Abstract
A central question in ecology is how to link processes that occur over different scales. The daily interactions of individual organisms ultimately determine community dynamics, population fluctuations and the functioning of entire ecosystems. Observations of these multiscale ecological processes are constrained by various technological, biological or logistical issues, and there are often vast discrepancies between the scale at which observation is possible and the scale of the question of interest. Animal movement is characterized by processes that act over multiple spatial and temporal scales. Second-by-second decisions accumulate to produce annual movement patterns. Individuals influence, and are influenced by, collective movement decisions, which then govern the spatial distribution of populations and the connectivity of meta-populations. While the field of movement ecology is experiencing unprecedented growth in the availability of movement data, there remain challenges in integrating observations with questions of ecological interest. In this article, we present the major challenges of addressing these issues within the context of the Serengeti wildebeest migration, a keystone ecological phenomena that crosses multiple scales of space, time and biological complexity.
This article is part of the theme issue 'Collective movement ecology'.
Keywords: scale, wildebeest, migration
1. Introduction
The challenge of understanding processes that operate at multiple scales is common to all scientific disciplines. Dynamics, such as decadal economic cycles, or the progression of scientific discoveries, are the accumulation of fine-scale events; daily struggles lead to results and findings, these results aggregate to form a body of knowledge, this knowledge defines a discipline and so on. Often, when studying natural or social systems a scale of observation is selected, and the challenge then arises when attempting to relate what is observed at one scale with the phenomena that emerge at another, and the feedback processes that occur among scales.
The issue of scale is universal, and the field of ecology is no exception. At the broadest level, ecosystems provide the services that are essential for our survival [1], but these services are the result of the myriad direct and indirect interactions among individual organisms. Understanding how ecosystem services emerge from, and feedback to influence, micro-scale processes is a central problem in ecology [2] and it is the key to understanding how best these services can be protected. Schneider [3] identified the three components that define the problem of scale in ecology; firstly, core ecological questions often concern population-level, macro-scale processes; secondly, observations are constrained to relatively fine-scale measurements and sampling; and third, when dealing with complex biological systems, processes do not scale simply from one level of description to another.
In the context of movement ecology, examples of large-scale ecological questions concern the potential impacts of the cessation of a migration, the amount of connectivity required to maintain a viable metapopulation, or the ecosystem impacts of reductions in animal movement [4,5,6]. These questions are addressed through monitoring and data collection on the movement of individual animals and groups [7,8]. Connecting these observations with population- or community-level processes is an unresolved task [9–11] and one that in essence involves extrapolating from second-by-second movements to annual migration patterns, from resource selection and risk avoidance to survival and fecundity rates [12], and from individual behaviours to population dynamics and persistence.
2. The Serengeti wildebeest migration
Wildebeest are an iconic example of a migratory species that plays a dominant role in the ecology of the area it inhabits. The annual migration of the blue wildebeest (Connochaetes taurinus) covers the entire range of the Greater Serengeti ecosystem, a round-trip that far exceeds the straight-line distance of 650 km, with data from GPS collars suggesting that the true distance covered is over 1500 km [13]. Herds head south from their dry season refuge in the Masai Mara (Kenya) as the short rains begin in early November, and spend the wet season (December–May) in the fertile southern short grass plains of Tanzania, defined by the extent of the volcanic ash soils and the mean annual precipitation. Calving season in February coincides with this period of peak primary production. Calves are highly precocial and will follow their mothers within hours [14]. The wildebeest migration is constantly moving, with females having an average daily displacement of 4.5 km [15], opting for high rainfall areas in the Western Corridor before continuing northwest to the Masai Mara by July. The dry season (August–November) is spent in the northern woodlands of the Serengeti National Park and the Masai Mara National Reserve, before the cycle begins again (see figure 1).
Figure 1.

The Serengeti wildebeest migration. The figure shows the annual movements of 8 female wildebeest that were collared and monitored between 1999 and 2001.
This mass migration of animals is not only an awe-inspiring visual spectacle but also plays a keystone role in the region's ecosystem. The migrants transport nutrients, consuming around 4500 tonnes of grass per day, which is constantly getting digested and relocated as they move around the ecosystem [16], and are a source of food for multiple species of predator and scavenger [14,17,6]. Movement enables the wildebeest population to be much more abundant than expected based on the environment [18]. In total, Serengeti wildebeest are about twice the biomass of the next 12 most abundant large mammals in the ecosystem combined [19]. By moving among seasonal areas, migratory wildebeest increase their access to food and therefore avoid being regulated by its availability at the local scale [20]. Furthermore, the natural rotational grazing system inherent in the annual migration facilitates compensatory growth in the grasses. The grasses grow more rapidly after being grazed, thereby increasing the total annual biomass of available forage [21]. Thirdly, by migrating en masse the population avoids becoming regulated by predators either by swamping the local resident predator population and thereby decreasing the per capita mortality rate, or by improving their detection of predators [22].
Without the annual migratory cycle much of the region's biodiversity would decline [23], as the passage of the 1.3 million migrants affects virtually every other ecological interaction from below ground nutrient cycles, to insects and avifauna abundance, to predator–prey interactions of resident herbivores and carnivores as well as the services this ecosystem provisions for the communities around it [24]. Hence, movement is the force that drives the ecosystem dynamics in the area and there would be fundamental changes in the ecology and its services if it were to stop [25].
The migration is inherently a multiscale phenomenon. Wildebeest aggregate in herds ranging from tens of individuals to up to 400 000 [26]. The highly synchronous calving of Serengeti wildebeest could be an emergent property of the seasonal environment where the cost of reproduction is high but only energetically possible in certain areas and during short periods of time [27], rather than be an adaptive response to predation. Breeding synchrony ultimately leads to all reproductively active females (about 450 000 animals) requiring the same resources at the same time. This leads to competition between individuals for limited resources in the local environment, forcing them to search further afield for adequate forage and water [19]. From a food-intake perspective, it would be more advantageous for individuals to remain solitary as they would be able to maximize their intake rate (i.e. the biomass per bite). However, from a safety point of view solitary animals are more exposed to predation; it is the balance between food and security operating at different scales and at different times that interact to form volatile fission–fusion dynamics of the herd. Therefore, movement decisions of individuals are influenced by multiple factors including physiology, social interactions, environmental cues, resource availability, memory and predation risk [15,27–29]. Disentangling these competing, hierarchical drivers of movement is a substantial task; however, it is a challenge that must be met in order to develop the evidence-based policy required to protect vital ecosystem services in the region.
3. From individual to collective movement
(a). Connecting tracks with cues
We are now obtaining unprecedented levels of data on the movement trajectories of animals, and moving towards collecting lifetime tracks of individuals of certain species [7]. A current major challenge is relating these data to the underlying environmental and/or social drivers of movement [30]. Without connecting movement decisions with the instantaneous conditions the animal was experiencing we are only able to make crude inference on remotely collected data such as response to season or large landscape-scale effects.
When trying to integrate animal movement data with cues and drivers there are two issues of scale. The first is the ability to collect data at a resolution that is relevant to the animal's decisions. Metrics relating to vegetation quality may be collected from satellite imagery [31–33]; however, these data are often at a far coarser scale than the movement trajectories [7,11] and taken at different times. Normally, there is a trade-off between increased spatial resolution and the amount of time-delay between the movement and the environmental observation [31]. This lack of temporal synchrony is not an issue when considering long-lasting features of the environment such as trails [34] but when dealing with vegetation that is growing rapidly and being consumed by large numbers of herbivores it may be a significant factor. The second issue of scale is that the individuals respond to their environment at different and often unknown scales [35], therefore even if it were possible to collect environmental data instantaneously, at any resolution, it is a priori unknown what the correct resolution to select should be; indeed, there probably is not a single correct resolution. Wildebeest may be responding to vegetation gradients [36] (i.e. the so-called green-wave hypothesis [37,38]), intermediate-range cues such as rain storms (discussed in [28]), risk factors relating to predation [15], memory [29] or a combination of these factors.
In the future, greater satellite resolution and on-demand coverage may help to resolve some of these issues, with disruptive technologies such as wide-area motion imagery (WAMI) offering a potential step-change in our ability to collect environmental data. Advances in on-board sensors that accompany GPS collars also facilitate the collection of social and environmental data [39–41].
The importance of traditional behavioural ecology in this context should not be underestimated, and the use of GPS collars has been criticized for their inability to provide behavioural and social context [11]. While GPS collars and satellite images are unrivalled in terms of sheer volume of data, detailed on-the-ground observations still remain an irreplaceable tool for understanding behaviour.
(b). Social context and interaction rules
While GPS collars on individual animals are providing tremendous insight into individual behaviour they rarely reveal any information about social interactions due to the difficulty associated with tracking a large proportion of a group (aside from some notable exceptions e.g. [34,42–44]). For ungulates the role of social interactions has been examined in the situation where collared animals have been released together [45], while signatures of collective behaviour have been detected by analysing the temporal autocorrelation of trajectories at the same spatial location [46,47]. For species such as wildebeest where herds number in the thousands and group membership is usually weakly cohesive and dynamic, GPS collars are not a viable method to investigate social interactions and alternate methods need to be found. This is imperative, as for social species such as wildebeest, the behaviour of conspecifics is probably as important as environmental cues. When herds potentially span kilometers, social interactions represent a mechanism of collective sensing that far exceeds the sensory range of an individual [28,48,49].
If movement decisions of wildebeest are collective decisions then studying individual behaviour will inevitably omit an important aspect of the migration. The first stage in addressing this deficit is creating a picture of interactions such as has been achieved in the study of other species [50–53]. Increasingly, aerial filming using platforms such as drones, blimps or balloons offer a partial solution [8]. Figure 2 shows the relative spatial distribution and orientation of near neighbours of migrating wildebeest based on data collected from a UAV-borne camera. These types of data provide an entirely different insight into movement dynamics as compared to long-term individual track data. Track lengths are of the order of seconds or minutes, instead of years, while hundreds of individuals may be simultaneously tracked for short amounts of time.
Figure 2.
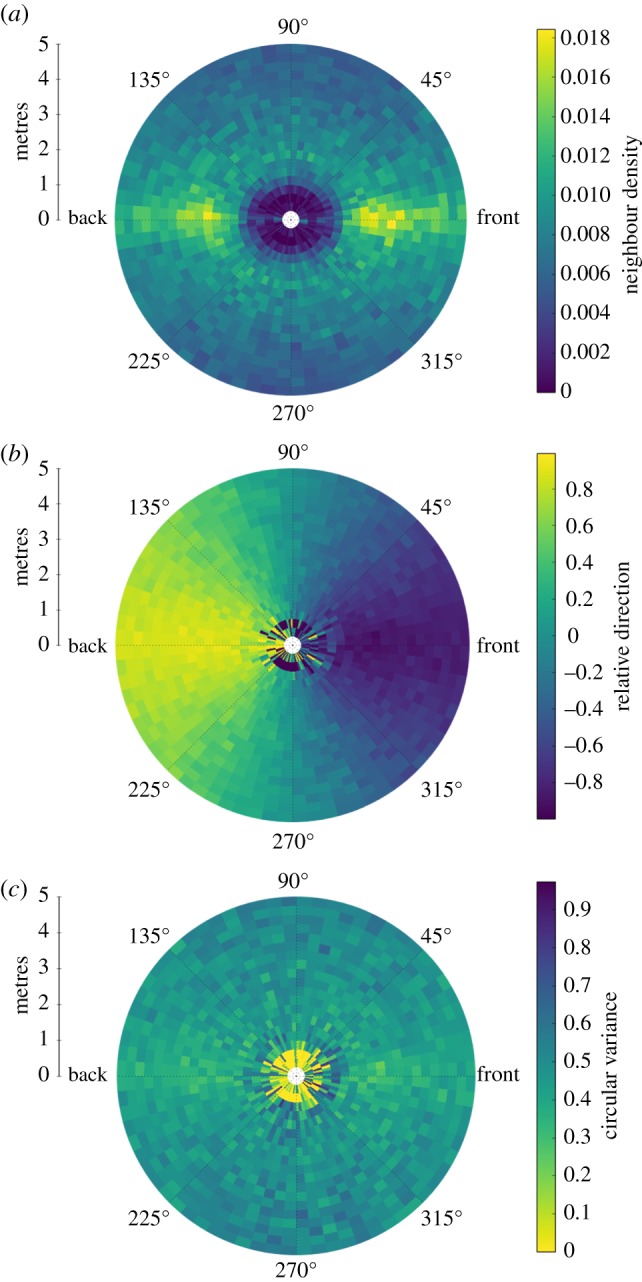
Fine-scale dynamics of wildebeest herds. Data are taken from UAV-borne video. (a) Spatial distribution of near-neighbours relative to a focal individual. Wildebeest display a well-defined inter-individual spacing with greater density to the front and rear of the focal individual. (b) Relative direction of neighbours. This is calculated as the dot product of neighbour heading vector with the vector towards the focal individual. Higher values indicate the neighbour is heading towards the focal individual, lower values mean the neighbour is moving away. (c) Circular variance in heading of near-neighbours. Variation in heading is greater either side of the focal individual. (Plots b and c effectively show the first and second moments, respectively, of the local distribution of neighbour headings.) Combined, these data suggest that wildebeest tend to align and follow each other in linear formations.
To fully leverage these novel data streams an approach is required that integrates different multiscale data collection techniques. To link long-term studies of individuals with fine timescale observations from other platforms it is necessary to identify collared animals within video images, something that is now possible due to the data logging capacity of modern aerial filming platforms [54].
(c). Conflict, mutual benefits and emergence
The interplay between the individual and the collective creates a feedback mechanism that amplifies or inhibits behaviour. In effect the social context is both driven by, and drives, the behaviour of individuals. These feedbacks operate over multiple time scales from the pressure to conform to directional choices in order to avoid predation, to the evolutionary dynamics of exploitation and information production in contexts such as vigilance [22] or navigation [55,56]. The net effect of the individual on the collective behaviour of the herd is probably a function of the behaviour of the initiator and the internal state of the recipient, such that persistent behaviour is required to motivate a large lethargic group, but a small inadvertent action can have large effects for an anxious highly vigilant group.
The unpredictable relationship between the actions of individuals and the behaviour of the collective is the focus of complex systems science. At the heart of this discipline is the notion that interactions at lower levels give rise to properties at the higher level, e.g. a collective phenomenon that might not have been predicted from observations of individuals in isolation [57]. For example, the lengthscale of the spatial structure of grazing wave fronts of wildebeest far exceeds the perception range of an individual. The structure is therefore an emergent phenomenon and the product of both the individual-level behaviour and the inter-individual interactions [58]. These concepts mean that observations of individual movement only provide a partial picture of the migration.
Without considering collective dynamics, observations may appear counterintuitive. For example, for wildebeest the correlation between movement speed and environment quality switches from negative to positive as the density of grazers increases. Hopcraft et al. [15] showed that for herds of wildebeest there was a positive relationship between speed and forage quality, i.e. animals moved faster through regions of high-quality resources rather than lingering to take advantage of a good resource patch as one would expect. This is in contrast to the classical view of resource selection considered at the individual level where animals should tend towards encampment in high-quality patches and rapidly pass through low-quality patches. One interpretation of these counter-intuitive results is that resources in high-quality patches are consumed more rapidly by large groups, thereby forcing individuals to eat rapidly and move on to the next patch in order to remain close to the leading edge of the grazing front.
This example illustrates some of the challenges in revealing the drivers of movement decisions that are influenced by multiscale environmental and social factors. Different drivers may lead to similar observed behaviour [59] and novel statistical methods [60] are required to distinguish socially driven movements from movements driven by common external cues [30] and for assessing social structure and the differing leadership roles individuals adopt [61–63]. Identifying how the subtle nuances and interactions between covariates can lead to totally different responses remains one of the largest challenges in the field.
4. Scaling across time and space
(a). Observation scale and hierarchical approaches
When we observe animal movement we necessarily do so at a certain range of scales. Often there is no single correct scale [2] at which to observe ecological phenomena. In some cases, a scale is arbitrarily chosen, while in others it is imposed. How data are collected will influence the phenomena that are observed, the questions addressed, and thus, possibly, the conclusions that are drawn [64].
As an example consider attaching a programmable GPS collar to a wildebeest. The collar has a finite battery life and so may produce a fixed number of reads at a controllable interval. If we are interested in how the animal responds to local cues, such as vegetation gradients we require an interval on the order of seconds or minutes. If we want to know how the animal responds to larger scale features we would select an interval of a day or more. If our question relates to whether the herds track seasonal variation we would require an interval of weeks, and finally if we wish to know about site fidelity and the annual return of individuals to particular areas we would require monthly, or less frequent, fixtures extending over as many years as possible. As we move from one end of the spectrum to another we sacrifice fine scale detail for temporal range, breadth for depth. When a process has self-similarity we may extrapolate straightforwardly from fine scale observations to longer distance movement properties [65]; however, for species such as wildebeest, different mechanisms drive behaviour at different scales [35], and no universal scaling law exists [66].
To understand these various multiscale and overlapping drivers, it is necessary to integrate data from different sources. Remote data from GPS collars can provide insight into long-term movement dynamics, the relocation of collared animals and repeated observations will reveal individual characteristics, while observations from aerial platforms provide information on fine scale second by second interactions. Through an iterative process where observations at one scale help to design observations at another in a hierarchical fashion, a holistic picture of movement can be created.
(b). Decision points and behavioural states
Much of the difficulty in understanding the different scales of movement arise from variability. Individuals within the herd are not identical, so models that assume homogeneity fail to capture core details about individual variation in response to certain factors, or apply the unique characteristics of a few collared animals to the entire population. Similarly, it is not possible to make detailed observations over the course of a few hours and extrapolate to movement over weeks or months, since behavioural variation or behavioural modes [67], such as ‘foraging’ or ‘encamped’ [68], have to be considered. Switching between these modes may be intermittent or occur multiple times a day as in the case of wildebeest alternating between grazing and marching bands. State-space models [69,70] and hidden Markov models [68] have been used to detect these different modes from individual trajectories. Research has shown these modes exist and that switching between them depends on environmental conditions [71,72]. While within these different states, animals are still responding to local cues and one another, the presence of these transition points indicate that there are some periods of time that more significantly impact overall movement patterns.
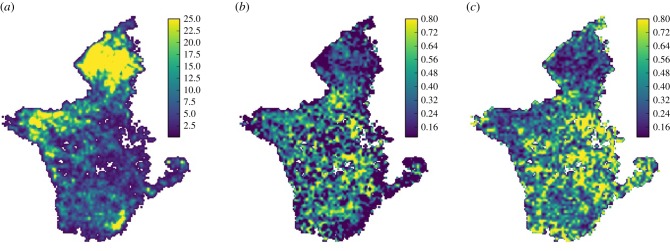
The first stage in an integrated multiscale approach is to identify the presence of behavioural states from long-term tracking data [60]. Covariates, such as landscape features or temporal drivers, associated with the transition from one behavioural state to another, may be inferred from higher resolution positional and environmental data (see figure 3 for data relating to the landscape level movements of the wildebeest herds). Finally, armed with information on the presence and predictors of transitions, fine scale observations can be made. In order to reveal the mechanisms underlying collective decision-making [73], these targeted, fine-scale observations require data collection methods akin to those associated with laboratory-based studies of movement [51,52,74] to be employed, from which detailed information about the interactions between individuals and their environment can be used to infer patterns of collective behaviour.
Figure 3.
Landscape level movement patterns of the Serengeti wildebeest. GPS collar data taken from 55 wildebeest in the period 2005–2016. (a) Wildebeest density over time. The heat map displays the regions where wildebeest are most concentrated throughout the year. (b) Individual directness. Plotted is the average dot product of previous heading with current heading for each individual. High values indicate wildebeest are travelling more directly through these regions. (c) Population-level coordination. The degree of alignment for all individuals in each region is plotted. This reveals the spatial locations where the population as a whole tends to move in the same heading (South-East of the region).
(c). Feedbacks between movement and the environment
While social feedbacks can rapidly reinforce fast time-scale processes such as startle responses [75] or departure events [76], the interaction between the environment and animal movement patterns creates a longer time-scale feedback. The constant migration of animals across a landscape creates a system of trails that reinforce and even dictate movement patterns. Trails are created through a process of positive feedback [77] and once formed can have a significant effect on the movement decisions of individuals [34,78].
Animal trails are found throughout the Serengeti and clearly influence the movement decisions of wildebeest (see figure 4). The tendency of wildebeest to follow these pathways presents both opportunities and difficulties when studying the migration. The effect of trails on fine-scale behaviour is hard to distinguish from social effects. Following a trail is an energy efficient strategy so will encourage wildebeest to form travelling lines, hence the spatial structure of herds, shown in figure 2, may well be a result of maintaining cohesion and trail following. When attempting to detect signatures of collective behaviour, changes in orientation that are caused by meandering paths may be interpreted as imitation. Care must be taken to include landscape features into movement models at this scale [30].
Figure 4.

Trail following behaviour of wildebeest. This image was taken from an aerial survey of the wildebeest population undertaken in 2009.
Trail networks may also be interpreted as a form of cultural memory [79]. The lifetime of these spatial patterns may well extend beyond the lifespan of individual wildebeest and probably provide indicators of optimal routes for less experienced individuals. The trails represent a form of stigmergy, usually associated with social insects [80], as they represent an indirect interaction between wildebeest that is mediated by the environment. In fact, wildebeest have scent glands on their hooves, and the continuous passage of individuals along trails has been hypothesized to facilitate navigation [81] as wildebeest are able to follow social cues without maintaining visual contact. Explicit incorporation of trails has been shown to improve the predictive power of movement models in other species such as baboons [34], hence trails are probably a significant factor that may be followed by individuals due to efficiency savings or as a navigational aide [82].
Greater resolution satellite imagery combined with automated computer vision techniques now offer the potential to remotely detect and monitor these trails, offering the possibility to detect features of the migration even in the absence of wildebeest.
(d). Memory, fidelity and spatial scale
Memory is a ubiquitous component of animal movement, influencing individual decision-making at various scales [83]. Memory's relative effect on movement can be difficult to distinguish from those of other sensory cues such as local forage quality, predation risk and conspecific attraction. In wild populations, inferences about memory are typically based on observing where individuals and conspecifics move in the past, and assuming that these locations (or prominent features there within) represent a known spatial reference [34,84]. For instance, relocation data suggest that a variety of organisms exhibit strong loyalty to specific sites or routes at the individual level (i.e. ‘site fidelity’) [85]. Site fidelity, however, is not necessarily memory-driven, and can arise because of either innate navigational programs or because the set of available sites is small. Stronger evidence that memory shapes recursive movements comes from experimental work that involves relocations or introductions to novel landscapes. Memory effects can be separated from other navigational mechanisms, such as those based on compass bearing or celestial orienteering [82].
Memory shapes movements in social animals in a number of ways. Navigational success appears to improve when groups are composed of older, more experienced individuals (e.g. [86,87]), presumably because experienced animals transfer information about sites or routes to inexperienced members [79,88]. This learning also appears to enable adaptive route changes in response to environmental change [89]. An animal's social context may determine whether memory-based movements decisions are the most profitable or efficient in the short term [90]. For instance, strong fidelity across years is hypothesized to facilitate the use of the most efficient routes or the sites with the most predictable resources [91]. However, spatial fidelity can come at the cost of food intake or predator avoidance when the quality of the site/route changes [92,93], and for animals moving in large groups, site fidelity may represent a particularly costly strategy because local resources become depleted quickly.
For migratory species, these conflicts play out across their annual cycles. Migration in wildebeest (and other larger herbivores) is classically described as an adaptive movement strategy enabling individuals to exploit ephemeral resource patches, such as those occurring after recent rainfall [15,28,36,94]. The large group sizes extend wildebeest perceptual ranges for locating high-quality forage up to 70 km [28]. However, even wildebeest exhibit spatial fidelity at annual scales. In a multi-year mark-recapture study involving several thousand individuals, adult wildebeest in the Tarangire Ecosystem, Tanzania returned with high frequency to the same wet season ranges each year (82–100%) [29]. Notably, despite mixing within herds with wildebeest from other ranges in the dry season, individuals tended to return to the same wet season range each year [29]. Thus, fidelity may play a stronger role in inter-annual movement decisions than social-group membership in wildebeest. Whether this same pattern holds in groups as large as the ones found in Serengeti, where the population is 100-fold larger than in Tarangire, remains to be tested.
5. Conclusion
The primary goals of movement ecology may be divided into two broad categories: first, to understand the drivers and mechanisms underlying patterns of movement; and second, to evaluate the consequences of movement for individuals, populations and communities [95,96]. The past several years have seen a rapid increase in the data available for addressing these questions. These data come from field observations and sampling, radio telemetry and satellite tracking [7,97], camera traps [98], aerial surveys and video footage [8]. In the context of understanding the causes of movement and the mechanisms underlying spatio-temporal movement patterns, research efforts have focused on connecting movement trajectories with local cues and drivers, examining temporal variation and behavioural modes, and understanding the role of social interactions and leadership. For broad-scale consequences of animal movement, the concept of mobile links [99] has been introduced and increasingly movement patterns are seen as equalizing or stabilizing forces within ecosystems [6].
While there have been advances in data collection and analysis methods, the next major challenge for movement ecology lies in integrating data from different sources and developing comprehensive descriptions of movement that encompass both its drivers and its consequences. Inevitably this is a problem of scale. Data collection methods impose constraints on the nature of the observations that can be made and controlled experiments are often impossible. This means that extrapolation must be made from one scale (the scale of observation) to another (the scale of the phenomena of interest).
There remain gaps in our knowledge of both the drivers and the consequences of the Serengeti wildebeest migration. The synchronized mass movement of thousands of wildebeest returning to the same locations on an annual basis raises questions about how this level of organization is achieved. On one hand, the mass migration could be the result of animals moving in response to an oscillating underlying abiotic gradient such as seasonal rainfall or soil fertility that determines the cyclical availability of grass. However, behavioural observations illustrate wildebeest are unpredictable, responding to environmental cues at multiple scales, and not operating as independent migrants but instead part of a complex social structure [14].
Efforts to disentangle the various drivers of the migration are focused on increasing the number of individuals that are monitored and gaining higher resolution data on environmental covariates. As the number of GPS tracking collars deployed on wildebeest increases, a picture of individual and temporal variation can be developed. Combining these data with statistical models and inference techniques will reveal the competing time-varying drivers that influence decisions. To complement these individual-based data, observations of collective behaviour are required. Aerial filming and computer vision methods are now providing the tools needed to collect and process these observations. Integrating these studies of collective movement with individual tracking [54] will allow us to detect which individuals are influencing decisions [100] and to understand how wildebeest herds collectively respond to their environments.
As in many studies of animal migration, the aim of understanding the Serengeti wildebeest migration is driven by the significant impact it has on the local ecosystem. The wildebeest affect every facet of the ecology in the region [6]. The migration facilitates other species of herbivore through successional grazing, migrants are transporters of disease, they impact vegetation dynamics and fire regimes, and are vital prey for carnivores [14,101]. Movement allows the population of wildebeest to persist at high levels and this vast biomass has huge impacts as it moves around the park [19]. In the Serengeti region, as elsewhere, greater understanding of the mechanisms that drive keystone ecological processes is vital due to increased human activity [102] and the need to make informed and effective management decisions [103,104].
Data accessibility
Data deposited in Enlighten: Research Data http://dx.doi.org/10.5525/gla.researchdata.566.
Competing interests
We declare we have no competing interests.
Funding
This research was supported by the Army Research Office Grant W911NF-14-1-0431. J.G.C.H. acknowledges support from the British Ecological Society large grant scheme and the European Union Horizon 2020 grant no 641918 supporting T.A.M. C.J.T. gratefully acknowledges support from a James S. McDonnell Foundation Studying Complex Systems Scholar Award. I.D.C. acknowledges support from NSF (IOS-1355061), ONR (N00014- 14-1-0635), ARO (W911NF-14-1-0431), the ‘Struktur- und Innovationsfonds für die Forschung (SI-BW)’ of the State of Baden-Württemberg, and the Max Planck Society.
References
- 1.Daily GC. et al. 2000. The value of nature and the nature of value. Science 289, 395–396. ( 10.1126/science.289.5478.395) [DOI] [PubMed] [Google Scholar]
- 2.Levin SA. 1992. The problem of pattern and scale in ecology: the Robert H. MacArthur award lecture. Ecology 73, 1943–1967. ( 10.2307/1941447) [DOI] [Google Scholar]
- 3.Schneider DC. 2001. The rise of the concept of scale in ecology. Bioscience 51, 545–553. ( 10.1641/0006-3568(2001)051%5B0545:TROTCO%5D2.0.CO;2) [DOI] [Google Scholar]
- 4.Bauer S, Hoye BJ. 2014. Migratory animals couple biodiversity and ecosystem functioning worldwide. Science 344, 1242552 ( 10.1126/science.1242552) [DOI] [PubMed] [Google Scholar]
- 5.Lundberg J, Moberg F. 2003. Mobile link organisms and ecosystem functioning: implications for ecosystem resilience and management. Ecosystems 6, 0087–0098. ( 10.1007/s10021-002-0150-4) [DOI] [Google Scholar]
- 6.Holdo RM, Holt RD, Sinclair ARE, Godley BJ, Thirgood S. 2011. Migration impacts on communities and ecosystems: empirical evidence and theoretical insights. In Animal Migration (eds Milner-Gulland EJ, Fryxell JM, Sinclair) AR, pp. 131–143. Oxford, UK: Oxford University Press. [Google Scholar]
- 7.Kays R, Crofoot MC, Jetz W, Wikelski M. 2015. Terrestrial animal tracking as an eye on life and planet. Science 348, aaa2478 ( 10.1126/science.aaa2478) [DOI] [PubMed] [Google Scholar]
- 8.Hughey LF, Hein AM, Strandburg-Peshkin A, Jensen FH. 2018. Challenges and solutions for studying collective animal behaviour in the wild. Phil. Trans. R. Soc. B 373, 20170005 ( 10.1098/rstb.2017.0005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sutherland WJ. 1996. From individual behaviour to population ecology. Oxford, UK: Oxford University Press on Demand. [Google Scholar]
- 10.Fryxell JM, Lundberg P. 1997. Individual behavior and community dynamics. New York, NY, USA: Springer. [Google Scholar]
- 11.Hebblewhite M, Haydon DT. 2010. Distinguishing technology from biology: a critical review of the use of GPS telemetry data in ecology. Phil. Trans. R. Soc. B 365, 2303–2312. ( 10.1098/rstb.2010.0087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthiopoulos J, Fieberg J, Aarts G, Beyer HL, Morales JM, Haydon DT. 2015. Establishing the link between habitat selection and animal population dynamics. Ecol. Monogr. 85, 413–436. ( 10.1890/14-2244.1) [DOI] [Google Scholar]
- 13.Thirgood S. et al. 2004. Can parks protect migratory ungulates? The case of the Serengeti wildebeest. Anim. Conserv. 7, 113–120. ( 10.1017/S1367943004001404) [DOI] [Google Scholar]
- 14.Estes R. 2014. The Gnu's world: Serengeti wildebeest ecology and life history. Berkeley, CA: University of California Press. [Google Scholar]
- 15.Hopcraft JGC, Morales JM, Beyer HL, Borner M, Mwangomo E, Sinclair ARE, Olff H, Haydon DT. 2014. Competition, predation, and migration: individual choice patterns of Serengeti migrants captured by hierarchical models. Ecol. Monogr. 84, 355–372. ( 10.1890/13-1446.1) [DOI] [Google Scholar]
- 16.Subalusky AL, Dutton CL, Rosi EJ, Post DM. 2017. Annual mass drownings of the Serengeti wildebeest migration influence nutrient cycling and storage in the Mara River. Proc. Natl Acad. Sci. USA 114, 7647–7652. ( 10.1073/pnas.1614778114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McNaughton SJ. 1985. Ecology of a grazing ecosystem: the Serengeti. Ecol. Monogr. 55, 259–294. ( 10.2307/1942578) [DOI] [Google Scholar]
- 18.Fryxell JM, Greever J, Sinclair ARE. 1988. Why are migratory ungulates so abundant? Am. Nat. 131, 781–798. ( 10.1086/284822) [DOI] [Google Scholar]
- 19.Hopcraft JGC, Holdo R, Mwangomo E, Mduma S, Thirgood S, Borner M, Fryxell JM, Olff H, Sinclair ARE. 2015. Why are wildebeest the most abundant herbivore in the Serengeti ecosystem? In Serengeti IV: sustaining biodiversity in a coupled human-natural system (eds Sinclair ARE, Metzger SAR, Mduma SAR, Fryxell JM), pp. 35–71. Chicago, IL, USA: University of Chicago Press. [Google Scholar]
- 20.Sinclair ARE. 2003. Mammal population regulation, keystone processes and ecosystem dynamics. Phil. Trans. R. Soc. Lond. B 358, 1729–1740. ( 10.1098/rstb.2003.1359) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McNaughton SJ, Banyikwa FF, McNaughton MM. 1997. Promotion of the cycling of diet-enhancing nutrients by african grazers. Science 278, 1798–1800. ( 10.1126/science.278.5344.1798) [DOI] [PubMed] [Google Scholar]
- 22.Robinson BG, Merrill EH. 2013. Foraging–vigilance trade-offs in a partially migratory population: comparing migrants and residents on a sympatric range. Anim. Behav. 85, 849–856. ( 10.1016/j.anbehav.2013.02.004) [DOI] [Google Scholar]
- 23.Dobson AP. et al. 2010. Road will ruin Serengeti. Nature 467, 272–273. ( 10.1038/467272a) [DOI] [PubMed] [Google Scholar]
- 24.Sinclair ARE, Metzger KL, Mduma SAR, Fryxell JM. 2015. Serengeti IV: sustaining biodiversity in a coupled human-natural system. Chicago, IL: University of Chicago Press. [Google Scholar]
- 25.Holdo RM, Fryxell JM, Sinclair ARE, Dobson A, Holt RD. 2011. Predicted impact of barriers to migration on the Serengeti wildebeest population. PLoS ONE 6, e16370 ( 10.1371/journal.pone.0016370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.TAWIRI. 2010. Aerial census in the Serengeti ecosystem—Wet Season 2010. Arusha, Tanzania: TAWIRI. [Google Scholar]
- 27.Rysava K, McGill RAR, Matthiopoulos J, Hopcraft JGC. 2016. Re-constructing nutritional history of Serengeti wildebeest from stable isotopes in tail hair: seasonal starvation patterns in an obligate grazer. Rapid Commun. Mass Spectrom. 30, 1461–1468. ( 10.1002/rcm.7572) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holdo RM, Holt RD, Fryxell JM. 2009. Opposing rainfall and plant nutritional gradients best explain the wildebeest migration in the Serengeti. Am. Nat. 173, 431–445. ( 10.1086/597229) [DOI] [PubMed] [Google Scholar]
- 29.Morrison TA, Bolger DT. 2012. Wet season range fidelity in a tropical migratory ungulate. J. Anim. Ecol. 81, 543–552. ( 10.1111/j.1365-2656.2011.01941.x) [DOI] [PubMed] [Google Scholar]
- 30.Calabrese JM, Fleming CH, Fagan WF, Rimmler M, Kaczensky P, Bewick S, Leimgruber P, Mueller T. 2018. Disentangling social interactions and environmental drivers in multi-individual wildlife tracking data. Phil. Trans. R. Soc. B 373, 20170007 ( 10.1098/rstb.2017.0007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neumann W, Martinuzzi S, Estes AB, Pidgeon AM, Dettki H, Ericsson G, Radeloff VC. 2015. Opportunities for the application of advanced remotely-sensed data in ecological studies of terrestrial animal movement. Mov. Ecol. 3, 8 ( 10.1186/s40462-015-0036-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bartlam-Brooks HLA, Beck PSA, Bohrer G, Harris S. 2013. In search of greener pastures: using satellite images to predict the effects of environmental change on zebra migration. J. Geophys. Res.: Biogeosci. 118, 1427–1437. ( 10.1002/jgrg.20096) [DOI] [Google Scholar]
- 33.Mueller T, Olson KA, Fuller TK, Schaller GB, Murray MG, Leimgruber P. 2008. In search of forage: predicting dynamic habitats of Mongolian gazelles using satellite-based estimates of vegetation productivity. J. Appl. Ecol. 45, 649–658. ( 10.1111/j.1365-2664.2007.01371.x) [DOI] [Google Scholar]
- 34.Strandburg-Peshkin A, Farine DR, Crofoot MC, Couzin ID. 2017. Habitat and social factors shape individual decisions and emergent group structure during baboon collective movement. Elife 6, e19505 ( 10.7554/eLife.19505) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson CJ, Parker KL, Heard DC, Gillingham MP. 2002. Movement parameters of ungulates and scale-specific responses to the environment. J. Anim. Ecol. 71, 225–235. ( 10.1046/j.1365-2656.2002.00595.x) [DOI] [Google Scholar]
- 36.Wilmshurst JF, Fryxell JM, Farm BP, Sinclair A, Henschel CP. 1999. Spatial distribution of Serengeti wildebeest in relation to resources. Can. J. Zool. 77, 1223–1232. ( 10.1139/z99-088) [DOI] [Google Scholar]
- 37.Drent R, Weijand B, Ebbinge B. 1978. Balancing the energy budget of arctic breeding geese throughout the annual cycle: a progress report. Verh. Ornithol. Ges. Bayern. 23, 239–264. [Google Scholar]
- 38.Bischof R, Loe LE, Meisingset EL, Zimmermann B, VanMoorter B, Mysterud A. 2012. A migratory northern ungulate in the pursuit of spring: jumping or surfing the green wave?. Am. Nat. 180, 407–424. ( 10.1086/667590) [DOI] [PubMed] [Google Scholar]
- 39.Cvikel N, Levin E, Hurme E, Borissov I, Boonman A, Amichai E, Yovel Y. 2015. On-board recordings reveal no jamming avoidance in wild bats. Proc. R. Soc. B: Biol. Sci. 282, 20142274 ( 10.1098/rspb.2014.2274) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rutz C, Troscianko J. 2012. Programmable, miniature video-loggers for deployment on wild birds and other wildlife. Methods Ecol. Evol. 4, 114–122. ( 10.1111/2041-210x.12003) [DOI] [Google Scholar]
- 41.Lidgard DC, Bowen WD, Jonsen ID, Iverson SJ. 2012. Animal-borne acoustic transceivers reveal patterns of at-sea associations in an upper-trophic level predator. PLoS ONE 7, e48962 ( 10.1371/journal.pone.0048962) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagy M, Akos Z, Biro D, Vicsek T. 2010. Hierarchical group dynamics in pigeon flocks. Nature 464, 890–893. ( 10.1038/nature08891) [DOI] [PubMed] [Google Scholar]
- 43.Voelkl B, Portugal SJ, Unsöld M, Usherwood JR, Wilson AM, Fritz J. 2015. Matching times of leading and following suggest cooperation through direct reciprocity during V-formation flight in ibis. Proc. Natl Acad. Sci. USA 112, 2115–2120. ( 10.1073/pnas.1413589112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagy M, Couzin ID, Fiedler W, Wikelski M, Flack A. 2018. Synchronization, coordination and collective sensing during thermalling flight of freely migrating white storks. Phil. Trans. R. Soc. B 373, 20170011 ( 10.1098/rstb.2017.0011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haydon DT, Morales JM, Yott A, Jenkins DA, Rosatte R, Fryxell JM. 2008. Socially informed random walks: incorporating group dynamics into models of population spread and growth. Proc. Biol. Sci. 275, 1101–1109. ( 10.1098/rspb.2007.1688) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dalziel BD, Le Corre M, Côté SD, Ellner SP. 2015. Detecting collective behaviour in animal relocation data, with application to migrating caribou. Methods Ecol. Evol. 7, 30–41. ( 10.1111/2041-210X.12437) [DOI] [Google Scholar]
- 47.Langrock R. et al. 2014. Modelling group dynamic animal movement. Methods Ecol. Evol. 5, 190–199. ( 10.1111/2041-210X.12155) [DOI] [Google Scholar]
- 48.Berdahl A, Torney CJ, Ioannou CC, Faria JJ, Couzin ID. 2013. Emergent sensing of complex environments by mobile animal groups. Science 339, 574–576. ( 10.1126/science.1225883) [DOI] [PubMed] [Google Scholar]
- 49.Couzin ID. 2007. Collective minds. Nature 445, 715 ( 10.1038/445715a) [DOI] [PubMed] [Google Scholar]
- 50.Ballerini M. et al. 2008. Interaction ruling animal collective behavior depends on topological rather than metric distance: evidence from a field study. Proc. Natl Acad. Sci. USA 105, 1232–1237. ( 10.1073/pnas.0711437105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katz Y, Tunstrøm K, Ioannou CC, Huepe C, Couzin ID. 2011. Inferring the structure and dynamics of interactions in schooling fish. Proc. Natl Acad. Sci. USA 108, 18 720–18 725. ( 10.1073/pnas.1107583108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herbert-Read JE, Perna A, Mann RP, Schaerf TM, Sumpter DJT, Ward AJW. 2011. Inferring the rules of interaction of shoaling fish. Proc. Natl Acad. Sci. USA 108, 18 726–187 31. ( 10.1073/pnas.1109355108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lukeman R, Li YX, Edelstein-Keshet L. 2010. Inferring individual rules from collective behavior. Proc. Natl Acad. Sci. USA 107, 12 576–12 580. ( 10.1073/pnas.1001763107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harvey RJ, Roskilly K, Buse C, Evans HK, Hubel TY, Wilson AM. 2016. Determining position, velocity and acceleration of free-ranging animals with a low-cost unmanned aerial system. J. Exp. Biol. 219, 2687–2692. ( 10.1242/jeb.139022) [DOI] [PubMed] [Google Scholar]
- 55.Torney CJ, Levin SA, Couzin ID. 2010. Specialization and evolutionary branching within migratory populations. Proc. Natl Acad. Sci. USA 107, 20 394–20 399. ( 10.1073/pnas.1014316107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guttal V, Couzin ID. 2010. Social interactions, information use, and the evolution of collective migration. Proc. Natl Acad. Sci. USA 107, 16 172–16 177. ( 10.1073/pnas.1006874107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parrish JK, Edelstein-Keshet L. 1999. Complexity, pattern, and evolutionary trade-offs in animal aggregation. Science 284, 99–101. ( 10.1126/science.284.5411.99) [DOI] [PubMed] [Google Scholar]
- 58.Gueron S, Levin SA. 1993. Self-organization of front patterns in large wildebeest herds. J. Theor. Biol. 165, 541–552. ( 10.1006/jtbi.1993.1206) [DOI] [Google Scholar]
- 59.Perna A, Grégoire G, Mann RP. 2014. On the duality between interaction responses and mutual positions in flocking and schooling. Mov. Ecol. 2, 22 ( 10.1186/s40462-014-0022-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hooten MB, Johnson DS, McClintock BT, Morales JM. 2017. Animal movement: statistical models for telemetry data. Boca Raton, FL: CRC Press. [Google Scholar]
- 61.Scharf HR, Hooten MB, Fosdick BK, Johnson DS, London JM, Durban JW. 2016. Dynamic social networks based on movement. Ann. Appl. Stat. 10, 2182–2202. ( 10.1214/16-AOAS970) [DOI] [Google Scholar]
- 62.Strandburg-Peshkin A, Papageorgiou D, Crofoot M, Farine D. 2018. Inferring influence and leadership in moving animal groups. 373, 20170006 ( 10.1098/rstb.2017.0006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Torney CJ, Lamont M, Debell L, Angohiatok RJ, Leclerc L-M, Berdahl AM. 2018. Inferring the rules of social interaction in migrating caribou. Phil. Trans. R. Soc. B 373, 20170385 ( 10.1098/rstb.2017.0385) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Plank MJ, Codling EA. 2009. Sampling rate and misidentification of Lévy and non-Lévy movement paths. Ecology 90, 3546–3553. ( 10.1890/09-0079.1) [DOI] [PubMed] [Google Scholar]
- 65.Berg HC. 1983. Random walks in Biology. Princeton, NJ, USA: Princeton University Press. [Google Scholar]
- 66.Benhamou S. 2013. Of scales and stationarity in animal movements. Ecol. Lett. 17, 261–272. ( 10.1111/ele.12225) [DOI] [PubMed] [Google Scholar]
- 67.Michelot T, Langrock R, Patterson TA. 2016. moveHMM: an R package for the statistical modelling of animal movement data using hidden Markov models. Methods Ecol. Evol. 7, 1308–1315. ( 10.1111/2041-210X.12578) [DOI] [Google Scholar]
- 68.Langrock R, King R, Matthiopoulos J, Thomas L, Fortin D, Morales JM. 2012. Flexible and practical modeling of animal telemetry data: hidden Markov models and extensions. Ecology 93, 2336–2342. ( 10.1890/11-2241.1) [DOI] [PubMed] [Google Scholar]
- 69.Patterson TA, Thomas L, Wilcox C, Ovaskainen O, Matthiopoulos J. 2008. State-space models of individual animal movement. Trends Ecol. Evol. 23, 87–94. ( 10.1016/j.tree.2007.10.009) [DOI] [PubMed] [Google Scholar]
- 70.Morales JM, Haydon DT, Frair J, Holsinger KE, Fryxell JM. 2004. Extracting more out of relocation data: building movement models as mixtures of random walks. Ecology 85, 2436–2445. ( 10.1890/03-0269) [DOI] [Google Scholar]
- 71.Fryxell JM, Hazell M, Börger L, Dalziel BD, Haydon DT, Morales JM, McIntosh T, Rosatte RC. 2008. Multiple movement modes by large herbivores at multiple spatiotemporal scales. Proc. Natl Acad. Sci. USA 105, 19 114–19 119. ( 10.1073/pnas.0801737105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McClintock BT, London JM, Cameron MF, Boveng PL. 2017. Bridging the gaps in animal movement: hidden behaviors and ecological relationships revealed by integrated data streams. Ecosphere 8, e01751 ( 10.1002/ecs2.1751) [DOI] [Google Scholar]
- 73.Deneubourg JL, Goss S. 1989. Collective patterns and decision-making. Ethol. Ecol. Evol. 1, 295–311. ( 10.1080/08927014.1989.9525500) [DOI] [Google Scholar]
- 74.Mann RP, Perna A, Strömbom D, Garnett R, Herbert-Read JE, Sumpter DJT, Ward AJ. 2012. Multi-scale inference of interaction rules in animal groups using Bayesian model selection. PLoS Comput. Biol. 8, e1002308 ( 10.1371/journal.pcbi.1002308) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 75.Rosenthal SB, Twomey CR, Hartnett AT, Wu HS, Couzin ID. 2015. Revealing the hidden networks of interaction in mobile animal groups allows prediction of complex behavioral contagion. Proc. Natl Acad. Sci. USA 112, 4690–4695. ( 10.1073/pnas.1420068112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Helm B, Piersma T, van der Jeugd H. 2006. Sociable schedules: interplay between avian seasonal and social behaviour. Anim. Behav. 72, 245–262. ( 10.1016/j.anbehav.2005.12.007) [DOI] [Google Scholar]
- 77.Helbing D, Keltsch J, Molnár P. 1997. Modelling the evolution of human trail systems. Nature 388, 47–50. ( 10.1038/40353) [DOI] [PubMed] [Google Scholar]
- 78.Couzin ID, Krause J. 2003. Self-organization and collective behavior in vertebrates. Adv. Study. Behav. 32, 1–75. ( 10.1016/S0065-3454(03)01001-5) [DOI] [Google Scholar]
- 79.Berdahl AM, Kao AB, Flack A, Westley PAH, Codling EA, Couzin ID, Dell AI, Biro D. 2018. Collective animal navigation and migratory culture: from theoretical models to empirical evidence. Phil. Trans. R. Soc. B 373, 20170009 ( 10.1098/rstb.2017.0009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dorigo M, Bonabeau E, Theraulaz G. 2000. Ant algorithms and stigmergy. Future Gener. Comput. Syst. 16, 851–871. ( 10.1016/S0167-739X(00)00042-X) [DOI] [Google Scholar]
- 81.Talbot LM, Talbot MH. 1963. The wildebeest in western Masailand, East Africa. Wildlife Monographs 12, 3–88. [Google Scholar]
- 82.Biro D, Freeman R, Meade J, Roberts S, Guilford T. 2007. Pigeons combine compass and landmark guidance in familiar route navigation. Proc. Natl Acad. Sci. USA 104, 7471–7476. ( 10.1073/pnas.0701575104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fagan WF. et al. 2013. Spatial memory and animal movement. Ecol. Lett. 16, 1316–1329. ( 10.1111/ele.12165) [DOI] [PubMed] [Google Scholar]
- 84.Bingman VP, Cheng K. 2005. Mechanisms of animal global navigation: comparative perspectives and enduring challenges. Ethol. Ecol. Evol. 17, 295–318. ( 10.1080/08927014.2005.9522584) [DOI] [Google Scholar]
- 85.Piper WH. 2011. Making habitat selection more ‘familiar’: a review. Behav. Ecol. Sociobiol. 65, 1329–1351. ( 10.1007/s00265-011-1195-1) [DOI] [Google Scholar]
- 86.Biro D, Sumpter DJ, Meade J, Guilford T. 2006. From compromise to leadership in pigeon homing. Curr. Biol. 16, 2123–2128. ( 10.1016/j.cub.2006.08.087) [DOI] [PubMed] [Google Scholar]
- 87.Mueller T, O'Hara RB, Converse SJ, Urbanek RP, Fagan WF. 2013. Social learning of migratory performance. Science 341, 999–1002. ( 10.1126/science.1237139) [DOI] [PubMed] [Google Scholar]
- 88.Thorup K, Bisson IA, Bowlin MS, Holland RA, Wingfield JC, Ramenofsky M, Wikelski M. 2007. Evidence for a navigational map stretching across the continental US in a migratory songbird. Proc. Natl Acad. Sci. USA 104, 18 115–18 119. ( 10.1073/pnas.0704734104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Teitelbaum CS, Converse SJ, Fagan WF, Böhning-Gaese K, O'Hara RB, Lacy AE, Mueller T. 2016. Experience drives innovation of new migration patterns of whooping cranes in response to global change. Nat. Commun. 7, 12793 ( 10.1038/ncomms12793) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Biro D, Meade J, Guilford T. 2004. Familiar route loyalty implies visual pilotage in the homing pigeon. Proc. Natl Acad. Sci. USA 101, 17 440–17 443. ( 10.1073/pnas.0406984101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Switzer PV. 1993. Site fidelity in predictable and unpredictable habitats. Evol. Ecol. 7, 533–555. ( 10.1007/BF01237820) [DOI] [Google Scholar]
- 92.Krebs JR. 1971. Territory and breeding density in the Great Tit, Parus Major L. Ecology 52, 2–22. ( 10.2307/1934734) [DOI] [Google Scholar]
- 93.Merkle JA, Cherry SG, Fortin D. 2015. Bison distribution under conflicting foraging strategies: site fidelity versus energy maximization. Ecology 96, 1793–1801. ( 10.1890/14-0805.1) [DOI] [PubMed] [Google Scholar]
- 94.Fryxell JM, Sinclair AR. 1988. Causes and consequences of migration by large herbivores. Trends Ecol. Evol. 3, 237–241. ( 10.1016/0169-5347(88)90166-8) [DOI] [PubMed] [Google Scholar]
- 95.Nathan R, Getz WM, Revilla E, Holyoak M, Kadmon R, Saltz D, Smouse PE. 2008. A movement ecology paradigm for unifying organismal movement research. Proc. Natl Acad. Sci. USA 105, 19 052–19 059. ( 10.1073/pnas.0800375105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fryxell JM, Berdahl AM. 2018. Fitness trade-offs of group formation and movement by Thomson's gazelles in the Serengeti ecosystem. Phil. Trans. R. Soc. B 373, 20170013 ( 10.1098/rstb.2017.0013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sasaki T, Mann RP, Warren KN, Herbert T, Wilson T, Biro D. 2018. Personality and the collective: bold homing pigeons occupy higher leadership ranks in flocks. Phil. Trans. R. Soc. B 373, 20170038 ( 10.1098/rstb.2017.0038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rowcliffe JM, MarcusRowcliffe J, Jansen PA, Kays R, Kranstauber B, Carbone C. 2016. Wildlife speed cameras: measuring animal travel speed and day range using camera traps. Remote Sens. Ecol. Conserv. 2, 84–94. ( 10.1002/rse2.17) [DOI] [Google Scholar]
- 99.Jeltsch F. et al. 2013. Integrating movement ecology with biodiversity research—exploring new avenues to address spatiotemporal biodiversity dynamics. Mov. Ecol. 1, 6 ( 10.1186/2051-3933-1-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.del M Delgado M, Miranda M, Alvarez SJ, Gurarie E, Fagan WF, Penteriani V, di Virgilio A, Morales JM. 2018. The importance of individual variation in the dynamics of animal collective movements. Phil. Trans. R. Soc. B 373, 20170008 ( 10.1098/rstb.2017.0008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sinclair A, Metzger K, Brashares JS, Nkwabi A, Sharam G, Fryxell JM. 2010. Trophic cascades in African savanna: Serengeti as a case study. In Trophic Cascades: Predators, Prey and the Changing Dynamics of Nature (eds Terborgh J, Estes JA), pp. 255–274. Washington DC, USA: Island Press. [Google Scholar]
- 102.Hardesty-Moore M. et al. 2018. Migration in the Anthropocene: how collective navigation, environmental system and taxonomy shape the vulnerability of migratory species. Phil. Trans. R. Soc. B 373, 20170017 ( 10.1098/rstb.2017.0017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wolanski E, Gereta E, Borner M, Mduma S. 1999. Water, migration and the serengeti ecosystem. Am. Sci. 87, 526–533. ( 10.1511/1999.42.838) [DOI] [Google Scholar]
- 104.Sinclair ARE, Hopcraft JGC, Olff H, Mduma SAR, Galvin KA, Sharam GJ. 2008. Historical and future changes to the Serengeti ecosystem. In Serengeti III: human impacts on ecosystem dynamics (eds Sinclair ARE, Packer C), pp. 7–46. Chicago, IL, USA: University of Chicago Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data deposited in Enlighten: Research Data http://dx.doi.org/10.5525/gla.researchdata.566.