Abstract
During recent years, long noncoding RNAs (lncRNAs) have been recognized as key regulators in the development and progression of human cancers, however, their roles in osteosarcoma metabolism are still not well understood. The present study aims to investigate the expression profiles and potential modulation of specific lncRNA(s) in osteosarcoma metabolism. The high-throughput Hiseq sequencing was performed to screen for abnormally expressed lncRNAs in osteosarcoma cells cultured under glucose starvation condition, and lncRNA HAND2-AS1 was eventually identified as one that was significantly up-regulated when compared with normal cultured cells. Mechanistic investigations indicated that knockdown of HAND2-AS1 abrogated the energy stress-induced effect on cell apoptosis and proliferation, and promoted osteosarcoma progression. Moreover, knockdown of HAND2-AS1 promoted glucose uptake, lactate production, and the expression level of a serious of enzymes that involved in energy metabolism. Subsequently, RNA pull-down and RNA immuneprecipitation revealed that, upon energy stress, HAND2-AS1 regulated osteosarcoma metabolism through sequestering FBP1 from binding to HIF1α, thereby releasing HIF1α expression and promoting the protein level. Taken together, our integrated approach reveals a regulatory mechanism by lncRNA HAND2-AS1 to control energy metabolism and tumor development in osteosarcoma. Thus, HAND2-AS1 may be a potential biomarker and therapeutic target for the repression of osteosarcoma metabolism.
Keywords: lncRNA HAND2-AS1, osteosarcoma, glucose starvation, HIF1α, FBP1
Introduction
Osteosarcoma is the most common primary bone malignancy in children and young adults, and accounts for approximately 60% of malignant bone tumors in the first two decades of life [1]. Approximately 80% of osteosarcoma patients have metastatic disease at the time of diagnosis, and metastasis is a consistent problem in tumor prognosis and treatment [2]. While the molecular mechanism of osteosarcoma has gained considerable attention, the mechanisms underlying its initiation and progression remain unclear. Thus, a good understanding of the molecular mechanism underlying osteosarcoma progression and metastasis is urgent to improve the diagnosis and effective therapy at the onset of the disease.
It has been recognized for a long time that the metabolism of cancer cells is significantly different from that of their normal counterparts. It is well accepted that glycolysis is an important process in react to energy stress, through which a large amount of glucose are consumed to convert to lactate. This unique metabolic phenotype is known as the Warburg effect, characterized by promoted glycolysis and decreased oxidative phosphorylation [3]. Conceptual progress has made us to better understand that the chronic and uncontrolled cell proliferation and metastasis are representative of the essence of osteosarcoma, which involves not only deregulated control of cell proliferation but also making corresponding adjustments of energy metabolism in order to accelerate cell growth and division.
The biological functions are regulated by various of transcription factors, such as HIF1α transcription factor, which has been widely reported to be overexpressed in cancer cells and commonly functions as a oncogene [4-6]. Previous studies reported that HIF1α regulated glucose metabolism through associating with lncRNAs, thereby promoting glycolysis or energy metabolism [7,8]. Under hypoxic conditions or within solid tumor microenvironments, specific genes were rapidly induced by HIF1α, and these genes then disrupted the HIF1α-VHL interaction, stabilized HIF1α, and increased expression of HIF1α-responsive genes such as glycolytic enzymes Glut1 and LDHA, thereafter promoting glycolysis in various cancer types [9].
With the advanced development of whole genome and transcriptome sequencing technologies and the ENCODE project, it is more and more clear that most of the genome DNA is represented in processed genes lacking of protein-coding capacity [10]. LncRNAs participate in several different biological processes including epigenetic regulation, nuclear import, cell cycle control, nuclear and cytoplasmic trafficking, imprinting, cell differentiation, alternative splicing, RNA decay, transcription and translation [11]. LncRNAs can regulate gene expression at post-transcriptional level [12] and modulate post-transcriptional gene silencing via the mRNA regulation [13]. However, the functional role of specific lncRNA(s) in glucose metabolism under energy stress condition is still not well known.
In current study, we investigated energy stress-regulated lncRNA regulation in osteosarcoma by performing the genome-wide sequencing in cells that were cultured under glucose starvation condition. In addition, we identified that, lncRNA HAND2-AS1, repressed HIF1α-mediated glycolysis and suppressed osteosarcoma tumorigenesis under glucose starvation condition.
Materials and methods
Cell culture
Human osteosarcoma cell lines MG-63, SAOS-2, U-2OS, HOS, SW1353 were obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). All of the cell lines were free of mycoplasma contamination (tested by the vendors using the MycoAlert kit from Lonza). No cell lines used in this study are found in the database of commonly misidentified cell lines (ICLAC and NCBI Biosample) based on short tandem repeats (STR) profiling performed by vendors. All osteosarcoma cell lines were maintained in DMEM medium (Invitrogen, Carlsbad, CA, USA) with 10% FBS (Sigma-Aldrich, St. Louis, MO, USA) at 37°C in 5% CO2 and 95% air. For glucose starvation experiments, cells were cultured in DMEM with different concentrations of glucose (1, or 25 mM) + 10% dialyzed FBS for 48 h, followed by functional experiments.
cDNA library construction and HiSeq sequencing analysis
Total RNA from five osteosarcoma cell lines that were culture with glucose starvation or normal condition was extracted by one-step extraction using a Trizol kit (Life Technologies, USA), and the purity and quantity of RNA were determined by UV spectrophotometry. cDNA library construction and sequencing were performed according to previously described methods [14].
Cell transfection
The small interfering RNAs (siRNAs) that target lncRNA HAND2-AS1 were synthesized and named as si-HAND2-AS1 (Ribo Bio Cooperation, Guangzhou, China) and embeded with GFP fluorescence for transfection quality control. The si-Negative Control (si-NC) was also provided by Genechem Corporation. Control siRNA and siRNA against HIF1α was purchased from OriGene. Forty-eight h after transfection, osteosarcoma cells were planted at a 24-well plate, then 100 nM of specific RNA oligoribonucleotides as well as negative controls were transfected into the cells with Lipofectamine 3000 (Invitrogen) according to the manufacturer’s instructions. The sequences of siRNAs are as follows: si-HAND2-AS1 #1: GAATCCACTTCAAACGGCTdTdT; si-HAND2-AS1 #2: TTTATTTTGGTAGTAAGGGdTdT; si-HAND2-AS1 #3: AACGUCGCCGGUAGUACG AdTdT; si-HIF1α: UCGACUAUCUGCUCCAAGUUCdTdT; si-NC (GFP): CAUCGGGACCUG GAGCGCACCdTdT.
TUNEL assay
TUNEL staining was performed to evaluate cell apoptosis. In brief, osteosarcoma cells were treated with 10 nM docetaxel combined with 20 μM Hemin or Znpp for 24 h and fixed by using 4% formaldehyde. Cells were fixed and stained with TUNEL kit according to the manufacturer’s instructions (Vazyme, TUNEL Bright-Red Apoptosis Detection Kit, A113). TUNEL-positive cells were counted under fluorescence microscopy (DMI4000B, Leica).
Cell viability assay
The changed cell viability after transfection or other treatment was assayed using the CCK8 Kit (Dojindo, Rockville, MD, USA). In brief, osteosarcoma cell lines were seeded into a 96-well plate and then treated with serial types of siRNAs for different time. After, cell cultures were treated with the CCK8 reagent and further cultured for 2 h. The optical density at 450 nm was measured with a spectrophotometer (Thermo Electron Corporation, MA, USA). The percentage of the control samples of each cell line was calculated thereafter.
Nuclear fractionation
Nuclear fractionation was performed with a PARISTM Kit (Ambion, Austin, TX). For nuclear fractionation, 1×107 cells were collected and re-suspended in the cell fraction buffer and incubated on ice for 10 min. After centrifugation, supernatant and nuclear pellet were preserved for RNA extraction using a cell disruption buffer according to the manufacturer’s instructions.
Quantitative real-time PCR
Total RNA was isolated from primary osteosarcoma cells using TRIzol reagent (Invitrogen). And then, the cDNA was synthesized from 200 ng extracted total RNA using the PrimeScript RT reagent Kit (Takara Bio Company, Shiga, Japan) and amplified by qRT-PCR with an SYBR Green Kit (Takara Bio Company) on an ABI PRISM 7500 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) with the housekeeping gene GAPDH as an internal control. The 2-ΔΔCt method was used to determine the relative quantification of gene expression levels. All the primers were synthesized by RiboBio, and the premier sequences are as follows: HAND2-AS1 (Forward): 5’-GGGTGTTTACGTAGACCAGAACC-3, (Reverse): 5’-CTTCCAAAAGCCTTCTGCCTTAG-3; HIF-1α (Forward) 5’-TCTAGACTCGAGTACAAGGCAGCAGAAAC-3’, (Reverse) 5’-TCTAGAGTTTGTGCAGTATTGTAGCC-3’; GAPDH (Forward): 5’-GCACCGTCAAGGCTGA GAAC-3, (Reverse): 5’-ATGGTGGTGAAGACGCCAGT-3. Each experiment was performed in triplicate.
RNA immunoprecipitation (RIP)
The Magna RIP RNA-Binding Protein Immunoprecipitation Kit (Millipore, Billerica, MA, USA) was used according to the manufacturer’s instructions. The RNAs were immunoprecipitated using anti-FBP1 (Cell Signaling Technology, Beverly, MA, USA) antibody. Total RNA and controls were also assayed to demonstrate that the detected signals were from RNAs specifically binding to FBP1. The final analysis was performed using qRT-PCR and shown as the fold enrichment. The RIP RNA fraction Ct value was normalized to the input RNA fraction Ct value.
Fluorescence in situ hybridization analysis (FISH)
Nuclear and cytosolic fraction separation was performed using a PARIS kit (Life Technologies), and RNA FISH probes were designed and synthesized by Bogu according to the manufacturer’s instructions. Briefly, cells were fixed in 4% formaldehyde for 15 min and then washed with PBS. The fixed cells were treated with pepsin and dehydrated through ethanol. The air-dried cells were incubated further with 40 nM of the FISH probe in hybridization buffer. After hybridization, the slide was washed, dehydrated and mounted with Prolong Gold Antifade Reagent with DAPI for detection. The slides were visualized for immunofluorescence with an Olympus fluorescence microscope with an attached CCD camera.
Western blots
Cell lysates were prepared with RIPA buffer containing protease inhibitors (Sigma). Protein concentrations were measured with the BCA Protein Assay according to the manufacturer’s manual (Beyotime Institute of Biotechnology). Equal amounts of protein were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and were transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA). Membranes were incubated overnight at 4°C with a 1:1000 solution of antibodies for HIF-1α, glucose transporter type 1 (Glut-1), hexokinase-2 (HK-2), ALDOC, MCT4 (Cell Signaling Technology). Then, the blots were immunostained with secondary antibody at room temperature for 1 h.
Statistical analysis
For glucose starvation versus normal cell lines, differences in mean expression were determined using Student’s t test. The correlation analysis was evaluated using the Spearman test. The results were considered statistically significant at P < 0.05. Error bars in figures represent SD. Statistical analyses were performed with GraphPad Prism (v5.01) software. The package plots and function heatmap in R software were used for mapping.
Results
Energy stress induces lncRNA HAND2-AS1 in osteosarcoma cells
To identify the candidate lncRNAs that regulate cancer cell metabolism, we performed a high-throughput Hiseq sequencing array by extracting RNAs from osteosarcoma cells cultured with glucose starvation or normal condition. As shown in Figure 1A, we identified a group of lncRNAs that are dysregulated in cells that experienced energy stress in contrast to normal cells. Among these, we found that lncRNA HAND2-AS1, an lncRNA transcribed antisense adjacent to Heart and Neural Crest Derivatives Expressed 2 (HAND2) in chromosome 4q33-34, was significantly up-regulated in glucose starved-cells; however, the HAND2-AS1 expression was not affected by glutamine starvation (Figure 1B). In addition, treatment with the glucose analog 2-deoxy-glucose (2DG), another energy stress inducer that inhibits hexokinase and blocks glycolysis, yielded similar results (Figure 1C). Therefore, our results showed that energy stress induces lncRNA HAND2-AS1 in osteosarcoma cells.
Figure 1.
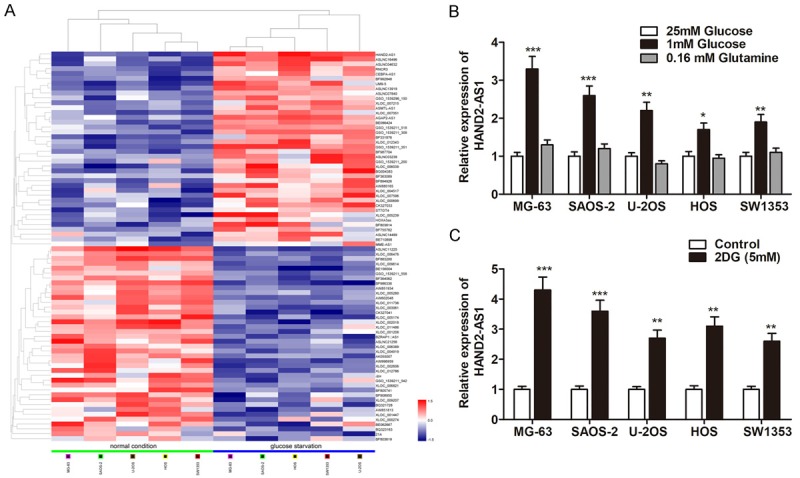
Energy stress induces lncRNA HAND2-AS1 in osteosarcoma cells. A. The heat map showed the top 50 most increased and decreased lncRNAs in osteosarcoma cell lines cultured under glucose starvation condition as compared to that cultured under normal condition by Hiseq. B. Bar graph shows the relative expression changes of HAND2-AS1 by qRT-PCR in osteosarcoma cells under different culture conditions for 48 h as indicated. C. The expression changes of HAND2-AS1 were determined in osteosarcoma cells treated with 2DG (5 mm) for 24 h.
Knockdown of lncRNA HAND2-AS1 relieved energy stress-mediated cell growth and apoptosis
We then investigated the functional role of lncRNA HAND2-AS1 in energy stress condition. MG-63 and SAOS-2 were used for further functional investigations, because they showed a relative higher HAND2-AS1 expression. Three specific siRNAs that could potently knockdown lncRNA HAND2-AS1 expression was generated, and si-HAND2-AS1 #1 was chosen for subsequent studies (Figure 2A). The CCK8 assay showed that knockdown of HAND2-AS1 had little effect on cell viability when the cells were cultured in 25 mM glucose containing medium, however, HAND2-AS1 knockdown significantly promoted cell viability when cells were treated with 1 mM glucose containing medium (Figure 2B and 2C). In addition, transfection with si-HAND2-AS1 showed no effect on cell-cycle pattern (data not shown). Furthermore, a significant increased colony formation ability was observed in cells transfected with si-HAND2-AS1 in contrast to control in osteosarcoma cells (Figure 2D). We then evaluated the role of HAND2-AS1 on cell apoptosis under glucose starvation situation. As shown in Figure 2E, HAND2-AS1 deficiency reversed the apoptosis caused by energy stress (1 mM glucose condition) as evidenced by the TUNEL assay.
Figure 2.
HAND2-AS1 deficiency reversed energy stress-regulated proliferation and apoptosis. (A) lncRNA HAND2-AS1 was downregulated by transfection of specific siRNAs in MG-63 and SAOS-2 cells. (B, C) MG-63 (A) and SAOS-2 (B) cells transfected with si-HAND2-AS1 or si-NC were cultured in glucose starvation or normal condition for different days as indicated, and then subjected to cell proliferation analysis. (D) Colony formation assay was performed after transfection of indicated siRNAs for 48 h in both osteosarcoma cells. (E) TUNEL assay was performed to detect the effect of HAND2-AS1 knockdown on cell apoptosis in MG-63 and SAOS-2 cells.
LncRNA HAND2-AS1 deficiency promotes glucose uptake and lactate production
After having observed the effect of HAND2-AS1 on cell growth and apoptosis under glucose starvation, we further investigated the underlying mechanism by which HAND2-AS1 exerts the suppressive role. We examined whether HAND2-AS1 knockdown had effects on the expression of a serious of enzymes that involved in energy metabolism. As shown in Figure 3A, knockdown of HAND2-AS1 significantly promoted the protein expression of enzymes that are involved in energy metabolism, such as Glut1, HK2, ALDOC and MCT4 when the cells were cultured in energy deficiency condition. Subsequently, we detected the lactate production and glucose uptake, and found that knockdown of HAND2-AS1 dramatically increased the lactate production and glucose uptake (Figure 3B and 3C). Finally, Seahorse experiment showed that si-HAND2-AS1 promoted the acidification rate of osteosarcoma cells (Figure 3D).
Figure 3.
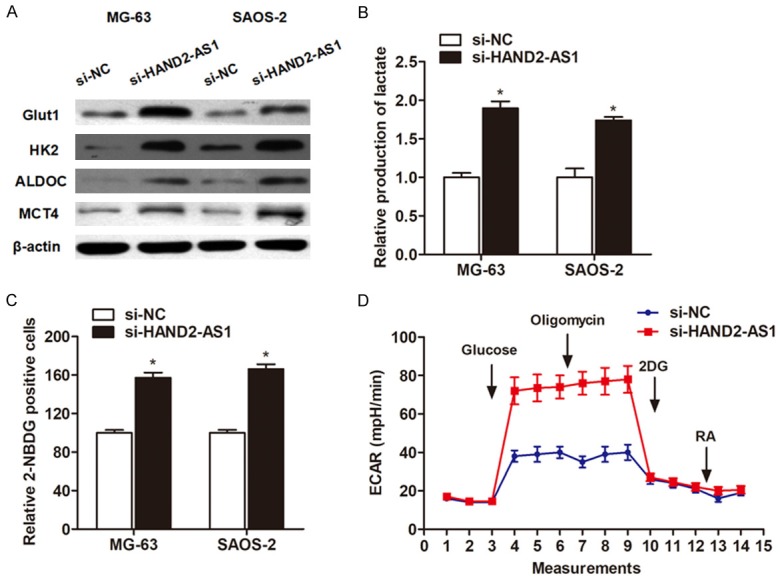
Knockdown of HAND2-AS1 induced glucose uptake and lactate production. (A) Western blot assay showed the expression change of enzymes involved in glucose metabolism after knockdown of HAND2-AS1. (B, C) Knockdown of HAND2-AS1 promoted lactate production (B) and glucose uptake (C). (D) Seahorse was performed to detect the effect of HAND2-AS1 on glucose metabolism.
LncRNA HAND2-AS1 regulates energy metabolism by inhibiting HIF1α
Glucose metabolism is largely regulated by the master transcription factors, including c-Myc, HIF1α and HIF2α. We detected the protein levels of c-Myc, HIF1α and HIF2α after knockdown of HAND2-AS1 in osteosarcoma cells under glucose starvation condition. As shown in Figure 4A, knockdown of HAND2-AS1 showed no effect on HIF1α protein level under normal culture condition, but promoted the protein expression level of HIF1α when cultured in glucose deficiency circumstance. In addition, HAND2-AS1 knockdown showed little effect on the expression change of c-Myc and HIF2α protein under glucose starvation condition (data not shown). Notably, HAND2-AS1 did not affect the HIF1α mRNA level, suggesting that HAND2-AS1 may regulate HIF1α at post-transcriptional level (Figure 4B).
Figure 4.
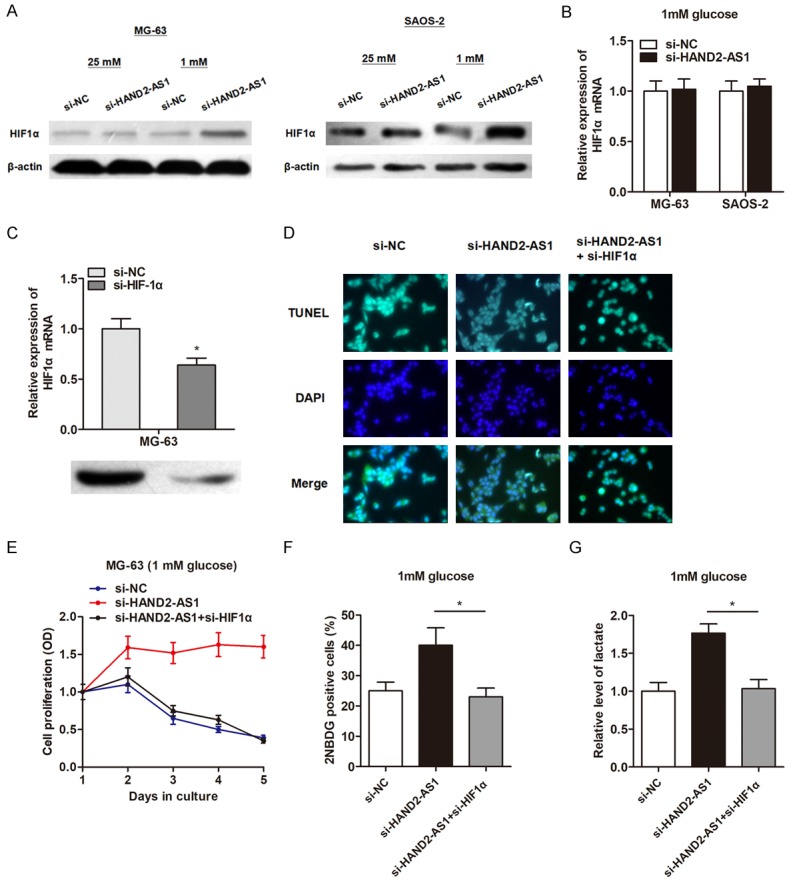
HAND2-AS1 regulates glucose metabolism by suppressing HIF1α level. (A) Si-RNA transfected osteosarcoma cells cultured under 25 mM or 1 mM glucose containing medium for 48 h, then subjected to western blot to detect the protein level of HIF1α. (B) qRT-PCR was performed to detect the mRNA level of HIF1α after transfection of si-HAND2-AS1 under glucose starvation condition. (C) Validation of knockdown effect of HIF1α after transfection of specific siRNAs in MG-63 cells. (D) TUNEL assay indicated that knockdown of HIF1α abrogated the si-HAND2-AS1 induced inhibition of cell apoptosis in MG-63 cells. (E) CCK8 assay indicated that co-transfection of si-HIF1α reversed the elevated proliferation rate caused by HAND2-AS1 knockdown in MG-63 cells under glucose starvation condition. (F, G) Co-transfection of si-HIF1α reversed glucose uptake (F) and lactate production (G) caused by HAND2-AS1 knockdown in MG-63 cells under glucose starvation condition.
Next, we determined whether HIF1α played a causal role during the HAND2-AS1-regulated glucose metabolism. HIF1α was down-regulated by transfection of specific siRNA (Figure 4C). Knockdown of HIF1α significantly reversed the repressed cell growth, increased cell apoptosis, enhanced glucose absorb and lactate production induced by HAND2-AS1 knockdown in 1 mM glucose culturing condition in MG-63 cells (Figure 4D-G). Collectively, our results suggest that HAND2-AS1 regulates glucose metabolism through inhibiting HIF1α levels at the post-transcriptional level.
LncRNA HAND2-AS1 sequesters FBP1 from binding to HIF1α mRNA
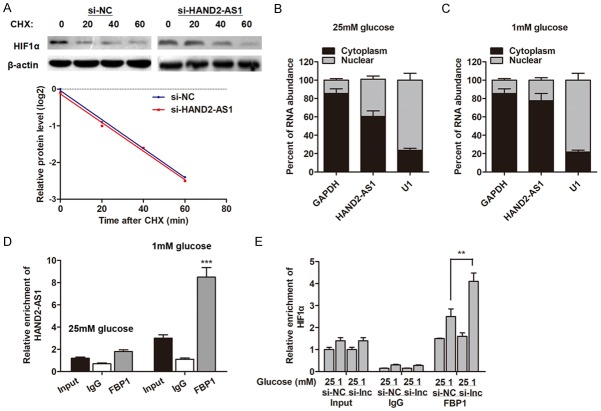
We then sought to define the underlying regulatory pathway by which HAND2-AS1 affects the expression level of HIFα. By treating with cycloheximide (CHX), we found that HAND2-AS1 did not affect the protein stability of HIF1α under glucose starvation condition (Figure 5A). Subsequently, RNA-pull down experiment was performed followed by mass spectrometry to search for the HAND2-AS1-associated proteins in the glucose starvation condition. As shown in Table 1, a list of correlative HAND2-AS1-associated proteins was identified. Then, we evaluated these potential regulator genes and sought to identify the specific protein that may regulate HIFα at the post-transcriptional level. Those assays validated Fructose-1, 6-bisphosphatase 1 (FBP1) as a potential interacted protein by HAND2-AS1. It was previously reported that FBP1 interacted with ARE areas within 3’UTR of HIF1α gene and activates HIF1α translation without influencing its mRNA level in clear cell renal cell carcinoma [15]. We then localized the expression pattern of HAND2-AS1 and found that HAND2-AS1 expressed in both nucleus and cytoplasm (Figure 5B). Furthermore, energy stress significantly promoted the proportion of HAND2-AS1 localization in cytoplasm (Figure 5C). In addition, RIP assay showed a direct interaction between HAND2-AS1 and FBP protein; energy stress (1 mM glucose condition) dramatically promoted the enrichment of HAND2-AS1 by FBP1 antibody (Figure 5D). HAND2-AS1 knockdown increased the interaction between FBP1 and HIF1α mRNA under glucose starvation condition (Figure 5E). Taken together, these data indicate that HAND2-AS1 serves as a competing endogenous RNA (ceRNA) for FBP1, therefore inhibiting HIF1α protein level under energy stress conditions.
Figure 5.
LncRNA HAND2-AS1 sequesters FBP1 from binding to HIF1α mRNA. (A) Control siRNA or si-HAND2-AS1 transfected MG-63 cells were cultured under glucose starvation condition for 48 h, then were added with 20 μg/ml Cycloheximide (CHX) for 0-80 min, then subjected to western blotting analysis. (B, C) Fractionation experiments revealed that HAND2-AS1 localized in both cytoplasm and nucleus under with 25 mM glucose culture condition (B), however, glucose starvation increased cytoplasmic localization of HAND2-AS1 (C). (D) Whole-cell lysates from MG-63 cells treated with 25 mM or 1 mM glucose containing medium, then RIP experiments were performed using an FBP1 antibody to immunoprecipitate RNA and primers to detect HAND2-AS1. (E) RIP assay was performed to detect the effect of HAND2-AS1 knockdown on the interaction between HIF1α and FBP1 under indicated situations.
Table 1.
Identification of HAND2-AS1 binding proteins by MS
| Protein | Beads | HAND2-AS1 | Ratio (HAND2-AS1/Beads) |
|---|---|---|---|
| FBP1 | 0 | 3 | NA |
| U2AF1 | 1 | 3 | 3 |
| NKRF | 0 | 3 | NA |
| EF1D | 0 | 3 | NA |
| AIMP2 | 0 | 3 | NA |
| ROA0 | 0 | 3 | NA |
| RO60 | 0 | 3 | NA |
| ARP2 | 0 | 3 | NA |
| STT3B | 0 | 3 | NA |
| PCH2 | 1 | 3 | 3 |
| MRP1 | 0 | 3 | NA |
| LAS1L | 0 | 3 | NA |
| ARF6 | 1 | 3 | 3 |
| PLST | 0 | 3 | NA |
| PSAL | 0 | 3 | NA |
| TTL12 | 0 | 3 | NA |
| ERLN1 | 0 | 3 | NA |
| NSF | 0 | 3 | NA |
| AKAP8 | 0 | 3 | NA |
| GSTO1 | 0 | 3 | NA |
| AP1B1 | 0 | 3 | NA |
| DPM1 | 0 | 3 | NA |
| PSDE | 1 | 3 | 3 |
| KTN1 | 1 | 3 | 3 |
Beads: spectral counts of proteins in beads only group; HAND2-AS1: spectral counts of proteins in HAND2-AS1 group; Ratio (HAND2-AS1/Beads): spectral count ratio of proteins comparing HAND2-AS1 group to beads only group; NA: not available.
Discussion
It is nowadays widely accepted that the obesity pandemic is caused by a combination of external (such as nutrition, sedentary lifestyle) and internal (genetic) components. To respond to these perturbations, cells use epigenetic regulation to modulate the expression of their genome. LncRNAs are at the center of this epigenetic regulation and add another regulatory layer in biological processes and pathophysiological conditions. In this study, we performed high-throughput sequencing array to identify the potential lncRNA (s) that may participated in the energy metabolism of osteosarcoma cells. We found that lncRNA HAND2-AS1 was dramatically upregulated in osteosarcoma cells that were cultured in glucose starvation condition. Mechanistic investigations revealed that knockdown of HAND2-AS1 inhibited energy stress-induced apoptosis, promoted cell growth, glucose uptake and lactate production through inhibiting HIF1α (Figure 6).
Figure 6.
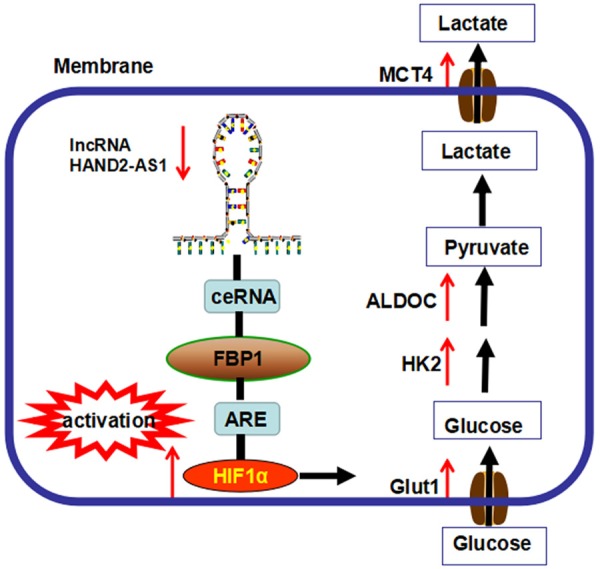
The functional model underlying the mechanism of lncRNA HAND2-AS1 on osteosarcoma metabolism.
A mounting body of evidence has continued to unequivocally demonstrate that cancer cells have altered metabolism [16]. One of the hallmark features of metabolic reprogramming in cancer cells is the enhanced glycolysis leading to lactate production even in the presence of oxygen [17]. However, how the altered metabolism occurs is still not well known. Previously, lncRNAs have been shown to play important roles during glucose metabolism by directly targeting glycolysis enzymes and indirectly targeting oncogenic signaling pathways [18]. By culturing the osteosarcoma cells under glucose starvation condition, we established the energy-stressed cells. HAND2-AS1 was finally identified as an altered expressed lncRNA in cells that cultured under glucose starvation condition based on the Hiseq profiling and qRT-PCR validation.
LncRNA HAND2-AS1 is located on chromosome 4q33-34 in a head-to-head orientation with Heart and Neural Crest Derivatives Expressed 2 (HAND2) gene. The genomic region encompassing HAND2-AS1 is known to generate multiple alternatively spliced transcripts. Two isoforms (5278 bp and 4186 bp) represent major transcript variants of HAND2-AS1 in primary neuroblastoma [19], and the isoform of HAND2-AS1 reported in this study is the one with 5278 bp. As far as we known, the biological role of HAND2-AS1 in cancer is largely unknown. A previous study by Yang et al. demonstrated that HAND2-AS1 inhibited invasion and metastasis in endometrioid endometrial carcinoma through inactivating neuromedin U [20]. They also found that lncRNA HAND2-AS1 and HAND2 gene were down-regulated by promoter DNA hypermethylation in endometrioid endometrial carcinoma, indicating that HAND2-AS1 and HAND2 had a concordant role in the progression of endometrioid endometrial carcinoma. We investigated the biological role of HAND2-AS1 in glycometabolism, and found that HAND2-AS1 deficiency dramatically promoted cell growth and suppressed apoptosis. Reprogrammed energy metabolism as an emerging hallmark of cancer acquires metabolic changes (such as the dysregulations of key enzymes of energy metabolism) in order to sustain rapid proliferation and cell growth and adapt to the tumor microenvironment [21-23]. We also detected whether HAND2-AS1 regulated glucose metabolism through affecting the enzymes that are involved in the glycometabolism process, and identified that a panel of genes including Glut1, Glut3, HK2, ALDOC, and MCT4 were regulated by HAND2-AS1. Together, we demonstrated that lncRNA HAND2-AS1 regulated glucose metabolism in osteosarcoma cells through affecting the expression of a serious of enzymes involved in the energy metabolism.
Many transcription factors play important roles in the energy metabolism process [24]. The most popular model proposed for lncRNA function probably is the one that lncRNAs regulate gene expression via recruiting transcription factors to specific loci [25,26]. This raises the possibility that HAND2-AS1 may regulate the transcription of target genes, such as HIF1α, HIF2α and c-Myc. This study identified that HAND2-AS1 exerted the regulatory function in energy metabolism through inhibiting HIF1α protein level. Increased HIF-1α levels have been found in many tumor types, accompanied by increased expression of HIF-1α target genes, including but not limited to VEGFA, PGK1, ANGPTL4, and HK2 [27]. The activation of HIF1α has been observed in osteosarcoma [28]; and clinically, osteosarcoma is commonly associated with abundant blood supply resulting from angiogenesis and glucose metabolism. Like other rapid growing solid tumors, osteosarcoma may develop a spatiotemporal hypoxic microenvironment during progression, hence activating HIF-1α. Our gain and loss functional assays validated that silence of HIF-1α in HAND2-AS1-knockdown cells to the level comparable to that in control cells reversed the expression change of glucose metabolism genes, indicating that HAND2-AS1 may functionally regulate HIF-1α under glucose starvation condition.
It is interesting that lncRNA HAND2-AS1 did not affect the expression level of HIF-1α mRNA or HIF-1α protein stability. To further explore how HAND2-AS1 regulates the protein level of HIF-1α, we performed RNA pulldown and mass spectrometry, and eventually identified that HAND2-AS1 physically interacted with FBP1 gene. Moreover, energy stress also promoted the proportion of HAND2-AS1 localized in cytoplasm and elevated the interaction between HAND2-AS1 and FBP1 gene. FBP1 is a key regulatory enzyme during the process of gluconeogenesis, which can block the glycolysis, by converting 1,6-fructose diphosphate to fructose-6-phosphate [29]. It has been reported that deletion of FBP1 expression is found in liver, gastric and colon cancers, which can accelerate glucose uptake and glycolysis [30,31]. Our data revealed an lncRNA network that, under energy stress, HAND2-AS1 interacted with FBP1, and might serve as a ceRNA to repress FBP1 from binding to HIF-1α mRNA, leading to suppression of HIF-1α protein levels, prevention of glucose uptake/lactate production and osteosarcoma carcinogenesis.
In conclusion, our study revealed an lncRNA-involved regulatory pathway to mediate HIF-1α levels under glucose starvation condition. First, energy stress induced lncRNA HAND2-AS1 expression in osteosarcoma cells. Second, knockdown of HAND2-AS1 promoted glucose metabolism under energy stress condition through interacting with FBP1 and promoting HIF-1α. Therefore, lncRNA HAND2-AS1 may be an important gene involved in osteosarcoma metabolism, restoration of HAND2-AS1 levels could be a future direction to overcome osteosarcoma cell proliferation and progression.
Acknowledgements
This research was supported by the National Natural Science Foundation of China 81272940, 81472507 to Jin Wang; the Science and Technology Program of Guangzhou 201504291231256 to Jin Wang; the Sun Yat-sen University Clinical Research 5010 Program 2012002, to Jin Wang; and the Ph.D. program foundation of the Ministry of Education of China (20130171110062 to Jin Wang).
Disclosure of conflict of interest
None.
References
- 1.Ma O, Cai WW, Zender L, Dayaram T, Shen J, Herron AJ, Lowe SW, Man TK, Lau CC, Donehower LA. MMP13, Birc2 (cIAP1), and Birc3 (cIAP2), amplified on chromosome 9, collaborate with p53 deficiency in mouse osteosarcoma progression. Cancer Res. 2009;69:2559–2567. doi: 10.1158/0008-5472.CAN-08-2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaste SC, Pratt CB, Cain AM, Jones-Wallace DJ, Rao BN. Metastases detected at the time of diagnosis of primary pediatric extremity osteosarcoma at diagnosis: imaging features. Cancer. 1999;86:1602–1608. doi: 10.1002/(sici)1097-0142(19991015)86:8<1602::aid-cncr31>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 3.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 4.Yao-Borengasser A, Monzavi-Karbassi B, Hedges RA, Rogers LJ, Kadlubar SA, Kieber-Emmons T. Adipocyte hypoxia promotes epithelial-mesenchymal transition-related gene expression and estrogen receptor-negative phenotype in breast cancer cells. Oncol Rep. 2015;33:2689–2694. doi: 10.3892/or.2015.3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iommarini L, Porcelli AM, Gasparre G, Kurelac I. Non-canonical mechanisms regulating hypoxia-inducible factor 1 alpha in cancer. Front Oncol. 2017;7:286. doi: 10.3389/fonc.2017.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sur S, Maurya AK, Roy A, Sharp TV, Pal DK, Panda CK. Over expression of HIF1alpha is associated with inactivation of both LimD1 and VHL in renal cell carcinoma: clinical importance. Pathol Res Pract. 2017;213:1477–1481. doi: 10.1016/j.prp.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Bhan A, Deb P, Shihabeddin N, Ansari KI, Brotto M, Mandal SS. Histone methylase MLL1 coordinates with HIF and regulate lncRNA HOTAIR expression under hypoxia. Gene. 2017;629:16–28. doi: 10.1016/j.gene.2017.07.069. [DOI] [PubMed] [Google Scholar]
- 8.Su X, Li G, Liu W. The long noncoding RNA cancer susceptibility candidate 9 promotes nasopharyngeal carcinogenesis via stabilizing HIF1alpha. DNA Cell Biol. 2017;36:394–400. doi: 10.1089/dna.2016.3615. [DOI] [PubMed] [Google Scholar]
- 9.Yang F, Zhang H, Mei Y, Wu M. Reciprocal regulation of HIF-1alpha and lincRNA-p21 modulates the Warburg effect. Mol Cell. 2014;53:88–100. doi: 10.1016/j.molcel.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Tukiainen T, Villani AC, Yen A, Rivas MA, Marshall JL, Satija R, Aguirre M, Gauthier L, Fleharty M, Kirby A, Cummings BB, Castel SE, Karczewski KJ, Aguet F, Byrnes A, Lappalainen T, Regev A, Ardlie KG, Hacohen N, MacArthur DG. Landscape of X chromosome inactivation across human tissues. Nature. 2017;550:244–248. doi: 10.1038/nature24265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang S, Qin C, Cao G, Xin W, Feng C, Zhang W. Systematic analysis of long noncoding RNAs in the senescence-accelerated mouse prone 8 brain using RNA sequencing. Mol Ther Nucleic Acids. 2016;5:e343. doi: 10.1038/mtna.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brockdorff N. Noncoding RNA and polycomb recruitment. RNA. 2013;19:429–442. doi: 10.1261/rna.037598.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li P, Zhang X, Wang H, Wang L, Liu T, Du L, Yang Y, Wang C. MALAT1 is associated with poor response to oxaliplatin-based chemotherapy in colorectal cancer patients and promotes chemoresistance through EZH2. Mol Cancer Ther. 2017;16:739–751. doi: 10.1158/1535-7163.MCT-16-0591. [DOI] [PubMed] [Google Scholar]
- 14.Boland JF, Chung CC, Roberson D, Mitchell J, Zhang X, Im KM, He J, Chanock SJ, Yeager M, Dean M. The new sequencer on the block: comparison of life technology’s proton sequencer to an illumina HiSeq for whole-exome sequencing. Hum Genet. 2013;132:1153–1163. doi: 10.1007/s00439-013-1321-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ning XH, Li T, Gong YQ, He Q, Shen QI, Peng SH, Wang JY, Chen JC, Guo YL, Gong K. Association between FBP1 and hypoxia-related gene expression in clear cell renal cell carcinoma. Oncol Lett. 2016;11:4095–4098. doi: 10.3892/ol.2016.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu C, Xue J, Zhu W, Jiao Y, Zhang S, Cao J. Warburg meets non-coding RNAs: the emerging role of ncRNA in regulating the glucose metabolism of cancer cells. Tumour Biol. 2015;36:81–94. doi: 10.1007/s13277-014-2875-z. [DOI] [PubMed] [Google Scholar]
- 19.Voth H, Oberthuer A, Simon T, Kahlert Y, Berthold F, Fischer M. Identification of DEIN, a novel gene with high expression levels in stage IVS neuroblastoma. Mol Cancer Res. 2007;5:1276–1284. doi: 10.1158/1541-7786.MCR-06-0258. [DOI] [PubMed] [Google Scholar]
- 20.Yang X, Wang CC, Lee WYW, Trovik J, Chung TKH, Kwong J. Long non-coding RNA HAND2-AS1 inhibits invasion and metastasis in endometrioid endometrial carcinoma through inactivating neuromedin U. Cancer Lett. 2018;413:23–34. doi: 10.1016/j.canlet.2017.10.028. [DOI] [PubMed] [Google Scholar]
- 21.Dang CV. Links between metabolism and cancer. Genes Dev. 2012;26:877–890. doi: 10.1101/gad.189365.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 23.Schulze A, Harris AL. How cancer metabolism is tuned for proliferation and vulnerable to disruption. Nature. 2012;491:364–373. doi: 10.1038/nature11706. [DOI] [PubMed] [Google Scholar]
- 24.Cun Y, Dai N, Li M, Xiong C, Zhang Q, Sui J, Qian C, Wang D. APE1/Ref-1 enhances DNA binding activity of mutant p53 in a redox-dependent manner. Oncol Rep. 2014;31:901–909. doi: 10.3892/or.2013.2892. [DOI] [PubMed] [Google Scholar]
- 25.Strauss J, Reyes-Dominguez Y. Regulation of secondary metabolism by chromatin structure and epigenetic codes. Fungal Genet Biol. 2011;48:62–69. doi: 10.1016/j.fgb.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng J, Bi C, Clark BS, Mady R, Shah P, Kohtz JD. The Evf-2 noncoding RNA is transcribed from the Dlx-5/6 ultraconserved region and functions as a Dlx-2 transcriptional coactivator. Genes Dev. 2006;20:1470–1484. doi: 10.1101/gad.1416106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amelio I, Melino G. The “sharp” blade against HIF-mediated metastasis. Cell Cycle. 2012;11:4530–4535. doi: 10.4161/cc.22820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Liu Y, Zou J, Yan L, Du W, Zhang Y, Sun H, Lu P, Geng S, Gu R, Zhang H, Bi Z. Tetrahydrocurcumin induces mesenchymal-epithelial transition and suppresses angiogenesis by targeting HIF-1alpha and autophagy in human osteosarcoma. Oncotarget. 2017;8:91134–91149. doi: 10.18632/oncotarget.19845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi L, He C, Li Z, Wang Z, Zhang Q. FBP1 modulates cell metabolism of breast cancer cells by inhibiting the expression of HIF-1alpha. Neoplasma. 2017;64:535–542. doi: 10.4149/neo_2017_407. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Wang Y, Li QG, Xue JJ, Wang Z, Yuan X, Tong JD, Xu LC. Downregulation of FBP1 promotes tumor metastasis and indicates poor prognosis in gastric cancer via regulating epithelial-mesenchymal transition. PLoS One. 2016;11:e0167857. doi: 10.1371/journal.pone.0167857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen M, Zhang J, Li N, Qian Z, Zhu M, Li Q, Zheng J, Wang X, Shi G. Promoter hypermethylation mediated downregulation of FBP1 in human hepatocellular carcinoma and colon cancer. PLoS One. 2011;6:e25564. doi: 10.1371/journal.pone.0025564. [DOI] [PMC free article] [PubMed] [Google Scholar]