Abstract
Anthropogenic landscape features such as urban parks and gardens, landfills and farmlands can provide novel, seasonally reliable food sources that impact wildlife ecology and distributions. In historically migratory species, food subsidies can cause individuals to forgo migration and form partially migratory or entirely sedentary populations, eroding a crucial benefit of migration: pathogen avoidance through seasonal abandonment of transmission sites and mortality of infected individuals during migration. Since many migratory taxa are declining, and wildlife populations in urban areas can harbour zoonotic pathogens, understanding the mechanisms by which anthropogenic resource subsidies influence infection dynamics and the persistence of migration is important for wildlife conservation and public health. We developed a mathematical model for a partially migratory population and a vector-borne pathogen transmitted at a shared breeding ground, where food subsidies increase the nonbreeding survival of residents. We found that higher resident nonbreeding survival increased infection prevalence in residents and migrants, and lowered the fraction of the population that migrated. The persistence of migration may be especially threatened if residency permits emergence of more virulent pathogens, if resource subsidies reduce costs of infection for residents, and if infection reduces individual migratory propensity.
This article is part of the theme issue ‘Anthropogenic resource subsidies and host–parasite dynamics in wildlife’.
Keywords: partial migration, mathematical model, host–pathogen dynamics, resource supplementation
1. Introduction
Land use change and urbanization increasingly lead to overlap between wildlife and areas of high human population density [1]. Wildlife that are able to persist at the wildlife–human interface often do so by capitalizing on novel food resources provided unintentionally, e.g. through crop-raiding or scavenging of human trash, via exotic fruiting or flowering plants planted ornamentally or occurring as widespread invasives, and intentional feeding in parks and gardens. Food subsidies alter many components of life history, for instance by increasing breeding duration, breeding success and survival during harsh seasons [2], and by decoupling predator–prey relationships [3,4]. As a result, wildlife often spend more time and attain high densities in provisioned areas, but with unintended negative consequences such as increased pathogen pressure [5,6] and risk of spillover of zoonotic pathogens [7–9].
Anthropogenic food sources tend to be more spatially and seasonally predictable than food encountered in natural habitats [10], causing many animals to change their distributions and movement patterns (e.g. [11,12]). In particular, animals that undergo seasonal, long-distance migrations between breeding and nonbreeding habitats have been shown to adopt novel migratory routes and shorter migrations [13,14], or even to abandon migration altogether and form sedentary populations [15–17]. In combination with range-wide threats of climate change and habitat loss, provisioning that reduces migratory propensity may contribute to declines in migratory behaviour across taxa, and loss of important ecosystem services provided by migratory animals (e.g. [18,19]).
In migratory species where some individuals are able to abandon migration and form sedentary populations, food abundance is one critical mechanism that can affect the proportion of residents to migrants [20]. In historically partially migratory populations, the ability to capitalize on novel food sources may be particularly flexible. For example, individuals that migrate to escape harsh overwinter conditions are able to escape competition for scarce resources and move to more climatically favourable conditions, while those that remain resident have the advantage of being able to compete for and secure territories before migrants arrive [21,22]. In these cases, residents benefit from reproductive success while migrants benefit from increased overwinter survival. With resource supplementation, however, residents are more likely to survive over winter and have a competitive advantage in spring, potentially outweighing the life-history benefit of maintaining migrants in the population, and outcompeting migrants.
A reduction in migratory propensity in response to food supplementation would seem beneficial for populations that no longer need to migrate to track seasonal resources or escape inhospitable environmental conditions [23]; these populations in theory avoid migration-caused mortality. A reduction in migratory propensity, however, could mean loss of a major benefit of migration: escape from predation [24,25] and pathogen pressure [26]. Mathematical models have been useful for exploring the mechanisms by which migrants experience reduced pathogen impacts, including seasonal escape from sites of high transmission potential by all migrants [27] or by highly susceptible host stages [28], disproportionate mortality of infected individuals during costly migrations [29], and recovery from infection on nonbreeding grounds [30]. In areas where food provisioning or other external forces increase sedentary behaviour in partially migratory or formerly fully migratory populations, however, these benefits of migration may be lost, leading to enhanced transmission and potential maintenance of more virulent pathogens.
Mathematical models have been developed to explain the evolution and maintenance of partially migratory populations [31–33] and the consequences of resource provisioning for local and spatial pathogen transmission [34,35]. We lack, however, synthetic studies that explore pathogen dynamics in partially migratory populations. Increases in resource availability and more favourable overwinter climates that increase nonbreeding season survival across taxa make such studies particularly timely. Here, we explored how increased nonbreeding resident survival, one potential direct consequence of resource supplementation, affects the persistence of migration and a shared pathogen in a partially migratory population where the breeding location is shared. Since residents frequently find mates and establish breeding territories before migrants return [36], we make the simplifying assumption that residents and migrants do not interbreed. We assume that offspring adopt the same strategy as their parents, and further assume that migrants and residents do not switch strategies, except in the case in which costs of infection cause migrants to adopt a resident strategy with some probability. These are reasonable assumptions given that residents tend to outcompete migrants for resources and territories, individuals often maintain a given migratory strategy over time, both facultative and obligate migration strategies can exist within a population, and in cases where switching occurs, it is more often migrant to resident than vice versa [37–40].
We explore the possibility of switching migratory strategies upon infection because infected individuals may have delayed migratory departure and shorter migrations [41,42], which could ultimately lead to residency. We expect reverting back to a migratory strategy to be less likely in the avian system with which we parametrize our model because infected individuals are unlikely to recover (e.g. from avian malarias [43]), and the likelihood of switching strategies multiple times is low given a relatively short lifespan in the wild [44]. In addition, with resource supplementation, we expect that individuals that are delayed from departing should benefit from abandoning migration, given high costs of infection on migration [45]. We do not here model fecundity costs of infection, and thus migrants that become resident due to infection have the same reproductive success as residents if they survive the nonbreeding season.
Our mathematical modelling framework explores how increased nonbreeding resident survival, our proxy for increases in resource provisioning, alters disease risk in partially migratory populations, and the role of migration in mediating pathogen prevalence. For a partially migratory population where the majority of individuals migrate prior to resource supplementation, we expected that (1) long-distance migration would minimize infection prevalence through migratory culling and seasonal escape from infected biting vectors in the breeding region; (2) increases in nonbreeding season survival of individuals that forgo migration would reduce the proportion of migrants in the population; and, therefore, (3) increasing residency, presumably induced by resource supplementation, would increase infection prevalence by eroding the population-level benefits of migration for reducing pathogen impacts.
2. Material and methods
We present a modelling framework for a partially migratory host population and a pathogen that is transmitted by a biting vector in the host breeding range. We focused on the case of ‘nonbreeding partial migration’, whereby individuals breed in the same location but spend the nonbreeding season apart [21]. We assumed that the major fitness effect of resource supplementation was to increase survival of residents during the nonbreeding season; this is consistent with increasing numbers of primarily migratory songbirds overwintering at north-temperate latitudes in gardens and cities, where they capitalize on provisioned food such as seeds and suet, and ornamental plantings that provide fruit, flowers or evergreen foliage that supports invertebrate prey [46,47]. The goal of our model was, therefore, to analyse how increased nonbreeding season survival in the breeding region, a likely consequence of resource supplementation [46,48], influences the coexistence of migrants and residents, and invasion and persistence of pathogens. The model was motivated by, and parametrized for, a partially migratory passerine bird for which migration distances have become increasingly shorter, but its general structure may be adapted for other partially migratory host taxa.
(a). Disease-free model
Let N = N(Y, τ) be the population size of the partial migrant in year Y and within-year time τ. For model tractability, we assumed a perfectly heritable migration strategy, with the number of migratory and resident individuals given by NM and NR, respectively. The breeding ground has a favourable season beginning at within-year time τ = τ0, and ending at τ = τ1, during which conditions are suitable for breeding, and migrants and residents experience the same per capita mortality rate, μb. We assumed that residents initiate breeding at the beginning of the favourable season, but that migrants initiate breeding with a small delay δ (i.e. at within-year time τ = τ0 + δ) due to constraints of migration timing. Migrants and residents cease breeding at τ1 and migrants depart, experiencing relatively high migratory mortality (μmig > μb) during a one-way migration of duration Tmig, followed by relatively low mortality at their wintering site (μw ≈ μb) for the duration of overwintering, Tw. In the unfavourable season (i.e. nonbreeding season, Tnb, of within-year duration Tnb = 1 − (τ1 − τ0)), residents experience relatively high nonbreeding mortality (μnb > μb) that declines with increasing resource supplementation.
Migrants and residents compete for resources on the breeding ground, and so the population is regulated by density dependence in offspring production. To avoid offspring contributing to reproduction in their hatch year, resident and migrant fecundity is proportional to the number of residents surviving the winter (NRB), and the number of returning migrants (NMB), respectively. We describe the population dynamics of migrants and residents through an annual cycle by systems of differential equations for each of the breeding and nonbreeding phases. When both migrants and residents are breeding (τ0 + δ < τ < τ1), the dynamics are given by
| 2.1a |
and
| 2.1b |
where the subscripts M and R refer to the migrant and resident populations, respectively, b0 and b1 are the density-independent and density-dependent reproductive rates, and μb is mortality during the breeding season. Prior to the arrival of the migrants (τ0 < τ < τ0 + δ), the residents' breeding dynamics are given by equation (2.1b), where N in the density-dependent reproduction term is simply NR because no migrants are present.
Outside of the breeding season, we assumed that migrants and residents experience density-independent mortality at rate μj, where j = [mig, w, nb], during periods of migration, migrant overwintering and resident nonbreeding (each with respective duration Tj). The probability that migrant or resident individuals survive each of these periods is therefore
| 2.2 |
Equation (2.2) can be used to relate the number of breeding migrants and residents in the current year to the respective number of residents and migrants at the end of the previous breeding season:
| 2.3a |
and
| 2.3b |
Together, equations ((2.1)–(2.3)) describe the interannual dynamics of the system.
(b). Host–pathogen dynamics
We followed Hall et al. [29] and assumed the focal pathogen is transmitted by a biting vector at the partial migrant's shared breeding ground whose activity period coincides with the favourable season (figure 1). Because we are modelling a vector in a temperate system, we describe the seasonal vector emergence rate, E(τ), by a quadratic function with peak emergence Emax in the middle of the favourable season:
| 2.4 |
and vectors experience per capita mortality at rate μv. Following Hall et al. [29], we further assumed that the partially migratory host species is the primary competent reservoir for the pathogen, such that infection of vectors depends only on the number of infected residents and migrants, and that the vector distributes bites among a larger community of vertebrates such that the biting rate per host is independent of the host's population size. Thus, the transmission rate from hosts to vectors and vice versa is described by two constants, βhv and βvh, respectively.
Figure 1.
Overview of vector, resident and migrant population dynamics throughout the annual cycle (a), and associated timing for each period (b). (a) Schematic of the population and infection dynamics of a partially migratory host population, where resident and migratory populations share their breeding grounds with a disease-causing vector. Each large rectangle represents the breeding, migratory and wintering range of the migrant. Squares represent the populations of susceptible (S) and infected (I) migrant and resident (subscripts M, R respectively) hosts, and circles represent susceptible and infected vectors (subscript V). Solid arrows represent transitions between states resulting from births, deaths and infection; dashed arrows indicate variables influencing transmission rates. (b) Seasonal presence of residents, migrants and vectors throughout the migratory range. Grey shaded regions represent the timing of habitat use by the migrant, black lines indicate seasonal presence of migrants, residents and adult vectors during their breeding (thick lines) or nonbreeding (thin lines) period. Solid grey vertical lines represent the timing of migratory departure and arrival, and the grey dashed line denotes the onset of breeding in resident hosts and vectors.
We modelled costs of infection to the host as proportionate increases in mortality rates, which may vary through the annual cycle for residents and migrants; the mortality rate of infected individuals in stage j is μj/(1 − cj), where cj in [0, 1] is the magnitude of the associated cost. Additionally, we considered the case where infection may cause migrants to abandon migration and join the resident population with probability p ≥ 0. Vectors do not incur costs of infection, but we accounted for any vector mortality between the vector acquiring the pathogen and becoming infectious by the probability of the vector surviving the latent period, θ.
Denoting the susceptible and infected populations of migrants, residents and vectors as Sk and Ik, where k = [M, R, V], the host–pathogen dynamics when residents and migrants co-occur with vectors (i.e. when τ0 + δ < τ < τ1) are described by the system of equations
| 2.5a |
| 2.5b |
| 2.5c |
| 2.5d |
| 2.5e |
| 2.5f |
where I = IM + IR, N = SM + SR + I. The number of breeding adult migrants and residents at the beginning of their respective breeding seasons (NMB, NRB) is obtained by calculating the number of susceptible and infected individuals that survive their nonbreeding period (i.e. the product of their population sizes at the end of the breeding period and their nonbreeding survival), such that
| 2.6a |
and
| 2.6b |
The nonbreeding survival of migrants (partitioned into survival at the overwintering site and two rounds of migration) and residents are defined in equation (2.3), with survival of infected individuals modified by the cost of infection to migration or overwintering mortality. Before the migrants return to the breeding site (τ0 < τ < τ0 + δ), the resident-vector–pathogen dynamics are described by (2.5a,b) with N replaced by NR, and by (2.5e,f) with I replaced by IR. We summarize the model structure in figure 1, and provide a list of model parameters, their definitions, and default values in table 1.
Table 1.
Summary of model variables, parameters, definitions and their initial or default values (for those parameters that were varied, the range is shown in brackets). All times are in units of years and rates in units of years−1.
| symbol |
definition | value | |
|---|---|---|---|
| variables | SM | susceptible migrants | 450 |
| IM | infected migrants | 50 | |
| SR | susceptible residents | 450 | |
| IR | infected residents | 50 | |
| SV | susceptible vectors | 0 | |
| IV | infected vectors | 0 | |
| phenology | τ0, τ1 | start and end of favourable season at breeding site (breeding period for residents and vectors) | 0.29 (≈mid-late Apr), 0.74 (≈mid-late Aug) |
| δ | delay between τ0 and migrant arrival | 0.042 (≈2 weeks) | |
| Trb | duration of resident breeding (= τ1 − τ0) | 0.45 (5.4 months) | |
| Tnb | duration of resident nonbreeding (= 1 − Trb) | 0.55 (6.6 months) | |
| Tmb | duration of migrant breeding (= Trb − δ) | 0.41 (4.9 months) | |
| Tmig | duration of one-way migration | 0.092 (1.1 months) | |
| Tw | duration of migrant wintering (= 1 − Tmb − 2Tmig) | 0.41 (4.9 months) | |
| demography | b0 | density-independent reproductive rate | 3.7 |
| b1 | density-dependent reproductive rate | 3.7 × 10−3 | |
| μb | breeding season resident and migrant mortality rate | 0.075 | |
| σnb | resident nonbreeding survival probability | 0.49 [0.35–0.6] | |
| σmig | migration survival probability (one-way) | 0.74 | |
| σw | migrant overwintering survival probability | 0.97 | |
| Emax | maximum vector emergence rate | 3.65 × 105 (100/day) | |
| μv | vector mortality rate | 20 (≈18 day lifespan) | |
| infection | βhv | transmission rate from host to vector | 0.02 |
| βvh | transmission rate from vector to host | 0.02 | |
| cb | cost of infection to breeding survival | 0.3 | |
| cnb | cost of infection to resident nonbreeding survival | 0.3 [0.1–0.8] | |
| cmig | cost of infection to migration survival | 0.8 [0.3–0.8] | |
| cw | cost of infection to migrant overwinter survival | 0.3 | |
| p | probability infected migrant becomes resident | 0 [0–0.5] | |
| θ | probability of vector surviving latent period | 0.67 | |
(c). Parametrization
We parametrized our models using American Robins (Turdus migratorius) as an example because many now remain resident in urban areas at more northerly latitudes, they are known to be fed on seasonally by arthropod vectors (mosquitoes, blackflies and ticks) that transmit pathogens including avian malaria, and they are competent reservoir hosts for zoonotic pathogens of human health concern, including West Nile virus and Lyme disease [49–51]. We provide default parameter values in table 1, and details of the parameter derivation in the electronic supplementary material.
(d). Analysis
As noted above, we assumed that the effect of resource supplementation was primarily to increase nonbreeding survival of the resident portion of the population. First, we varied resident nonbreeding survival to examine how the population sizes of migrants and residents, and migratory propensity (the fraction of the population that migrates, NM/N), respond to resource supplementation in the absence of pathogens, and in the presence of pathogens that inflict different costs on survival across the annual cycle. When pathogens were present, we also recorded infection prevalence in the host population (I/N) and in migratory and resident hosts (IM/NM, IR/NR, respectively).
We considered the following scenarios for pathogens with differing costs of infection to compare to disease-free scenarios:
(i) Baseline: moderate costs of infection for both migrants and residents (infected individuals experience a 30% reduction in expected lifespan) distributed proportionally through the seasonal mortality rates; cj = 0.3 for j = [b, mig, w, nb], and all infected migrants attempt migration (p = 0);
(ii) Migratory virulence: elevated costs of infection for migrants during migration (cmig varied up to a maximum of 0.8, representing a pathogen too virulent to persist in a wholly migratory population);
(iii) Nonbreeding tolerance: the cost of infection on nonbreeding resident survival is reduced by resource supplementation (cnb varied down to 0.1);
(iv) Nonbreeding virulence: harsh environmental conditions in winter cause elevated costs of infection for resident nonbreeding survival (cnb varied up to 0.8, representing a pathogen too virulent to persist in a wholly resident population); and
(v) Disease-induced residency: infected migrants abandon migration and become resident with probability p ≤ 0.5.
We ran all models in R [52] using the deSolve package [53]. Where maximum and minimum values are output in models, for simplicity we present minimum host population sizes, minimum migratory propensity, and maximum host infection prevalence. We chose these cases because we consider them of most relevance to conservation, allowing us to identify potential conditions under which food-subsidized sedentary populations could lead to undesirable outcomes such as loss of migration or increased risk of zoonotic spillover.
3. Results
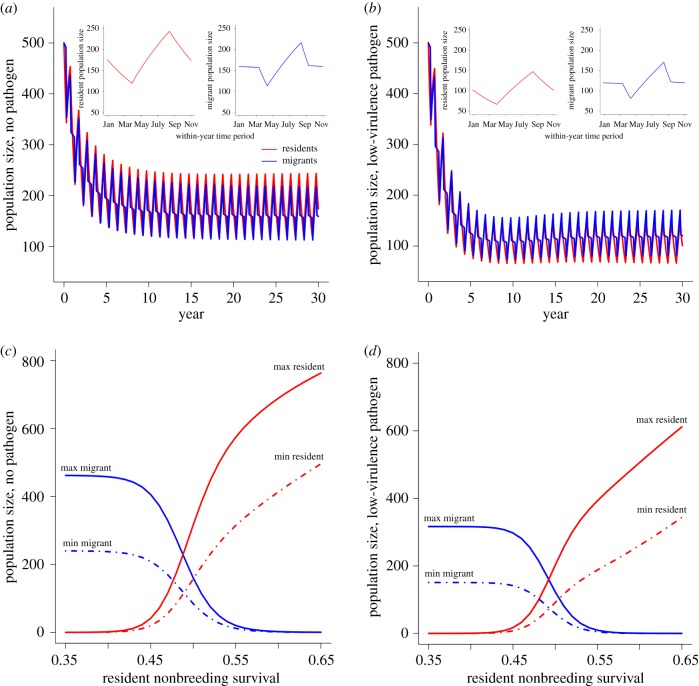
After an initial transient phase of approximately 20 years, host and pathogen dynamics settled into an annually repeating pattern (henceforth referred to as ‘equilibrium’) in which population size increased during the breeding season and declined across the nonbreeding portions of the year (figure 2a,b); provided at least some residents, migrants and pathogen were present initially, the equilibrium pattern was independent of initial conditions. In the disease-free case, when resident nonbreeding survival was increased from low values that prevent long-term persistence of resident populations (σnb ≈ 0.35), the migratory population declined and the resident population increased until migrants were competitively excluded (at σnb ≈ 0.6; figure 2c).
Figure 2.
Resident and migrant population sizes (red and blue lines, respectively) for disease-free (left column) and infection with a low-virulence pathogen (call = 0.3, right column). (a,b) Population trajectory over a 30-year period, showing dynamics settling into an equilibrium annually repeating pattern; insets show within-year resident and migrant population sizes at equilibrium. (c,d) Maximum (solid lines) and minimum (dot-dashed lines) resident and migrant population sizes at equilibrium as a function of resident nonbreeding survival (σnb).
(a). Scenarios
(i). Baseline (cj = 0.3 throughout the annual cycle)
In the presence of a moderately virulent pathogen that reduced both resident and migrant survival equally across the annual cycle, the range of values of resident nonbreeding survival (σnb) for which residents and migrants coexisted was similar to the disease-free case (figure 2c,d). The maximum and minimum population sizes of residents and migrants, however, were lower (figure 2d). Maximum and minimum host population sizes through the equilibrium annual cycle responded in a qualitatively similar way to increases in σnb, justifying our inclusion in the results of only cases that we consider of most relevance for conservation: minimum host population sizes, minimum migratory propensity and maximum host infection prevalence.
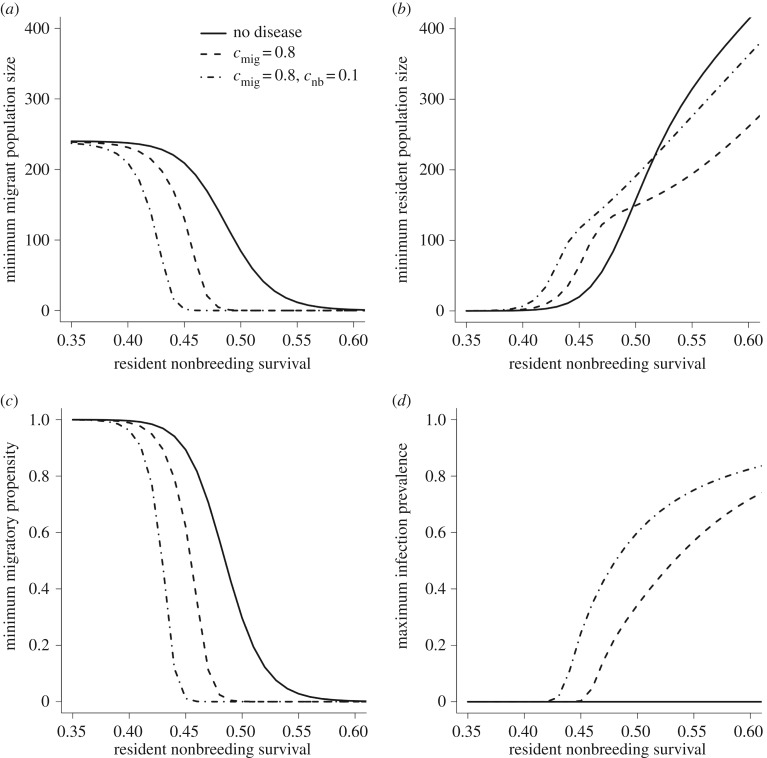
(ii). Migratory virulence (cmig > baseline)
When the cost of infection on migration was high and resident nonbreeding survival was too low for resident populations to persist, the pathogen was unable to establish, maximizing migrant population size (figure 3). Increasing nonbreeding season survival allowed resident hosts to establish and sustain parasite transmission, and migration was lost from the population at lower values of resident nonbreeding survival than in the baseline or disease-free cases (figure 3a,c and electronic supplementary material, figure S1a). Infection prevalence increased quickly with σnb as the number of migrants declined, and the role of migratory culling in reducing prevalence was diminished (figure 2d). At modest increases in nonbreeding survival, resident population sizes were high relative to the disease-free case, due to disease-induced mortality in migrants reducing breeding-season competition; however, at higher levels of σnb where the host population was dominated by residents, equilibrium resident population sizes were lower than the disease-free scenario (figure 3b).
Figure 3.
Minimum population size of (a) migrants and (b) residents, (c) minimum fraction of the population that migrates (i.e. migratory propensity) and (d) maximum infection prevalence across a range of resident nonbreeding survival values, σnb. Results are shown for three model scenarios: disease-free (solid lines); infection induces high survival costs during migration only (cmig = 0.8; dashed lines); and infection causes high migratory mortality and food provisioning lowers costs of infection for residents in the nonbreeding season (cmig = 0.8, cnb = 0.1; dotted-and-dashed lines). All other parameter values are listed in table 1.
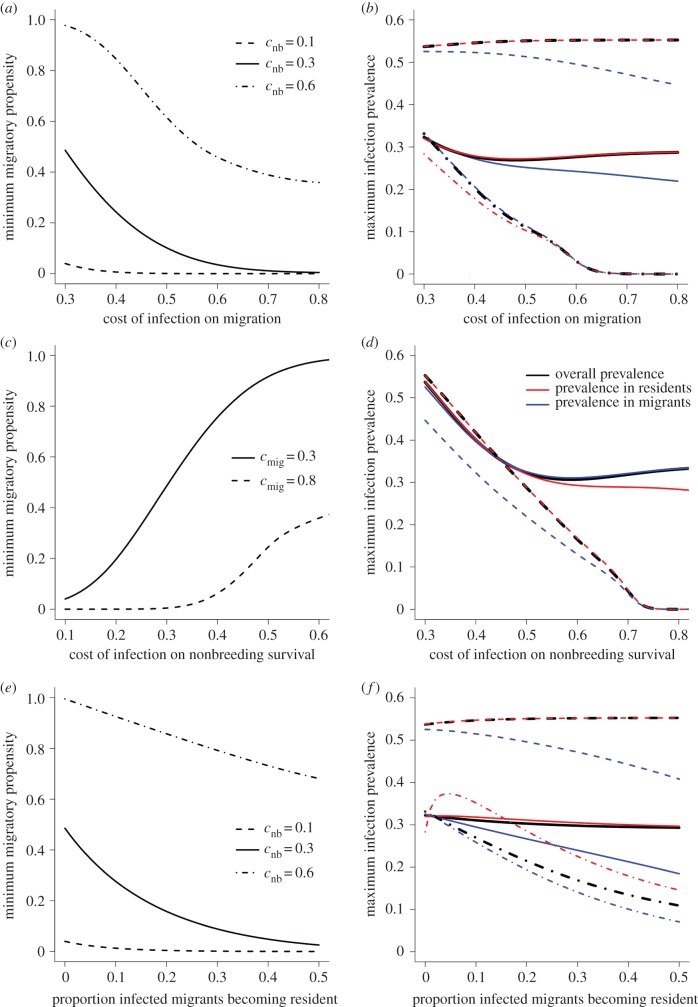
For a fixed value of nonbreeding survival that permitted disease-free coexistence of residents and migrants (σnb = 0.49), the proportion of the population that migrates declined as the cost of infection on migration increased (figure 4a). When costs of infection to nonbreeding survival were moderate (cnb = 0.3), infection prevalence across all hosts was minimized at intermediate values of migratory virulence, reflecting the switch from a decreasing relationship between prevalence and virulence when the host population was dominated by migrants, to an increasing but saturating relationship as the resident population increased (figure 4b, bold lines).
Figure 4.
Minimum fraction of the population that migrates (i.e. migratory propensity) and maximum expected infection prevalence for (a,b) varying levels of costs of infection on migration, cmig, (c,d) varying levels of costs of infection on nonbreeding resident survival, cnb, and (e,f) when a proportion of infected migrants forgo migration. Resident nonbreeding survival, σnb, = 0.49 in all cases, and all c values not shown in figure = 0.3.
(iii). Nonbreeding tolerance (cnb < baseline)
When we reduced the cost of infection on resident nonbreeding survival, a potential consequence of resource supplementation, residents established at lower values of σnb and migrants were lost from the population (electronic supplementary material, figure S1a,b). This effect was exacerbated when the pathogen caused high migratory virulence, and the range of σnb values for which coexistence of migrants and residents occurred was lower (figure 3a–c). Migratory populations were generally smaller (figure 3a), and overall infection prevalence was higher (figure 3d) when overwinter survival of infected residents was higher; this is the result of larger resident populations (figure 3b) negatively impacting migrants both through direct competition and through disease-mediated apparent competition.
(iv). Nonbreeding virulence (cnb > baseline)
When nonbreeding survival of infected residents was low, reflecting that infected individuals may be particularly vulnerable to harsh environmental conditions such as cold snaps, formation of persistent resident populations only occurred at substantially lower levels of natural overwinter mortality (i.e. the population was wholly migratory for larger values of σnb relative to the baseline; electronic supplementary material, figure S1a–c). For a fixed value of σnb permitting disease-free coexistence, migratory propensity increased with higher costs of infection on nonbreeding resident survival (figure 4c). For a pathogen causing moderate migratory virulence, infection prevalence was minimized at intermediate nonbreeding virulence; a weak positive relationship between overall prevalence and high resident nonbreeding virulence is explained by a feedback between disease-induced mortality of residents releasing the migratory population from competition, allowing elevated pathogen transmission in migrants that spills over into residents (figure 4d). Pathogens causing high virulence during migration and the resident nonbreeding season were typically unable to persist (figure 4b,d)
(v). Disease-induced residency (p > 0)
When infection made migrants more likely to forgo migration and become resident, migratory propensity was typically lower (figure 4e), and migrants were lost from the population at lower values of resident nonbreeding survival (electronic supplementary material, figure S1c). The net effect of disease-induced residency on population sizes and infection prevalence in migrants and residents depended on nonbreeding costs to infected residents. When we assumed that provisioning would reduce nonbreeding virulence, migrant population size and infection prevalence decreased, while resident population size and prevalence increased, with increasing probability of infection-induced residency (electronic supplementary material, figure S1a,b; figure 4f). For pathogens causing high nonbreeding virulence in residents, when nonbreeding survival was low (σnb < 0.45), disease-induced residency increased migrant population sizes by reducing infection prevalence (and associated migratory culling) in the migrant population (electronic supplementary material, figure S1a,d). When nonbreeding resident survival was increased, competition with residents reduced migrant population size, but overall infection prevalence remained low as increasing numbers of infected migrants recruited to the resident population and experienced high nonbreeding virulence (electronic supplementary material, figure S1a,d; figure 4f).
4. Discussion
We investigated mechanisms by which anthropogenic resource subsidies can influence migratory propensity and infection prevalence in a partially migratory population in which individuals have a shared breeding location and spend the nonbreeding season apart. We found that improved nonbreeding survival of residents, a potential consequence of food subsidies, can cause declines in migrant populations. Our results emerge via disease-induced and density-dependent declines that play out in the real world through increased competition for breeding resources and elevated exposure to pathogens. Increased pathogen exposure in migrants can occur as a result of the loss of key mechanisms of pathogen avoidance in migratory populations: migratory escape (seasonal departure from transmission sites reducing seasonal overlap with disease-causing agents) and migratory culling (high mortality of infected individuals during migration, or of individuals that abandon migration and succumb to harsh environmental conditions in the unfavourable season). The benefits of migratory escape are diminished by provisioning, however, because residents experience an extended pathogen transmission season relative to migrants and improved overwinter survival, resulting in higher infection prevalence in biting vectors when migrants return to their breeding grounds. Migratory culling or abandonment of migration by infected individuals can fail to reduce infection prevalence in migrants because provisioned resident populations can reach relatively high abundance and sustain transmission of pathogens, including those that would be too virulent to be maintained in wholly migratory populations.
Our work suggests several non-exclusive mechanisms by which resource supplementation can result in high disease burdens in partially migratory populations: (i) extended overlap of residents with a seasonally transmitted pathogen that increases the transmission window; (ii) increased resident population sizes that support more transmission; and (iii) increased survival of infected residents (and migratory ‘dropouts’) between transmission seasons to initiate seasonal epidemics. Several other lines of theoretical and empirical evidence support provisioning increasing host–pathogen overlap. In monarch butterflies (Danaus plexippus), planting of non-native milkweeds that do not die back in winter leads to year-round monarch breeding and ingestion of infective spores of the protozoan Ophryocystis elektroscirrha by their larvae; the resulting sedentary populations experience higher infection prevalence than migratory populations [15,54]. Additionally, increased aggregation of wildlife in food-provisioned areas has been demonstrated theoretically to increase pathogen transmission [34], and may be associated with larger outbreaks of Hendra virus in urban-living flying foxes (Pteropus spp.) [16]. Finally, food supplementation has been proposed to modulate costs of infection through improved immune defense in urban-feeding kit foxes (Vulpes macrotis mutica) [55] and provisioned house finches (Haemorhous mexicanus) [56]. Such mechanisms could also be important for transmission of potentially zoonotic pathogens in migratory waterfowl where infection reduces migratory propensity (e.g. [41]), but which successfully overwinter at more northerly latitudes in response to agricultural subsidies [57].
We modelled our framework for investigating vector-borne disease dynamics after a food-supplemented avian partial migrant. Given the assumptions underlying our model, the qualitative results that migrants declined, residents increased and overall prevalence increased when provisioning increased resident nonbreeding survival were not entirely surprising. We did not, however, have clear quantitative predictions about the magnitude of effects of differential costs of infection through the annual cycle of residents and migrants, or migratory dropout, and we did not a priori expect the nonlinear relationships between these variables and infection prevalence (e.g. figure 4). Ours is the first study to our knowledge to outline general predictions for resource supplementation altering migratory propensity and pathogen impacts, and to verify these with a mathematical model. This framework could readily be adapted and applied to other partially migratory taxa for which resource supplementation may affect migratory behaviour (e.g. [58]) and vector-borne parasites (e.g. ungulates and tick-borne diseases), to interacting residents and migrants of different species, and to scenarios where residents, migrants and parasites overlap in one or more parts of the migratory range. Our findings may also apply more generally to other modes of long-distance animal movement that may emerge in populations that also exhibit resident and migratory strategies, such as nomadism [59], or to directly or environmentally transmitted pathogens, provided that migrants and residents only aggregate at densities permitting transmission at their shared breeding site. Patterns of increased infection risk associated with sedentary behaviour have been attributed to other anthropogenic or environmental drivers of reduced migratory propensity; for example, climate change that results in shortened migrations and longer transmission windows [28] and barriers to migration [29].
In our model, we focused on effects of resource subsidies on residents during a temperate-latitude nonbreeding period (improvements to survival and reductions to costs of infection). We assumed that harsh environmental conditions prior to provisioning were most likely to limit establishment of resident populations and survival of infected individuals, and that availability of natural (i.e. non-supplemented) resources was the primary driver of breeding success and higher survival during the favourable season. Given that partial migration is a naturally occurring phenomenon, however, the mechanism by which we model it is likely independent of the potential for increases in pathogen prevalence with higher resident nonbreeding survival. Negative effects of food subsidies on migratory population size may not occur if both residents and migrants experience benefits from food subsidies during the breeding season, if migrants experience additional carryover effects that offset the delay in breeding initiation relative to residents [48], or if food subsidies in other parts of the migratory range improve survival away from the breeding sites. In cases where supplemental feeding stimulates explosive breeding in hosts, or where the cost of infection for both migrants and residents is low, and is even lower for nonbreeding residents with food supplementation (e.g. cnb = 0.1, all other c = 0.3, unpubl. simulations), population cycles for a highly transmissible, highly virulent pathogen might be expected. In addition, the relationship between food subsidies and increases in nonbreeding survival could saturate or lead to population cycles if resources or increased population sizes attract predators. Nevertheless, these positive effects of food supplementation may still be associated with increases in infection prevalence that may be relevant to zoonotic spillover [34].
We considered a pathogen that causes lifelong infection, as is often seen in short-lived songbirds infected by haemospiridian parasites [60]. Food subsidies could reduce infection prevalence if associated improvements to host immune defense allow them to recover more rapidly [34]. Alternatively, sedentary behaviour promoted by provisioning could erode another proposed benefit of migration: migratory recovery from infection [30]. Our model does not account for interannual variability in the harshness of environmental conditions in the nonbreeding season, or for the possibility of frequent extra-pair mating interfering with the temporal separation of mate acquisition, even in otherwise highly monogamous species. If food subsidies promote the formation of sedentary populations and draw in infected individuals from migratory populations, occasional climatic extremes such as cold snaps could extirpate resident populations and reduce pathogen transmission. However, if ephemeral resident populations also have a large negative effect on migrant abundance following mild winters, resource subsidies could threaten the persistence of the partial migrant and, therefore, act as an ecological trap.
In sum, our work suggests that animal migration, already considered to be a threatened phenomenon [18], may be negatively impacted through elevated transmission of virulent pathogens associated with food subsidies that allow resident populations to form. In extreme cases, this could lead to the irreversible loss of migratory phenotypes, with unforeseen consequences for ecosystem services [19]. Since food supplementation can increase infection prevalence of low-virulence pathogens in residents and migrants, it has the potential to influence zoonotic spillover risk not only at food-provisioned sites, but throughout the migratory range [35]. Our work presents a valuable framework for ecologists, highlights important consequences for wildlife managers, and motivates further empirical research on how food subsidies influence migratory decisions, survival, pathogen exposure risk and costs of infection in imperilled migrants and partial migrants that are known reservoirs for zoonoses.
Supplementary Material
Supplementary Material
Acknowledgements
We are grateful to Sonia Altizer for helpful discussions during early development of ideas and models for this study, and two anonymous reviewers for their helpful comments and suggestions.
Data accessibility
A version of the R code that can be used to run all models is included in this article's electronic supplementary material.
Authors' contributions
L.M.B. and R.J.H. contributed equally to conception and execution of the ideas, models and code for this manuscript, and drafted and revised the final manuscript.
Competing interests
The authors declare no competing interests.
Funding
This work was supported by a National Science Foundation (NSF) Postdoctoral training grant no. 1402287 in Mathematical Biology to L.M.B., and a NSF Ecology and Evolution of Infectious Disease grant no. DEB-1518611 to R.J.H.
References
- 1.Radeloff VC, Hammer RB, Stewart SI, Fried JS, Holcomb SS, McKeefry JF. 2005. The wildland–urban interface in the United States. Ecol. Appl. 15, 799–805. ( 10.1890/04-1413) [DOI] [Google Scholar]
- 2.Robb GN, McDonald RA, Chamberlain DE, Reynolds SJ, Harrison TJE, Bearhop S. 2008. Winter feeding of birds increases productivity in the subsequent breeding season. Biol. Lett. 4, 220–223. ( 10.1098/Rsbl.2007.0622) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodewald AD, Kearns LJ, Shustack DP. 2011. Anthropogenic resource subsidies decouple predator–prey relationships. Ecol. Appl. 21, 936–943. ( 10.1890/10-0863.1) [DOI] [PubMed] [Google Scholar]
- 4.Fischer JD, Cleeton SH, Lyons TP, Miller JR. 2012. Urbanization and the predation paradox: the role of trophic dynamics in structuring vertebrate communities. BioScience 62, 809–818. ( 10.1525/bio.2012.62.9.6) [DOI] [Google Scholar]
- 5.Murray MH, Becker DJ, Hall RJ, Hernandez SM. 2016. Wildlife health and supplemental feeding: a review and management recommendations. Biol. Conserv. 204, 163–174. ( 10.1016/j.biocon.2016.10.034) [DOI] [Google Scholar]
- 6.Becker DJ, Streicker DG, Altizer S. 2015. Linking anthropogenic resources to wildlife–pathogen dynamics: a review and meta-analysis. Ecol. Lett. 18, 483–495. ( 10.1111/ele.12428) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradley CA, Altizer S. 2007. Urbanization and the ecology of wildlife diseases. Trends Ecol. Evol. 22, 95–102. ( 10.1016/J.Tree.2006.11.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weaver SC. 2013. Urbanization and geographic expansion of zoonotic arboviral diseases: mechanisms and potential strategies for prevention. Trends Microbiol. 21, 360–363. ( 10.1016/j.tim.2013.03.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plowright RK, et al. 2015. Ecological dynamics of emerging bat virus spillover. Phil. Trans. R. Soc. B 282, 20142124 ( 10.1098/rspb.2014.2124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oro D, Genovart M, Tavecchia G, Fowler MS, Martínez-Abraín A. 2013. Ecological and evolutionary implications of food subsidies from humans. Ecol. Lett. 16, 1501–1514. ( 10.1111/ele.12187) [DOI] [PubMed] [Google Scholar]
- 11.Ciucci P, Boitani L, Francisci F, Andreoli G. 1997. Home range, activity and movements of a wolf pack in central Italy. J. Zool. 243, 803–819. ( 10.1111/j.1469-7998.1997.tb01977.x) [DOI] [Google Scholar]
- 12.López-López P, García-Ripollés C, Urios V. 2014. Food predictability determines space use of endangered vultures: implications for management of supplementary feeding. Ecol. Appl. 24, 938–949. ( 10.1890/13-2000.1) [DOI] [PubMed] [Google Scholar]
- 13.Teitelbaum CS, Converse SJ, Fagan WF, Böhning-Gaese K, O'Hara RB, Lacy AE, Mueller T. 2016. Experience drives innovation of new migration patterns of whooping cranes in response to global change. Nat. Commun. 7, 12793 ( 10.1038/ncomms12793) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berthold P, Helbig AJ, Mohr G, Querner U. 1992. Rapid microevolution of migratory behaviour in a wild bird species. Nature 360, 668–670. ( 10.1038/360668a0) [DOI] [Google Scholar]
- 15.Satterfield DA, Maerz JC, Altizer S. 2015. Loss of migratory behaviour increases infection risk for a butterfly host. Proc. R. Soc. B 282, 20141734 ( 10.1098/rspb.2014.1734) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plowright RK, Foley P, Field HE, Dobson AP, Foley JE, Eby P, Daszak P. 2011. Urban habituation, ecological connectivity and epidemic dampening: the emergence of Hendra virus from flying foxes (Pteropus spp.). Proc. R. Soc. B 278, 3703–3712. ( 10.1098/rspb.2011.0522) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atwell JW, O'Neal DM, Ketterson ED. 2011. Animal migration as a moving target for conservation: intra-species variation and responses to environmental change, as illustrated in a sometimes migratory songbird. Envtl. L. 41, 289. [PMC free article] [PubMed] [Google Scholar]
- 18.Wilcove DS, Wikelski M. 2008. Going, going, gone: is animal migration disappearing? PLoS Biol. 6, e188 ( 10.1371/journal.pbio.0060188) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bauer S, Hoye BJ. 2014. Migratory animals couple biodiversity and ecosystem functioning worldwide. Science 344, 1242552 ( 10.1126/science.1242552) [DOI] [PubMed] [Google Scholar]
- 20.Nilsson AL, Lindstroem A, Jonzen N, Nilsson SG, Karlsson L. 2006. The effect of climate change on partial migration—the blue tit paradox. Glob. Change Biol. 12, 2014–2022. ( 10.1111/j.1365-2486.2006.01237.x) [DOI] [Google Scholar]
- 21.Chapman BB, Brönmark C, Nilsson JÅ, Hansson LA. 2011. The ecology and evolution of partial migration. Oikos 120, 1764–1775. ( 10.1111/j.1600-0706.2011.20131.x) [DOI] [Google Scholar]
- 22.Lundberg P. 1988. The evolution of partial migration in birds. Trends Ecol. Evol. 3, 172–175. ( 10.1016/0169-5347(88)90035-3) [DOI] [PubMed] [Google Scholar]
- 23.Dingle H. 1996. Migration: the biology of life on the move. New York, NY: Oxford University Press. [Google Scholar]
- 24.Fryxell JM, Sinclair ARE. 1988. Causes and consequences of migration by large herbivores. Trends Ecol. Evol. 3, 237–241. ( 10.1016/0169-5347(88)90166-8) [DOI] [PubMed] [Google Scholar]
- 25.Zaret TM, Suffern JS. 1976. Vertical migration in zooplankton as a predator avoidance mechanism. Limnol. Oceanogr. 21, 804–813. ( 10.4319/lo.1976.21.6.0804) [DOI] [Google Scholar]
- 26.Altizer S, Bartel R, Han BA. 2011. Animal migration and infectious disease risk. Science 331, 296–302. ( 10.1126/science.1194694) [DOI] [PubMed] [Google Scholar]
- 27.Hall RJ, Altizer S, Bartel RA. 2014. Greater migratory propensity in hosts lowers pathogen transmission and impacts. J. Anim. Ecol. 83, 1068–1077. ( 10.1111/1365-2656.12204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krkošek M, Gottesfeld A, Proctor B, Rolston D, Carr-Harris C, Lewis MA. 2007. Effects of host migration, diversity and aquaculture on sea lice threats to Pacific salmon populations. Proc. R. Soc. B 274, 3141–3149. ( 10.1098/rspb.2007.1122) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall RJ, Brown LM, Altizer S. 2016. Modeling vector-borne disease risk in migratory animals under climate change. Integr. Comp. Biol. 56, 353–364. ( 10.1093/icb/icw049) [DOI] [PubMed] [Google Scholar]
- 30.Shaw AK, Binning SA.. 2016. Migratory recovery from infection as a selective pressure for the evolution of migration. Am. Nat. 187, 491–501. [DOI] [PubMed] [Google Scholar]
- 31.Shaw AK, Levin SA. 2011. To breed or not to breed: a model of partial migration. Oikos 120, 1871–1879. ( 10.1111/j.1600-0706.2011.19443.x) [DOI] [Google Scholar]
- 32.Reluga TC, Shaw AK. 2015. Resource distribution drives the adoption of migratory, partially migratory, or residential strategies. Theoret. Ecol. 8, 437–447. ( 10.1007/s12080-015-0263-y) [DOI] [Google Scholar]
- 33.Griswold CK, Taylor CM, Norris DR. 2011. The equilibrium population size of a partially migratory population and its response to environmental change. Oikos 120, 1847–1859. ( 10.1111/j.1600-0706.2011.19435.x) [DOI] [Google Scholar]
- 34.Becker DJ, Hall RJ. 2014. Too much of a good thing: resource provisioning alters infectious disease dynamics in wildlife. Biol. Lett. 10, 20140309 ( 10.1098/rsbl.2014.0309) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Becker DJ, Hall RJ. 2016. Heterogeneity in patch quality buffers metapopulations from pathogen impacts. Theoret. Ecol. 9, 197 ( 10.1007/s12080-015-0284-6) [DOI] [Google Scholar]
- 36.Grist H, Daunt F, Wanless S, Burthe SJ, Newell MA, Harris MP, Reid JM. 2017. Reproductive performance of resident and migrant males, females and pairs in a partially migratory bird. J. Anim. Ecol. 86, 1010–1021. ( 10.1111/1365-2656.12691) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kokko H. 1999. Competition for early arrival in migratory birds. J. Anim. Ecol. 68, 940–950. ( 10.1046/j.1365-2656.1999.00343.x) [DOI] [Google Scholar]
- 38.O'Connor R. 1990. Some ecological aspects of migrants and residents. In Bird migration: the physiology and ecophysiology (ed. Gwinner E.), pp. 175–182. New York, NY: Springer. [Google Scholar]
- 39.Krebs JR. 1982. Territorial defence in the great tit (Parus major): do residents always win? Behav. Ecol. Sociobiol. 11, 185–194. ( 10.1007/BF00300061) [DOI] [Google Scholar]
- 40.Brodersen J, Chapman BB, Nilsson PA, Skov C, Hansson L-A, Brönmark C. 2014. Fixed and flexible: coexistence of obligate and facultative migratory strategies in a freshwater fish. PLoS ONE 9, e90294 ( 10.1371/journal.pone.0090294) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Gils JA, Munster VJ, Radersma R, Liefhebber D, Fouchier RAM, Klaassen M. 2007. Hampered foraging and migratory performance in swans infected with low-pathogenic avian influenza A virus. PLoS ONE 2, e184 ( 10.1371/journal.pone.0000184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson PT, Stanton DE, Forshay KJ, Calhoun DM. 2017. Vertically challenged: how disease suppresses Daphnia vertical migration behavior. Limnol. Oceanogr. ( 10.1002/lno.10676) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Applegate JE. 1971. Spring relapse of Plasmodium relictum infections in an experimental field population of English Sparrows (Passer domesticus). J Wildlife Dis 7, 37–42. ( 10.7589/0090-3558-7.1.37) [DOI] [PubMed] [Google Scholar]
- 44.Vanderhoff N, Pyle P, Patten MA, Sallabanks R, James FC. 2016. American Robin (Turdus migratorius). In The birds of North America (ed. Rodewald PG.). Ithaca, NY: Cornell Lab of Ornithology; (See 10.2173/bna.462) [DOI] [Google Scholar]
- 45.Hawley DM, Altizer SM. 2011. Disease ecology meets ecological immunology: understanding the links between organismal immunity and infection dynamics in natural populations. Function. Ecol. 25, 48–60. ( 10.1111/j.1365-2435.2010.01753.x) [DOI] [Google Scholar]
- 46.Robb GN, McDonald RA, Chamberlain DE, Bearhop S. 2008. Food for thought: supplementary feeding as a driver of ecological change in avian populations. Front. Ecol. Environ. 6, 476–484. ( 10.1890/060152) [DOI] [Google Scholar]
- 47.Suhonen J, Jokimäki J, Kaisanlahti-Jokimäki M-L, Hakkarainen H, Huhta E, Inki K, Suorsa P. 2009. Urbanization and stability of a bird community in winter. Ecoscience 16, 502–507. ( 10.2980/16-4-3280) [DOI] [Google Scholar]
- 48.Greig EI, Wood EM, Bonter DN. 2017. Winter range expansion of a hummingbird is associated with urbanization and supplementary feeding. Proc. R. Soc. B 284, 20170256 ( 10.1098/rspb.2017.0256) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kilpatrick AM, Kramer LD, Jones MJ, Marra PP, Daszak P. 2006. West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biol. 4, e82 ( 10.1371/journal.pbio.0040082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Komar N. 2003. West Nile virus: epidemiology and ecology in North America. Adv. Virus Res. 61, 185–234. ( 10.1016/S0065-3527(03)61005-5) [DOI] [PubMed] [Google Scholar]
- 51.Richter D, Spielman A, Komar N, Matuschka F-R. 2000. Competence of American robins as reservoir hosts for Lyme disease spirochetes. Emerg. Infect. Dis. 6, 133 ( 10.3201/eid0602.000205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.R-Core-Team. 2016. R: A language and environment for statistical computing. Vienna, Austria. [Google Scholar]
- 53.Soetaert K, Petzoldt T, Setzer RW. 2010. Solving differential equations in R: Package deSolve. J. Stat. Softw. 33, 1–25. ( 10.18637/jss.v033.i09)20808728 [DOI] [Google Scholar]
- 54.Bartel RA, Oberhauser KS, de Roode JC, Altizer SM. 2011. Monarch butterfly migration and parasite transmission in eastern North America. Ecology 92, 342–351. ( 10.1890/10-0489.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cypher BL, Frost N. 1999. Condition of San Joaquin kit foxes in urban and exurban habitats. J. Wildlife Manage. 63, 930–938. ( 10.2307/3802807) [DOI] [Google Scholar]
- 56.Dhondt AA, Dhondt KV, Hawley DM, Jennelle CS. 2007. Experimental evidence for transmission of Mycoplasma gallisepticum in house finches by fomites. Avian Pathol. 36, 205–208. ( 10.1080/03079450701286277) [DOI] [PubMed] [Google Scholar]
- 57.Fox A, Madsen J, Boyd H, Kuijken E, Norriss D, Tombre I, Stroud D. 2005. Effects of agricultural change on abundance, fitness components and distribution of two arctic-nesting goose populations. Global Change Biol. 11, 881–893. ( 10.1111/j.1365-2486.2005.00941.x) [DOI] [Google Scholar]
- 58.Jones JD, Kauffman MJ, Monteith KL, Scurlock BM, Albeke SE, Cross PC. 2014. Supplemental feeding alters migration of a temperate ungulate. Ecol. Appl. 24, 1769–1779. ( 10.1890/13-2092.1) [DOI] [PubMed] [Google Scholar]
- 59.Singh NJ, Börger L, Dettki H, Bunnefeld N, Ericsson G. 2012. From migration to nomadism: movement variability in a northern ungulate across its latitudinal range. Ecol. Appl. 22, 2007–2020. ( 10.1890/12-0245.1) [DOI] [PubMed] [Google Scholar]
- 60.Applegate JE, Beaudion RL. 1970. Mechanism of spring relapse in avian malaria: effect of gonadotropin and corticosterone treatment. J. Wildlife Dis. 6, 443–447. ( 10.7589/0090-3558-6.4.443) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
A version of the R code that can be used to run all models is included in this article's electronic supplementary material.