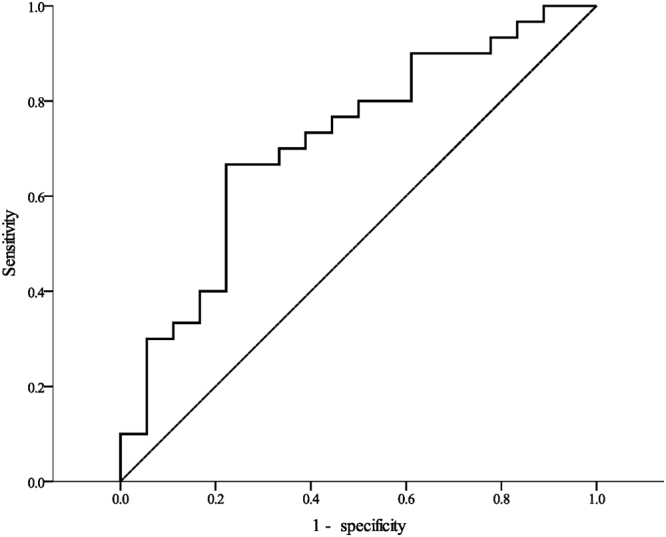
Receiver operating characteristic (ROC) analysis curve for serum BDNF level to differentiate a reduction in pulse pressure after a one-hour rest (area under the curve 0.715, 95%CI = 0.563–0.867).
Discussion
The main finding in our study was that higher levels of serum BDNF levels in female nurses during night shift work predicted a greater reduction in pulse pressure after a short morning rest when rotating back to night shift work. Arterial stiffening describes the loss of the arterial wall’s elastic capacity to buffer the blood flow from ventricular ejection34. Widening pulse pressure, resulting from a surge of systolic pressure against a non-compromised arterial wall followed by a low diastolic pressure, reflects the severity of arterial stiffness35. Arterial stiffness may involve not only the extracellular destruction of the vessel wall36,37, but also dysfunction of vascular smooth muscle cells38. Tropomyosin-related kinase receptor B (TrKB), a high-affinity receptor for BDNF, is expressed in the vascular smooth muscles39,40. Inactivation of the BDNF-TrkB signaling pathway in vascular smooth muscle cells may impair coronary vascular integrity and function41. Following treatment with empagliflozin, a sodium glucose co-transporter 2 inhibitor, higher BDNF expression was associated with the reduction of coronary arterial fibrosis in a diabetic mouse model42.
Night shift work interrupts the circadian rhythm and may impair vascular functions controlled by autonomic activity43. Increased arterial stiffness with decreased vascular activity, estimated using the reactive hyperemia index, has been found in subjects currently performing night shift work when compared with those not currently working night shifts44. Although the high blood pressure experienced during the work period normally decreases after a rest period, a delayed recovery of the high blood pressure during rest periods is more common in female nurses working night shifts than in those working day shifts45. High blood pressure may be the result of circadian disruption when rotating to night shift work. Kubo et al.46 also reported a reduction in the coronary flow reserve of the left anterior descending artery in female nurses the morning after working a night shift.
Regular coffee consumption increases BDNF expression in the in the mouse hippocampus47; however, the change in circulating BDNF was not reported. In the present study, coffee-consumption frequency did not significantly affect the serum BDNF (21.5 ± 5.3 ng/mL in the subjects who consumed coffee <1 time per month vs. 21.7 ± 7.5 ng/mL in the subjects who consumed coffee ≥1 time per month). Although the intake of a single-dose of coffee fruit extract was also reported to increase the circulating BDNF levels of healthy subjects within 120 min48, serum BDNF was assessed in a fasting state in the present study. This may explain why coffee consumption was not associated with serum BDNF levels in the present study. In contrast, it has been reported that arterial stiffness might be induced by either acute or chronic coffee consumption49–51. Souza et al.52 also reported that a single dose of coffee might increase post-exercise systolic blood pressure. The impact of coffee consumption on pulse pressure recovery after rest following a night shift should be further investigated.
We collected serum BDNF data in the morning after a night shift when the participants had worked the night shift for three weeks, and we measured the morning blood pressure after a night shift in female nurses who had rotated to a new series of night shifts within one week of the rotation. We found that high morning serum BDNF levels during stable night shift work could predict the quick recovery of a widening pulse pressure after a one-hour rest in the female nurses who were beginning a new night-shift schedule. However, the present study had certain limitations. First, the study only included young female nurses. Despite the delayed recovery of blood pressure in men performing night shift work53, the impact of shift work on mental health has been reported to differ between genders54. Therefore, our findings in female nurses cannot be applied men. In addition, our findings cannot be applied to postmenopausal women since a lower circulating BDNF concentration has been observed in postmenopausal females than in young females55,56. Second, menstrual cycle information was not recorded for analysis in the present study. An increase in circulating BDNF during the luteal phase compared with the follicular phase has been reported in woman with normal menstrual cycles56,57. Additionally, Dunne et al.58 reported lower blood pressure during the luteal phase than during the in follicular phase, and Mills et al.59 reported a difference in blood-pressure recovery after stress between the luteal phase and follicular phase. Therefore, the menstrual cycle could be a confounding factor in studies of young females. Third, we did not measure the serum BDNF levels when blood pressure was assessed because of the relationship between BDNF and vascular functions consistently reported in previous studies39–41,60–62. Fourth, the change in diastolic blood pressure was not statistically significant after rest. We did not further examine the underlying causes of differences in recovery between systolic and diastolic blood pressures. Finally, we did not explore the mechanism linking BDNF and pulse-pressure recovery after night shift work; moreover, we did not assess the long-term cardiovascular outcomes in subjects with high pulse pressures after a one-hour rest period.
In conclusion, the serum BDNF concentration measured during a stable period of night shift work predicted a reduction in pulse pressure in female nurses after one hour of rest when they were rotating to a new period of night shifts. These findings may indicate a possible modulatory effect of circulating BDNF on the recovery of pulse pressure in female nurses after they have worked rotating night shifts. Further studies are necessary to investigate the underlying mechanisms linking serum BDNF during night shift work to the long-term cardiovascular prognosis.
Acknowledgements
This work was supported by grants from Taichung Veterans General Hospital, Taichung, Taiwan (grant number TCVGH-1030104D, TCVGH-1063501E) and the Ministry of Science and Technology, Taiwan (grant number MOST 105-2314-B-075A-003). The statistical analysis was performed by the Biostatistics Task Force of Taichung Veterans General Hospital, Taichung, Taiwan. We thank the Nursing Department of Taichung Veterans General Hospital for their support.
Author Contributions
I.L., D.C. and W.H.S. contributed to the study design. I.L. and W.L. participated in collecting the data. I.L. participated in the analysis and interpretation of the data. I.L. drafted the manuscript. W.H.S. revised the manuscript. I.L. had full access to the data in the study. I.L. is the guarantor. All authors critically revised the manuscript for important intellectual content.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pan A, Schernhammer ES, Sun Q, Hu FB. Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med. 2011;8:e1001141. doi: 10.1371/journal.pmed.1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chin W, Guo YL, Hung YJ, Yang CY, Shiao JS. Short sleep duration is dose-dependently related to job strain and burnout in nurses: a cross sectional survey. Int J Nurs Stud. 2015;52:297–306. doi: 10.1016/j.ijnurstu.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Kawachi I, et al. Prospective study of shift work and risk of coronary heart disease in women. Circulation. 1995;92:3178–3182. doi: 10.1161/01.CIR.92.11.3178. [DOI] [PubMed] [Google Scholar]
- 4.Brown DL, et al. Rotating night shift work and the risk of ischemic stroke. Am J Epidemiol. 2009;169:1370–1377. doi: 10.1093/aje/kwp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu F, et al. Total and cause-specific mortality of U.S. nurses working rotating night shifts. Am J Prev Med. 2015;48:241–252. doi: 10.1016/j.amepre.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Togo, F., Yoshizaki, T. & Komatsu, T. Association between depressive symptoms and morningness-eveningness, sleep duration and rotating shift work in Japanese nurses. Chronobiol Int, 1–11, 10.1080/07420528.2016.1273942 (2017). [DOI] [PubMed]
- 7.Ozdemir PG, et al. The influence of shift work on cognitive functions and oxidative stress. Psychiatry Res. 2013;210:1219–1225. doi: 10.1016/j.psychres.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 8.Fonken LK, Nelson RJ. Dim light at night increases depressive-like responses in male C3H/HeNHsd mice. Behav Brain Res. 2013;243:74–78. doi: 10.1016/j.bbr.2012.12.046. [DOI] [PubMed] [Google Scholar]
- 9.Numakawa T, et al. The role of brain-derived neurotrophic factor in comorbid depression: possible linkage with steroid hormones, cytokines, and nutrition. Front Psychiatry. 2014;5:136. doi: 10.3389/fpsyt.2014.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Celik Guzel E, et al. Can low brain-derived neurotrophic factor levels be a marker of the presence of depression in obese women? Neuropsychiatr Dis Treat. 2014;10:2079–2086. doi: 10.2147/NDT.S72087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hashizume M, et al. Serum brain-derived neurotrophic factor level in elderly women depression: a community-based study. Prog Neuropsychopharmacol Biol Psychiatry. 2015;56:109–116. doi: 10.1016/j.pnpbp.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Varambally S, et al. Low serum brain derived neurotrophic factor in non-suicidal out-patients with depression: Relation to depression scores. Indian J Psychiatry. 2013;55:S397–399. doi: 10.4103/0019-5545.116311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeh SH, et al. Effects of music aerobic exercise on depression and brain-derived neurotrophic factor levels in community dwelling women. Biomed Res Int. 2015;2015:135893. doi: 10.1155/2015/135893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee IT, et al. Brain-derived neurotrophic factor, but not body weight, correlated with a reduction in depression scale scores in men with metabolic syndrome: a prospective weight-reduction study. Diabetol Metab Syndr. 2014;6:18. doi: 10.1186/1758-5996-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mikoteit T, et al. Improved Alertness Is Associated with Early Increase in Serum Brain-Derived Neurotrophic Factor and Antidepressant Treatment Outcome in Major Depression. Neuropsychobiology. 2015;72:16–28. doi: 10.1159/000437439. [DOI] [PubMed] [Google Scholar]
- 16.Gupta R, Gupta K, Tripathi AK, Bhatia MS, Gupta LK. Effect of Mirtazapine Treatment on Serum Levels of Brain-Derived Neurotrophic Factor and Tumor Necrosis Factor-alpha in Patients of Major Depressive Disorder with Severe Depression. Pharmacology. 2016;97:184–188. doi: 10.1159/000444220. [DOI] [PubMed] [Google Scholar]
- 17.Alomari MA, Khabour OF, Maikano A, Alawneh K. Vascular function and brain-derived neurotrophic factor: The functional capacity factor. Vasc Med. 2015;20:518–526. doi: 10.1177/1358863X15598390. [DOI] [PubMed] [Google Scholar]
- 18.Lee IT, et al. The synergistic effect of vascular cell adhesion molecule-1 and coronary artery disease on brain-derived neurotrophic factor. Clin Chim Acta. 2017;466:194–200. doi: 10.1016/j.cca.2017.01.026. [DOI] [PubMed] [Google Scholar]
- 19.Jiang H, Liu Y, Zhang Y, Chen ZY. Association of plasma brain-derived neurotrophic factor and cardiovascular risk factors and prognosis in angina pectoris. Biochem Biophys Res Commun. 2011;415:99–103. doi: 10.1016/j.bbrc.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 20.Hashimoto K. Sigma-1 receptor chaperone and brain-derived neurotrophic factor: emerging links between cardiovascular disease and depression. Prog Neurobiol. 2013;100:15–29. doi: 10.1016/j.pneurobio.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Safar, M. E., Cloarec-Blanchard, L. & London, G. M. Arterial alterations in hypertension with a disproportionate increase in systolic over diastolic blood pressure. J Hypertens Suppl14, S103–109, discussion S109-110 (1996). [DOI] [PubMed]
- 22.Benetos A, et al. Pulse pressure: a predictor of long-term cardiovascular mortality in a French male population. Hypertension. 1997;30:1410–1415. doi: 10.1161/01.HYP.30.6.1410. [DOI] [PubMed] [Google Scholar]
- 23.Domanski MJ, Davis BR, Pfeffer MA, Kastantin M, Mitchell GF. Isolated systolic hypertension: prognostic information provided by pulse pressure. Hypertension. 1999;34:375–380. doi: 10.1161/01.HYP.34.3.375. [DOI] [PubMed] [Google Scholar]
- 24.Fang J, Madhavan S, Alderman MH. Pulse pressure: a predictor of cardiovascular mortality among young normotensive subjects. Blood Press. 2000;9:260–266. doi: 10.1080/080370500448641. [DOI] [PubMed] [Google Scholar]
- 25.Gasowski J, et al. Pulsatile blood pressure component as predictor of mortality in hypertension: a meta-analysis of clinical trial control groups. J Hypertens. 2002;20:145–151. doi: 10.1097/00004872-200201000-00021. [DOI] [PubMed] [Google Scholar]
- 26.Protogerou AD, et al. Longitudinal Changes in Mean and Pulse Pressure, and All-Cause Mortality: Data From 71,629 Untreated Normotensive Individuals. Am J Hypertens. 2017;30:1093–1099. doi: 10.1093/ajh/hpx110. [DOI] [PubMed] [Google Scholar]
- 27.Thomas D, et al. Value of reserve pulse pressure in improving the risk stratification of patients with normal myocardial perfusion imaging. Eur Heart J. 2013;34:2074–2081a. doi: 10.1093/eurheartj/eht102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inrig JK, et al. Decreased pulse pressure during hemodialysis is associated with improved 6-month outcomes. Kidney Int. 2009;76:1098–1107. doi: 10.1038/ki.2009.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steptoe A, Donald AE, O’Donnell K, Marmot M, Deanfield JE. Delayed blood pressure recovery after psychological stress is associated with carotid intima-media thickness: Whitehall psychobiology study. Arterioscler Thromb Vasc Biol. 2006;26:2547–2551. doi: 10.1161/01.ATV.0000242792.93486.0d. [DOI] [PubMed] [Google Scholar]
- 30.Steptoe A, Kivimaki M, Lowe G, Rumley A. & Hamer, M. Blood Pressure and Fibrinogen Responses to Mental Stress as Predictors of Incident Hypertension over an 8-Year Period. Ann Behav Med. 2016;50:898–906. doi: 10.1007/s12160-016-9817-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mathiassen SE, Hallman DM, Lyskov E, Hygge S. Can cognitive activities during breaks in repetitive manual work accelerate recovery from fatigue? A controlled experiment. PLoS One. 2014;9:e112090. doi: 10.1371/journal.pone.0112090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matthews DR, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 33.National Kidney, F. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis39, S1–266 (2002). [PubMed]
- 34.Avolio A. Arterial stiffness. Pulse. 2013;1:14–28. doi: 10.1159/000348620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007;50:1–13. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 36.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–943. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- 37.Powell JT, Vine N, Crossman M. On the accumulation of D-aspartate in elastin and other proteins of the ageing aorta. Atherosclerosis. 1992;97:201–208. doi: 10.1016/0021-9150(92)90132-Z. [DOI] [PubMed] [Google Scholar]
- 38.Schmitt M, et al. Basal NO locally modulates human iliac artery function in vivo. Hypertension. 2005;46:227–231. doi: 10.1161/01.HYP.0000164581.39811.bd. [DOI] [PubMed] [Google Scholar]
- 39.Donovan MJ, et al. Neurotrophin and neurotrophin receptors in vascular smooth muscle cells. Regulation of expression in response to injury. Am J Pathol. 1995;147:309–324. [PMC free article] [PubMed] [Google Scholar]
- 40.Otani K, Okada M, Yamawaki H. Expression pattern and function of tyrosine receptor kinase B isoforms in rat mesenteric arterial smooth muscle cells. Biochem Biophys Res Commun. 2015;467:683–689. doi: 10.1016/j.bbrc.2015.10.084. [DOI] [PubMed] [Google Scholar]
- 41.Anastasia A, et al. Trkb signaling in pericytes is required for cardiac microvessel stabilization. PLoS One. 2014;9:e87406. doi: 10.1371/journal.pone.0087406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin B, et al. Glycemic control with empagliflozin, a novel selective SGLT2 inhibitor, ameliorates cardiovascular injury and cognitive dysfunction in obese and type 2 diabetic mice. Cardiovasc Diabetol. 2014;13:148. doi: 10.1186/s12933-014-0148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamasaki F, Schwartz JE, Gerber LM, Warren K, Pickering TG. Impact of shift work and race/ethnicity on the diurnal rhythm of blood pressure and catecholamines. Hypertension. 1998;32:417–423. doi: 10.1161/01.HYP.32.3.417. [DOI] [PubMed] [Google Scholar]
- 44.Jankowiak S, et al. Current and cumulative night shift work and subclinical atherosclerosis: results of the Gutenberg Health Study. Int Arch Occup Environ Health. 2016;89:1169–1182. doi: 10.1007/s00420-016-1150-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lo SH, et al. Working the night shift causes increased vascular stress and delayed recovery in young women. Chronobiol Int. 2010;27:1454–1468. doi: 10.3109/07420528.2010.498067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kubo T, et al. Comparison of coronary microcirculation in female nurses after day-time versus night-time shifts. Am J Cardiol. 2011;108:1665–1668. doi: 10.1016/j.amjcard.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 47.Costa MS, et al. Caffeine improves adult mice performance in the object recognition task and increases BDNF and TrkB independent on phospho-CREB immunocontent in the hippocampus. Neurochem Int. 2008;53:89–94. doi: 10.1016/j.neuint.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 48.Reyes-Izquierdo T, et al. Modulatory effect of coffee fruit extract on plasma levels of brain-derived neurotrophic factor in healthy subjects. Br J Nutr. 2013;110:420–425. doi: 10.1017/S0007114512005338. [DOI] [PubMed] [Google Scholar]
- 49.Mahmud A, Feely J. Acute effect of caffeine on arterial stiffness and aortic pressure waveform. Hypertension. 2001;38:227–231. doi: 10.1161/01.HYP.38.2.227. [DOI] [PubMed] [Google Scholar]
- 50.Karatzis E, et al. Acute effects of caffeine on blood pressure and wave reflections in healthy subjects: should we consider monitoring central blood pressure? Int J Cardiol. 2005;98:425–430. doi: 10.1016/j.ijcard.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 51.Vlachopoulos C, Panagiotakos D, Ioakeimidis N, Dima I, Stefanadis C. Chronic coffee consumption has a detrimental effect on aortic stiffness and wave reflections. Am J Clin Nutr. 2005;81:1307–1312. doi: 10.1093/ajcn/81.6.1307. [DOI] [PubMed] [Google Scholar]
- 52.Souza AA, Silva RS, Silva TF, Tavares RL, Silva AS. Influence of different doses of coffee on post-exercise blood pressure response. Am J Cardiovasc Dis. 2016;6:146–152. [PMC free article] [PubMed] [Google Scholar]
- 53.Su TC, et al. Elevated blood pressure, decreased heart rate variability and incomplete blood pressure recovery after a 12-hour night shift work. J Occup Health. 2008;50:380–386. doi: 10.1539/joh.L7056. [DOI] [PubMed] [Google Scholar]
- 54.Bara AC, Arber S. Working shifts and mental health–findings from the British Household Panel Survey (1995–2005) Scand J Work Environ Health. 2009;35:361–367. doi: 10.5271/sjweh.1344. [DOI] [PubMed] [Google Scholar]
- 55.Lommatzsch M, et al. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol Aging. 2005;26:115–123. doi: 10.1016/j.neurobiolaging.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 56.Begliuomini S, et al. Influence of endogenous and exogenous sex hormones on plasma brain-derived neurotrophic factor. Hum Reprod. 2007;22:995–1002. doi: 10.1093/humrep/del479. [DOI] [PubMed] [Google Scholar]
- 57.Cubeddu A, et al. Brain-derived neurotrophic factor plasma variation during the different phases of the menstrual cycle in women with premenstrual syndrome. Psychoneuroendocrinology. 2011;36:523–530. doi: 10.1016/j.psyneuen.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 58.Dunne FP, Barry DG, Ferriss JB, Grealy G, Murphy D. Changes in blood pressure during the normal menstrual cycle. Clin Sci. 1991;81:515–518. doi: 10.1042/cs0810515. [DOI] [PubMed] [Google Scholar]
- 59.Mills PJ, Berry CC. Menstrual cycle, race and task effects on blood pressure recovery from acute stress. J Psychosom Res. 1999;46:445–454. doi: 10.1016/S0022-3999(99)00015-X. [DOI] [PubMed] [Google Scholar]
- 60.Takeda K, et al. BDNF protects human vascular endothelial cells from TNFalpha-induced apoptosis. Biochem Cell Biol. 2013;91:341–349. doi: 10.1139/bcb-2013-0005. [DOI] [PubMed] [Google Scholar]
- 61.Donovan MJ, et al. Brain derived neurotrophic factor is an endothelial cell survival factor required for intramyocardial vessel stabilization. Development. 2000;127:4531–4540. doi: 10.1242/dev.127.21.4531. [DOI] [PubMed] [Google Scholar]
- 62.Meuchel LW, Thompson MA, Cassivi SD, Pabelick CM, Prakash YS. Neurotrophins induce nitric oxide generation in human pulmonary artery endothelial cells. Cardiovasc Res. 2011;91:668–676. doi: 10.1093/cvr/cvr107. [DOI] [PMC free article] [PubMed] [Google Scholar]