Abstract
Lipoprotein lipase (LPL) mediates hydrolysis of triglycerides (TGs) to supply free fatty acids (FFAs) to tissues. Here, we show that LPL activity is also required for hematopoietic stem progenitor cell (HSPC) maintenance. Knockout of Lpl or its obligatory cofactor Apoc2 results in significantly reduced HSPC expansion during definitive hematopoiesis in zebrafish. A human APOC2 mimetic peptide or the human very low-density lipoprotein, which carries APOC2, rescues the phenotype in apoc2 but not in lpl mutant zebrafish. Creating parabiotic apoc2 and lpl mutant zebrafish rescues the hematopoietic defect in both. Docosahexaenoic acid (DHA) is identified as an important factor in HSPC expansion. FFA-DHA, but not TG-DHA, rescues the HSPC defects in apoc2 and lpl mutant zebrafish. Reduced blood cell counts are also observed in Apoc2 mutant mice at the time of weaning. These results indicate that LPL-mediated release of the essential fatty acid DHA regulates HSPC expansion and definitive hematopoiesis.
Lipoprotein lipase (LPL) hydrolyzes triglycerides to supply free fatty acids (FFAs) to muscle for energy and adipocytes for storage. Here, the authors demonstrate that Lpl and its product, the FFA docosahexaenoic acid (DHA) are required for haematopoietic stem progenitor cell expansion during zebrafish embryogenesis.
Introduction
Lipoprotein lipase (LPL) is a major lipase in the vasculature responsible for hydrolysis of triglycerides (TGs) carried by TG-rich lipoproteins and supplying free fatty acids (FFAs) to tissues1. Apolipoprotein C-II (APOC2) is an obligatory cofactor required for LPL activity2. Human patients with APOC2 or LPL deficiency, or deficiency in glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 (GPIHBP1), the LPL vascular anchor, develop severe hypertriglyceridemia and chylomicronemia1, 3. Recent data indicating that plasma TG levels predict cardiovascular risk4, 5 have revived scientific community’s interest in regulation of LPL activity. Study of LPL activity in mice was initially impeded by post-natal lethality of systemic Lpl knockout6, 7. Tissue-specific LPL deficiency in adipose tissue resulted in decreased FFA uptake but increased endogenous synthesis of non-essential FFAs8. Heart-specific Lpl knockout mice showed cardiac dysfunction despite a compensatory increase in glucose utilization9. Similar cardiac phenotypes were observed in human patients with LPL deficiency10. Tissue-specific Lpl overexpression studies suggested that LPL is a key enzyme responsible for tissue-specific insulin sensitivity and lipid metabolism11, 12. These studies implicate LPL-mediated TG hydrolysis and release of FFAs as a key regulator of many physiologic processes in specific tissue contexts. We recently reported the development of systemic Apoc2 mutant mice, characterized by moderate-to-severe hypertriglyceridemia13, which will be used in future studies to investigate related phenotypes.
Zebrafish models have emerged as a new powerful tool to study lipid metabolism14. A hyperlipidemia response to feeding regimens, cholesteryl ester transfer protein (CETP) expression15, pliancy to genetic modifications, and the optical transparency of larval zebrafish significantly facilitate these studies. We have recently reported a chylomicronemia and hypertriglyceridemia phenotype in apoc2 knockout zebrafish16. In the present study, we have developed an lpl knockout zebrafish, which have a similar hypertriglyceridemia phenotype. Remarkably, both apoc2 and lpl mutant zebrafish display profound anemia and defects in hematopoietic stem progenitor cell (HSPC) maintenance and differentiation. Parabiosis of apoc2 and lpl mutants rescues the defective HSPC expansion in both mutants, indicating the importance of circulating FFAs. Docosahexaenoic acid (DHA) is selectively reduced in apoc2 zebrafish mutants. Injections of exogenous DHA in an FFA form, but not the DHA esterified into a TG, rescues the HSPC defects in apoc2 and lpl mutants. In addition, we report anemia in young Apoc2 mutant mice. These findings may have important therapeutic implications for using DHA as a dietary supplement to treat anemia and/or expand HSCs ex vivo.
Results
Loss of apoc2 function in zebrafish results in anemia
Red blood cells of adult apoc2 mutant zebrafish were characterized by hypochromia and decreased hemoglobin staining (Fig. 1a), and the total blood cell count in apoc2 mutants was significantly lower than in WT zebrafish (Fig. 1b). Decreased blood cell numbers, increased numbers of immature erythrocytes and weak hemoglobin staining were also observed in 6.3 days post-fertilization (dpf) zebrafish larvae, but not in 52 h post-fertilization (hpf) embryos (Fig. 1c–e and Supplementary Movies 1 and 2).
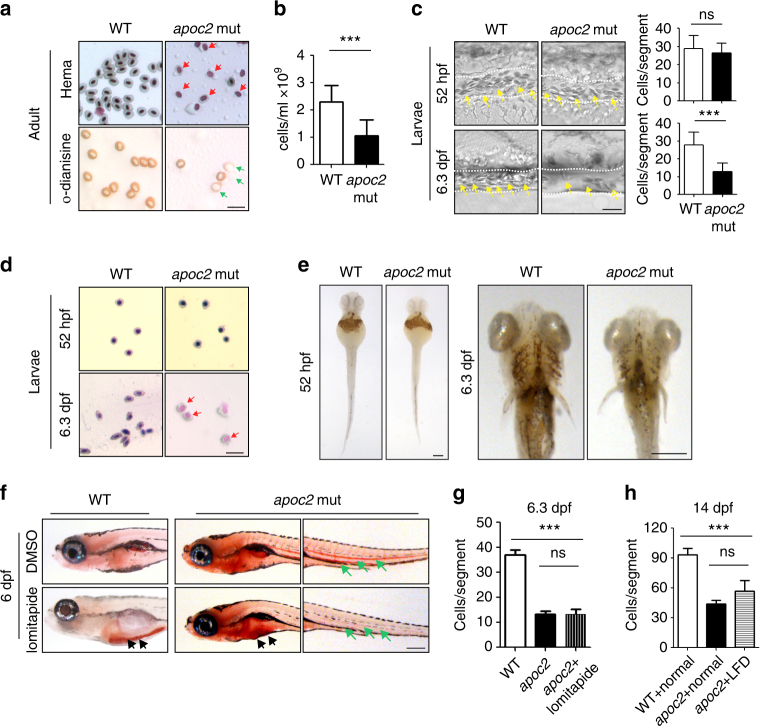
Fig. 1.
Anemia in apoc2 mutant zebrafish. a Wright–Giemsa (Hema) and o-dianisine staining of peripheral blood cells from adult (18–20-month-old) male wild-type (WT) and apoc2 mutant zebrafish. b Quantitative results of peripheral blood cell count (n = 11 for WT and n = 9 for apoc2 mutant groups). c Representative bright field images and quantitative results of blood cell (yellow arrows) count in the caudal vein (outlined with white dashed lines) of WT and apoc2 mutants at 52 hpf (n = 8 in WT and n = 9 in apoc2 mutant groups) and 6.3 dpf (n = 11 for WT and n = 13 for apoc2 mutant groups). See also Supplementary Movies 1 and 2. d Wright–Giemsa staining of blood smears from WT and apoc2 mutants at 52 hpf and 6.3 dpf. Immature erythrocytes containing larger and less condensed nuclei are indicated with red arrows in a and d. Immature erythrocytes with weaker hemoglobin staining are indicated with green arrows in a. e o-Dianisine staining of 52 hpf and 6.3 dpf WT and apoc2 mutant embryos. f Effect of lomitapide: WT and apoc2 mutants were treated with 5 μM lomitapide starting from 2 dpf until embryos were fixed at 6 dpf for ORO staining. Black arrows point to intestinal lipid accumulation and green arrows to circulating lipids. g Blood cell counts in the caudal vein of WT, apoc2 mutants and apoc2 mutants treated with lomitapide at 6.3 dpf (n = 5 in each group). h Blood cell counts in the caudal vein of 14 dpf WT, apoc2 mutants fed with normal diet and apoc2 mutants fed with low-fat diet (LFD) starting at 5 dpf (n = 4 in WT and apoc2 mut groups each; n = 5 in apoc2 + LFD group). Scale bars, 20 μm in a, c, and d, 100 μm in e, and 200 μm in f. Mean ± SEM; ***P < 0.001 (Student’s t test)
The hematopoiesis phenotype in apoc2 knockout zebrafish could be due to hyperlipidemia, i.e., high levels of non-hydrolyzed TG, and/or due to diminished FFA supply. To test whether hematopoietic defects are a direct result of hyperlipidemia, we treated apoc2 mutants with lomitapide, an inhibitor of microsomal triglyceride transfer protein, which reduces very low-density lipoprotein (VLDL) formation and is used as a lipid-lowering drug for treatment of familial hypercholesterolemia patients. Lomitapide has been reported to reduce hyperlipidemia in zebrafish as well17. Treatment with lomitapide starting from 2 dpf reduced hyperlipidemia but did not rescue anemia in apoc2 mutant zebrafish (Fig. 1f, g). Similarly, feeding apoc2 mutants a low-fat diet reduced hyperlipidemia but did not have a significant effect on anemia (Supplementary Fig. 1 and Fig. 1h).
lpl mutants show defective hematopoiesis
Zebrafish apoc2 is expressed in the yolk and intestine, but lpl is expressed in the head and in the caudal hematopoietic tissue (CHT) at 2 dpf, the latter is a hematopoietic organ during embryonic development (Supplementary Fig. 2 and Fig. 2a). To further evaluate the role of LPL activity in hematopoiesis, we mutated the zebrafish lpl gene using CRISPR-Cas9 and obtained a line with a 2 nt deletion in exon 4 of the lpl gene, resulting in-frame-shift and a pre-stop codon. This mutation may result in a truncated mRNA or in-frame exon skipping and alternative splicing18. To address the possibility of an alternative splicing, we cloned cDNA from the lpl mutant and found no alternative transcripts. Furthermore, we confirmed the presence of only one transcript carrying the 2 nt deletion that encodes a truncated, loss-of-function Lpl protein lacking its heparin-binding domain (Fig. 2b and Supplementary Fig. 3A–C). Interestingly, there was a compensatory increase in apoc2 expression in the lpl mutants, and a compensatory increase in lpl expression in the apoc2 mutants (Fig. 2c). However, because APOC2 does not have a catalytic activity and LPL is not functional without APOC2, both mutants developed hypertriglyceridemia (Fig. 2d and Supplementary Fig. 3D–E). Importantly, we found a similar anemia and hypochromia phenotype in adult and 6.3 dpf larval lpl mutants as those found in apoc2 mutants (Fig. 2e–i).
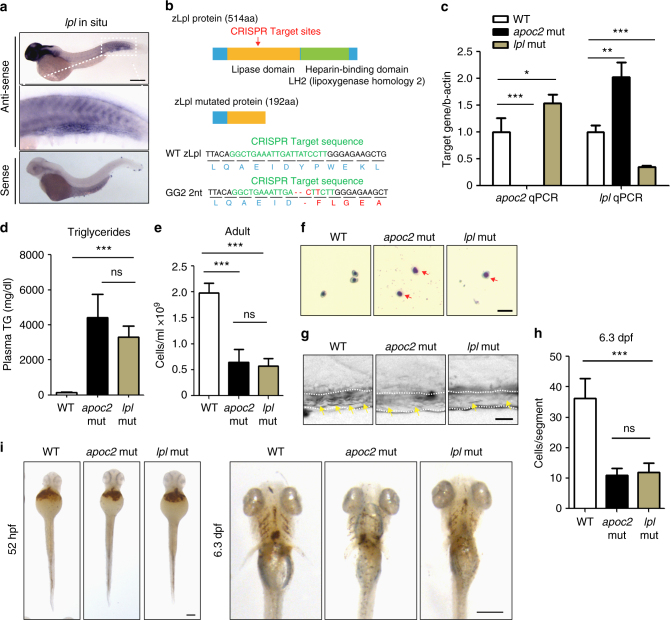
Fig. 2.
Hematopoietic defects in lpl mutants. a In situ hybridization with lpl antisense and sence probes in WT embryos at 2 dpf. b Diagram of lpl CRISPR target sites and the predicted truncated protein caused by the 2 nt deletion, which results in a codon shift and premature translation termination. c qPCR results of lpl and apoc2 mRNA expression in WT, apoc2 and lpl mutants at 5 dpf (n = 3 in each group). d Plasma TG levels in adult (9–15-month-old) male WT, apoc2 and lpl mutants (n = 5 in each group). e Peripheral blood cell count in adult (15-month-old) male WT, apoc2 and lpl mutants (n = 5 in each group). f Wright–Giemsa staining of blood smears from 6.3 dpf WT, apoc2 and lpl mutants. g, h Representative images and quantitative results of blood cell (yellow arrows) count in the caudal vein (outlined with white dashed lines) of WT, apoc2 and lpl mutants at 6.3 dpf (n = 5 in WT and apoc2 mut groups each; n = 6 in lpl mut group). i o-Dianisine staining of 52 hpf and 6.3 dpf WT, apoc2 and lpl mutant embryos. Scale bars, 200 μm in a; 20 μm in f, g, and 100 μm in i. Quantitative results are mean ± SEM; *P < 0.05 and ***P < 0.001 (Student’s t test)
HSPC expansion is disrupted in apoc2 and lpl mutants
Similar to mammals, zebrafish have two major waves of hematopoiesis, primitive and definitive19–21. The transitive primitive hematopoiesis begins at 11 hpf and produces erythroid and myeloid cells from mesoderm-derived hemangioblasts that persist for the first several days post fertilization. The definitive hematopoiesis is attained via HSPCs, which are specified from hemogenic endothelium comprising the ventral aspect of the dorsal aorta (VDA) at 20 hpf and then migrate to the CHT, where HSPCs undergo expansion and differentiate into mature blood cells at 2–4 dpf.
Interestingly, apoc2 and lpl mutants did not have any detectable defects in total blood cell count or hemoglobin content at 52 hpf, but these measurements were decreased at 6.3 dpf (Figs. 1c–e and 2i). Because erythrocytes are mostly derived from primitive hematopoiesis at 52 hpf and largely from definitive hematopoiesis at 6.3 dpf, we hypothesized that loss of apoc2 may affect definitive hematopoiesis and thus cause anemia at later larval stages and in adults. To test this hypothesis, we performed whole mount in situ hybridization with probes for specific hematopoietic markers at different stages.
At 20 hpf, wild-type and apoc2 mutant zebrafish had similar expression of gata1 and beta-globin (erythropoiesis), and pu.1 (myelopoiesis) (Supplementary Fig. 4A), suggesting no detectable defects in primitive hematopoiesis. Furthermore, wild-type and apoc2 mutants showed similar expression of HSPC markers runx1 in the VDA at 26 hpf and cmyb in the VDA and CHT at 52 hpf (Supplementary Fig. 4B), implicating that HSPC specification and migration were not affected. However, at 80 hpf, expression of the HSPC markers runx1/cmyb and the blood lineage markers beta-globin (erythropoiesis) and rag1 (lymphopoiesis) was significantly decreased in apoc2 mutants compared to wild type (Supplementary Fig. 4C), suggesting that HSPC expansion and differentiation were affected. The HSPC expansion defect was not due to a moderately delayed angiogenesis in the apoc2 mutant16, as the defects persisted in angiogenesis-synchronized apoc2 mutant embryos (Supplementary Fig. 5). As expected from results shown in Fig. 1g, lomitapide did not rescue the defect in runx1/cmyb and beta-globin expression in apoc2 mutants (Supplementary Fig. 6). There was no increase in apoptosis in the CHT region of apoc2 mutants (Supplementary Fig. 7). Comparing apoc2 mutants with lpl mutants, we confirmed that in both animal models, the hematopoietic defect commenced at the HSPC expansion (80 hpf) but not the specification or migration (26–50 hpf) stage of definitive hematopoiesis (Fig. 3a, b).
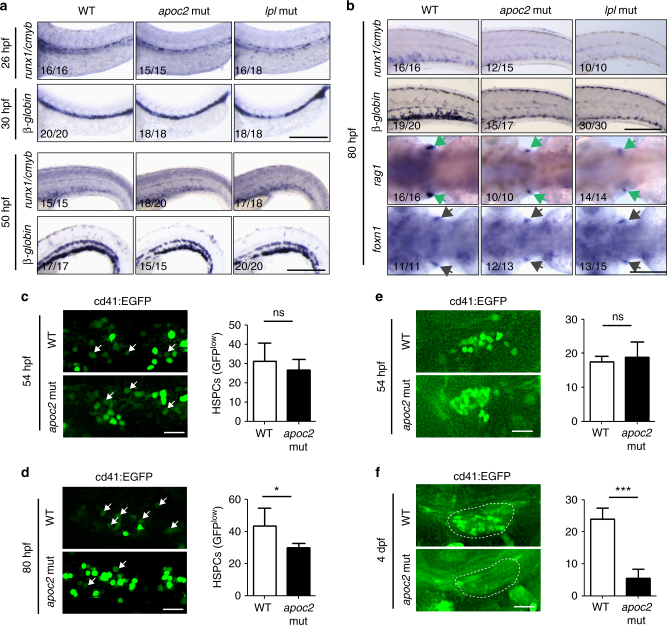
Fig. 3.
Hematopoietic defects in apoc2 and lpl mutants occur during HSPC expansion. a In situ hybridization with cmyb/runx1 and β-globin probes in WT, apoc2 and lpl mutants at 26 hpf, 30 hpf and 50 hpf. b In situ hybridization with cmyb/runx1, β-globin, rag1 (green arrows) and foxn1 (black arrows) probes in WT, apoc2 and lpl mutants at 80 hpf. foxn1 is a thymus development marker, used as a control. c, d Representative images and numbers of GFPlow cells (HSPCs, white arrows) in the CHT region of cd41:EGFP transgenic WT and apoc2 mutants at 54 and 80 hpf (n = 10 in WT and n = 8 in apoc2 mutant groups at 54 hpf; n = 6 in each group at 80 hpf). e, f Representative images and numbers of GFP-positive cells in the thymus region at 54 hpf (n = 9 in WT and n = 8 in apoc2 mutant groups) and at 4 dpf (n = 8 in each group). Scale bars, 200 μm in a and b; 50 μm in c–f. Mean ± SEM; *P < 0.05 and ***P < 0.001 (Student’s t test)
To visualize HSPCs in real-time, we used cd41:EGFP transgenic zebrafish in which HSPCs display weak EGFP fluorescence and become EGFP bright upon differentiation into thrombocyte progenitors in the CHT22, 23. In agreement with the in situ hybridization results, at 54 hpf, there were similar number of EGFPlow cells in the CHT region of wild-type and apoc2 mutants. When the same clutch of zebrafish was re-examined at 80 hpf, the numbers of HSPCs (EGFPlow) were significantly decreased in the CHT region of apoc2 mutants when compared to wild type (Fig. 3c, d). Consistent with the CHT data, numbers of EGFP-positive HSPCs that migrated from the CHT to the thymus, where they differentiate into lymphoid cells, decreased dramatically from 54 hpf to 4 dpf (Fig. 3e, f).
LPL-mediated triglyceride hydrolysis regulates hematopoiesis
If the hematopoietic defect in apoc2 mutants manifests from 50 hpf to 80 hpf, then restoration of apoc2 function starting at 2 dpf should rescue the hematopoiesis phenotype. Indeed, injecting the human APOC2 mimetic peptide CII-a, but not its inactive analog CII-i, rescued the hyperlipidemia and anemia phenotype at 6.3 dpf (Fig. 4a–d) and, importantly, restored both cmyb/runx1 and beta-globin expression at 3.3 dpf (Fig. 4e) in apoc2 mutants.
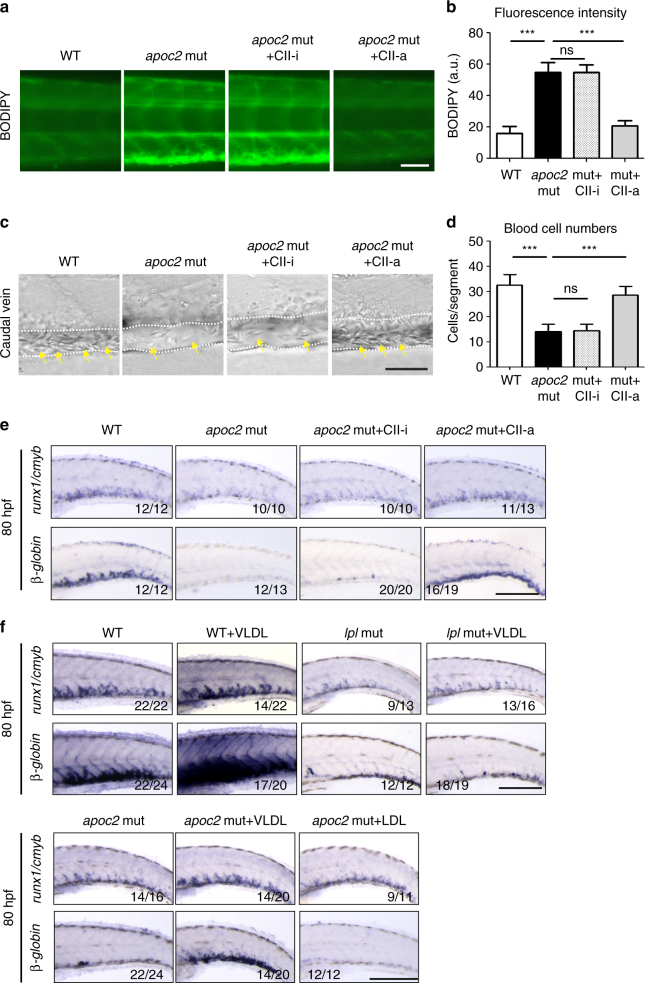
Fig. 4.
APOC2 mimetic peptide and VLDL rescue anemia in apoc2 mutants. a, b Representative images of BODIPY staining and quantitative results of BODIPY fluorescence intensity in WT, apoc2 mutants and the apoc2 mutants injected with APOC2 mimetic peptides (CII-a, active; CII-i, inactive) at 6.3 dpf (n = 5 in each group). c, d Representative images of blood cells in the caudal vein region and quantitative results of blood cell count in WT, apoc2 mutants and the apoc2 mutants injected with APOC2 mimetic peptides at 6.3 dpf (n = 6 in WT and mut + CII-i goups each; n = 8 in apoc2 mut group; n = 7 in mut + CII-a group). e In situ hybridization with cmyb/runx1 and β-globin probes in WT, apoc2 mutants and the apoc2 mutants injected with APOC2 mimetic peptides at 80 hpf. Embryos were injected with peptides at 2 dpf. f In situ hybridization with cmyb/runx1 and β-globin probes in WT, apoc2 and lpl mutants, including those injected with VLDL or LDL at 2 dpf. Embryos were analyzed at 80 hpf. Scale bars, 50 μm in a and c; 200 μm in e and f. Mean ± SEM; ***P < 0.001 (Student’s t test)
We next investigated whether TG hydrolysis, irrespective of hypertriglyceridemia, could affect normal hematopoiesis. Injection of human VLDL into WT embryos at 2 dpf enhanced expression of HSPC and erythrocyte markers at 80 hpf, suggesting that oversupply of the TG substrate for Lpl-mediated hydrolysis promotes, rather than inhibits, HSPC expansion and erythroid differentiation in the CHT region at this specific stage (Fig. 4f). Importantly, VLDL (which carries APOC2), but not LDL (which does not contain APOC2), rescued the defective hematopoiesis in apoc2 mutants when injected at 2 dpf (Fig. 4f). However, VLDL injection did not rescue the hematopoiesis defects in lpl mutants (Fig. 4f) because these zebrafish did not have functional Lpl to be activated by VLDL-delivered APOC2.
Together, these data suggest that hyperlipidemia per se does not cause a hematopoietic defect in apoc2 mutants and that ineffective plasma TG hydrolysis in apoc2 and lpl mutants is the cause of defective HSPC expansion and differentiation in the CHT region.
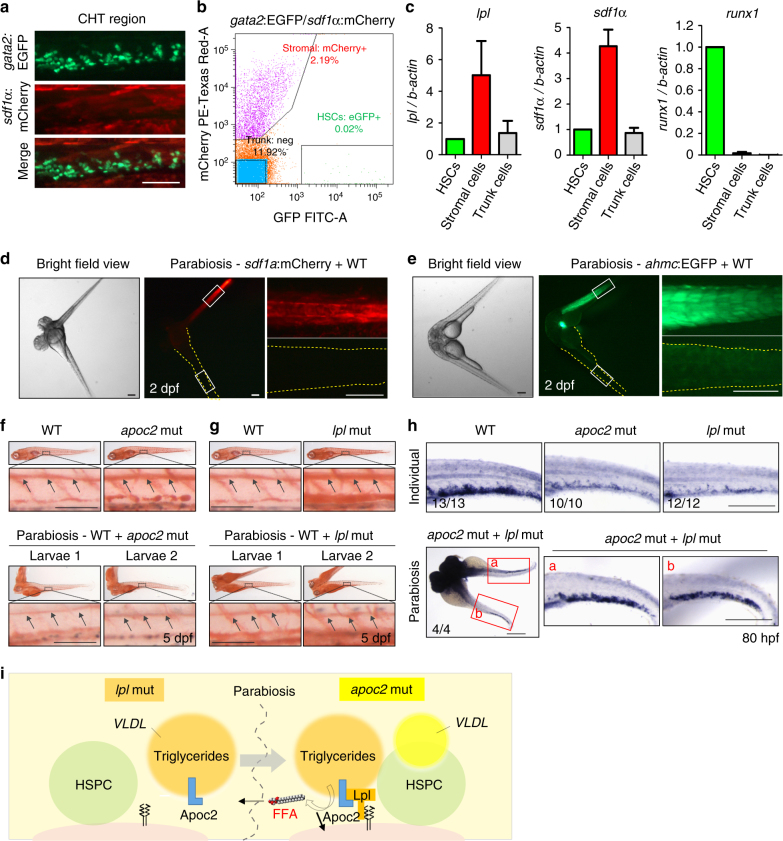
HSPCs anchor to mesenchymal stromal cells after arrival in the CHT region and this interaction is crucial for HSPCs maintenance and differentiation24. To determine which cell types express lpl, we sorted out HSPCs and stromal cells from the zebrafish expressing EGFP driven by the gata2 promoter (HSPCs) and mCherry driven by sdf1α promoter (stromal cells; Fig. 5a, b). The sorting results were confirmed by testing mRNA expression of runx1 and sdf1a in isolated fractions (Fig. 5c). Because there were 100-fold more stromal cells than HSPCs (Fig. 5b) and because stromal cells, but not HSPCs, highly expressed lpl (Fig. 5c), we concluded that stromal cells are the major source of lpl expression in the CHT.
Fig. 5.
Parabiosis of apoc2 and lpl mutants rescues defective hematopoiesis. a The CHT region of gata2:EGFP, sdf1a:mCherry double-positive embryos at 2.5 dpf. b Flow cytometry of gata2:EGFP and sdf1a:mCherry positive cells isolated from the CHT region. c RT-qPCR analysis of FACS-sorted gata2:EGFP and sdf1a:mCherry positive cells, using lpl, runx1 and sdf1α primers. Mean ± SD of two independent experiments. d, e Parabiosis of a sdf1α:mCherry or a ahmc:EGFP with a WT embryo. Right-hand panels are enlarged segments showed in white quadrangles in left-hand panels. Yellow dashed lines trace WT embryos’ boundaries. f, g Rescue of hyperlipidemia in apoc2 or lpl mutants by parabiosis with WT embryos. Upper panels: two separated embryos (WT and apoc2 or lpl). Lower panels: larva 1 and larva 2 from a parabiosis pair (WT with apoc2 or WT with lpl). Black arrows point to ORO staining in the lumen of blood vessels. h In situ hybridization with runx1/cmyb probe in WT, individual apoc2 and lpl mutants, and parabiotic apoc2 and lpl mutants at 80 hpf. Scale bars, 50 μm in f, g, and 200 μm in a, d, e and h. i Diagram of lipoprotein metabolism in parabiotic lpl and apoc2 mutants. In the lpl mutant, no Lpl is expressed. However, VLDL secreted by the lpl mutant (orange) delivers Apoc2 through the shared circulation to the apoc2 mutant, in which Lpl is expressed but its own VLDL (yellow) contains no Apoc2. VLDL from the lpl mutant compensates lack of Apoc2 in the apoc2 mutant and the reconstituted Apoc2/Lpl catalyzes hydrolysis of TG to release FFAs into the shared circulation, which in turn rescue the hematopoiesis defect in both mutants
To characterize in vivo the requirement for both Apoc2 and Lpl in supporting hematopoiesis, we employed a parabiotic zebrafish protocol25. After fusion of a sdf1α:mCherry or a ahmc:EGFP transgenic embryo, in which the EGFP is expressed in the cardiac and skeletal muscle cells at early stages, with a WT embryo, we found no detectable tissue expression of mCherry or EGFP in the WT embryo, indicating no stromal or muscle cell exchange (Fig. 5d, e). Fusion of WT embryos with either apoc2 or lpl mutants rescued the hyperlipidemia phenotype in both (Fig. 5f, g), suggesting shared blood circulation in parabiotic zebrafish. Interestingly, fusing apoc2 mutants with lpl mutants rescued cmyb/runx1 expression in the CHT region of both (Fig. 5h). These data suggest that under parabiotic settings, Apoc2 from the lpl mutant and Lpl from the apoc2 mutant could reconstitute the Apoc2/Lpl pathway. We thus propose that the Apoc2/Lpl-mediated release of FFAs into shared circulation rescues hematopoiesis in both mutants, even though the Lpl deficiency persists in the CHT niche in lpl mutants (Fig. 5i).
Free fatty acid DHA regulates hematopoiesis in zebrafish
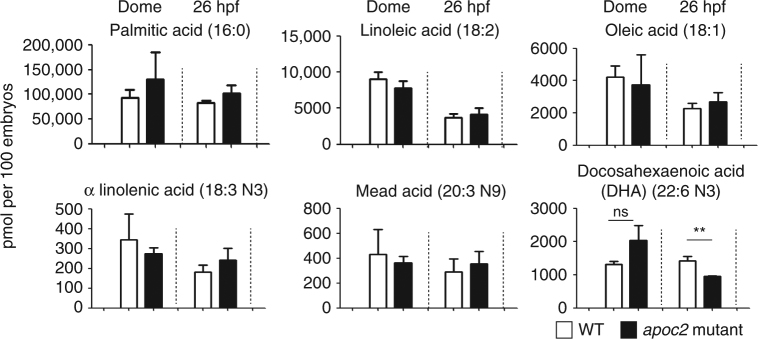
Although our data suggest that LPL-mediated hydrolysis of TGs regulates HSPC proliferation in zebrafish, surprisingly, we did not find a reduction in the total levels of FFAs in total body homogenates of apoc2 mutants compared to WT (Supplementary Fig. 8, bottom-right graph). This can be explained by a compensatory FFA synthesis in apoc2 mutants. Indeed, levels of FFAs that can be made de novo, trended higher or were not changed in apoc2 mutants. However, levels of DHA (22:6n3), which in zebrafish can only be derived from TG hydrolysis, were significantly lower in 26 hpf embryos (Fig. 6 and Supplementary Fig. 8).
Fig. 6.
Reduced DHA levels in apoc2 mutants. FFAs in WT and apoc2 mutants at dome and 26 hpf stages (n = 3; each replicate is a pool of 20 embryos); mean ± SEM; **P < 0.01 (Student’s t test)
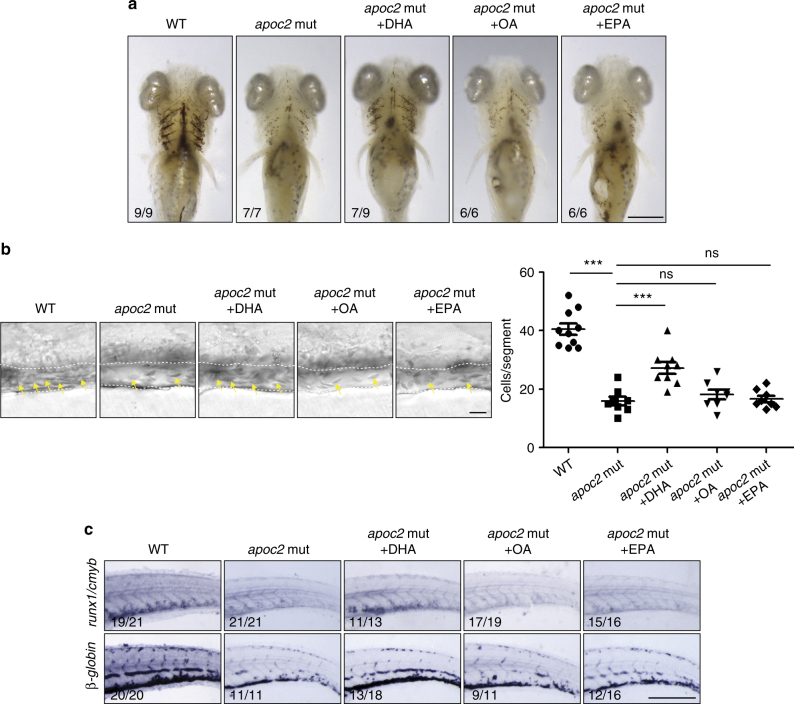
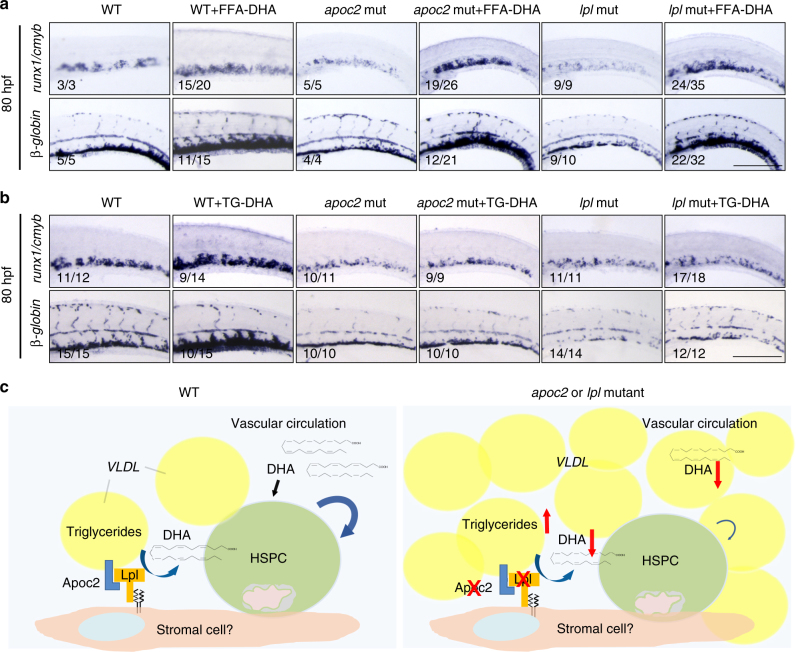
To investigate whether DHA plays a specific role in HSPC expansion in the CHT region, we injected DHA, as well as another n3 polyunsaturated fatty acid (PUFA) eicosapentaenoic acid (EPA; 20:5n3) or monounsaturated oleic acid (OA; 18:1n9) (all solubilized with BSA) into apoc2 mutants at 2 dpf. DHA, but not OA or EPA, rescued red blood cell hypochromia, hemoglobin levels, blood cell counts, and expression of runx1/cmyb and beta-globin (Supplementary Fig. 9 and Fig. 7a–c). To test whether DHA must be in a free fatty acid form (FFA-DHA) in order to rescue the hematopoiesis phenotype, in a separate experiment, we injected embryos with FFA-DHA or with tridocosahexaenoin, a TG with three esterified DHA acyl chains (TG-DHA; delivered as POPC micelles). FFA-DHA enhanced expression of runx1/cmyb and beta-globin in WT embryos and rescued hematopoietic phenotypes in apoc2 and lpl mutants (Fig. 8a). Remarkably, injection of TG-DHA enhanced runx1/cmyb and beta-globin expression only in WT zebrafish but did not have any effect on the phenotypes of apoc2 or lpl mutants (Fig. 8b), which are defective in TG hydrolysis. POPC alone did not have any effect on runx1/cmyb and beta-globin expression in WT zebrafish (Supplementary Fig. 10). Taken together, these results suggest that DHA is a major functional FFA released from TGs through the APOC2/LPL pathway that plays an important role in HSPC expansion during definitive hematopoiesis in zebrafish (Fig. 8c).
Fig. 7.
DHA rescues hematopoiesis in apoc2 mutants. a–c apoc2 mutant embryos were injected with free fatty acid docosahexaenoic acid (DHA), oleic acid (OA), or eicosapentaenoic acid (EPA) at 48 hpf. a o-Dianisine staining of 6.3 dpf larvae. b Representative bright field images and quantitative results of blood cell (yellow arrows) count in the caudal vein (outlined with white dashed lines) at 6.3 dpf. Mean ± SEM; n = 10 (WT), n = 8 (apoc2 mut, apoc2 mut + OA, and apoc2 mut + EPA), and n = 9 (apoc2 mut + DHA). ***P < 0.001 (Student’s t test). c In situ hybridization with cmyb/runx1 and β-globin probes. Scale bars, 100 μm in a; 50 μm in b; and 200 μm in c
Fig. 8.
FFA-DHA but not TG-DHA rescues hematopoiesis in apoc2 and lpl mutants. a, b In situ hybridization with runx1/cmyb and β-globin probes in WT, apoc2 and lpl mutants injected with FFA-DHA (a) or TG-DHA (b) at 2 dpf; embryos were fixed at 80 hpf for in situ hybridization. Scale bars, 200 μm. c Schematic representation of the working hypothesis. VLDL delivers both the TG substrate and Apoc2, an obligatory activator of Lpl to the hematopoietic niche. Lpl, expressed on stromal and/or endothelial cells, catalyzes hydrolysis of TG to produce FFAs. Among FFAs released by the Apoc2/Lpl catalysis, the essential fatty acid DHA supports normal hematopoiesis. apoc2 and lpl mutant zebrafish in which TG hydrolysis is blocked, have a defect in HSPC maintenance and differentiation. Administration of DHA as a free fatty acid, but not DHA esterified into a TG, rescues the hematopoiesis defect in apoc2 and lpl mutant zebrafish
Anemia in Apoc2 mutant mice
In mouse bone marrow, HSPCs reside in a complex niche, including stromal cells26. In in vitro experiments, stromal cells are used to support HSPCs maintenance, proliferation, and differentiation. While APOC2 is associated with circulating VLDL, LPL is a cell surface-associated protein highly expressed in tissues dependent on FFA supply for energy (cardiac and skeletal muscle) or storage (adipose)27–29. To examine which bone marrow cell types express Lpl, we performed RT-qPCR and found that Lpl was not expressed in mouse HSPCs (defined for the purposes of this work as Lin−cKit+ Sca1+) or in other Lin− bone marrow cells (Supplementary Fig. 11). However, in agreement with the zebrafish FACS sorting/RT-qPCR data (Fig. 5b, c), stromal cells capable of HSPC support (OP9 cell line) expressed high levels of LPL (Supplementary Fig. 11). Thus, the effect of LPL activity on HSPC maintenance is non-cell autonomous.
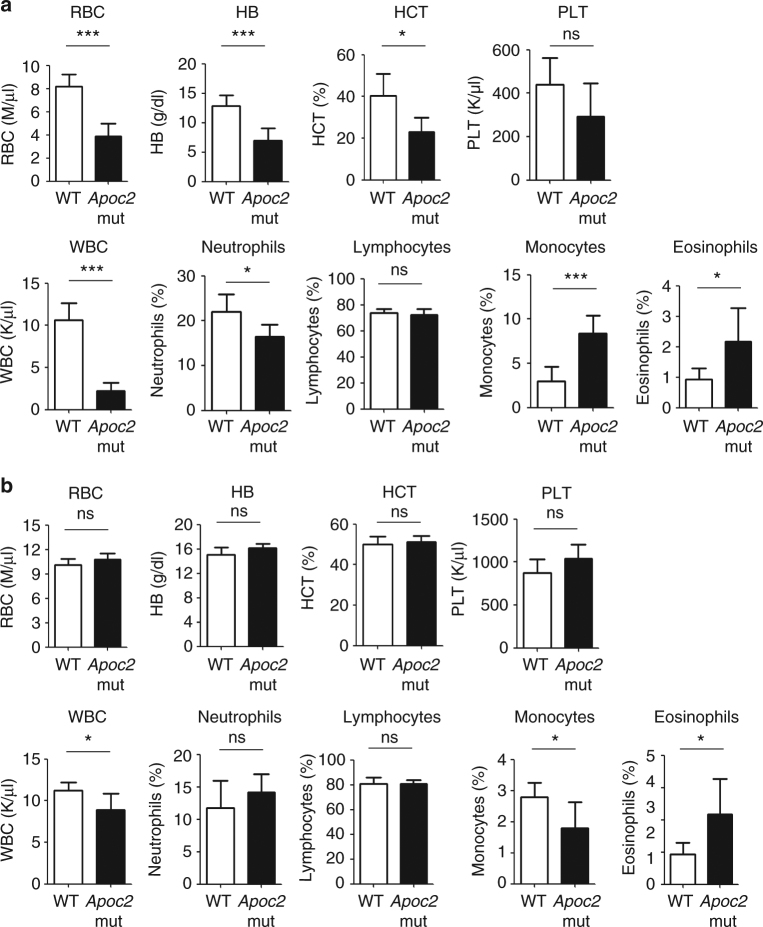
While whole-body LPL knockout mice die soon after birth, homozygous Apoc2 mutant mice, in which three amino acids are deleted, survive and develop hypertriglyceridemia (757.5 ± 281.2 mg/dl)13. We found that Apoc2 mutant mice had much less white and red blood cells compared to wild-type mice, when assessed at the time of weaning (4 weeks) (Fig. 9a). However, the profound anemia phenotype was alleviated in adult Apoc2 mutant mice (3–4 months), though mutant mice still had significantly less white blood cells (Fig. 9b). These data suggest that LPL activity may have an important role in mammalian hematopoiesis as well.
Fig. 9.
Complete blood count in WT and Apoc2 mutant mice. a Blood samples from WT mice and Apoc2 mutant mice were analyzed at the time of weaning (28–31 days old). WT, n = 7 (four males and three females); Apoc2 mutants, n = 5 (two males and three females). No apparent differences between sexes were observed in each group. b Blood samples from WT mice and Apoc2 mutant mice were analyzed at the age of 4–5 months. n = 5 in each group, all males. Results are mean ± SEM; *P < 0.05 and ***P < 0.001 (Student’s t test). WBC white blood cells, RBC red blood cells, HB hemoglobin, HCT hematocrit, PLT platelets. Absolute numbers of WBC and percentages (%) of neutrophils, lymphocytes, monocytes and eosinophils are shown in lower row of graphs in a and b
Discussion
Our work with apoc2 and lpl mutant zebrafish resulted in an observation of profound anemia in these LPL activity deficient zebrafish (Figs. 1 and 2), which was not previously discovered in relevant LPL mouse models. We found that although primitive hematopoiesis and early HSPC specification and migration were not affected in the apoc2 and lpl mutants, there was decreased HSPC expansion in the CHT niche (Fig. 3a, b). With the cd41:EGFP transgenic fish line, we found HSPCs, but not differentiated thrombocytes, were decreased during its expansion in the CHT region, which resulted in decreased HSPCs migration to the thymus (Fig. 3c–f). Restoration of Apoc2 function starting at 2 dpf rescued the hematopoietic defects (Fig. 4a–e), confirming the late onset of the requirement for LPL activity in hematopoiesis. The CHT niche is highly vascularized, which facilitates delivery of TG by VLDL. VLDL also carries Apoc2, the activator of Lpl. Thus, injections of human VLDL, supplying both the TG substrate and the APOC2, rescued the HSPCs defect in apoc2 mutants but not in lpl mutants, and increased hematopoietic markers in WT zebrafish (Fig. 4f).
Our in situ hybridization data indicated that lpl was highly expressed in the CHT region (Fig. 2a) and FACS sorting/RT-qPCR results suggested stromal cells were the major source of lpl expression (Fig. 5a–c). In agreement with the zebrafish results, mouse studies demonstrated that stromal cells, but not HSPCs, expressed Lpl (Supplementary Fig. 11), suggesting that HSPCs rely on systemic and/or stromal cell-derived LPL activity. The non-cell autonomous character of LPL activity in regulation of HSPC maintenance complicates mouse studies because of the post-natal lethality of systemic Lpl knockout. Although mice with the complete loss of APOC2 are not available, Apoc2 mutant mice with a three amino acid deletion, which leads to the retention of the signal peptide and the lack of APOC2 on TG-rich lipoproteins, are viable and show moderate hypertriglyceridemia13, suggesting this mutation is hypomorphic and that Apoc2 mutant mice can be used as a model of partial LPL deficiency. The finding of anemia in young Apoc2 mutant mice (Fig. 9a) suggests that our discovery of the role of LPL activity in zebrafish hematopoiesis is relevant to mammalian biology. There is a report of lower hemoglobin levels and anemia observed in 10 out of 14 infants with LPL deficiency30, but to the best of our knowledge, there are no reports of anemia in adult human patients with LPL or APOC2 deficiency. This is in agreement with our findings of the anemia phenotype being resolved in adult Apoc2 mutant mice (Fig. 9b). One reason for anemia resolution in adults could be an increased expression of other lipases, such as hepatic lipase, endothelial lipase, or phospholipase A2, which hydrolyze TG or phospholipid substrates, releasing FFA-DHA into the circulation to support HPSC maintenance. Changes in diet, from fat rich to carbohydrate rich, in mammals, but not in zebrafish may also play a role.
Previous studies have reported a moderate delay in developmental angiogenesis in apoc2 mutant or knockdown zebrafish embryos, which rapidly recovered in older larvae16, 31. It has been suggested that increased levels of apoB negatively regulate developmental angiogenesis in zebrafish31. To evaluate the possibility that the hematopoietic defects observed in apoc2 mutants may be due to the increased apoB-driven delay in angiogenesis in hyperlipidemic zebrafish, we synchronized angiogenesis in WT and apoc2 mutant embryos and found that the hematopoietic defects persisted in apoc2 mutants (Supplementary Fig. 5). Furthermore, inhibition of apoB lipoprotein production by lomitapide did not rescue anemia in apoc2 mutants (Supplementary Fig. 6) in which LPL function remained defective. These results suggest that the LPL deficiency and not delayed angiogenesis or increased apoB levels is responsible for the hematopoietic defect in apoc2 mutants. Restoring LPL activity in parabiotic apoc2 and lpl mutants rescued the hematopoietic defect in both (Fig. 5).
We found that the essential fatty acid DHA was specifically downregulated in apoc2 mutant embryos (Fig. 6 and Supplementary Fig. 8). This is in agreement with a report that DHA levels were decreased in the hypothalamus of neuron-specific LPL-deficient mice32. DHA supplementation increases ex vivo expansion of CD34+ cells derived from umbilical cord or peripheral blood and enhances generation of megakaryocytes33, 34. Diets enriched in fish oil have been reported to promote hematopoiesis in mice, the effect that the authors attributed to MMP12-dependent remodeling of the hematopoietic niche35. Maternal dietary supplementation of n3 PUFAs, which contain 56% of DHA, has been shown to increase numbers of CD34+ hematopoietic progenitor cells36. A clinical trial is under way to test whether diet supplementation with DHA during the second to third trimester of pregnancy could improve the viability of stem cells derived from umbilical cord blood37.
However, as we now understand from our studies, supply of the essential fatty acid DHA or its precursors with the diet is necessary but not sufficient to promote hematopoiesis. The DHA needs to be present in an FFA form to be active in the hematopoietic niche, but diet-supplied DHA is esterified in the digestive system to be transported by TG-rich lipoproteins38. Thus, LPL activity is necessary to release DHA from the TGs. This is demonstrated in our experiments showing that injections of DHA in the form of FFA, but not in the esterified TG form, rescue the hematopoietic defects in apoc2 and lpl mutants (Fig. 8a, b). Both FFA and TG forms of DHA promote hematopoiesis in the WT zebrafish in which Lpl function is preserved (Fig. 8). The effect seems to be specific to DHA since another n3 PUFA, EPA, or the monounsaturated OA did not rescue hematopoietic defect in apoc2 mutants (Fig. 7 and Supplementary Fig. 9).
The exact cellular mechanism of FFA-DHA-mediated HSPC maintenance remains to be elucidated. It may include activation of PPARα/δ or upregulation of genes involved in FAO and mitochondrial biogenesis39–42. In addition, enzymatic oxidation of DHA might be important for its function since several enzymes capable of oxidizing DHA, such as soluble epoxide hydrolase, 12/15-lipoxygenase, or cytochrome P450 epoxygenase, have been reported to regulate HSPCs function43–45. Enzymatic products of FFA arachidonic acid (20:4n6) oxidation, prostaglandin E2 (PGE2), and epoxyeicosatrienoic acids, have been shown to regulate HSC homing and engraftment45, 46. Clinical applications for PGE2 in umbilical cord blood HSC transplantation have been suggested47, 48. Feeding mice with a mix of n6 and n3 PUFAs, including DHA, stimulated hematopoiesis and thrombopoiesis, as well as engraftment of donor cells49.
In summary, our studies uncovered an important role of LPL activity in regulation of HSPC maintenance and definitive hematopoiesis. The mechanism includes LPL-mediated release of the essential fatty acid DHA to support HSPC maintenance. These findings may have important therapeutic implications, both in terms of dietary recommendations and in optimizing conditions for ex vivo HSPC expansion.
Methods
Ethics statement
All animal experiments were performed according to the NIH guidelines and were approved by the University of California, San Diego Institutional Animal Care and Use Committee (protocols S07266 and S04155). Human plasma, used for VLDL and LDL isolation, was obtained from normal volunteers who provided written informed consent according to a protocol approved by the UC San Diego Human Research Protection Program (project #71402).
Zebrafish and mouse maintenance
Adult zebrafish, wild-type (AB strain) and apoc2 and lpl mutants (on the AB background), were maintained at 28 °C, 14-h-light/10-h-dark cycle and fed brine shrimp twice a day. The low-fat diet (LFD) was prepared by extracting lipid from zebrafish GP100–200 micron larval diet (Brine Shrimp Direct, Utah) with diethyl ether. Zebrafish embryos or larvae younger than 5 days post fertilization (dpf) were kept at 28.5 °C in E3 solution (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, 0.33 mM MgSO4). The pigment development of early larvae was inhibited by adding 0.003% N-phenylthiourea (Sigma, Cat. 222909) to the E3 medium. apoc2 mutants16 and lpl mutants zebrafish were generated in our lab. cd41:EGFP and ahmc:EGFP transgenic zebrafish were kindly provided by David Traver and Neil Chi (both at UCSD), respectively. Apoc2 mutant mice were created in Remaley’s lab13 and were fed regular chow diet after weaning. All animal studies were approved by the University of California, San Diego Institutional Animal Care and Use Committee.
Wright–Giemsa and o-dianisidine staining
Peripheral blood cells were collected from euthanized zebrafish by tail amputation in adults and by heart puncture in embryos or larvae. Blood smears were made on superfrost/plus slides (Fisher, Cat.12-550-15) and fixed in 100% methanol for 15 s. To visualize erythrocyte morphology, cells were stained using a Hema3 kit (Fisher Diagnostics, Cat. 123–869, an analog to Wright–Giemsa method). To stain for hemoglobin, cells were incubated with an o-dianisidine staining buffer (0.6 mg/ml o-dianisidine, 0.01 M sodium acetate, pH 4.5, 0.65% H2O2, and 40% ethanol (vol/vol)) for 15 min in the dark. The slides were imaged with a BZ9000 Keyence microscope. For the whole mount o-dianisidine staining, dechorionated and euthanized embryos or larvae were incubated with the o-diansidine staining buffer for 15 min in the dark, and the embryos or larvae were photographed with a Leica CTR5000 microscope. The images shown in Figs. 1 and 2 are representative results from at least two independent experiments, with at least three embryos or larvae in each group per experiment.
Peripheral blood cell count
To count blood cells, anesthetized zebrafish embryos or larvae were laterally mounted in 1.0% low-melting agarose in a 50-mm glass bottom dishes (MatTek, Cat. P50G-0-14-F) and the blood flow video were recorded using a BZ9000 Keyence microscope. Three frames were extracted from the video and blood cells were counted manually in each frame, the average number was used for statistical analysis. Blood was collected from adult male zebrafish through tail amputation and peripheral blood cells were counted using a haemocytometer after 1:2000 dilution in PBS.
In situ hybridization probe synthesis
Digoxigenin (DIG)-labeled oligonucleotides were synthesized using an in vitro transcription system (Roche, Cat. 11175025910). gata1, cmyb, runx1, and rag1 probes were synthesized from linearized plasmids with T7 polymerase. beta-globin, pu.1, and foxn probes were synthesized from PCR templates with SP6 polymerase. The PCR templates were amplified from cDNA with specific primers, which were 5′-cgttgctgtcgttctgttta-3′ and 5′-gatttaggtgacactatagttagtggtactgtcttccca-3′ for beta-globin (beta embryonic 1.1), 5′-atctatcgaccaccaatgga-3′ and 5′-gatttaggtgacactataggcgaaggtgttaatgcaaag-3′ for pu.1 and 5′-agtgtagatggaagtcctgt-3′ and 5′-gatttaggtgacactatagttctccaccttctcaaagca-3′ for foxn. The sequence in italic encodes the SP6 promoter.
Whole-mount in situ hybridization
Whole-mount in situ hybridization (WISH) was performed as described50. In brief, embryos were fixed in 4% paraformaldehyde (PFA) overnight at 4 °C and dehydrated sequentially with methanol in PBST (25, 50, and 75%) and stored in 100% methanol at −20 °C. On the day of WISH, embryos were rehydrated in PBST (0.1% Tween-20 in PBS), treated with proteinase K and re-fixed in 4% PFA. After a wash in PBST, the embryos were incubated with hybridization buffer (HB), which contains 50% formamide, 5× saline sodium citrate (SSC), 500 µg/ml torula yeast tRNA, 50 µg/ml heparin, 0.1% Tween-20, and 9 mM citric acid (pH 6.5) for 1 h and then with HB containing DIG-labeled probes overnight at 68 °C. Afterward, embryos were washed sequentially with 2× SSC in HB (25, 50, and 75%) and 0.2× SSC at 68 °C, and then with 0.2× SSC in PBST (75, 50, and 25%) at room temperature (RT). After the wash, embryos were incubated in the blocking buffer (PBST with 2% heat-inactivated goat serum and 2 mg/ml bovine serum albumin) for 1 h at RT and then with blocking buffer containing an alkaline phosphatase (AP)-conjugated anti-DIG antibody (Roche, Cat. 11093274910, 1:5000 dilution) overnight at 4 °C. To visualize the signals, embryos were washed six times with PBST for 15 min and then three times with an AP reaction buffer (100 mM Tris, pH 9.5, 50 mM MgCl2, 100 mM NaCl, 0.1% Tween-20) for 5 min at RT. The signals were developed by incubating the embryos with a BM purple AP substrate (Roche, Cat. 11442074001) or an AP reaction buffer containing NBT/BCIP substrate (Roche, 11681451001). The reaction was terminated using a stop buffer (1× PBS, pH 5.2, 1 mM EDTA, 0.1% Tween-20) and the embryos were photographed with a Leica CTR5000 microscope. In situ results shown in Fig. 2d are representative data from three independent experiments. The results in other figures are representative data from two to five independent experiments. The images shown in figures are from the same experiment and the embryo or larva numbers (presented phenotype/total) in each group are indicated in the panels.
Live imaging
Anesthetized zebrafish embryos were mounted in 1% low-melting point agarose (Fisher, BP1360-100) containing 0.02% tricaine (Sigma, Cat. A5040) and imaged using a BZ9000 Keyence fluorescent microscope.
Embryonic injections
The human APOC2 mimetic peptides C-II-a and C-II-I were reported in previous work16, 51. Six nl of C-II-a or C-II-i (2 mg/ml), 8 nl of VLDL (2.1 mg/ml) or LDL (2.9 mg/ml), 10 nl of DHA (docosahexaenoic acid):BSA (bovine serum albumin) (3 mg/ml:100 mg/ml) or OA (oleic acid):BSA (3.3 mg/ml:129 mg/ml), or 10 nl of POPC:TG-DHA (2 mg/m l:1.2 mg/ml) or POPC (2 mg/ml) liposome were injected into 2 dpf stage embryos through the sinus venosus using a FemtoJet micro-injector (Eppendorf).
Oil red O staining and BODIPY staining
Oil red O (ORO) staining was conducted according to published protocols16. Briefly, embryos were fixed in 4% PFA for 2 h, washed three times in PBS, incubated in 0.3% ORO solution for 2 h, and then washed with PBS before imaging. For BODIPY staining, live larvae were immersed in E3 medium containing 0.1 µg/ml BODIPY 505/515 (Invitrogen, Cat. D-3921) for 1 h in dark and then rinsed with E3 medium before imaging. The images shown in figures are representative results from two to five independent experiments, with at least three embryos or larvae in each group per experiment.
CRISPR-Cas9-mediated Lpl knockout in zebrafish
pT3TS-zCas9 and T7-gRNA plasmids were from Chen lab52 through Addgene. Following the published protocol52, nls-zCas9-nls mRNA was synthesized with an mMESSAGE mMACHINE T3 kit (ThermoFisher, AM1348) and recovered with lithium chloride precipitation. lpl gRNA was generated using a MEGAshortscript T7 kit (ThermoFisher, AM1354) and purified using a mirVana miRNA isolation kit (ThermoFisher, AM1560). The zebrafish lpl genomic target sequence was 5′-ggctgaaattgattatccttGGG-3′, in which the first 20 nt was the gRNA template and the last 3 nt was protospacer adjacent motif (PAM) required for CRISPR/Cas9 function. 30 pg lpl gRNA and 150 pg nls-zCas9-nls mRNA were injected into 1–2 cells stage embryos. Genomic DNA (gDNA) was extracted from whole embryos or from adult tail tissue using a KAPA Express Extract Kit (KAPA Biosystems, Cat. KR0383). The gDNA fragment containing the target site was amplified using KOD DNA polymerase (EMD Millipore, Cat. 71086) and digested with T7 endonuclease (NEB, Cat. M0302). Primers used for PCR amplification of lpl gDNA fragment were 5′-aacatcagcctcctacacaa-3′ and 5′-tcactcgtttctcatgcgaa-3′.
Quantitative RT-PCR
RNA was isolated from 5 dpf zebrafish embryos using an RNeasy kit (Qiagen, Cat. 74104) and cDNA was reverse transcribed using an EcoRry Premix (Takara-Clontech, Cat. 639543). Quantitative PCR (Kapa SYBR FAST qPCR kit, Cat. KK4602) was performed using a Rotor Gene Q qPCR machine (Qiagen). Primers used in qRT-PCR were 5′-ggcttctgctctgtatgg-3′ and 5′-ggctctgaccttgttgat-3′ for zebrafish β-actin, 5′- atgaacaagatactggctat -3′ and 5′-ttgatggtctctacatatcc-3′ for zebrafish apoc2, 5′-gcacggcagttcattcaa-3′ and 5′- gtcagattctaccattccagtt-3′ for zebrafish lpl, 5′-cgtcttcacaaaccctcctcaa-3′ and 5′-gctttactgcttcatccggct-3′ for zebrafish runx1, and 5′-ccaacagcagcaggtctaa-3′ and 5′-tggtggtctggtggtctt-3′ for zebrafish sdf1α.
Generation of parabiotic zebrafish embryos
The parabiosis experiment followed the protocol published in ref. 25. Briefly, embryos for parabiosis were transferred to a glass Petri dish and dechorionated with forceps at the 256-cell stage. Drops of 4% methylcellulose were laid in rows at the bottom of a plastic Petri dish and covered with HCR (116M NaCl, 2.9 mM KCl, 10 mM CaCl2, and 5 mM Hepes) containing antibiotics (2.5 μg/ml ampicillin, 0.5 μg/ml kanamycin, and 10 U/ml penicillin–streptomycin). Small wells were made on top of the methylcellulose drops using the tip of a glass pipette. Then, two individual dechorionated embryos, which developed to the sphere stage but no later than the dome stage, were transferred to these small wells and gently pressed together with a tiny round-end iron needle. At the attaching site of two embryos, a few cells were removed using a sharp glass micropipette. If necessary, the two embryos were moved again to press the wounds against each other. To avoid any shaking that could separate the two blastulae, the attached embryos were left under the microscope for 20–30 min. Once the attachment was secured, the methylcellulose around the embryos was removed as much as possible. Finally, the plates were fully filled with HCR containing antibiotics and transferred to a 28.5 °C incubator. Next day, HCR medium was replaced with E3 medium containing antibiotics and residual methylcellulose was removed.
Drug treatment
Lomitapide (Cayman Chem, Cat. 10009610) powders were dissolved in DMSO at the concentration of 1 mM and embryos were treated with 5 μM lomitapide from 2 dpf to 3.3 dpf. DMSO treatment was used as control.
Gas chromatography – mass spectrometry (GC-MS)
Pooled embryos or larvae at the dome, 26 h post fertilization (hpf), or 6 dpf stages were homogenated in PBS (10 μl per embryo or larva). Fifty μl of the homogenate was used for free fatty acid extraction, and free fatty acid GC-MS was conducted at the LIPID MAPS Lipidomics Core at UC San Diego53, 54.
Apoptosis assay
Zebrafish embryos (3.3 dpf) were fixed in 4% PFA overnight at 4 °C. Embryos were digested with proteinase K (10 μg/ml) for 30 min and then re-fixed with 4% PFA at RT for 20 min. After the PBST wash, the embryos were incubated with TUNEL reaction mixture (In Situ Cell Death Detection Kit, TMRed, Roche, Cat.12156792910) at 37 °C for 1 h. Positive control embryos were digested with Dnase I for 15 min. Negative control embryos were incubated with Label solution without terminal transferase. After the PBST wash, embryos were imaged with a BZ9000 Keyence fluorescent microscope.
Preparation of injected materials
C-II-a and C-II-i mimetic peptides51 were dissolved in PBS to a concentration of 2 mg/ml; VLDL (2.1 mg/ml) and LDL (2.9 mg/ml) were from healthy donors and isolated using a standard ultracentrifugation protocol in UC San Diego lipid core. DHA:BSA were made by dissolving 12 mg DHA (Cayman, Cat. 90310), which was first dried under argon, in 4 ml of 100 mg/ml BSA solution (Sigma, Cat. A8806). Oleic acid:BSA was from Sigma (Cat. O3008). Fatty acid concentrations were measured using an HR Series NEFA-HR method (Wako, Cat. 999–34691, 991–34891, 993–35191) and BSA was measured using Lowry assay (Biorad, Cat. 500–0116). To make POPC:TG-DHA and POPC liposomes, chloroform solubilized POPC (Avanti, Cat. 850457) and tridocosahexaenoin (TG-DHA) (Larodan Cat. 33–2260) were mixed at 2 mg POPC with 1.2 mg TG-DHA or 2 mg POPC alone. The mixtures were dried under argon in round bottom glass tubes and then 1 ml PBS was added and incubated at RT for 30 min. Then, POPC:TG-DHA or POPC in PBS was sonicated in a water bath ultrasound machine (VWR, Model 75D) three times for 10 min, until the solution became uniform and translucent.
Mouse complete blood count
Blood samples (~50 μl) were collected into EDTA tubes (BD, Cat. 365974) from mouse tail. To minimize clotting, the tubes were flicked immediately after blood collection and the samples were sent to the UCSD Veterinary Diagnostic Laboratory for CBC analysis within 2 h.
FACS sorting of HSPCs and stromal cells
EGFP and mCherry double-positive embryos were picked up from progenies of the cross between gata2:EGFP and sdf1a:mCherry zebrafish at 2.5 dpf. Caudal hematopoietic tissues (CHT) were cut out and washed with PBS. CHT from about 200 embryos were digested with 1 ml Trypsin-EDA (Corning, Cat. 25-052-CI) containing 10 μg/ml collagenase (Worthington Biochem, Cat. MOP123) at 35 °C. FBS (Omega Science, Cat. FB-01) was added to a final concentration of 5% to stop the reaction. Cells were spun down at 350 × g for 5 min, re-suspended in PBS and stained with Aqua-LIVE/DEAD kit (ThermoFisher, Cat. L34965). Cells were spun down, washed once with PBS, and re-suspended in 0.5 ml staining buffer (PBS containing 0.5% BSA). After filtration through a 70 μm cartridge, cells were sorted using a BD FACSAria II machine. Live EGFP single-positive cells, mCherry single-positive cells, and EGFP/mCherry double-negative cells were defined as HSPCs, stromal cells, and trunk cells, respectively.
Bone marrow cells were collected from mouse femur and tibia and depleted from red blood cells with a red blood cell lysis buffer (eBioscience, Cat. 00–4333). Cells were spun down at 350 × g for 5 min, re-suspended in the staining buffer to 10 million per ml, and stained with Lin-APC (BD, Cat. 55807, 1:1000 dilution), cKit-PE (eBioscience, Cat. 12-1172-82, 1:300 dilution), and Sca1-PEcy7 (eBioscience, Cat. 25-5981-81, 1:2500 dilution) antibodies on ice for 15 min. Cells were spun down and washed once with PBS and re-suspended in the staining buffer to 3–5 million per ml. Cells were sorted using a BD FACSAria II machine and fluorescence minus one (FMO) and single stain controls were used to calibrate the threshold in the FACS sorting. Cells with lineage negative staining (Lin−) were further selected for positive Sca1 and c-Kit staining (Sca1+ and c-Kit+), and these cells were defined as LSK.
Data availability
The authors declare that all data supporting the findings of this study are available within the article and its supplementary information files or from the corresponding author on reasonable request.
Electronic supplementary material
Description of Additional Supplementary Files
Acknowledgements
This study was supported by grants HL135737, HL136275, and HL088093 (Y.I.M.) from the National Institutes of Health and 16POST27250126 (C.L.) from the American Heart Association. The UCSD School of Medicine Microscopy Core is supported by grant P30 NS047101 from the NIH. Equipment is supported by Jennifer Santini.
Author contributions
Studies were designed and planned by C.L., Y.I.M, and D.T.; experiments and data analysis were performed by C.L., T.H., D.L.S., H.W., B.L.V., and J.K.; C.L. and Y.I.M. wrote the manuscript; R.L.K., A.T.R, T.M.R., and D.T. contributed to study discussions and manuscript revisions.
Competing interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-03775-y.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Merkel M, Eckel RH, Goldberg IJ. Lipoprotein lipase: genetics, lipid uptake, and regulation. J. Lipid Res. 2002;43:1997–2006. doi: 10.1194/jlr.R200015-JLR200. [DOI] [PubMed] [Google Scholar]
- 2.Baggio G, et al. Apolipoprotein C-II deficiency syndrome. Clinical features, lipoprotein characterization, lipase activity, and correction of hypertriglyceridemia after apolipoprotein C-II administration in two affected patients. J. Clin. Invest. 1986;77:520–527. doi: 10.1172/JCI112332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fong LG, et al. GPIHBP1 and plasma triglyceride metabolism. Trends Endocrinol. Metab. 2016;27:455–469. doi: 10.1016/j.tem.2016.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jorgensen AB, Frikke-Schmidt R, Nordestgaard BG, Tybjaerg-Hansen A. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N. Engl. J. Med. 2014;371:32–41. doi: 10.1056/NEJMoa1308027. [DOI] [PubMed] [Google Scholar]
- 5.Dewey FE, et al. Inactivating variants in ANGPTL4 and risk of coronary artery disease. N. Engl. J. Med. 2016;374:1123–1133. doi: 10.1056/NEJMoa1510926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinstock PH, et al. Severe hypertriglyceridemia, reduced high density lipoprotein, and neonatal death in lipoprotein lipase knockout mice. Mild hypertriglyceridemia with impaired very low density lipoprotein clearance heterozygotes. J. Clin. Invest. 1995;96:2555–2568. doi: 10.1172/JCI118319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coleman T, et al. COOH-terminal disruption of lipoprotein lipase in mice is lethal in homozygotes, but heterozygotes have elevated triglycerides and impaired enzyme activity. J. Biol. Chem. 1995;270:12518–12525. doi: 10.1074/jbc.270.21.12518. [DOI] [PubMed] [Google Scholar]
- 8.Weinstock PH, et al. Lipoprotein lipase controls fatty acid entry into adipose tissue, but fat mass is preserved by endogenous synthesis in mice deficient in adipose tissue lipoprotein lipase. Proc. Natl Acad. Sci. USA. 1997;94:10261–10266. doi: 10.1073/pnas.94.19.10261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Augustus AS, et al. Loss of lipoprotein lipase-derived fatty acids leads to increased cardiac glucose metabolism and heart dysfunction. J. Biol. Chem. 2006;281:8716–8723. doi: 10.1074/jbc.M509890200. [DOI] [PubMed] [Google Scholar]
- 10.Khan RS, Schulze PC, Bokhari S, Goldberg IJ. A sweet heart: increased cardiac glucose uptake in patients with lipoprotein lipase deficiency. J. Nucl. Cardiol. 2011;18:1107–1110. doi: 10.1007/s12350-011-9442-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim JK, et al. Tissue-specific overexpression of lipoprotein lipase causes tissue-specific insulin resistance. Proc. Natl Acad. Sci. USA. 2001;98:7522–7527. doi: 10.1073/pnas.121164498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levak-Frank S, et al. Induced mutant mouse lines that express lipoprotein lipase in cardiac muscle, but not in skeletal muscle and adipose tissue, have normal plasma triglyceride and high-density lipoprotein-cholesterol levels. Proc. Natl Acad. Sci. USA. 1999;96:3165–3170. doi: 10.1073/pnas.96.6.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakurai T, et al. Creation of apolipoprotein C-II (ApoC-II) mutant mice and correction of their hypertriglyceridemia with an ApoC-II mimetic peptide. J. Pharmacol. Exp. Ther. 2016;356:341–353. doi: 10.1124/jpet.115.229740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang L, Liu C, Miller YI. Zebrafish models of dyslipidemia: relevance to atherosclerosis and angiogenesis. Transl. Res. 2014;163:99–108. doi: 10.1016/j.trsl.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JY, Seo J, Cho KH. Aspartame-fed zebrafish exhibit acute deaths with swimming defects and saccharin-fed zebrafish have elevation of cholesteryl ester transfer protein activity in hypercholesterolemia. Food Chem. Toxicol. 2011;49:2899–2905. doi: 10.1016/j.fct.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Liu C, et al. Apoc2 loss-of-function zebrafish mutant as a genetic model of hyperlipidemia. Dis. Model Mech. 2015;8:989–998. doi: 10.1242/dmm.019836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeituni EM, et al. Endoplasmic reticulum lipid flux influences enterocyte nuclear morphology and lipid-dependent transcriptional responses. J. Biol. Chem. 2016;291:23804–23816. doi: 10.1074/jbc.M116.749358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lalonde S, et al. Frameshift indels introduced by genome editing can lead to in-frame exon skipping. PLoS ONE. 2017;12:e0178700. doi: 10.1371/journal.pone.0178700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rowe RG, Mandelbaum J, Zon LI, Daley GQ. Engineering hematopoietic stem cells: lessons from development. Cell Stem Cell. 2016;18:707–720. doi: 10.1016/j.stem.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clements WK, Traver D. Signalling pathways that control vertebrate haematopoietic stem cell specification. Nat. Rev. Immunol. 2013;13:336–348. doi: 10.1038/nri3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stachura DL, Traver D. Cellular dissection of zebrafish hematopoiesis. Methods Cell. Biol. 2016;133:11–53. doi: 10.1016/bs.mcb.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 22.Bertrand JY, Kim AD, Teng S, Traver D. CD41+cmyb+precursors colonize the zebrafish pronephros by a novel migration route to initiate adult hematopoiesis. Development. 2008;135:1853–1862. doi: 10.1242/dev.015297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin HF, et al. Analysis of thrombocyte development in CD41-GFP transgenic zebrafish. Blood. 2005;106:3803–3810. doi: 10.1182/blood-2005-01-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamplin OJ, et al. Hematopoietic stem cell arrival triggers dynamic remodeling of the perivascular niche. Cell. 2015;160:241–252. doi: 10.1016/j.cell.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demy DL, et al. Generating parabiotic zebrafish embryos for cell migration and homing studies. Nat. Methods. 2013;10:256–258. doi: 10.1038/nmeth.2362. [DOI] [PubMed] [Google Scholar]
- 26.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–334. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davies BS, Beigneux AP, Fong LG, Young SG. New wrinkles in lipoprotein lipase biology. Curr. Opin. Lipidol. 2012;23:35–42. doi: 10.1097/MOL.0b013e32834d0b33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang H, Eckel RH. Lipoprotein lipase: from gene to obesity. Am. J. Physiol. Endocrinol. Metab. 2009;297:E271–E288. doi: 10.1152/ajpendo.90920.2008. [DOI] [PubMed] [Google Scholar]
- 29.Goldberg IJ, Eckel RH, Abumrad NA. Regulation of fatty acid uptake into tissues: lipoprotein lipase- and CD36-mediated pathways. J. Lipid Res. 2009;50(Suppl):S86–S90. doi: 10.1194/jlr.R800085-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feoli-Fonseca JC, Levy E, Godard M, Lambert M. Familial lipoprotein lipase deficiency in infancy: clinical, biochemical, and molecular study. J. Pediatr. 1998;133:417–423. doi: 10.1016/S0022-3476(98)70280-X. [DOI] [PubMed] [Google Scholar]
- 31.Avraham-Davidi I, et al. ApoB-containing lipoproteins regulate angiogenesis by modulating expression of VEGF receptor 1. Nat. Med. 2012;18:967–973. doi: 10.1038/nm.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang H, et al. Deficiency of lipoprotein lipase in neurons modifies the regulation of energy balance and leads to obesity. Cell. Metab. 2011;13:105–113. doi: 10.1016/j.cmet.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siddiqui NF, Shabrani NC, Kale VP, Limaye LS. Enhanced generation of megakaryocytes from umbilical cord blood-derived CD34(+) cells expanded in the presence of two nutraceuticals, docosahexanoic acid and arachidonic acid, as supplements to the cytokine-containing medium. Cytotherapy. 2011;13:114–128. doi: 10.3109/14653241003588858. [DOI] [PubMed] [Google Scholar]
- 34.Dhenge A, Limbkar K, Melinkeri S, Kale VP, Limaye L. Arachidonic acid and Docosahexanoic acid enhance platelet formation from human apheresis-derived CD34+cells. Cell Cycle. 2017;16:979–990. doi: 10.1080/15384101.2017.1312233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xia S, et al. Fish oil-rich diet promotes hematopoiesis and alters hematopoietic niche. Endocrinology. 2015;156:2821–2830. doi: 10.1210/en.2015-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Denburg JA, et al. Fish oil supplementation in pregnancy modifies neonatal progenitors at birth in infants at risk of atopy. Pediatr. Res. 2005;57:276–281. doi: 10.1203/01.PDR.0000148279.72611.1D. [DOI] [PubMed] [Google Scholar]
- 37.Martini I, et al. Optimization of the viability of stem cells derived from umbilical cord blood after maternal supplementation with DHA during the second or third trimester of pregnancy: study protocol for a randomized controlled trial. Trials. 2014;15:164. doi: 10.1186/1745-6215-15-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams KJ. Molecular processes that handle -- and mishandle -- dietary lipids. J. Clin. Invest. 2008;118:3247–3259. doi: 10.1172/JCI35206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kimura R, et al. DHA attenuates postprandial hyperlipidemia via activating PPARalpha in intestinal epithelial cells. J. Lipid Res. 2013;54:3258–3268. doi: 10.1194/jlr.M034942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Vogel-van den Bosch HM, et al. PPARalpha-mediated effects of dietary lipids on intestinal barrier gene expression. BMC Genomics. 2008;9:231. doi: 10.1186/1471-2164-9-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu K, et al. Differential activation of peroxisome proliferator-activated receptors by eicosanoids. J. Biol. Chem. 1995;270:23975–23983. doi: 10.1074/jbc.270.41.23975. [DOI] [PubMed] [Google Scholar]
- 42.Poudyal H, Panchal SK, Diwan V, Brown L. Omega-3 fatty acids and metabolic syndrome: effects and emerging mechanisms of action. Prog. Lipid Res. 2011;50:372–387. doi: 10.1016/j.plipres.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 43.Fromel T, et al. Soluble epoxide hydrolase regulates hematopoietic progenitor cell function via generation of fatty acid diols. Proc. Natl Acad. Sci. USA. 2012;109:9995–10000. doi: 10.1073/pnas.1206493109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kinder M, et al. Hematopoietic stem cell function requires 12/15-lipoxygenase-dependent fatty acid metabolism. Blood. 2010;115:5012–5022. doi: 10.1182/blood-2009-09-243139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li P, et al. Epoxyeicosatrienoic acids enhance embryonic haematopoiesis and adult marrow engraftment. Nature. 2015;523:468–471. doi: 10.1038/nature14569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.North TE, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–1011. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cutler C, et al. Prostaglandin-modulated umbilical cord blood hematopoietic stem cell transplantation. Blood. 2013;122:3074–3081. doi: 10.1182/blood-2013-05-503177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goessling W, et al. Prostaglandin E2 enhances human cord blood stem cell xenotransplants and shows long-term safety in preclinical nonhuman primate transplant models. Cell Stem Cell. 2011;8:445–458. doi: 10.1016/j.stem.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Limbkar K, et al. Oral feeding with polyunsaturated fatty acids fosters hematopoiesis and thrombopoiesis in healthy and bone marrow-transplanted mice. J. Nutr. Biochem. 2017;47:94–105. doi: 10.1016/j.jnutbio.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 50.Thisse, C., Thisse, B., Schilling, T. F. & Postlethwait, J. H. Structure of the zebrafish snail1 gene and its expression in wild-type, spadetail and no tail mutant embryos. Development119, 1203–1215 (1993). [DOI] [PubMed]
- 51.Amar MJ, et al. A novel apolipoprotein C-II mimetic peptide that activates lipoprotein lipase and decreases serum triglycerides in apolipoprotein E-knockout mice. J. Pharmacol. Exp. Ther. 2015;352:227–235. doi: 10.1124/jpet.114.220418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jao LE, Wente SR, Chen W. Efficient multiplex biallelic zebrafish genome editing using a CRISPR nuclease system. Proc. Natl Acad. Sci. USA. 2013;110:13904–13909. doi: 10.1073/pnas.1308335110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Quehenberger O, Armando A, Dumlao D, Stephens DL, Dennis EA. Lipidomics analysis of essential fatty acids in macrophages. Prostaglandins Leukot. Essent. Fat. Acids. 2008;79:123–129. doi: 10.1016/j.plefa.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Quehenberger O, Armando AM, Dennis EA. High sensitivity quantitative lipidomics analysis of fatty acids in biological samples by gas chromatography-mass spectrometry. Biochim. Biophys. Acta. 2011;1811:648–656. doi: 10.1016/j.bbalip.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The authors declare that all data supporting the findings of this study are available within the article and its supplementary information files or from the corresponding author on reasonable request.