Abstract
Anthropogenic food provisioning of wildlife can alter the frequency of contacts among hosts and between hosts and environmental sources of pathogens. Despite the popularity of garden bird feeding, few studies have addressed how feeders influence host contact rates and disease dynamics. We experimentally manipulated feeder density in replicate aviaries containing captive, pathogen-naive, groups of house finches (Haemorhous mexicanus) and continuously tracked behaviours at feeders using radio-frequency identification devices. We then inoculated one bird per group with Mycoplasma gallisepticum (Mg), a common bacterial pathogen for which feeders are fomites of transmission, and assessed effects of feeder density on house finch behaviour and pathogen transmission. We found that pathogen transmission was significantly higher in groups with the highest density of bird feeders, despite a significantly lower rate of intraspecific aggressive interactions relative to the low feeder density groups. Conversely, among naive group members that never showed signs of disease, we saw significantly higher concentrations of Mg-specific antibodies in low feeder density groups, suggesting that birds in low feeder density treatments had exposure to subclinical doses of Mg. We discuss ways in which the density of garden bird feeders could play an important role in mediating the intensity of Mg epidemics.
This article is part of the theme issue ‘Anthropogenic resource subsidies and host–parasite dynamics in wildlife'.
Keywords: garden bird feeders, disease transmission, house finch (Haemorhous mexicanus), Mycoplasma gallisepticum, mycoplasmal conjunctivitis, supplementary feeding
1. Introduction
Anthropogenic resource provisioning of wildlife, whether intentional or not [1], can alter the dynamics of hosts and pathogens in ways that impact disease transmission [2]. Food provisioning can influence disease transmission via several mechanisms, including behaviourally altering contact rates among hosts or between hosts and environmental fomites, and physiologically changing host susceptibility or infectiousness [2,3]. A meta-analysis found that wildlife provisioning generally augments host aggregation and contact rate, resulting in higher rates of infection [2]. However, in some cases, provisioning was associated with dietary changes that improved body condition and led to lower infection rates [2]. Overall, while there is growing support that provisioning impacts disease dynamics via both behavioural and physiological mechanisms, there have been few controlled, experimental studies examining how resource provisioning alters host behaviour, infectiousness and disease dynamics.
One of the most widespread forms of anthropogenic food supplementation is domestic garden bird feeding, with some estimates indicating that over half of the households in the United States and United Kingdom feed wild birds (reviewed in [4]). However, despite their widespread popularity, little is known about the health effects of bird feeders for wild birds. Bird feeders have been hypothesized to facilitate disease transmission in several emerging host–pathogen systems, yet causative links remain rare. However, two recent experimental studies varied the presence or absence of bird feeders and examined effects on parasite prevalence in wild birds. Wilcoxen et al. [5] found a significantly higher clinical disease prevalence among wild birds captured at forested sites with feeders relative to birds captured at sites without supplemental food. Because birds at supplemented sites also showed multiple signs of increased physiological health (higher antioxidant levels and body condition, reduced stress, etc.), the higher disease prevalence detected at sites with feeders was likely a result of increased rates of contact between hosts and/or hosts and fomites [5]. Galbraith et al. [6] similarly manipulated the presence of feeders and found marginal positive effects of supplemental food on condition metrics for two commonly captured bird species; however, the detected effects of bird feeders on parasite prevalence were highly parasite- and host-specific. Finally, feeders have been indirectly linked with the transmission of several epidemic pathogens of songbirds. Since the mid 2000s, European greenfinches (Chloris chloris) and common chaffinches (Fringilla coelebs) have suffered severe epidemics of the protozoal parasite Trichomonas gallinae, which are thought to have been exacerbated by garden bird feeding [7,8]. Salmonella outbreaks impact multiple species of songbirds, and are also frequently tied to garden bird feeders [9]. Additionally, bird feeder use has been linked to the transmission of mycoplasmal conjunctivitis in house finches (Haemorhous mexicanus) [10,11], but to date no manipulative studies have examined how bird feeders alter the dynamics of this host–pathogen system.
Mycoplasmal conjunctivitis, caused by the bacterial pathogen Mycoplasma gallisepticum (hereafter referred to as Mg), was first detected in house finches in eastern North America in the mid 1990s [12,13]. Shortly following emergence, Mg was associated with house finch population declines of up to 60% in some regions [14]. Since then, house finch populations in eastern North America have experienced annual epidemics of Mg during their non-breeding season [15], when finches forage in mixed-sex flocks, frequently visiting garden feeders [16]. Mycoplasmal conjunctivitis in house finches is characterized by conjunctival inflammation and exudate, depressed motor activity [17], including reduced anti-predator behaviours [18], and higher mortality of diseased birds in the wild [19]. The pathogen is transmitted by direct contact or indirect contact with contaminated environmental fomites such as bird feeders [11]. The extent of time that an individual spends perched on feeders has been positively linked to the likelihood of acquiring and transmitting Mg [10], suggesting that feeders are important for disease dynamics in this host–pathogen system. Additionally, the presence of tube-type bird feeders in gardens has been linked to an increase in Mg prevalence in the wild [20]. However, elucidating the causative role of bird feeders for Mg transmission dynamics, and the mechanisms by which feeders alter disease spread, requires experimental manipulation.
In this study, we tested how different densities of tube-style bird feeders influenced Mg transmission within captive groups of house finches, and whether behavioural or physiological mechanisms might underlie effects of feeders on Mg dynamics. We used radio-frequency identification device (RFID) equipped feeders and fitted all birds with passive integrative transponder (PIT) tags to monitor foraging behaviours and social interactions at feeders. We predicted that higher feeder density would result in increased access to feeder ports, leading to more time on feeders and longer feeding bouts. Because time spent on feeders has been positively associated with Mg transmission [10], we predicted increased pathogen transmission in higher feeder density groups. Additionally, we predicted that limited access to feeder ports would lead to more aggressive displacements in the lower feeder density groups than in the higher feeder density groups. These aggressive interactions could serve as a potential mode of direct transmission for Mg [10]. We also tested whether the infectiousness of the index birds (defined as those directly inoculated with equal Mg doses to initiate group epidemics) differed for birds from high versus low density feeder treatments—a potential physiological mechanism by which feeder density might alter Mg transmission. However, we did not expect index birds to show differences in infectiousness across feeder density treatment, as prior work has shown that neither time spent on bird feeders [10] nor experimentally elevated aggression [21] influences infectiousness in this host–pathogen system. Finally, we measured body mass throughout the course of the epidemic because prior work indicates that feeder density alters body mass in house finches [22]. We predicted that birds with higher feeder densities would have higher body mass due to higher food intake and/or lower metabolic costs of intraspecific competition.
2. Material and methods
(a). Field captures and pre-experiment housing
Hatch-year house finches (N = 108) were captured June–September 2014 in Blacksburg and Radford, VA. Immediately following capture, birds were weighed on a balance to 0.1 g and ringed with an aluminium band with a unique ID number. All birds were quarantined for two weeks (see electronic supplementary material) and only birds that showed no clinical signs of mycoplasmal conjunctivitis (see ‘Tracking transmission' below) and were negative for Mg-specific antibodies (see ‘Quantifying-Mg-specific antibodies' below) were used in experimental groups.
Before placement in experimental groups, all birds were given a unique combination of colour bands for visual identification, and a PIT tag containing a unique 9-digit identifier. Each 0.1 g PIT tag (approx. 0.5% of body weight) was fastened to the colour bands on the right leg using coloured electrical tape matching the underlying band colours [23].
(b). Experimental housing
On experimental day −17 (i.e. 17 days prior to introduction of Mg), all birds were moved into one of 12 experimental groups in identical outdoor aviary compartments (5.5 × 2.5 × 2.4 m; see electronic supplementary material). Each group was provided with four (0.46 m long) wooden dowel perches, a synthetic evergreen tree, a heat lamp, ad libitum water in a plastic dish, and two tube-shaped feeders containing ad libitum pelleted diet (Daily Maintenance Diet, Roudybush Inc., Woodland, CA). Each feeder had a single accessible port that was monitored by an RFID device that recorded the number and duration of feeder visits [24]. Each antenna was connected to a reader which logged one data point per second [10] from 06.00 to 19.00 Eastern Standard Time for the duration of the study. On experimental day −7 (table 1), the six groups assigned to high feeder density treatment (see ‘Experimental design') were provided with two additional feeders for the remainder of the study.
Table 1.
Experimental timeline.
| day | activity |
|---|---|
| −17 | move to aviaries, establish groups, begin logging behaviour |
| −7 | establish experimental feeder densities |
| 0 | pre-inoculation sample (mass, eye score, conjunctival swab), inoculation |
| 3, 6, 9, 12, 15, 18, 21, 24 | sample (mass, eye score, conjunctival swab) |
| 27 | final sample (mass, eye score, eye swab, blood sample) |
(c). Experimental design
To assess the effects of feeder density on house finch behaviour and Mg transmission, we varied the density of bird feeders (high or low) within 12 captive groups of house finches of equal size (table 2; N = 6 groups per density treatment). High feeder density groups had access to four feeders, each with one available feeding port, and the low feeder density groups had access to two feeders, also each with one available port. Consistent with free-living flocks during the non-breeding season [15,25], all groups were mixed-sex (three or four females: four to six males) and initially consisted of nine randomly assigned birds. However, mortality during the acclimation period left two groups with only eight birds. In order to balance competition for resources across treatments, we chose to remove one bird randomly from two additional groups before initiating the experiment, thus ensuring that we had an equal number of eight- and nine-bird groups across the two experimental treatments.
Table 2.
Experimental design of the study. All groups contained eight or nine house finches of mixed sex.
| treatment | low feeder density (two feeders per group) | high feeder density (four feeders per group) |
|---|---|---|
| experimental epidemics | N = 5 groups | N = 5 groups |
| sentinel groups | N = 1 group | N = 1 group |
Foraging and social behaviours at feeders were monitored continuously throughout the study (table 1). Food was provided ad libitum, with feeders topped-up daily and food available from all feeders at all times. Our treatments thus did not differ in food availability per se, but rather competition for access to food. Within 10 of the 12 experimental groups (table 2), we initiated Mg epidemics by inoculating a single bird per group (the ‘index bird’) and tracked transmission for 27 days (table 1). In two ‘sentinel groups', a single bird per group was sham-treated with medium alone (see below) and all group members were tracked as in experimental groups. Finally, we tracked the disease course of index birds, which were directly inoculated with identical pathogen doses, as a metric of potential physiological effects of feeder density.
(d). Inoculation
Because prior work [10] showed that the time an index bird spends on a feeder, which is positively correlated with social status, predicts the extent of transmission in experimental epidemics, we selected birds of intermediate dominance status from each group as index birds. We used behaviours from experimental days −7 to −1 to quantify dominance hierarchies and identify birds of intermediate status in each group (see ‘Extracting behavioural metrics using radio-frequency identification data' below).
On experimental day 0 (20 November 2014), we inoculated one index bird from each experimental group with 70 µl of inoculum containing approximately 2.46 × 108 colour changing units of Mg in Frey's medium (2010.003-1-3P 10/25/2010; D.H. Ley, North Carolina State University, College of Veterinary Medicine, Raleigh). We chose an Mg isolate of relatively low virulence to maximize variation in transmission among treatment groups [26]. Inoculum was distributed approximately equally via micropipette across both conjunctiva. A single individual from each sentinel group was sham-inoculated with an equal volume of Frey's medium alone.
(e). Tracking transmission
To track transmission, we sampled all birds every 3 days from day 0 to 27 post-inoculation (PI). At each sampling point, we captured birds using hand-held butterfly nets specific to each treatment group. To quantify visible pathology, birds were given an ‘eye score' on a 0–3 scale encompassing swelling, presence of exudate and eversion of the conjunctiva [27]. Eye scores were determined blind to treatment by three of the authors (S.C.M., J.S.A. and D.M.H.) and one technician. Blindness was maintained because sampling was done randomly with respect to treatment, and treatment identity was not listed on the data sheets. All birds were captured and placed in paper lunch bags, and moved into a separate room prior to sampling; samplers then pulled birds out of bags and scored eyes without knowledge of group origin or treatment.
To sample for the presence and quantity of pathogen, we swabbed each conjunctival sac using sterile cotton swabs. This entailed rotating tryptose phosphate broth (TPB)-saturated swabs along the inner conjunctiva for 5 s, then swirling the swabs into a microcentrifuge tube containing 300 µl of TPB, and wringing out the swab on the tube's inner wall. A separate swab was used for each conjunctiva, but the contents were pooled into the same tube for a given bird and sampling day. We also recorded each bird's mass (to 0.1 g) at every sampling time point.
(f). Quantifying Mg-specific antibodies
We quantified Mg-specific antibodies on the last sampling day (day 27, table 1) to assess induction of Mg-specific antibodies during experimental epidemics. Samples (approx. 100 µl total; approx. 1% body mass) were collected by puncturing the wing vein using a sterile 26-gauge needle and collecting blood into heparin-coated capillary tubes. All samples were kept on ice until centrifugation (see electronic supplementary material) within 4 h of collection. Plasma was then separated and frozen at −20°C for later use in enzyme-linked immunosorbent assays (ELISA) for Mg-specific antibodies [28]. To control for inter-assay variation, all ELISA values were calculated as the ratio of the sample absorbance to that of the positive control.
(g). Extracting behavioural metrics using radio-frequency identification data
Using RFID data, we extracted two general categories of behavioural metrics: foraging behaviours and ‘at-feeder' inter-individual interactions. We chose these categories because we hypothesized that bird feeder density would likely alter these behaviours, and they have previously predicted disease transmission in the house finch–Mg system [10]. To test whether feeder density influenced foraging behaviours of house finches, we quantified the average amount of time individuals spent on all available feeders per day, the average length of feeding bouts, and the relative feeder preference of index birds (electronic supplementary material, table S1). Displacements at the feeders, likely representing aggression, were defined as any time two individuals were logged at the same feeder port within 2 s of one another [10]. We used Elo scores to rank each bird in terms of its propensity to displace others during the pre-inoculation phase. Elo scores integrate the number of times a bird displaces others, is displaced by others, and the relative rank of each individual at the time of a given displacement [29,30]. Finally, we calculated what we term ‘following latency', a metric pertaining only to non-index group members, and defined as the average length of time between an index bird departing a feeding port and a group member replacing it.
(h). Statistical analyses
All statistics were run in R (version 3.3.2) [31] and models used a Gaussian distribution, unless otherwise noted. For all models, we initially included all pairwise interactions among fixed effects, but only retained them in the final models if they were significant (p ≤ 0.05). Where interactions were significant, we present only the results of the interactions; see electronic supplementary material, table S2 for results of full models.
(i). Feeder density and transmission success
We used generalized linear models with a binomial error distribution to assess relationships between feeder density and transmission success. Our response variable, transmission success, was defined in two ways. Our primary definition of a transmission event was any time that a group member (non-index bird) showed signs of visible pathology. For this model, our combined response variable was the number of naive group members that showed signs of pathology (=non-zero eye score), and the number of naive group members that showed no signs of pathology [32]. Our second definition of a transmission event relied on quantitative polymerase chain reaction (qPCR) results, which are subject to frequent low-level contamination [33]. Therefore, we used a conservative definition of infection established by Adelman et al. [10]: if a naive group member had greater than or equal to 1349 copies of the pathogen present in the conjunctiva at any time point post-infection, we considered that as a successful transmission event. For this model, our combined response variable was the number of naive group members that met our pathogen load cut-off, and the number of naive group members that did not. Our predictor variables for both transmission success models were feeder density (high or low) and sex of the index bird. We included sex of the index bird because prior work in same-sex groups indicated potential sex differences in transmission success of Mg [10].
To assess relationships between feeder density and serum antibody concentrations of group members that did not show pathology during the experiment, we used a linear mixed model. We included group identity as a random effect, and feeder density and sex as fixed effects. Our response variable was Mg serum antibody concentration.
(j). Feeder density and disease metrics in index birds
We used linear mixed models to assess relationships between feeder density and disease metrics in index birds. We included feeder density, sex and experimental day (table 1) as fixed effects, and bird identity as a random effect to account for the non-independence of repeated measurements. Our response variables were conjunctival pathology (scores summed across both eyes and rounded to nearest integer, fitted using a Poisson distribution) and pathogen load (log10 transformed prior to analysis).
To assess relationships between feeder density and serum antibody concentrations of index birds at the conclusion of the study, we used a linear mixed model. Our response variable was Mg serum antibody concentration. Our predictor variables were sex and feeder density, and group was included as a random effect.
(k). Feeder density and behaviour
We used linear mixed models to assess relationships between feeder density and behaviours (analysed in separate models) of index birds and non-index group members (analysed separately). Behavioural data were separated by week PI to account for any temporal effects of feeder density and/or infection. For the three index bird models, response variables included average time spent on the feeder per day (log10 transformed prior to analysis), average feeding bout length (log10 transformed prior to analysis) and number of aggressive interactions. We included feeder density (high or low), sex and week (5-level factor: week 0 = inoculation week) as fixed effects, and bird identity as a random effect in all models. For our four models assessing group member behaviour, the same response variables were used as in the index bird models, and we included an additional model with following latency (negative binomial distribution) as the response variable. Our group member models included group identity as an additional random effect to account for the non-independence of group members.
Index birds' relative preference for their ‘preferred' feeder was generated by first calculating the proportion of time an individual spent on each available feeder, then identifying the feeder with the highest value as preferred. We then defined relative preference as the proportion of time spent on the preferred feeder divided by the proportion of time expected if equal time was spent on each available feeder (high feeder density: 0.25; low feeder density: 0.5). We compared relative preference across treatments using a t-test.
(l). Body mass
We used linear mixed models to assess relationships between feeder density and mass of group members and index birds (analysed separately). For all models, we included feeder density, sex and day (10-level factor) as fixed effects, and bird identity as a random effect. For group members, we included group identity as an additional random effect, as described above. Our response variable for both models was mass.
3. Results
(a). Feeder density and transmission success
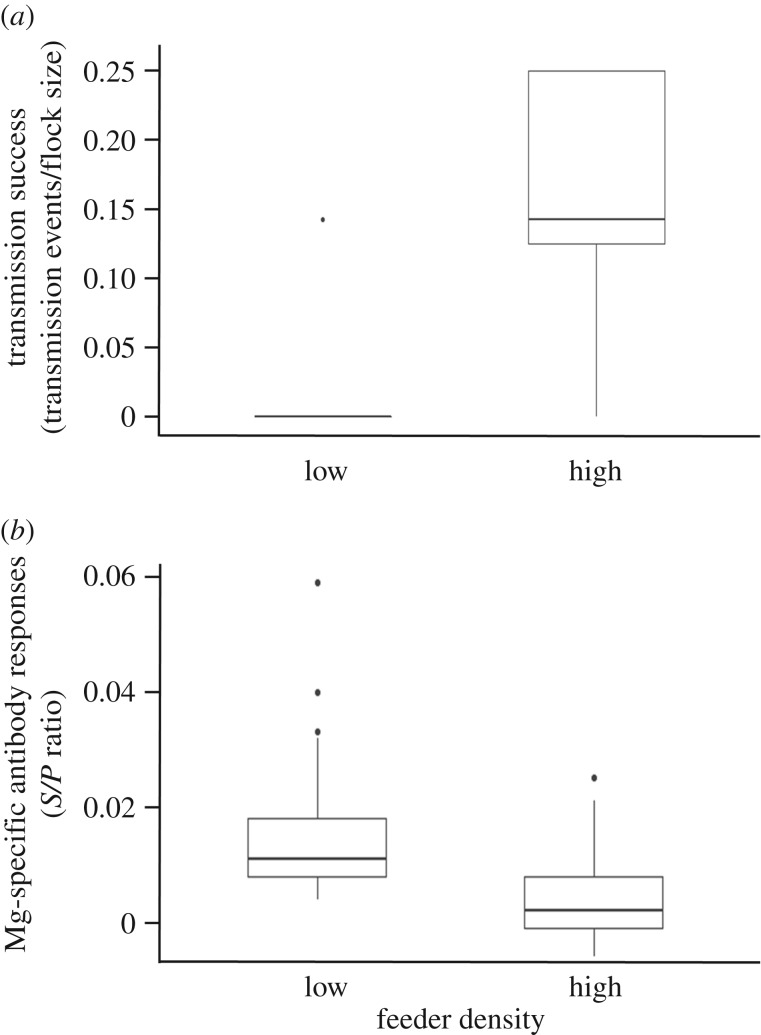
The transmission success of Mg (=proportion of naive group members that developed pathology) was significantly higher in groups with high feeder densities (feeder density high: β = 2.14 ± 1.12, Z = 1.91, p = 0.036; figure 1a). We found a similar, but non-significant pattern when we defined transmission via a conservative pathogen load cut-off (feeder density high: β = 0.87 ± 0.76, F1,9 = 1.10, p = 0.087). Sex of the index bird was not a significant predictor of transmission success in either model (sexF: β < 0.38 ± 1.14, F1,9 < 1.10, p > 0.19). All transmission events were detected between day 12 and day 24 PI.
Figure 1.
High feeder density groups (N = 5) had significantly higher pathogen transmission success (number of naive group members that showed pathology divided by the total number of group members) than the low feeder density groups (N = 5; (a)) Only one low-density group showed evidence of disease transmission. At the termination of the experiment (day 27), group members that never showed any signs of pathology from the low feeder density groups had significantly more circulating MG-specific antibodies than those in the high feeder density groups (b). Antibody responses were calculated as the ratio of the sample absorbance to that of the positive control (S/P ratio) in order to control for inter-assay ELISA variation.
Overall, rates of transmission were low, despite the fact that birds remained in constant contact over the entire experiment. Only 7/91 naive birds (7.69%) showed signs of pathology, and 9/91 (9.89%, including those with pathology) were considered infected using our pathogen load cut-off. While no birds in our sentinel groups showed visible pathology, 5/18 sentinel birds showed transient low-level pathogen loads at some point during the study and 1/18 sentinel birds met our conservative definition of ‘transmission' for naive group members. This suggests that eye score may be a more robust proxy for transmission than qPCR results, which are subject to contamination [33].
At the termination of the experiment (day 27), naive group members that never showed any signs of pathology varied in their Mg-specific antibody concentrations by treatment, with birds in the low feeder density groups having significantly higher antibody concentrations than those in the high feeder density groups (β = −0.011 ± 0.0023, F1,68 = 22.7, p < 0.0001; figure 1b).
(b). Feeder density and disease metrics in index birds
Index birds from the high and low feeder density groups showed statistically indistinguishable levels of pathology and pathogen load over the course of infection (pathology: β = 0.31 ± 0.33, Z-score = 0.93, p = 0.36; pathogen load: β = 0.25 ± 0.86, F1,39 = 0.60, p = 0.47), indicating that index birds from the high feeder density groups were not more infectious than those in the low feeder density groups. Index females had significantly lower eye scores than males (sexF: β = −0.84 ± 0.38, Z1,49 = −2.23, p = 0.026), but sex was not a significant predictor of pathogen load (β = −0.95 ± 0.94, F1,39 = 3.44, p = 0.11). Index birds from the low and high feeder density groups did not differ in antibody concentrations at the conclusion of this study (sex: β = 0.0067 ± 0.015, F1,9 = 0.20, p = 0.67; feeder density: β = 0.0051 ± 0.014, F1,9 = 0.14, p = 0.72).
(c). Feeder density and behaviour
(i). Daily time on feeders
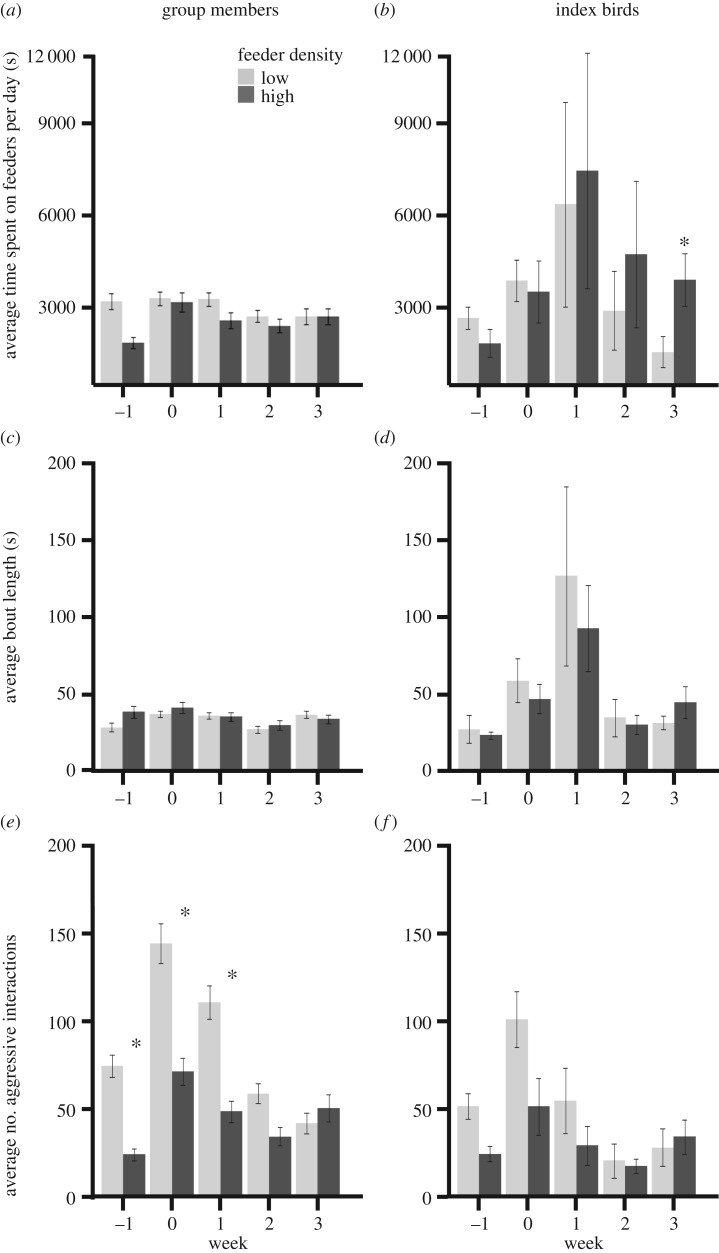
Time spent on the feeders by group members per day did not significantly differ across feeder densities (β = −0.23 ± 0.16, F1,379 = 2.01, p = 0.19), but did vary by week (F1,379 = 5.22, p = 0.00044; electronic supplementary material, table S2). Non-index birds spent significantly more time on the feeders the week of inoculation than they did the week before (Tukey least squares (LS) means: p = 0.0002; figure 2a). For index birds, we found temporally variable effects of feeder density on the average time spent on feeders per day (feeder density * week: F1,49 = 2.74, p = 0.046; electronic supplementary material, table S2). Index birds in high feeder density groups spent significantly more time on the feeders during week 3 (Tukey LS means: week 3 p = 0.032, all other weeks p-values greater than 0.22; figure 2b).
Figure 2.
Foraging and aggressive behaviours for group members (a,c,e) and index birds (b,d,f) at low (light grey) or high (dark grey) feeder density throughout the course of an experimental epidemic. Asterisks indicate weeks with significant differences across feeder densities in post hoc tests (see Results). Feeding behaviours were log10 transformed for analysis, but raw data are shown here. Error bars represent standard error. Asterisks indicate a significant difference between treatment groups.
(ii). Feeding bout length
Feeder density treatment had a significant but temporally inconsistent effect on the average feeding bout length of group members (feeder density * week: F1,377 = 3.42, p = 0.009; figure 2c). However, feeding bout length did not significantly differ in post hoc tests between treatment groups for any week (Tukey LS means: p > 0.085). For index birds, the average feeding bout length did not significantly differ by feeder density treatment either as a main effect (β = 0.0061 ± 0.13, F1,49 = 0.0021, p = 0.96) or in interaction with week, but did significantly differ by week (F1,49 = 10.0, p < 0.0001; figure 2d). Feeding bouts were significantly longer the week after inoculation (week 1) than all other weeks, with the exception of inoculation week (Tukey LS means: week 0 p = 0.13, all other p-values less than 0.006; figure 2d). Sex was not a significant predictor of either average time spent feeding per day or average bout length for group members (β = −1.15 ± 2.95, F1,379 = 0.15, p = 0.70) or index birds (β = 0.022 ± 0.14, F1,49 = 0.024, p = 0.88).
(iii). Relative feeder preference of index birds
Index birds in both the high and low feeder density groups foraged at all feeders available to them throughout the experiment. Index birds in the high feeder density groups had, on average, a stronger preference for their ‘most preferred' feeder than those in the low feeder density groups (mean preference high feeder density = 1.68; mean preference low feeder density = 1.11; p = 0.042; electronic supplementary material, figure S1).
(iv). Aggressive interactions
Feeder density had a significant but temporally variable effect on the number of aggressive interactions that group members experienced at feeders (feeder density * week: F1,359 = 20.0, p < 0.0001; electronic supplementary material, table S2). Consistent with our predictions, significantly more displacements occurred at feeders in the low feeder density treatment than the high feeder density treatment, for the first three weeks of the experiment (Tukey LS means: weeks 2 and 3 p > 0.20, weeks −1 to 1 p < 0.021; figure 2e). Feeder density had a similar time-specific effect on aggressive interactions in index birds (week * feeder density F1,49 = 3.24, p = 0.025; electronic supplementary material, table S2). However, the only significant differences in aggressive interactions involving index birds across feeder densities occurred during the week of inoculation (Tukey LS means: week 0 p = 0.006, all other weeks p > 0.095), perhaps due to the much smaller sample size of index birds relative to group members (figure 2f). Sex was not a significant predictor of the number of aggressive interactions individuals experienced (group members sexF: β = −0.044 ± 5.64, F1,359 = 0.0001, p = 0.99; index sexF: β = 10.93 ± 14.1, F1,49 = 0.60, p = 0.46)
(v). Following latency
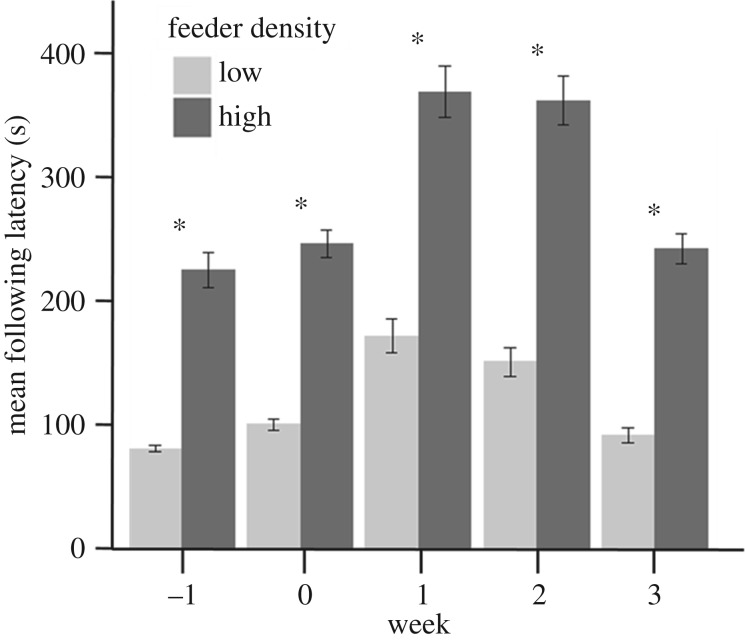
The effects of feeder density on ‘following latency' (=time between an index bird leaving its position on a feeder port and a group member replacing it) varied with feeder density, with individuals in the low feeder density groups having shorter following latencies (feeder density: β = 0.90 ± 0.27, Z1,24077 = 22.85, p < 0.0001; figure 3). Thus, birds in the low feeder density groups tended to feed at the same port as an index bird more quickly than those in the high feeder density groups. Additionally, following latencies varied over time (electronic supplementary material, table S2). Sex was not a significant predictor of following latency (sexF: β = 0.11 ± 0.79, 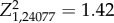 , p = 0.16).
, p = 0.16).
Figure 3.
Group members in the low feeder density treatment had shorter average following latencies (=time between an index bird leaving a feeder port and a group member replacing it) than birds in the high feeder density groups. Error bars represent standard error. Asterisks indicate a significant difference between treatment groups.
(d). Body mass
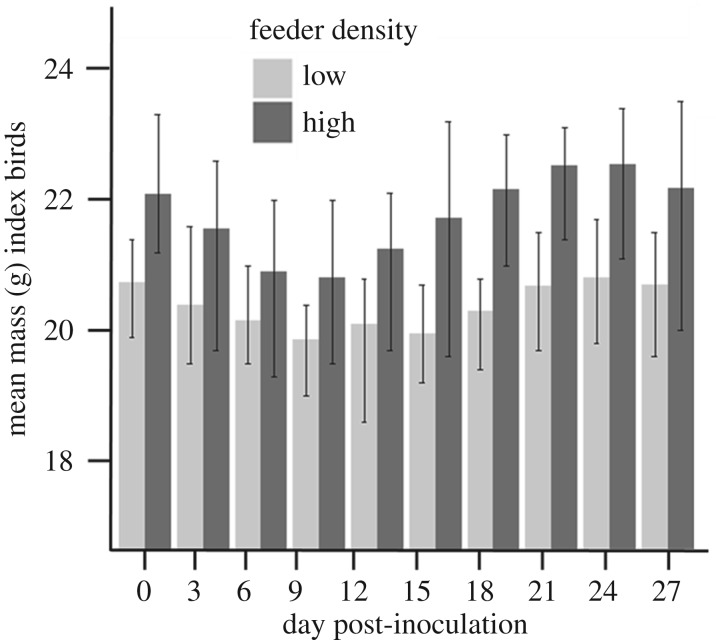
Feeder density was not a significant predictor of body mass for group members (feeder density: β = 0.061 ± 0.39, F1,759 = 0.0253, p = 0.88), but day PI significantly predicted group member body mass (day PI: β = 0.013 ± 0.0019, F1,759 = 11.2, p < 0.0001). For index birds, feeder density had time-specific effects on body mass. Index birds in the high feeder density treatment weighed more, on average, than those in the low feeder density, but the magnitude of this discrepancy varied with time (feeder density * day PI: β = 0.028 ± 0.013, F1,99 = 5.01, p = 0.028; figure 4; electronic supplementary material, table S2). Mass of index birds at initial capture did not differ between feeder treatments (feeder density low: β = 0.51 ± 26, F1,85 = 3.91, p = 0.051).
Figure 4.
Index birds in the high feeder density groups weighed more, on average, than those in the low feeder density groups throughout the experiment, but the magnitude of this discrepancy varied with time. Error bars represent standard error.
4. Discussion
We found that an increased density of bird feeders enhanced the incidence of mycoplasmal conjunctivitis during experimental epidemics. While previous studies have linked the presence of tube-type bird feeders with mycoplasmal conjunctivitis prevalence in wild house finch populations [20], this is the first study to experimentally vary the availability of feeders and examine effects on Mg transmission. Additionally, we found that group members in the low feeder density groups, which had very low rates of detectable infection and disease, had significantly higher concentrations of Mg-specific antibodies at the termination of the experiment than those in the high feeder density groups. This discrepancy suggests that exposure to Mg was largely subclinical at lower feeder densities. Taken together, our results suggest potential links between the density of garden bird feeders and epidemics of conjunctivitis in free-living house finch populations. However, because our study was done in captivity such that we could specifically isolate the effects of feeder density on Mg transmission, it is unclear how the patterns we detected would extrapolate to free-living populations.
Although we saw differences in disease transmission between the high and low feeder density groups, the effects of feeder density on the foraging behaviours previously shown to be important for Mg acquisition and transmission [10] were not straightforward. We predicted that higher feeder densities would lead to group members spending greater amounts of time on bird feeders, but we did not see a significant effect of feeder density on the time group members spent on the feeder. Index birds from high feeder density treatments did spend longer average amounts of time on feeders in week 3 PI (figure 2b), which was consistent with our predictions and may partly explain the higher rates of disease transmission in groups with high feeder densities. Furthermore, index birds in the high feeder density treatment maintained higher body mass than those in the low feeder density treatment, despite having equal mass at capture. These results are consistent with past work which found higher body condition (mass scaled for body size) in free-living birds foraging at sites with bird feeders present [5]. The higher body mass of index birds at high feeder densities, despite only weak effects of feeder density on the time index birds spent on feeders, suggests that index birds at high feeder densities may have consumed more food than at low feeder densities, potentially resulting in more pathogen deposition while feeding. It is also possible, however, that some other physiological process (e.g. differences in metabolism or body mass regulation based on the perception of food availability), rather than differences in food intake, drove this discrepancy in mass.
Similar to the patterns detected for the time spent on the feeder, we did not detect consistent treatment differences in the average length of feeding bouts, another behaviour that could potentially influence Mg exposure or deposition at the feeders. Thus, differences in feeding bout length are unlikely to be responsible for the observed discrepancy in transmission success. Overall, we did not find strong or consistent effects of feeder density on foraging behaviours, suggesting that rates of contacts with feeders alone do not explain the detected differences in disease transmission. We did see a trend towards increased feeding bout lengths and the time spent feeding by index birds the week after inoculation (week 1, figure 2b,c), corresponding with peak infection. This shift in behaviour has been documented in free-living birds [34] and is likely due to lethargy during infection, with sick birds taking longer to fly away from feeders during a bout, but not necessarily spending more time directly contacting feeder ports while foraging. Because our RFID approach detected only the time spent sitting on feeders and did not determine what birds were doing while at perches, sickness behaviours of index birds may have obscured any such differences in direct physical contact with feeder surfaces. Recent developments in tracking technology for captive birds now allow much more detailed data to be collected across entire aviaries [35]. These advances will allow capture of much finer detail on behavioural shifts associated with changing infection state.
One possible mechanism underlying the higher transmission rate detected in high feeder density groups is simply the higher number of potential environmental sources of Mg (i.e. feeders) available. Conversely, higher feeder densities for equal group sizes could be predicted to lead to ‘dilution' of Mg deposition across feeders, if index birds in both the high and low feeder density groups were depositing roughly equivalent copies of Mg on feeders. Unfortunately, we were not able to quantify the extent of Mg deposition on feeders because sampling the feeders would remove Mg and potentially hinder transmission. We do know that index birds in both treatments used, and potentially deposited Mg on, all of the feeders available (electronic supplementary material, figure S1). However, the preference for an index bird to feed at the most ‘preferred' feeder was strongest at high feeder densities, potentially resulting in unequal pathogen deposition across the available fomites. Future studies could quantify the rate of pathogen deposition onto feeding ports across feeder densities to test for augmentation or dilution of pathogen levels on feeder surfaces as a result of idiosyncratic feeding preferences by infectious individuals.
Our feeder density treatment did cause predicted changes in intraspecific aggression, with birds in the low feeder density treatment engaging in more aggressive displacements at the limited feeder ports than those in the high feeder density groups. These short-term indirect contacts at the feeders, and potentially direct contacts through the process of an agonistic displacement, did not, however, translate to higher disease transmission. In fact, we significantly found lower rates of clinical disease at low feeder density, suggesting that, in contrast to other wildlife diseases [36], aggressive interactions are not likely to drive transmission success in the finch–Mg system. Consistent with the higher levels of aggression detected, we also found that finches in the low feeder density groups exhibited shorter following latencies. This is likely due to the fact that heightened competition for limited resource access leads to quick turnover at feeder ports. However, given that rates of clinical disease were lowest in groups with the longest following latencies, it appears that closely following an index bird at the same feeder port is also not an important risk factor for acquiring conjunctivitis.
The course of pathology and pathogen load in index birds did not differ across our feeder treatments, indicating that detected differences in transmission were not due to physiological mechanisms such as differences in infectiousness. However, we found that among group members that never showed clinical signs, birds in the low feeder density groups showed higher Mg-specific antibody concentrations than group members in the high density feeder groups. This result suggests that there were feeder-density-specific differences in either physiological response to or exposure to Mg. It is possible that competition for limited feeder ports at the low feeder density increased the likelihood of exposure to subclinical doses of Mg. Another possibility is that social stress surrounding competition for limited feeder access caused a physiologically distinct response to similar exposure doses in the low feeder density groups. However, serum antibody concentrations did not differ for index birds from high versus low feeder densities, suggesting that physiological differences alone are unlikely to explain the detected patterns. The behavioural mechanisms generating this difference, and the extent to which these subinfectious doses might provide meaningful immunological protection to individuals [33], are an exciting area for further study.
Overall, our results suggest that bird feeders may play an important but complex role in the dynamics of Mg spread in house finches, with a low density of bird feeders more likely to result in subclinical exposure and a higher density of feeders more likely to cause disease. However, relative to past studies [26,27,37], we had very low rates of disease transmission overall; thus, our ability to uncover the behavioural mechanisms involved in successful transmission was limited. Additionally, while this captive study allowed us to directly manipulate feeder density and pathogen exposure, experimental manipulations of feeder density in the wild are sorely needed to understand the role of feeder density when birds can self-assemble into flocks, and move freely across the local landscape in response to resource density. Although there is only limited information on house finch spatial movements in the non-breeding season, about 35% of house finches studied in Ithaca, NY moved more than 1000 m between their roost site and daytime foraging sites [38], suggesting that most free-living flocks likely visit multiple gardens in a given day. Furthermore, within our study, the quantity and quality of food remained consistent, whereas wild birds likely experience significant variation in the quality and quantity of food available to them in gardens (reviewed in [4]). Additionally, free-living house finches regularly interact with other bird species at feeders that are largely not competent hosts of Mg [39,40], potentially limiting the extent of indirect contact between sick and healthy house finches if these species effectively serve as ‘dilution' hosts [41]. Finally, our manipulations of feeder density per se did not allow us to address how the presence of feeders (relative to only natural food sources) alters Mg transmission. For example, while our study showed a decrease in rates of aggression with an increasing density of feeders, the presence of feeders in the wild (relative to no supplemental food) likely augments host contact rates by providing high-value point-source resources. Finally, the abundance of free-living house finches is likely linked to garden feeder abundance [42], providing another potential mechanism by which bird feeder density can contribute to the dynamics of Mg transmission [43] that our study with constant group sizes could not address.
This study highlights the potentially complex impacts of bird feeder density on disease transmission in a naturally occurring host–pathogen system. The distinct effects of feeder density on the severity of disease spread in captive groups versus rates of subclinical exposure suggest that, at least under some conditions, bird feeders could play important and potentially paradoxical roles in the extent of Mg epidemics among free-living house finches. However, experiments in free-living finches are sorely needed to extrapolate our captive findings to the much more complex social, temporal and spatial dynamics of free-living finches. With more than 50.2 million Americans feeding birds [44], it is critical to understand the implications of garden bird feeders for the health of common feeder visitors such as house finches.
Supplementary Material
Acknowledgements
We thank Laila Kirkpatrick, Johanel Caceres, Courtney Youngbar, John Hallagan, Juan Mateo Botero, Dorian Jackson and Ariel Leon for field and laboratory assistance; William Hopkins for allowing us to use the aviary facilities; and Eli Bridge, David Bonter and John DeCoste for RFID expertise. We also thank two anonymous reviewers for their thoughtful suggestions.
Ethics
This study was conducted under the following permits: Virginia Tech Institutional Animal Care and Use Committee, Virginia Department of Game and Inland Fisheries (50352), and the United States Fish and Wildlife Service (MB158404-1).
Data accessibility
Field and captive experiment data has been deposited in the Dryad repository [45].
Authors' contributions
S.C.M., J.S.A., D.R.F. and D.M.H. designed the experiment. S.C.M., J.S.A. and D.M.H. executed observations. S.C.M., C.A.T. and J.S.A. performed the analyses. All authors contributed to writing the paper.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the National Science Foundation (IOS-1054675 to D.M.H.), and a Virginia Tech Sigma Xi Student Research Grant (awarded to S.C.M).
References
- 1.Oro D, Genovart M, Tavecchia G, Fowler MS, Martínez-Abraín A. 2013. Ecological and evolutionary implications of food subsidies from humans. Ecol. Lett. 16, 1501–1514. ( 10.1111/ele.12187) [DOI] [PubMed] [Google Scholar]
- 2.Becker DJ, Streicker DG, Altizer S. 2015. Linking anthropogenic resources to wildlife-pathogen dynamics: a review and meta-analysis. Ecol. Lett. 18, 483–495. ( 10.1111/ele.12428) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray MH, Becker DJ, Hall RJ, Hernandez SM. 2016. Wildlife health and supplemental feeding: a review and management recommendations. Biol. Conserv. 204, 163–174. ( 10.1016/j.biocon.2016.10.034) [DOI] [Google Scholar]
- 4.Reynolds SJ, Galbraith JA, Smith JA, Jones DN. 2017. Garden bird feeding: insights and prospects from a north-south comparison of this global urban phenomenon. Front. Ecol. Evol. 5, 1–15. ( 10.3389/fevo.2017.00024) [DOI] [Google Scholar]
- 5.Wilcoxen TE, et al. 2015. Effects of bird-feeding activities on the health of wild birds. Conserv. Physiol. 3, 1–13. ( 10.1093/conphys/cov058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galbraith JA, Stanley MC, Jones DN, Beggs JR. 2017. Experimental feeding regime influences urban bird disease dynamics. J. Avian Biol. 48, 700–713. ( 10.1111/jav.01076) [DOI] [Google Scholar]
- 7.Robinson R, et al. 2010. Emerging infectious disease leads to rapid population declines of common British birds. PLoS ONE 5, e12215 ( 10.1371/journal.pone.0012215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawson B, Robinson R, Colvile KM, Peck KM, Chantrey J, Pennycott TW, Simpson VR, Toms MP, Cunningham AA. 2012. The emergence and spread of finch trichomonosis in the British Isles. Phil. Trans. R. Soc. B 367, 2852–2863. ( 10.1098/rstb.2012.0130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tizard I. 2004. Salmonellosis in wild birds. Semin. Avian Exot. Pet Med. 13, 50–66. ( 10.1053/j.saep.2004.01.008) [DOI] [Google Scholar]
- 10.Adelman JS, Moyers SC, Farine DR, Hawley DM. 2015. Feeder use predicts both acquisition and transmission of a contagious pathogen in a North American songbird. Proc. R. Soc. B 282, 20151429 ( 10.1098/rspb.2015.1429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhondt AA, Dhondt KV, Hawley DM, Jennelle CS. 2007. Experimental evidence for transmission of Mycoplasma gallisepticum in house finches by fomites. Avian Pathol. 36, 205–208. ( 10.1080/03079450701286277) [DOI] [PubMed] [Google Scholar]
- 12.Ley DH, Berkhoff JE, Mclaren JM. 1996. Mycoplasma gallisepticum isolated from house finches (Carpodacus mexicanus) with conjunctivitis. Avian Dis. 40, 480–483. ( 10.2307/1592250) [DOI] [PubMed] [Google Scholar]
- 13.Fischer JR, Stallknecht DE, Luttrell P, Dhondt AA, Converse KA. 1997. Mycoplasmal conjunctivitis in wild songbirds: the spread of a new contagious disease in a mobile host population. Emerg. Infect. Dis. 3, 69–72. ( 10.3201/eid0301.970110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hochachka WM, Dhondt AA. 2000. Density-dependent decline of host abundance resulting from a new infectious disease. Proc. Natl Acad. Sci. USA 97, 5303–5306. ( 10.1073/pnas.080551197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Altizer S, Hochachka WM, Dhondt AA. 2004. Seasonal dynamics of mycoplasmal conjunctivitis in eastern North American house finches. J. Anim. Ecol. 73, 309–322. ( 10.1111/j.0021-8790.2004.00807.x) [DOI] [Google Scholar]
- 16.Hill GE. 1993. House finch (Carpodacus mexicanus). Birds North Am . Online ( 10.2173/bna.46) [DOI] [Google Scholar]
- 17.Kollias GV, Sydenstricker KV, Kollias HW, Ley DH, Hosseini PR, Connolly V, Dhondt AA. 2004. Experimental infection of house finches with Mycoplasma gallisepticum. J. Wildl. Dis. 40, 79–86. ( 10.7589/0090-3558-40.1.79) [DOI] [PubMed] [Google Scholar]
- 18.Adelman JS, Mayer C, Hawley DM. 2017. Infection reduces anti-predator behaviors in house finches. J. Avian Biol. 48, 519–528. ( 10.1111/jav.01058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faustino CR, Jennelle CS, Connolly V, Davis AK, Swarthout EC, Dhondt AA, Cooch EG. 2004. Mycoplasma gallisepticum infection dynamics in a house finch population: seasonal variation in survival, encounter and transmission rate. J. Anim. Ecol. 73, 651–669. ( 10.1111/j.0021-8790.2004.00840.x) [DOI] [Google Scholar]
- 20.Hartup BK, Mohammed HO, Kollias GV, Dhondt AA. 1998. Risk factors associated with mycoplasmal conjunctivitis in house finches. J. Wildl. Dis. 34, 281–288. ( 10.7589/0090-3558-34.2.281) [DOI] [PubMed] [Google Scholar]
- 21.Adelman JS, Moore IT, Hawley DM. 2015. House finch responses to Mycoplasma gallisepticum infection do not vary with experimentally increased aggression. J. Exp. Zool. Part A Ecol. Integr. Physiol. 323, 39–51. ( 10.1002/jez.1894) [DOI] [PubMed] [Google Scholar]
- 22.Hawley DM, Lindström K, Wikelski M. 2006. Experimentally increased social competition compromises humoral immune responses in house finches. Horm. Behav. 49, 417–424. ( 10.1016/j.yhbeh.2005.09.003) [DOI] [PubMed] [Google Scholar]
- 23.Bonter DN, Zuckerberg B, Sedgwick CW, Hochachka WM. 2013. Daily foraging patterns in free-living birds: exploring the predation–starvation trade-off. Proc. R. Soc. B 280, 20123087 ( 10.1098/rspb.2012.3087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bridge ES, Bonter DN. 2011. A low-cost radio frequency identification device for ornithological research. J. Field Ornithol. 82, 52–59. ( 10.1111/j.1557-9263.2010.00307.x) [DOI] [Google Scholar]
- 25.Thompson W. 1960. Agonistic behavior in the house finch. Part I: annual cycle and display patterns. Condor 62, 245–271. ( 10.2307/1365516) [DOI] [Google Scholar]
- 26.Williams PD, Dobson AP, Dhondt KV, Hawley DM, Dhondt AA. 2014. Evidence of trade-offs shaping virulence evolution in an emerging wildlife pathogen. J. Evol. Biol. 27, 1271–1278. ( 10.1111/jeb.12379) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sydenstricker K, Dhondt A, Hawley DM, Jennelle CS, Kollias HW, Kolliasa GV. 2006. Characterization of experimental Mycoplasma gallisepticum infection in captive house finch flocks. Avian Dis. 50, 39–44. ( 10.1637/7403-062805R.1) [DOI] [PubMed] [Google Scholar]
- 28.Hawley DM, Grodio J, Frasca S, Kirkpatrick L, Ley DH. 2011. Experimental infection of domestic canaries (Serinus canaria domestica) with Mycoplasma gallisepticum: a new model system for a wildlife disease. Avian Pathol. 40, 321–327. ( 10.1080/03079457.2011.571660) [DOI] [PubMed] [Google Scholar]
- 29.Neumann C, Duboscq J, Dubuc C, Ginting A, Irwan AM, Agil M, Widdig A, Engelhardt A. 2011. Assessing dominance hierarchies: validation and advantages of progressive evaluation with Elo-rating. Anim. Behav. 82, 911–921. ( 10.1016/j.anbehav.2011.07.016) [DOI] [Google Scholar]
- 30.Sánchez-Tojar A, Schroeder J, Farine DR. In press. A practical guide for inferring reliable dominance hierarchies and estimating their uncertainty. J. Anim. Ecol. ( 10.1111/1365-2656.12776) [DOI] [PubMed] [Google Scholar]
- 31.R Development Core Team. 2014. R: a language and environment for statistical computing.https://www.r-project.org/.
- 32.Crawley MJ. 2013. The R book, 2nd edn Chichester, UK: John Wiley & Sons. [Google Scholar]
- 33.Leon AE, Hawley DM. 2017. Host responses to pathogen priming in a natural songbird host. Ecohealth 14, 793–804. ( 10.1007/s10393-017-1261-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hawley DM, Davis AK, Dhondt AA. 2007. Transmission-relevant behaviours shift with pathogen infection in wild house finches (Carpodacus mexicanus). Can. J. Zool. 85, 752–757. ( 10.1139/Z07-053) [DOI] [Google Scholar]
- 35.Alarcon-Nieto G, Graving J, Klarevas-Irby JA, Maldonado-Chaparro A, Mueller I, Farine DR. 2017. An automated barcode tracking system for behavioural studies in birds. bioRχiv ( 10.1101/201590) [DOI] [Google Scholar]
- 36.Hamede RK, McCallum H, Jones M. 2013. Biting injuries and transmission of Tasmanian devil facial tumour disease. J. Anim. Ecol. 82, 182–190. ( 10.1111/j.1365-2656.2012.02025.x) [DOI] [PubMed] [Google Scholar]
- 37.Luttrell M, Stallknecht D, Fischer JR, Sewell C, Kleven S. 1998. Natural Mycoplasma gallisepticum infection in a captive flock of house finches. J. Wildl. Dis. 34, 289–296. ( 10.7589/0090-3558-34.2.289) [DOI] [PubMed] [Google Scholar]
- 38.Dhondt AA, Driscoll MJL, Swarthout ECH. 2007. House finch Carpodacus mexicanus roosting behaviour during the non-breeding season and possible effects of mycoplasmal conjunctivitis. Ibis 149, 1–9. ( 10.1111/j.1474-919X.2006.00588.x) [DOI] [Google Scholar]
- 39.Farmer KL, Hill GE, Roberts SR. 2005. Susceptibility of wild songbirds to the house finch strain of Mycoplasma gallisepticum. J. Wildl. Dis. 41, 317–325. ( 10.7589/0090-3558-41.2.317) [DOI] [PubMed] [Google Scholar]
- 40.Dhondt AA, Dhondt KV, McCleery BV. 2008. Comparative infectiousness of three passerine bird species after experimental inoculation with Mycoplasma gallisepticum . Avian Pathol. 37, 635–640. ( 10.1080/03079450802499100) [DOI] [PubMed] [Google Scholar]
- 41.Ostfeld RS, Keesing F. 2011. Biodiversity and disease risk: the case of Lyme disease. Conserv. Biol. 14, 722–728. ( 10.1046/j.1523-1739.2000.99014.x) [DOI] [Google Scholar]
- 42.Fischer JD, Miller JR. 2015. Direct and indirect effects of anthropogenic bird food on population dynamics of a songbird. Acta Oecologica 69, 46–51. ( 10.1016/j.actao.2015.08.006) [DOI] [Google Scholar]
- 43.Becker DJ, Hall RJ. 2014. Too much of a good thing: resource provisioning alters infectious disease dynamics in wildlife. Biol. Lett. 10, 20140309 ( 10.1098/rsbl.2014.0309) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.U.S. Department of the Interior, U.S. Fish and Wildlife Service, U.S. Department of Commerce, U.S. Census Bureau. 2011. 2011 National survey of fishing, hunting, and wildlife-associated recreation https://www.census.gov/prod/2012pubs/fhw11-nat.pdf)
- 45.Moyers SC, Adelman JS, Farine DR, Thomason CA, Hawley DM. 2018. Feeder density enhances house finch disease transmission in experimental epidemics Dryad Digital Repository. ( 10.5061/dryad.fv584) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Moyers SC, Adelman JS, Farine DR, Thomason CA, Hawley DM. 2018. Feeder density enhances house finch disease transmission in experimental epidemics Dryad Digital Repository. ( 10.5061/dryad.fv584) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Field and captive experiment data has been deposited in the Dryad repository [45].