Abstract
Based on previous findings that cyclooxygenase-2 (COX-2) is a critical molecule in chondrocyte differentiation and skeletal repair, we hypothesized that COX-2 deficiency or inhibition affects the ossification of vertebral endplates (VEP) and degeneration of intervertebral discs (IVD) and thus is involved in the pathogenesis of low back pain (LBP). We aimed to delineate the COX-2 working mechanism and its interacting molecules, and to explore the effect of NSAIDs and selective COX-2 inhibitor on degenerative spinal diseases. Here, lumbar spinal samples harvested from Cox-2 mutant (Cox-2-/-) and wild type (WT) mice were used for histological examinations. Nucleus pulposus (NP) cells isolated from rat were treated with PGE-2. Mouse endplate chondrocytes (mEC) isolated from mice were treated with a recombinant sonic hedgehog (Shh) protein. A mouse IVD organ culture system was established and treated COX-2 inhibitor Celecoxib. Human lumbar endplate chondrocytes were cultured and treated with Celecoxib. Immunohistochemical (IHC) studies were done in the human and mouse VEP samples. Radiographic and histological examinations revealed delayed VEP ossification in Cox-2-/- mice compared to WT ones. Decreased PGE-2 expression was found to promote Shh expression in rat NP cells, while Shh increased noggin expression in mEC. IHC showed that noggin expression was increased while pSmad1 expression decreased in the VEP of Cox-2-/- mice. Human VEP samples from patients with severe IVD degeneration showed decreased expression of Shh and noggin and increased expression of COX-2 and pSmad1 compared with milder cases. In cultured mouse IVDs and human endplate chondrocytes, Celecoxib enhanced expression of Shh and noggin and decreased Smad1 phosphorylation. In conclusion, COX-2/PGE-2 axis plays an important role in VEP ossification and IVD degeneration through crosstalk with Shh and BMP signaling pathways. These findings may facilitate clinical use of COX-2 inhibitor to prevent LBP progression.
Keywords: Intervertebral disc, endplate, degeneration, ossification, cyclooxygenase
Introduction
Low back pain (LBP) is a common symptom and its incidence keeps increasing as the population ages [1]. Emerging data indicate that intervertebral disc (IVD) degeneration play a critical role in its pathogenesis. Advanced MRI techniques have demonstrated a positive correlation between the pathological changes in vertebral endplates (VEP) adjacent bone (Modic changes) and the severity of LBP, which is consistent with the results from the modified Pfirrmann grading system [2-4].
Prostaglandin E2 (PGE-2), a major inflammatory mediator, takes its effect on musculoskeletal system through its receptors EP1-EP4 [5-8]. Cyclooxygenases (COX) are the rate-limiting enzymes for PGE-2 synthesis. While COX-1 is constitutively expressed in most tissues and cells, the expression of COX-2 is temporally and spatially restricted [9]. Previous studies show that fracture healing is delayed in Cox-2 mutant (Cox-2-/-) mice partially due to aberrant chondrocyte differentiation. Bone marrow mesenchymal stromal cells (BMSCs) isolated from Cox-2-/- mice are unable to form bone nodules, while addition of either PGE-2 or EP4 agonist can rescue such defect [10,11]. The role of COX-2 and PGE-2 in the pathogenesis of osteoarthritis (OA) remains elusive. In a mouse OA model, inhibition of COX-2 activity does not show evident chondroprotective effect [12], while in another study, use of a COX-2 inhibitor Celecoxib in mechanically stimulated porcine mandibular chondrocytes down-regulates the expression of certain MMPs and ADAMTS5, and up-regulates the expression of type II collagen (Col2) and aggrecan [13].
Lumbar IVD degeneration eventually may lead to rupture of the annulus fibrous (AF) and herniation of the nucleus pulposus (NP). Physical and biological stimuli to nerve roots incite local and radiating pain. In addition, disregulated ossification and calcification of VEP may exacerbate IVD degeneration by interruption of nutrient supplies [14]. Therefore, premature VEP ossification can be used to assess the severity of IVD degeneration. LBP with mild IVD degeneration has been routinely treated with non-steroidal anti-inflammatory drugs (NSAIDs). To reduce the adverse effects of NSAIDs, selective COX-2 inhibitor such as celecoxib has become increasingly popular in LBP treatment [15]. Interestingly, Celecoxib can also prevent ectopic ossification after total hip replacement, probably through inhibition of osteoblast differentiation [16,17].
BMP-2 is a critical molecule involved in physiological and pathological processes in the skeletal system. It binds to its receptors on chondrocytes and osteoblasts causing phosphorylation of Smad1, 5, and 8 [18,19]. We recently showed that BMP-2 induces Cox-2 expression and PGE-2 production, and regulates chondrocyte differentiation by inducing ATF4 phosphorylation [20]. Noggin, an extracellular inhibitor for BMPs, prevents activation of BMP signaling pathway through competitive binding to their receptors [21]. In addition to BMP signaling pathway, Hedgehog (Hh) pathway including ligands such as Sonic Hh (Shh) and Indian Hh (Ihh) also plays an important role in the development of skeletal tissues by regulating differentiation of chondrocytes and osteoblasts [22]. Shh inhibits BMP-4 function by increasing Noggin expression, whereas another study shows that expression of BMP-2 is independent of Hh signaling. Clearly, more studies are needed to delineate the interdependent relationship among COX-2, BMP and Hh pathways in musculoskeletal tissues [23-25].
Based on previous studies, we hypothesized that COX-2 deficiency affect VEP chondrocyte differentiation and play a role in the IVD degeneration and LBP, and that the severity of spinal degeneration correlate with the expression level of COX-2. We thus performed relevant experiments to confirm our hypotheses and to determine the molecular mechanisms underlying such changes with the focus on the interaction among COX-2, BMP and Shh signaling pathways. We expect that the findings made in our present study will lend some novel insights to LBP pathogenesis and the effect of selective COX-2 inhibitor on IVD degeneration.
Materials and methods
Patients
Patients (n=23, 10 men, 13 women, average age: 62 years old) with LBP due to IVD degeneration and spinal instability were treated with single-segment fusion after removal of VEP-IVD. All patients signed the form of consent and all procedures were approved by the Ethnic Committee of the People’s Hospital of Nanjing Medical University, China. The MRI examination before surgery showed that 9 patients had Modic changes and 14 patients did not have.
Animals
All animal studies were conducted with the approval of the Committee on Animal Resources in the University of Rochester and the Committee of laboratory Animal Use in Nanjing Medical University. Mice.: The lumbar spine samples were collected from both Cox-2-/- and their WT littermates at different ages (3, 5, 7 months, n=6 in each group and each time point). After careful removal of soft tissue, the samples were subjected to radiographic examination using Faxitron machine (Faxitron Corporation, Wheeling, IL). Then, the samples were fixed and decalcified for histological and immunohistochemical studies. Rats.: The lumbar spine samples were collected from 6-month-old Wistar rats after sacrifice. After careful removal of soft tissue, L1-L5 spinal columns were kept sterile for NP cell isolation.
Isolation of primary rat NP cells
Lumbar spine samples were harvested from 5-month-old Wistar rats, and the jelly-like NP was collected and digested with 0.25% trypsin (Life Technologies, Grand Island, NY) at 37°C for 30 min. After wash and centrifuge, the pellet was further digested with 0.1% type II collagenase (Sigma-Aldrich; St. Louis, MO) at 37°C for 3 hrs. The debris were removed by filtering through a 100 µm sieve and the NP cells were re-suspended for subsequent culture in DMEM containing 10% FBS. At about 80% confluence, the NP cells were starved in serum free medium for 12 hrs prior to PGE2 treatment. The RNA and protein samples were harvested at 12 and 24 hrs, respectively.
Isolation of primary mouse endplate chondrocytes (mEC)
The mEC were isolated from the cartilaginous endplates of the lumbar vertebrae of 5-month-old C57BL/6J WT mice. Soft tissue was removed by digesting with pronase (0.2% in PBS, Roche, Indianapolis, IN) at 37°C, shaking for 45 min and digested with collagenase D (Roche, 0.3% in serum-free medium) for 60 min at 37°C. After wash, cartilage tissue blocks were further digested with collagenase D for 5 hrs at 37°C. Purified mEC were cultured on DMEM containing 10% FBS (Invitrogen; Carlsbad, CA), and then treated with a mouse recombinant Shh protein (10-6 M, Sigma-Aldrich). The RNA and protein samples were collected 12 and 24 hrs after treatment by RT-PCR and western blotting, respectively.
Isolation and culture of degenerated human intervertebral disc endplate chondrocytes
Surgical human cartilage endplates were placed in sterile PBS. Soft tissue was removed by digesting with pronase (0.2% in PBS, Roche, Indianapolis, IN) at 37°C, shaking for 45 min and digested with collagenase D (Roche, 0.3% in serum-free medium) for 60 min at 37°C. After wash, cartilage tissue blocks were further digested with collagenase D for 5 hrs at 37°C. Purified chondrocytes were cultured on DMEM containing 10% FBS (Invitrogen; Carlsbad, CA), the culture solution was changed every other day. When growing to 80% confluence, the chondrocytes were starved in a serum-free medium for 12 hrs. Cells in the control group were treated with 0.1% DMSO, while cells in the Celecoxib group were treated with a selective COX-2 inhibitor Celecoxib (Sigma-Aldrich) at a concentration of 300 mg/ml. The RNA samples were collected 12 hrs after treatment by RT-PCR.
In vitro organ culture of mouse IVDs
C57BL/6 WT mice of 5-month-old were sacrificed and the lumbar spine samples (L4-L6) were collected. All samples were cultured in petri dishes in DMEM/F12 medium containing 20% FBS [26]. A selective COX-2 inhibitor Celecoxib (300 ng/ml, Sigma-Aldrich) [27] was added to the dishes with DMSO as vehicle control. The medium and drugs were changed every 24 hours and the treatment lasted for 3 and 7 days, respectively. Then, these cultured samples were collected for subsequent studies.
Real-time PCR (RT-PCR)
RNA was extracted with a kit from Qiagen (Valencia, CA) and reversely transcribed into cDNA with SuperScript First Strand Synthesis System (Invitrogen). RT-PCR was performed with the relevant mouse, rat and humanprimers and β-actin was used as an internal reference gene. The expression of each gene was normalized to β-actin and the results were represented as the mean and standard deviation (SD).
Western blotting analysis
Western blotting analysis was performed as previously described [20]. The Shh and Noggin antibodies were purchased from Abcam (Cambridge, MA, USA).
Histological and immunohistochemical (IHC) studies
Human VEP samples, the lumbar spine samples from both Cox-2-/- and WT mice, and the in vitro cultured IVD samples were fixed with formalin and decalcified in 5% EDTA. After processing, the samples were embedded in paraffin and the sections (5 µm) were cut for subsequent analysis. Histological examinations were performed after hematoxylin/eosin (HE) and Alcian blue staining. IHC studies were performed as previously described [20]. The following antibodies were used in the studies: COX-2 (Cayman Chemical, Ann Arbor, MI), phosphorylated Smad1 and VEGFR2 (Cell Signaling Technology, Danvers, MA), Noggin and Shh (Abcam).
Statistical analysis
All data was expressed as the mean ± SD. Students’ T test was performed using the software SPSS version 13.0 (SPSS Inc.; Chicago, IL, USA) with a P value < 0.05 denoting significant difference.
Results
Delayed VEP ossification in Cox-2-/- mice
Radiographic examination of the lumbar spine samples were performed and analyzed by 3 independent researchers and that result showed that compared to the WT ones, IVD spaces were much clearer in Cox-2-/- mice (Figure 1A and 1B). Such difference was most significant in 5-month-old mice. We thus focused our experiments on the 5 month samples. Histological examination showed that normal ossification of VEP with formation of bone marrow cavities seen in WT mice was absent in Cox-2-/- mice of the same age (Figure 1C and 1D). Alcian blue staining showed that the shape of the growth plate in WT mice was irregular, indicative of progressive ossification. VEP ossification, as evidenced by loss of cartilage matrix staining, was more evident in WT mice than their Cox-2-/- littermates. In contrast, the shape of growth plates was regular and VEP ossification was not apparent in Cox-2-/- mice (Figure 1E and 1F). The results suggest that COX-2 deficiency delays the VEP ossification. We then decided to study the relationship between the level of COX-2 expression and human IVD degeneration.
Figure 1.
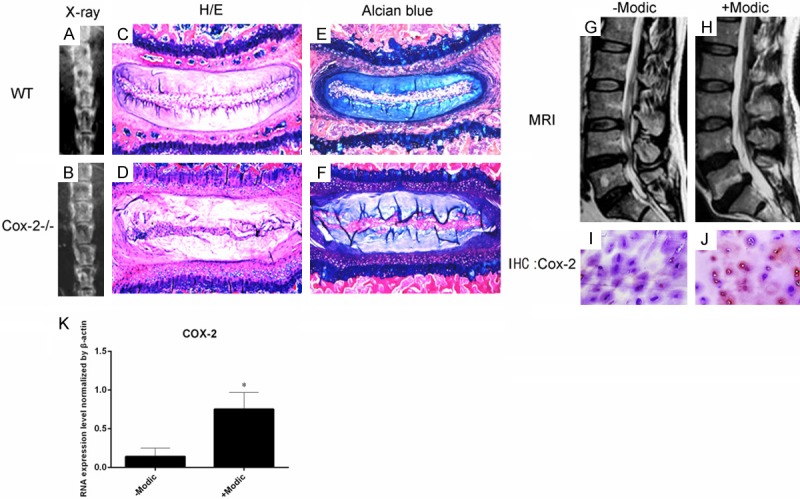
Radiographic examination demonstrated that IVD spaces were much clearer in Cox-2-/- mice than in their WT littermates (A, B). Histological analysis with H/E (C, D) and Alcian blue (E, F) staining showed that cartilaginous VEP in mice, unlike that in humans, was in continuation with growth plate. While apparent ossification with bone marrow formation was observed in the VEP of WT mice, such phenomenon was not evident in Cox-2-/- mice. Human VEP samples were divided into two groups based on MRI examination: group 1 samples from patients with Modic changes and severe IVD degeneration (H), and group 2 samples from the patients without Modic changes and mild IVD degeneration (G). IHC studies showed intense immunoreactivity to COX-2 in the group 1 samples (J), while such signal was negligible in group 2 samples (I). RT-PCR assay showed higher COX-2 expression in patients’ end-plate cartilage in group 2 than in group 1 (K).
COX-2 expression level correlated with the severity of IVD degeneration
Human VEP samples were collected from the patients suffering from IVD degeneration with or without Modic changes undergoing spinal fusion surgery. Our previous study has shown a correlative relationship between Modic changes and Pfirrmann grading system with respects to the severity of IVD degeneration in lumbar spine [3]. We thus divided the patient VEP samples into two groups: group 1 samples from patients with Modic changes and more severe IVD degeneration such as sequestration, and group 2 samples from patients without Modic changes and milder IVD degeneration such as protrusion as shown by MRI (Figure 1G and 1H). Our IHC results showed that COX-2 expression was more significant in group 1 compared to group 2 samples (Figure 1I and 1J). RT-PCR assay showed higher COX-2 expression in patients’ endplate cartilage in group 1 than in group 2 (Figure 1K).
PGE2 inhibited Shh expression in rat NP cells
Based on these observations, we reasoned that reduced PGE-2 production in IVD cells due to COX-2 deficiency may affect VEP ossification. Primary rat NP cells were treated with PGE-2 at different doses (10-9-10-7 M), and the related genes were examined by RT-PCR. PGE-2 treatment did not cause significant changes in the expression of TGF-β superfamily members, different BMPs and VEFGs (Figure 2A-C). Additionally, the changes in the expression of Il-1β, Tnf-α, Col2a1, Aggrecan, Adamts5, and Mmp-13 were not evident (Figure 2D). We then examined the possible changes in the ligands of Hh signaling pathway because this pathway is closely related to chondrocyte hypertrophy and ossification. Our RT-PCR results showed that PGE-2 did not affect the expression of Ihh but downregulated the Shh expression in a dose-dependent manner (Figure 3A). Western blotting analysis confirmed that PGE-2 (10-7 M) decreased the Shh protein expression in these cells (Figure 3B). Consistent with these in vitro findings, Shh expression was enhanced in the NP and VEP of Cox-2-/- mice compared to their WT littermates (Figure 3C).
Figure 2.
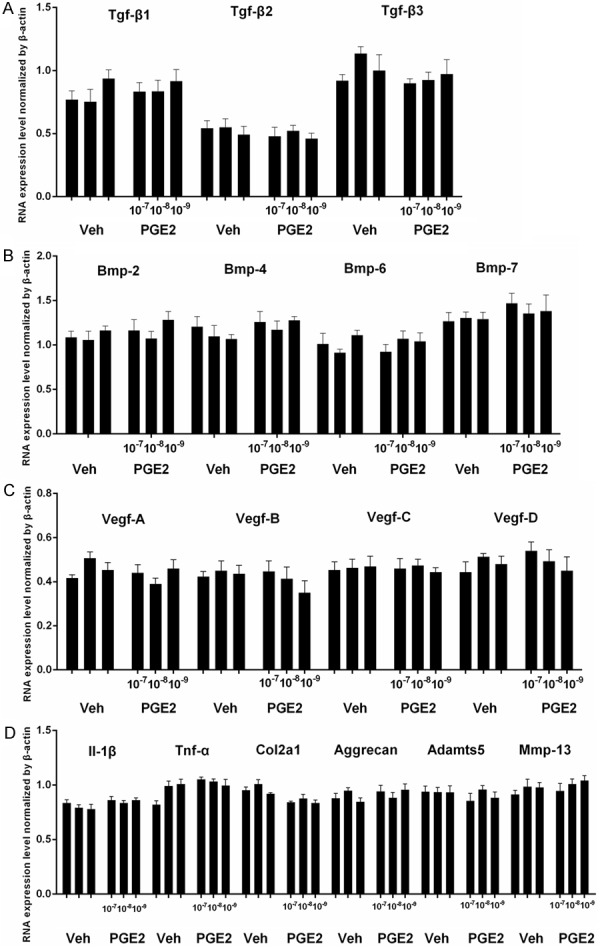
Rat nucleus pulposus (NP) cells were treated with PGE-2 at different concentrations (10-9-10-7 M) and the expressions of the TGF-β superfamily members were determined by RT-PCR. The results showed that PGE-2 did not have evident effect on TGFβ, β2, β3 (A), and BMP-2, -4, -6 and -7 (B). PGE-2 did not have significant effect on VEGFA-D (C). In addition, PGE-2 treatment did not significantly change the expression of pro-inflammatory cytokines IL-1β and TNF-α, two major matrix proteins Col-2 and aggrecan, and two matrix degrading enzymes Adamts5 and Mmp-13 (D). *, P < 0.05. Error bars, S.D.
Figure 3.
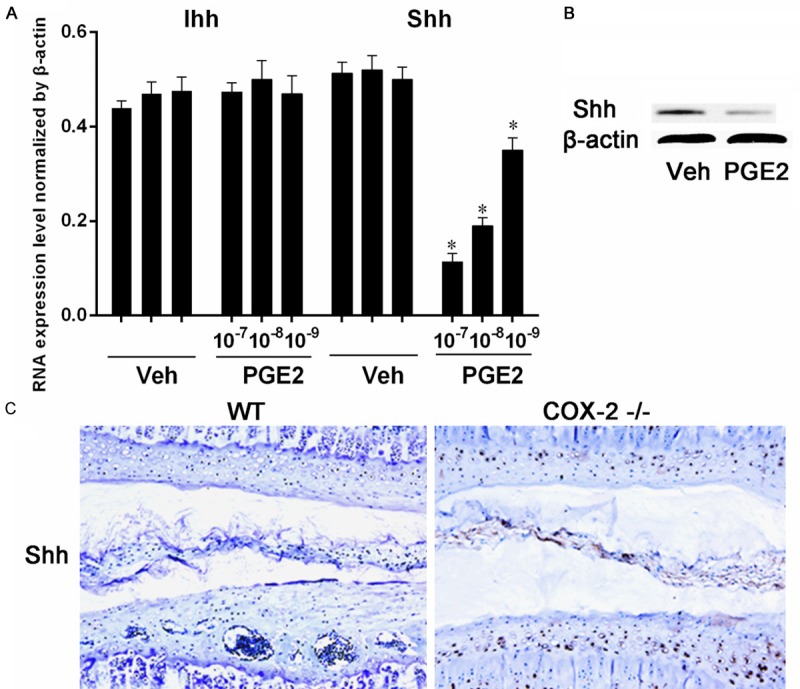
Rat NP cells were treated with PGE-2 and the expression of Hh molecules were analyzed with RT-PCR. The expression of Ihh was not changed after PGE-2 treatment. However, PGE-2 significantly suppressed Shh expression of (A). Western blotting analysis showed that PGE-2 suppressed the protein expression of Shh (B). IHC study of the IVD samples with an antibody to Shh showed a much stronger immunostaining in Cox-2-/- mice than in WT ones (C). *, P < 0.05. Error bars, S.D.
Shh increased noggin expression in mEC; COX-2 deficiency increased noggin expression and inhibited the activation of BMP2 and VEGF pathways
Based on previous studies and observations made so far, we decided to examine the effect of Shh protein on BMP signaling pathway in VEP chondrocytes as this pathway is critical for chondrocyte differentiation. The mEC were treated with a recombinant sonic hedgehog (Shh) protein. While Shh did not have significant effect on the BMP signaling molecules, it did increase noggin expression in mEC at both mRNA and protein levels (Figure 4A and 4B). Accordingly, noggin expression was increased in the VEP and NP of Cox-2-/- mice, probably due to enhanced Shh expression. As an extracellular inhibitor of BMPs family, noggin suppresses activation of BMP-Smad pathway. Our IHC study showed Smad1 phosphorylation was repressed in the VEP of Cox-2-/- mice, suggesting inactivation of BMP-Smad pathway. As BMP signaling is also closely related to angiogenesis, we did IHC staining with a phosphorylated VEGFR2 (pVEGFR2) antibody in mouse VEP samples to assess the activation status of VEGF pathway. Our results showed that compared to their WT littermates, the staining of pVEGFR2 was much weaker in Cox-2-/- mice, implying a suppressed VEGF-mediated angiogenesis (Figure 4C).
Figure 4.
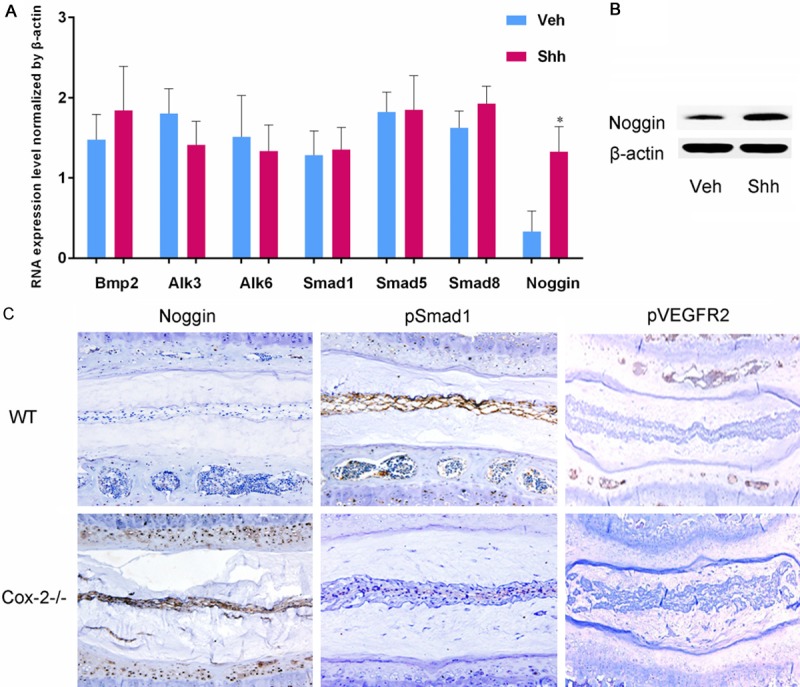
Mouse EC were treated with a recombinant mouse Shh protein for 12 or 24 hrs for RT-PCR and western blotting, respectively. Shh increased the expression of noggin at both mRNA and protein levels (A, B). IHC staining revealed intense noggin immunoreactivity in Cox-2-/- mice, while the noggin positive cells were hardly seen in WT ones. In addition, IHC staining showed numerous phosphorylated Smad1 (pSmad1) and pVEGFR2 positive cell in the IVD of WT mice, while such positive cells were rarely seen in the samples from Cox-2-/- mice (C).
Expression of Shh, noggin and pSmad1 in human VEP samples
To correlate our findings made in mice and in vitro studies with human IVD degeneration, we performed IHC studies to compare the expression of the above mentioned molecules in group 1 and group 2 VEP samples. In group 1 samples with Modic changes and severe IVD degeneration, the expression of Shh (Figure 5A and 5B) and Noggin (Figure 5C and 5D) was repressed compared to group 2 samples without Modic changes and mild IVD degeneration. In contrast, pSmad1 staining was more intense in group 1 samples than group 2 ones (Figure 5E and 5F). However, RT-PCR showed lower Shh and Noggin expression in patients’ end-plate chondrocytes in group 1 in relative to in group 2, while higher pSmad1 expression was detected in group 1 than in group 2 (Figure 5G).
Figure 5.
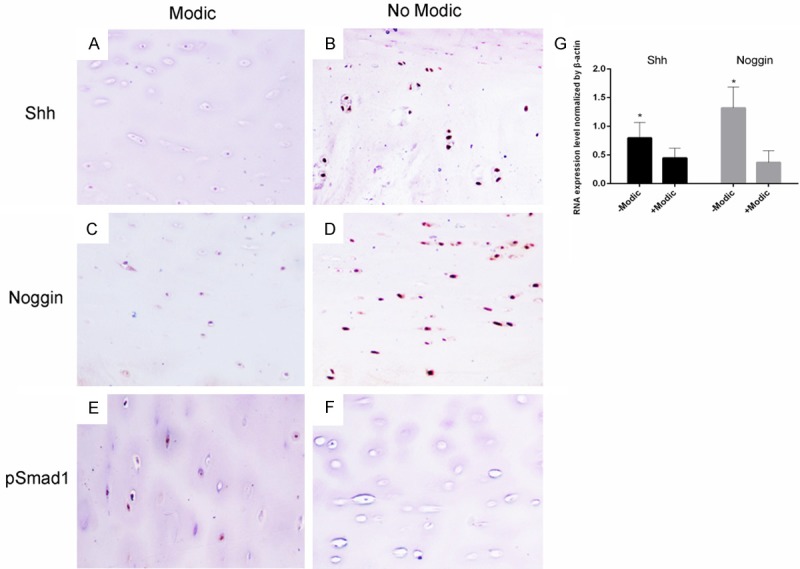
Human IVD samples with (group 1) or without Modic (group 2) changes harvested during surgery were subjected to IHC staining with the antibodies to Shh, noggin and pSmad1. Shh (A, B) and noggin (C, D) positive cells were detected in the group 2 samples, but not in the group 1 samples. In contrast, the group 1, but not group 2 samples, showed positive staining for pSmad1 (E, F). However, RT-PCR showed lower Shh and Noggin expression in patients’ end-plate chondrocytes in group 1 than in group 2, while higher pSmad1 expression was detected in group 1 than in group 2 (G). *, P < 0.05. Error bars, S.D.
Celecoxib increased Shh and noggin expression and suppressed Smad1 phosphorylation
Previous studies show that COX-2 is closely involved in the process of chondrocyte differentiation and skeletal repair. To determine whether exogenous COX-2 inhibitor has a similar effect to deficiency of endogenous COX-2, we established an organ culture model of mouse IVDs with VEPs. These in vitro cultured mouse IVD-VEPs were treated with either Celecoxib or vehicle. Our studies showed that the morphology of the cultured samples remained largely intact 7 days after treatment (Figure 6A and 6B), and that Celecoxib effectively suppressed COX-2 expression (Figure 6C and 6D). IHC staining showed that Celecoxib increased the protein expression of both Shh (Figure 6E and 6F) and noggin (Figure 6G and 6H), while the number of pSmad1 positive cells were reduced after treatment (Figure 6I and 6J). In addition, Celecoxib treatment was found to cause an elevation of Shh and Noggin expression and a reduction of pSmad1 expression in patients’ endplate chondrocyte as compared to vehicle, as revealed by RT-PCR assay (Figure 6K).
Figure 6.
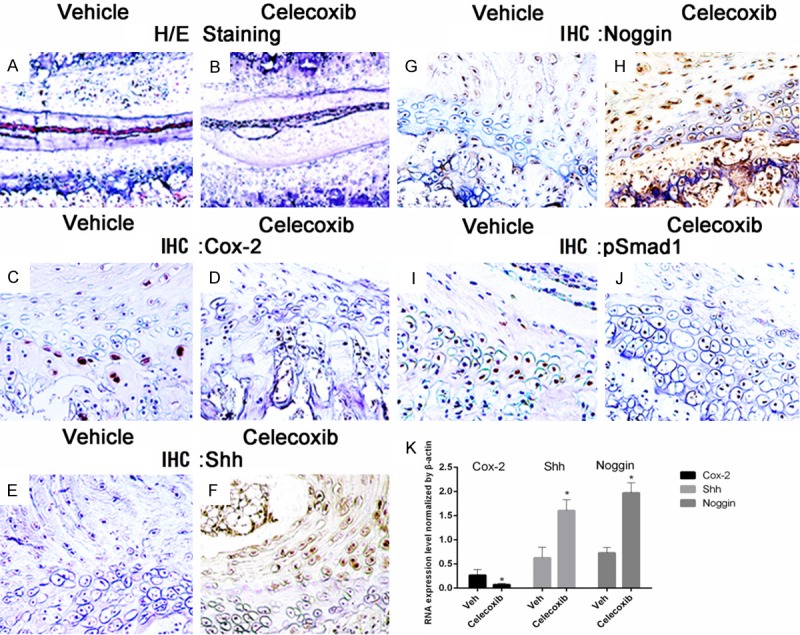
Mouse lumbar IVD samples were cultured in vitro in the presence or absence of COX-2 inhibitor Celecoxib for 7 days. Histological examination showed that Celecoxib treatment did not cause evident changes in the morphology of cultured IVD (A, B). However, IHC analysis demonstrated that Celecoxib significantly suppressed COX-2 expression (C, D). In addition, Celecoxib increased the protein expression of both Shh (E, F) and Noggin (G, H), and inhibited Smad1 phosphorylation in IVD and EP (I, J). In addition, Celecoxib treatment increased Shh and Noggin expression and decreased pSmad1 expression in patients’ endplate chondrocyte in relative to vehicle, as revealed by RT-PCR assay (K). *, P < 0.05. Error bars, S.D.
Discussion
Our studies for the first time demonstrate that endogenous COX-2 deficiency in Cox-2-/- mice causes delayed VEP ossification (Figure 7). In humans, VEP calcification and ossification contributes to LBP pathogenesis by inducing IVD degeneration through interruption of nutrient supplies from vertebral bodies to IVD. We thus decided to investigate the mechanisms underlying such changes for in-depth understanding of IVD degeneration. More importantly, as NSAIDs and COX-2 specific inhibitor are widely used in LBP treatment, elucidation of their effect on VEP ossification may improve our understanding of IVD pathologies. Based on previous observations made in other groups and our own, we focused on possible crosstalk among COX-2, TGF-β superfamily members and Hh signaling pathway. To our surprising, the only positive result from our RT-PCR screening was PGE-2 mediated inhibition of Shh expression in rat NP cells. In fact, Shh is a critical molecule in IVD development [28]. We postulated that the factors produced by NP cells in IVD may regulate VEP chondrocyte differentiation via a paracrine manner. Previous studies have shown that BMP-2 is an important molecule for chondrocyte differentiation and noggin is an extracellular inhibitor of BMP signaling pathway, and that there is an interdependent relationship between Shh and BMP-noggin network [29-32]. Thus, we took use of the mEC to investigate the effect of Shh on noggin production in the chondrocytes. In vitro studies demonstrate that Shh upregulates the expression of noggin in mEC. Consistent with these findings, noggin expression is increased in Cox-2-/- mice, at least partially due to lack of PGE2 mediated inhibition of Shh expression. Naturally, BMP-Smad pathway is deactivated in such mice as shown by scarce immunoreactivity to pSmad1. Similar to that in growth plate where chondrocyte hypertrophy and apoptosis often is followed by blood vessel invasion, normal activation of VEGF signaling is also a concomitant response accompanying BMP-Smad activation [33]. As such, the phosphorylation of VEGF receptor with ensuing activation of this pathway is inhibited in Cox-2-/- mice. In human VEP samples, IHC study has shown a positive correlation between the level of COX-2 expression and the severity of IVD degeneration as denoted by the absence or presence of Modic changes. Further, treatment of the cultured IVD-VEP and human endplate chondrocytes with Celecoxib resulted in similar changes to that in the Cox-2-/- mice. Collectively, these findings suggest that inhibition of COX-2 activity delays VEP ossification and prevents progression of IVD degeneration.
Figure 7.
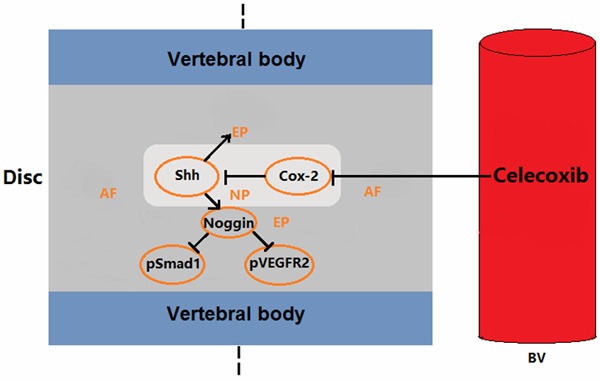
Proposed model: A reduced expression of endogenous COX-2 promoted Shh expression in the intervertebral disc nucleus pulposus, while Shh increased Noggin expression in endplate, which led to reduced pSmad1 and pVEGFR2 expression, and alleviation of ossification of the endplate cartilage. The exogenous COX-2 inhibitor was found to partially suppress COX-2 expression, promote Shh and Noggin expression and decrease pSmad1 expression.
TGF-β superfamily members including different BMPs are closely involved in heterotrophic ossification, chondrocyte maturation and skeletal repair. COX-2 and its metabolite PGE-2 are also important in osteochondrogenesis and fracture healing. Our previous studies have demonstrated a complex crosstalk between BMP and COX-2 signaling [20,34]. In the present study, we explored the effect of COX-2 deficiency on mouse VEP ossification and established a molecular cascade involving BMP-2, COX-2 and Hh signaling events. We found a critical molecule, noggin, linking BMP-2 and COX-2 signaling pathways. The end result of COX-2 deficiency in mouse spine is the suppression of the BMP-Smad1 pathway in IVD and the delay in the VEP ossification, suggesting a possible clinical use in early LBP. Shh may also become a therapeutic target for spinal degeneration.
Adult IVDs are the largest hypoxic organ in humans, which helps maintain VEP as cartilaginous tissue. While it is widely accepted that vascular invasion is a causative factor for IVD degeneration [35], the detailed mechanisms underlying these processes are not completely understood. Previous studies show that hypoxia induces COX-2 expression causing subsequent neoangiogenesis, and that fracture healing is delayed in Cox-2-/- mice due to impaired endochondral bone formation and angiogenesis [10,11,36]. In another report, treatment of rat periodontitis with a COX-2 inhibitor causes suppression of VEFG expression and prevents bone loss [37]. Binding of VEGFs to the tyrosine kinase receptors causes their phosphorylation with subsequent activation of the downstream signaling [38]. Scarce pVEGFR2 immunoreactivity in the VEP of Cox-2-/- mice implies that COX-2 deficiency leads to inhibition of VEGF signaling and neoangiogenesis, thus alleviating IVD degeneration. These observations are somewhat contradictory to our in vitro findings that PGE2 has negligible effect on the expression of VEGFs in rat NP cells. It is plausible to postulate that a cell specific response may exist, and that in vivo and in vitro responses may differ. More mechanistic studies are warranted to unveil the crosstalk among COX-2, BMPs, and VEGFs in the IVD degeneration.
Shh is a critical molecule for the development of limbs, somites and neural tube, and Shh mutant mice develop severe limb deformities [39,40]. Our results show that PGE-2 inhibits Shh expression in a dose-dependent manner while it does not affect Ihh expression. We also reveal a reverse correlation between Shh and the severity of IVD degeneration. Previous studies have shown that Shh enhances osteoblast proliferation and differentiation, therefore promoting bone repair. Transient inhibition of Shh causes premature yet permanent closure of growth plate [41,42]. Interestingly, overexpression of Shh in the prostate cancer cells increases the expression of noggin and BMP-7 [43]. In our studies, the treatment of mEC with a recombinant Shh protein increases noggin expression. COX-2 deficiency and decreased PGE-2 production in Cox-2-/- mice results in the loss of the PGE-2 mediated inhibition of Shh expression, leading to upregulation of noggin and inhibition of BMP-Smad1 activation. Collectively, the results suggest that noggin is an important mediator in the COX2/PGE2/Shh/BMP-2 axis during VEP ossification and IVD degeneration.
Currently, selective COX-2 inhibitors such as Celecoxib are widely used in LBP because of their efficacy in pain relieving and relatively less adverse effects. The additional beneficial effects of COX-2 inhibition revealed in our present study provide further support for their clinical use in the early stages of IVD degeneration through inhibition of VEP ossification.
Acknowledgements
We thank Professor Yrjo T. Konttinen, University of Helsinki, Finland, for constructive discussion, Dr. ArashRafiq for language revision, and Ms. Ming Xue for technical support. This study was supported by National Natural and Science Foundation (81472080, 81520108018, 81572149) and an NIH grant (AR048681, AR055915 and AR054465).
Disclosure of conflict of interest
None.
Authors’ contribution
QD and YR performed IHC and organ culture of IVD. HL and SY did cell isolation, RT-PCR and Western blotting. YZ and HA participated in data collection and interpretation, and manuscript revision. RJO and DC participated in conception of study, and revision of manuscript. TFL and GY participated in study design and manuscript preparation. Each author have read and approved its publication in the current version. Dr. Guoyong Yin and Tian-Fang Li have full access to all of data presented in this manuscript and are responsible for the integrity and interpretation of data.
Abbreviations
- COX-2
cyclooxygenase 2
- LBP
low back pain
- IVD
intervertebral disc
- VEP
vertebral endplate
- NP
nucleus pulposus
- mEC
mouse endplate chondrocytes
- BMP
bone morphogenetic protein
- Shh
sonic hedgehog
References
- 1.Waddell G. Low back pain: a twentieth century health care enigma. Spine. 1996;21:2820–2825. doi: 10.1097/00007632-199612150-00002. [DOI] [PubMed] [Google Scholar]
- 2.Modic MT, Steinberg PM, Ross JS, Masaryk TJ, Carter JR. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166:193–199. doi: 10.1148/radiology.166.1.3336678. [DOI] [PubMed] [Google Scholar]
- 3.Yu LP, Qian WW, Yin GY, Ren YX, Hu ZY. MRI assessment of lumbar intervertebral disc degeneration with lumbar degenerative disease using the pfirrmann grading systems. PLoS One. 2012;7:e48074. doi: 10.1371/journal.pone.0048074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burke JG, Watson RW, McCormack D, Dowling FE, Walsh MG, Fitzpatrick JM. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Joint Surg. 2002;84B:196–201. doi: 10.1302/0301-620x.84b2.12511. [DOI] [PubMed] [Google Scholar]
- 5.Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- 6.Li TF, Zuscik MJ, Ionescu AM, Zhang X, Rosier RN, Schwarz EM, Drissi H, O’Keefe RJ. PGE2 inhibits chondrocyte differentiation through PKA and PKC signaling. Exp Cell Res. 2004;300:159–169. doi: 10.1016/j.yexcr.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 7.Kawaguchi H, Pilbeam CC, Harrison JR, Raisz LG. The role of prostaglandins in the regulation of bone metabolism. Clin Orthop. 1995;313:36–46. [PubMed] [Google Scholar]
- 8.Yoshida K, Oida H, Kobayashi T, Maruyama T, Tanaka M, Katayama T, Yamaguchi K, Segi E, Tsuboyama T, Matsushita M, Ito K, Ito Y, Sugimoto Y, Ushikubi F, Ohuchida S, Kondo K, Nakamura T, Narumiya S. Stimulation of bone formation and prevention of bone loss by prostaglandin E EP4 receptor activation. Proc Natl Acad Sci USA. 2002;99:4580–4585. doi: 10.1073/pnas.062053399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB, Lipsky PE. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–1073. [PubMed] [Google Scholar]
- 10.Zhang X, Schwarz EM, Young DA, Puzas JE, Rosier RN, O’Keefe RJ. Cyclooxygenase-2 regulates mesenchymal cell differentiation into the osteoblast lineage and is critically involved in bone repair. J Clin Invest. 2002;109:1405–1415. doi: 10.1172/JCI15681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie C, Liang B, Xue M, Lin AS, Loiselle A, Schwarz EM, Guldberg RE, O’Keefe RJ, Zhang X. Rescue of impaired fracture healing in Cox-2-/- mice via activation of prostaglandin E2 receptor subtype 4. Am J Pathol. 2009;175:772–785. doi: 10.2353/ajpath.2009.081099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukai A, Kamekura S, Chikazu D, Nakagawa T, Hirata M, Saito T, Hosaka Y, Ikeda T, Nakamura K, Chung UI, Kawaguchi H. Lack of a chondroprotective effect of cyclooxygenase 2 inhibition in a surgically induced model of osteoarthritis in mice. Arthritis Rheum. 2012;64:198–203. doi: 10.1002/art.33324. [DOI] [PubMed] [Google Scholar]
- 13.Su SC, Tanimoto K, Tanne Y, Kunimatsu R, Hirose N, Mitsuyoshi T, Okamoto Y, Tanne K. Celecoxib exerts protective effects on extracellular matrix metabolism of mandibular condylar chondrocytes under excessive mechanical stress. Osteoarthritis Cartilage. 2014;22:845–851. doi: 10.1016/j.joca.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 14.Yuan W, Che W, Jiang YQ, Yuan FL, Wang HR, Zheng GL, Li XL, Dong J. Establishment of intervertebral disc degeneration model induced by ischemic sub-endplate in rat tail. Spine J. 2015;15:1050–1059. doi: 10.1016/j.spinee.2015.01.026. [DOI] [PubMed] [Google Scholar]
- 15.Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A, Makuch R, Eisen G, Agrawal NM, Stenson WF, Burr AM, Zhao WW, Kent JD, Lefkowith JB, Verburg KM, Geis GS. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis. JAMA. 2000;284:1247–1255. doi: 10.1001/jama.284.10.1247. [DOI] [PubMed] [Google Scholar]
- 16.Nakai K, Tanaka S, Sakai A, Nagashima M, Tanaka M, Otomo H, Nakamura T. Cyclooxygenase-2 selective inhibition suppresses restoration of tibial trabecular bone formation in association with restriction of osteoblast maturation in skeletal reloading after hindlimb elevation of mice. Bone. 2006;39:83–92. doi: 10.1016/j.bone.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 17.Saudan M, Saudan P, Perneger T, Riand N, Keller A, Hoffmeyer P. Celecoxib versus ibuprofen in the prevention of heterotopic ossification following total hip replacement. J Bone Joint Surg. 2007;89B:155–159. doi: 10.1302/0301-620X.89B2.17747. [DOI] [PubMed] [Google Scholar]
- 18.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi T, Lyons KM, McMahon AP, Kronenberg HM. BMP signaling stimulates cellular differentiation at multiple steps during cartilage development. Proc Natl Acad Sci U S A. 2005;102:18023–18027. doi: 10.1073/pnas.0503617102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li TF, Yukata K, Yin G, Sheu T, Maruyama T, Jonason JH, Hsu W, Zhang X, Xiao G, Konttinen YT, Chen D, O’Keefe RJ. BMP-2 induces ATF4 phosphorylation in chondrocytes through a COX-2/PGE2 dependent signaling pathway. Osteoarthritis Cartilage. 2014;22:481–489. doi: 10.1016/j.joca.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Capdevila J, Johnson RL. Endogenous and ectopic expression of noggin suggests a conserved mechanism for regulation of BMP function during limb and somite patterning. Dev Biol. 1998;197:205–217. doi: 10.1006/dbio.1997.8824. [DOI] [PubMed] [Google Scholar]
- 22.Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- 23.Drossopoulou G, Lewis KE, Sanz-Ezquerro JJ, Nikbakht N, McMahon AP, Hofmann C, Tickle C. A model for anteroposterior patterning of the vertebrate limb based on sequential long- and short-range Shh signalling and Bmp signalling. Development. 2000;127:1337–1348. doi: 10.1242/dev.127.7.1337. [DOI] [PubMed] [Google Scholar]
- 24.Guimond JC, Lévesque M, Michaud PL, Berdugo J, Finnson K, Philip A, Roy S. BMP-2 functions independently of SHH signaling and triggers cell condensation and apoptosis in regenerating axolotl limbs. BMC Dev Biol. 2010;10:15. doi: 10.1186/1471-213X-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirsinger E, Duprez D, Jouve C, Malapert P, Cooke J, Pourquié O. Noggin acts downstream of Wnt and Sonic Hedgehog to antagonize BMP4 in avian somite patterning. Development. 1997;124:4605–4614. doi: 10.1242/dev.124.22.4605. [DOI] [PubMed] [Google Scholar]
- 26.Risbud MV, Izzo MW, Adams CS, Arnold WW, Hillibrand AS, Vresilovic EJ, Vaccaro AR, Albert TJ, Shapiro IM. An organ culture system for the study of the nucleus pulposus: description of the system and evaluation of the cells. Spine. 2003;28:2652–2658. doi: 10.1097/01.BRS.0000099384.58981.C6. [DOI] [PubMed] [Google Scholar]
- 27.Kawamori T, Rao CV, Seibert K, Reddy BS. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer Res. 1998;58:409–412. [PubMed] [Google Scholar]
- 28.Winkler T, Mahoney EJ, Sinner D, Wylie CC, Dahia CL. Wnt signaling activates Shh signaling in early postnatal intervertebral discs, and re-activates Shh signaling in old discs in the mouse. PLoS One. 2014;9:e98444. doi: 10.1371/journal.pone.0098444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woo WM, Zhen HH, Oro AE. Shh maintains dermal papilla identity and hair morphogenesis via a Noggin-Shh regulatory loop. Genes Dev. 2012;26:1235–1246. doi: 10.1101/gad.187401.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bastida MF, Sheth R, Ros MA. A BMP-Shh negative-feedback loop restricts Shh expression during limb development. Development. 2009;136:3779–3789. doi: 10.1242/dev.036418. [DOI] [PubMed] [Google Scholar]
- 31.Qiao C, Zhang K, Sun B, Liu J, Song J, Hu Y, Yang S, Sun H, Yang B. Sustained release poly (lactic-co-glycolic acid) microspheres of bone morphogenetic protein 2 plasmid/calcium phosphate to promote in vitro bone formation and in vivo ectopic osteogenesis. Am J Transl Res. 2015;7:2561–2572. [PMC free article] [PubMed] [Google Scholar]
- 32.Brazil DP, Church RH, Surae S, Godson C, Martin F. BMP signalling: agony and antagony in the family. Trends Cell Biol. 2015;25:249–264. doi: 10.1016/j.tcb.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 33.Magne D, Julien M, Vinatier C, Merhi-Soussi F, Weiss P, Guicheux J. Cartilage formation in growth plate and arteries: from physiology to pathology. Bioessays. 2005;27:708–716. doi: 10.1002/bies.20254. [DOI] [PubMed] [Google Scholar]
- 34.Clark CA, Li TF, Kim KO, Drissi H, Zuscik MJ, Zhang X, O’Keefe RJ. Prostaglandin E2 inhibits BMP signaling and delays chondrocyte maturation. J Orthop Res. 2009;27:785–792. doi: 10.1002/jor.20805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antoniou J, Steffen T, Nelson F, Winterbottom N, Hollander AP, Poole RA, Aebi M, Alini M. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 1996;98:996–1003. doi: 10.1172/JCI118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao L, Wu Y, Xu Z, Wang H, Zhao Z, Li Y, Yang P, Wei X. Involvement of COX-2/PGE2 signalling in hypoxia-induced angiogenic response in endothelial cells. J Cell Mol Med. 2012;16:1840–1855. doi: 10.1111/j.1582-4934.2011.01479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oliveira TM, Sakai VT, Machado MA, Dionísio TJ, Cestari TM, Taga R, Amaral SL, Santos CF. COX-2 inhibition decreases VEGF expression and alveolar bone loss during the progression of experimental periodontitis in rats. J Periodontol. 2008;79:1062–1069. doi: 10.1902/jop.2008.070411. [DOI] [PubMed] [Google Scholar]
- 38.Roskoski R Jr. VEGF receptor protein-tyrosine kinases: structure and regulation. Biochem Biophys Res Commun. 2008;375:287–291. doi: 10.1016/j.bbrc.2008.07.121. [DOI] [PubMed] [Google Scholar]
- 39.Kimura H, Ng JM, Curran T. Transient inhibition of the Hedgehog pathway in young mice causes permanent defects in bone structure. Cancer Cell. 2008;13:249–260. doi: 10.1016/j.ccr.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 40.Chiang C, Litingtung Y, Harris MP, Simandl BK, Li Y, Beachy PA, Fallon JF. Manifestation of the limb prepattern: limb development in the absence of sonic hedgehog function. Dev Biol. 2001;236:421–435. doi: 10.1006/dbio.2001.0346. [DOI] [PubMed] [Google Scholar]
- 41.Horikiri Y, Shimo T, Kurio N, Okui T, Matsumoto K, Iwamoto M, Sasaki A. Sonic hedgehog regulates osteoblast function by focal adhesion kinase signaling in the process of fracture healing. PLoS One. 2013;8:e76785. doi: 10.1371/journal.pone.0076785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuasa T, Kataoka H, Kinto N, Iwamoto M, Enomoto-Iwamoto M, Iemura S, Ueno N, Shibata Y, Kurosawa H, Yamaguchi A. Sonic hedgehog is involved in osteoblast differentiation by cooperating with BMP-2. J Cell Physiol. 2002;193:225–232. doi: 10.1002/jcp.10166. [DOI] [PubMed] [Google Scholar]
- 43.Shaw A, Gipp J, Bushman W. Exploration of Shh and BMP paracrine signaling in a prostate cancer xenograft. Differentiation. 2010;79:41–47. doi: 10.1016/j.diff.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


