Abstract
Objective: To investigate the mechanism of aerobic exercise in the relief of vascular cognitive impairment (VCI). Materials and methods: Latency of Water Maze test was measured at sham, 2VO, 2VO+EX groups. miR-503 and BDNF mRNA levels were detected by quantitative real-time PCR. Protein levels of NF-κB and BDNF were detected by Western blot. Hippocampal neuron cell apoptosis was detected by flow cytometry. Luciferase reporter assay was conducted to investigate the effect of miR-503 on BDNF. Results: Latency of Water Maze test in 2VO group was longer than Sham group, while exercise shortened the latency. The expressions of NF-κB and miR-503 in 2VO group were higher than Sham group, while exercise downregulated the expressions. BDNF level in 2VO group were downregulated than Sham group, while exercise upregulated the levels. We also found NF-κB, miR-503 levels were upregulated and BDNF level was downregulated in OGD-treated hippocampal neuron cells. In addition, OGD increased the expression of NF-κB and miR-503, and the expression of miR-503 was downregulated when treated with NF-κB inhibitor (PDTC). Moreover, we confirmed BDNF was a direct target of miR-503. OGD decreased the expression of BDNF, while miR-503 inhibitor reversed this effect. And we proved OGD induced cell apoptosis via NF-κB/miR-503/BDNF. Finally, in rats injected with miR-503 inhibitor, latency of Water Maze test was shortened, miR-503 expression was downregulated, and BDNF level was upregulated. While in rats injected with miR-503 mimic, the results were the opposite. Conclusion: Aerobic exercise relieved VCI via NF-κB/miR-503/BDNF pathway.
Keywords: Aerobic exercise, vascular cognitive impairment, hippocampal neuron cells, NF-κB, miR-503, BDNF
Introduction
Vascular cognitive impairment (VCI) describes a series of cognitive disorders containing from mild cognitive impairment to dementia, which is commonly caused by Alzheimer’s disease (AD) or vascular dementia (VasD) [1]. As the aging population is increasing worldwide, AD and VasD become more and more serious social problems, therefore, more attentions should be paid on VCI. In the previous reports, nutritional, pharmacological and immunologic strategies were used in relieving VCI in patients, however, these strategies had limited roles in VCI [2,3]. Recently, researchers proved that physical fitness could improve cognition in old patients with cognitive impairment [4-7], providing new insights for the future study of VCI.
Nuclear factor kappa B (NF-κB) is a kind of transcription factor that binds to the enhancer element of the immunoglobulin kappa light-chain of activated B cells, and contains five members, namely p65 (RelA), RelB, c-Rel, NF-κB1 and NF-κB2 [8]. It has been reported that p65 immunoactivity level was increased in AD patients, and NF-κB played important roles in chronic neurodegenerative disorders, including AD, Parkinson’s diseases, and epilepsy [9]. Moreover, Koo et al [10] found that TLR2/MyD88/NF-κB pathway altered in neuroprotective effects in a murine model of Parkinson’s disease produced by treadmill exercise. However, molecular pathways downstream of NF-κB in treadmill exercise treated neurodegenerative disorders were still not clear.
MicroRNAs (miRNAs) are non-coding short-chain RNA which can regulate cell proliferation and differentiation, protein synthesis and the progress of diseases [11]. Studies have showed that miR-34a-5p, miR-106, miR-665 were involved in cognitive impairment [12-14]. Soumya et al [15] found that miR-503 was remarkably downregulated in IGF-1 treated embryonic striatal stem cells where striatum was considered to be the central processing unit of cognitive function of the brain. Xie et al [16] discovered that NS5A inhibited NF-κB activation to decrease the expression of miR-503, indicating that NF-κB positively regulated miR-503.
Brain-derived neurotrophic factor (BDNF) is a kind of neurotrophins that has potential role in the disease pathology or the treatment of AD and Huntington’s disease [17]. Researchers have shown that BDNF played important role in cognitive function. For example, Cui et al [14] reported that cognitive function could be impaired by regulating BDNF when exposed to irradiation. Suzuki et al [18] proved that the reduction of BDNF was related with cognitive dysfunction. Zhang et al [19] observed that metabolic adverse effects of olanzapine aggravated cognitive dysfunction through an interaction between BDNF and TNF-α. What’s more, bioinformatics software showed there were combination sites between miR-503 and BDNF. Thus, we speculated that exercise relieved cognitive impairment via NF-κB/miR-503/BDNF.
In this study, we measured the expression of NF-κB, miR-503 and BDNF in hippocampus tissues and hippocampal neuron cells among different groups, and explore the exact mechanism of aerobic exercise in VCI.
Materials and methods
Establishment of rat model of vascular dementia
Sixty male Sprague-Dawley (SD) rats were purchased from Shanghai Laboratory Animal Center of Chinese Academy of Science (Shanghai, China). Rats (about 6 months and 700 g) were divided into three groups: sham control group (n=10); 2 vessel occlusion (2VO; hypoperfusion) control group (n=10); 2VO+exercise (2VO+EX) group (n=10). Rats were anesthetized by isoflurane (4% induction; 2.5% maintenance) in 70/30% nitric oxide/oxygen, then underwent bilateral common carotid artery occlusion (cerebral hypoperfusion; 2VO) or sham surgery.
In 2VO and 2VO+EX groups, a midline neck incision was made, and the left and right common carotid arteries were separated from surrounding muscles and adjacent nerve tract. Every artery in 2VO and 2VO+EX groups was ligated with 4-0 silk, examined for lack of patency and returned to the neck cavity. In sham surgery, neck incision and isolation of arteries without ligation were made similarly. Local 2% Xylocaine was used in the midline neck incision. Then, rats were placed in a standard cage on a heating blanket until recovery.
After surgery was done, treadmill exercise was conducted 30 minutes every day (15 m/min) for four weeks from the third week to the seventh week in 2VO+EX group. Water Maze test was used to determine the latency that rats found the platform for four days from the eighth week. All procedures were approved by the Ethics Committee of the Tianjin Medical University.
Cell culture and transfection
The hippocampus tissues were removed from the newborn rat, then were dissected and triturated, and seeded into cell plates which were precoated with 10 μg/mL poly-D-lysine (Sigma, USA). They were incubated in the culture media that contained neurobasal medium (Gibco, USA) with 10% fetal bovine serum (Gibco, USA) and 2% B27 supplement (Invitrogen, USA). Three days later, cell proliferation was inhibited by 5 μM cytosine arabinoside (Sigma, USA). The neurons were used at 14 days after plating for the following experiments. NF-κB inhibitor (PDTC), miR-503 mimic and pcDNA-BDNF were transfected into hippocampal neuron cells using Lipofectamine 2000 transfection reagent (Invitrogen, Carlsbad, USA) according to the manufacturer’s instructions.
Quantitative real-time PCR
Total RNA was separated from hippocampus tissues and hippocampal neuron cells using TRIzol (Invitrogen, Carlsbad, USA) according to the manufacturer’s instructions. miR-503 and BDNF expressions of miRNAs in tissues and cells were measured by TaqMan microRNA Reverse Transcription Kit and the TaqMan Universal Master Mix II (Applied Biosystems, USA). β-actin was used as an internal control. All reactions were performed in triplicate, and mRNA expressions were measured by 2-step cycle protocol. Data were analyzed by ABI Prism 7300 SDS software (Applied Biosystems). The relative expression of miR-503 and BDNF gene were calculated by comparative method 2-ΔΔCt.
Western blot analysis
Total protein samples were homogenized and extracted from hippocampus tissues or hippocampal neuron cells by RIPA buffer. Protein concentrations of these extracts were determined by BCA Protein Assay kit (Pierce Biotechnology, USA) after centrifugation at 12,000 rpm for 15 min. Proteins were separated on 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel and transferred to a polyvinylidene difluoride (PVDF) membrane (Thermo Scientific, USA) which was blocked with 5% non-fat dried milk for 2 h. Primary monoclonal antibodies against NF-κB (Cell Signaling Technology, USA), BDNF (Cell Signaling Technology, USA), β-actin (Cell Signaling Technology, USA), and second antibody corresponding horseradish peroxidase-conjugated secondary antibody (Invitrogen, USA) were used in this Western blot analysis. Bands were quantified using enhanced chemiluminescence system (ECL, GE Healthcare), and protein expressions of NF-κB and BDNF were normalised to the β-actin level.
Luciferase reporter assays
The 3’UTR of BDNF containing BDNF-miR-503 response element was inserted into the psiCHECK-2 vector (Promega) using XhoI/NotI cuts. A mutant 3’-UTR of BDNF was synthesised by PCR. 293T cells were transfected with psiCHECK-2_WT.BDNF or psiCHECK-2_Mut.BDNF. For Luciferase reporter assay, microRNAs (miR-503 mimic, miR-503 inhibitor, or negative control) and reporter plasmid were cotransfected using Lipofectamine 2000 (Invitrogen) for 48 h. Finally, luciferase activity was measured by dual Luciferase detection system (Promega).
Annexin V/PI double staining method
Cell apoptosis was observed by annexin V/PI double staining method. Hippocampal neuron cells from seven groups (OGD, OGD+NF-κB inhibitor, OGD+NF-κB inhibitor+NC, OGD+NF-κB inhibitor+miR-503 mimic, OGD+NF-κB inhibitor+miR-503 mimic+pcDNA, OGD+NF-κB inhibitor+miR-503 mimic+pcDNA-BDNF) were collected. 1 × Annexin V binding buffer was added to make a final concentration of 6 × 105/mL. Annexin V and PI solution were added for staining for 15 min, and apoptosis was evaluated by flow cytometry. The apoptotic cells were detected by flow cytometry (FACSCanto II, BD Biosciences, USA). Percentage of apoptosis rate (%) = (number of apoptotic cells/number of all cells) × 100%.
Intracerebroventricular injection
miRNAs were injected according to previously report [20]. Rats were anesthetized and placed in a stereotaxic apparatus (Shanghai Biowill Biological Instruments, Shanghai, China). The coordinates for intracerebroventricular injection into left lateral ventricle were as follows: 0.8 mm posterior to the bregma, 1.5 mm lateral to the midline, and 4.5 mm ventral to the surface of the skull. miR-503 inhibitor or mimic (1 μl, Invitrogen, USA) were diluted with the equal volume of transfection reagent Lipofectamine 2000 (Invitrogen, USA) and cultured at room temperature for 15 min. The mixture of RNA and transfection reagent was intracerebroventricular injected into the rat brain.
Statistical analysis
We used SPSS 17.0 for the data analysis. Student’s t test was used for all analyses, with P<0.05 considered statistically significant.
Results
The establishment of rat model of vascular dementia
To investigate the effect of NF-κB, miR-503, BDNF and aerobic exercise on vascular dementia, we established rat model of vascular dementia and divided them into Sham, 2VO, and 2VO+EX groups. As shown in Figure 1A, the latency of Water Maze test in 2VO group was longer than Sham group, while the latency in 2VO+EX group was shorter than 2VO group. Also, we observed that the expressions of NF-κB and miR-503 in 2VO group were higher than Sham group, while the expressions of NF-κB and miR-503 in 2VO+EX group were downregulated than 2VO group (Figure 1B). mRNA and protein levels of BDNF in 2VO group were downregulated than Sham group, while mRNA and protein levels of BDNF in 2VO+EX group were upregulated than 2VO group (Figure 1C). These finding suggested that rat model of vascular dementia was successfully established.
Figure 1.
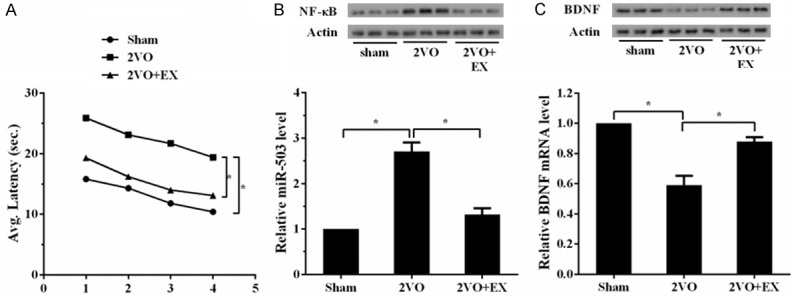
The establishment of rat model of vascular dementia. Rats were divided into three groups: sham control group (n=10), 2VO control group (n=10), and 2VO+EX group (n=10). Water Maze test was used at the eighth week after surgery. Then, rats were sacrificed and hippocampus tissues were obtained to measure the expression of NF-κB, miR-503 and BDNF. A. Latency of Water Maze test in 2VO group was longer than Sham group, while latency in 2VO+EX group was shorter than 2VO group. B. The expressions of NF-κB and miR-503 in 2VO group were higher than Sham group. While the expressions of NF-κB and miR-503 in 2VO+EX group were downregulated than 2VO group. C. mRNA and protein levels of BDNF in 2VO group were downregulated than Sham group. While mRNA and protein levels of BDNF in 2VO+EX group were upregulated than 2VO group.
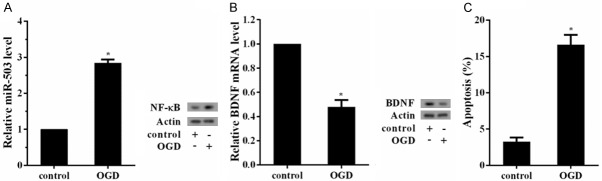
Upregulation of NF-κB, miR-503 levels and downregulation of BDNF in oxygen-glucose deprivation (OGD) treated hippocampal neuron cells
Hippocampal neuron cells were treated with OGD to simulate vascular dementia in vitro. We found the expressions of NF-κB and miR-503 were upregulated in OGD group (Figure 2A). mRNA and protein levels of BDNF were downregulated in OGD group (Figure 2B). Moreover, apoptosis rate was increased in OGD group (Figure 2C). These findings indicated that NF-κB and miR-503 were upregulated, BDNF were downregulated, and apoptosis rate was increased in vascular dementia.
Figure 2.
Upregulation of NF-κB, miR-503 levels and downregulation of BDNF in oxygen-glucose deprivation (OGD) treated hippocampal neuron cells. Hippocampal neuron cells were divided into control group and OGD group (Cells were exposed to OGD for 1 h). The expressions of miR-503 and BDNF and cell apoptosis were measured 24 h later. A. The expressions of NF-κB and miR-503 were upregulated in OGD group. B. mRNA and protein levels of BDNF were downregulated in OGD group. C. Apoptosis rate was increased in OGD group.
OGD regulated the expression of miR-503 via NF-κB
To verify whether OGD regulated miR-503 via NF-κB, we measured miR-503 expression in cells treated with NF-κB inhibitor (PDTC). As shown in Figure 3A, OGD increased the expression of NF-κB. When treated with NF-κB inhibitor, the expression of NF-κB was downregulated. We also found OGD increased the expression of miR-503. When treated with NF-κB inhibitor, the expression of miR-503 was downregulated, indicating that OGD regulated the expression of miR-503 via NF-κB (Figure 3B).
Figure 3.
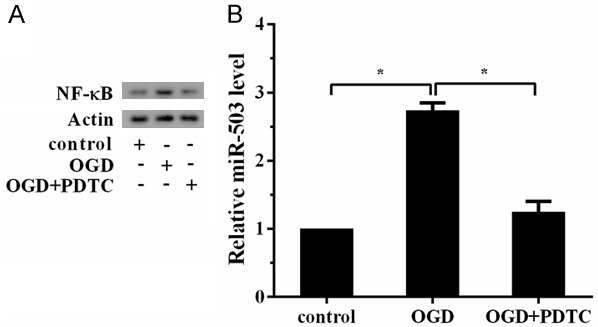
OGD regulated the expression of miR-503 via NF-κB. Hippocampal neuron cells were divided into three groups: control group, OGD group, OGD+NF-κB inhibitor (PDTC, 10 μM). A. OGD increased the expression of NF-κB. When treated with NF-κB inhibitor, the expression of NF-κB was downregulated. B. OGD increased the expression of miR-503. When treated with NF-κB inhibitor, the expression of miR-503 was downregulated.
BDNF was a direct target of miR-503
Regulation of miR-503 on BDNF was confirmed by luciferase reporter gene. Bioinformatics software showed the combination sites of miR-503 and BDNF (Figure 4A). After transfected with miR-503 mimic in WT and MUT cell lines, the activity of BDNF 3’-UTR in WT cell lines was decreased, and there was no significant difference in MUT cell lines (Figure 4B). Besides, mRNA and protein levels of BDNF in WT cell lines were decreased. After transfected with miR-503 inhibitor in WT and MUT cell lines, the activity in WT cell lines was increased, and there was no significant difference in MUT cell lines (Figure 4C). And mRNA and protein levels of BDNF in WT cell lines were increased. These findings proved that BDNF was a direct target of miR-503.
Figure 4.
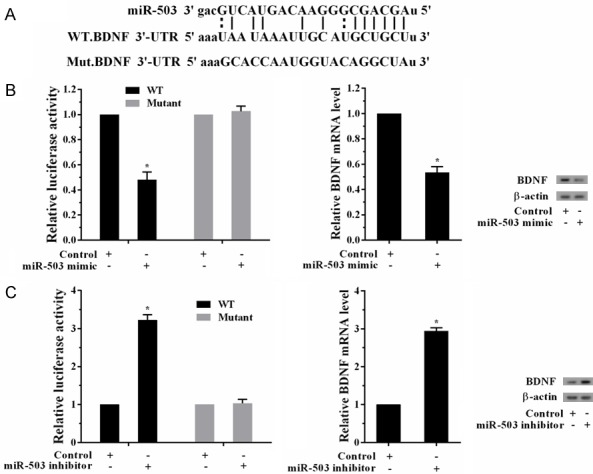
BDNF is a direct target of miR-503. A. Bioinformatics software showed the combination sites of miR-503 and BDNF to construct the BDNF mRNA (WT and MUT) cell lines. B. The activity of BDNF 3’-UTR was measured after transfected with miR-503 mimic in WT and MUT cell lines. The activity in WT cell lines was decreased, and there was no significant difference in MUT cell lines. mRNA and protein levels of BDNF in WT cell lines were decreased. C. The activity of BDNF 3’-UTR was measured after transfected with miR-503 inhibitor in WT and MUT cell lines. The activity in WT cell lines was increased, and there was no significant difference in MUT cell lines. mRNA and protein levels of BDNF in WT cell lines were increased.
OGD regulated the expression of BDNF via miR-503
Since BDNF was a direct target of miR-503, we assumed that OGD could regulate BDNF via miR-503. We found OGD increased the expression of miR-503, while miR-503 inhibitor reversed this effect (Figure 5A). OGD decreased mRNA and protein levels of BDNF, while miR-503 inhibitor reversed this effect, indicating that OGD regulated the expression of BDNF via miR-503 (Figure 5B).
Figure 5.
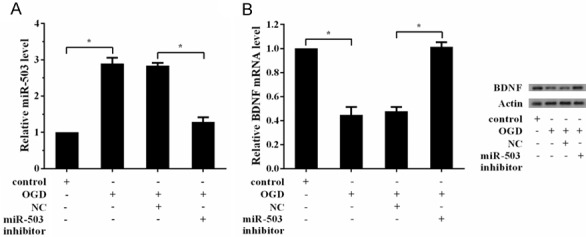
OGD regulated the expression of BDNF via miR-503. Hippocampal neuron cells were divided into four groups: control group, OGD group, OGD+NC group, and OGD+miR-503 inhibitor group. A. OGD increased the expression of miR-503, while miR-503 inhibitor reversed this effect. B. OGD decreased the expression of BDNF, while miR-503 inhibitor reversed this effect.
OGD induced cell apoptosis via NF-κB/miR-503/BDNF
To prove the exact signaling pathway of hippocampal neuron cell apoptosis induced by OGD, we divided the cells into seven groups. We observed OGD increased cell apoptosis, and NF-κB inhibitor decreased cell apoptosis. Then, miR-503 mimic increased cell apoptosis again, and pcDNA-BDNF further decreased cell apoptosis (Figure 6), suggesting OGD induced cell apoptosis via NF-κB/miR-503/BDNF.
Figure 6.
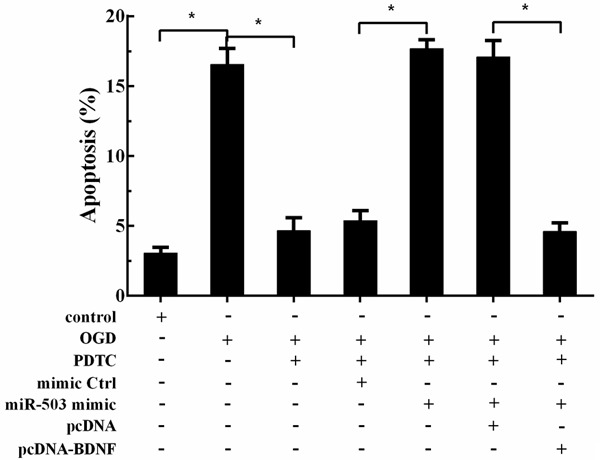
OGD induced cell apoptosis via NF-κB/miR-503/BDNF. Hippocampal neuron cells were divided into seven groups: control group, OGD group, OGD+NF-κB inhibitor group, OGD+NF-κB inhibitor+NC group, OGD+NF-κB inhibitor+miR-503 mimic group, OGD+NF-κB inhibitor+miR-503 mimic+pcDNA group, and OGD+NF-κB inhibitor+miR-503 mimic+pcDNA-BDNF group. OGD increased cell apoptosis, NF-κB inhibitor decreased cell apoptosis, miR-503 mimic increased cell apoptosis again, and pcDNA-BDNF further decreased cell apoptosis.
Aerobic exercise relieved vascular cognitive impairment via NF-κB/miR-503/BDNF pathway
To further investigate the exact mechanism of aerobic exercise relieved vascular cognitive impairment, miR-503 inhibitor or mimic were injected into the ventricle during 2VO surgery. After injected with miR-503 inhibitor, the latency of Water Maze test in miR-503 inhibitor group was shorter than control group (Figure 7A). The expression of miR-503 in miR-503 inhibitor group was downregulated, while mRNA and protein levels of BDNF were upregulated. After injected with miR-503 mimic, the latency of Water Maze test in miR-503 mimic group was longer than control group (Figure 7B). The expression of miR-503 in miR-503 mimic group was upregulated, while mRNA and protein levels of BDNF were downregulated.
Figure 7.
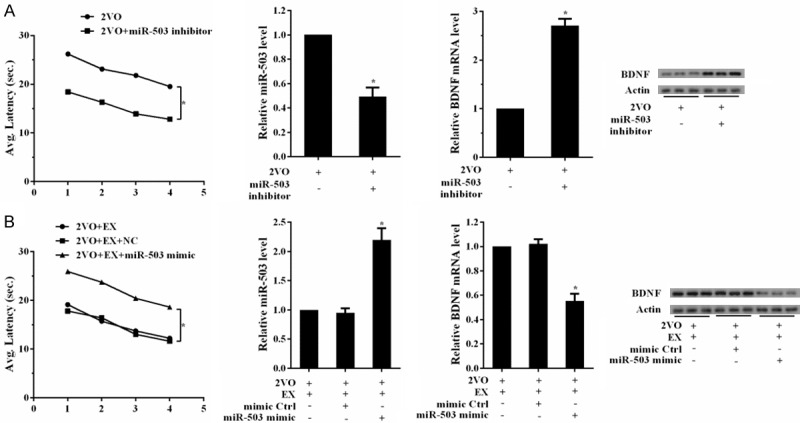
Aerobic exercise relieved vascular cognitive impairment via NF-κB/miR-503/BDNF pathway. A. miR-503 inhibitor was injected into the ventricle during 2VO surgery. Rats were divided into two groups: 2VO+NC, 2VO+miR-503 inhibitor groups. Water Maze test was used at the sixteenth week after surgery. Hippocampus tissues were obtained to measure the expression of NF-κB, miR-503 and BDNF. The latency of Water Maze test in miR-503 inhibitor group was shorter than control group. The expression of miR-503 in miR-503 inhibitor group was downregulated, while mRNA and protein levels of BDNF were upregulated. B. Rats were divided into three groups: 2VO+EX, 2VO+EX+mimic control, 2VO+miR-503 mimic+EX groups. Water Maze test was used at the eighth week after surgery. Hippocampus tissues were obtained to measure the expression of NF-κB, miR-503 and BDNF. The latency of Water Maze test in miR-503 mimic group was longer than control group. The expression of miR-503 in miR-503 mimic group was upregulated, while mRNA and protein levels of BDNF were downregulated.
Discussion
The aerobic exercise plays a critical role in the relief of cognitive impairment. As shown in previous reports, aerobic exercise could improve memory of middle-aged rats [21], and improve health status for patients with VCI and dementia [22]. In the present study, we found that NF-κB and miR-503 expressions were upregulated in rats underwent 2 vessel occlusion surgery, and BDNF expression was downregulated. In vitro experiments, we also found the expression trends of NF-κB, miR-503 and BDNF in OGD-treated hippocampal neuron cells were the same in vivo. We further observed OGD regulated the expression of miR-503 via NF-κB, BDNF was a direct target of miR-503, and OGD regulated the expression of BDNF via miR-503. Taken together, these results indicated that aerobic exercise relieved VCI via NF-κB/miR-503/BDNF, and proved the downstream molecules of NF-κB were miR-503 and BDNF.
Numerous studies demonstrated that increased NF-κB could induce cognitive impairment, and NF-κB pathway could be prevented by pharmacological intervention [23-25]. However, because of the high cost of pharmacotherapy, patients prefer treatments that cost less. Hence, revealing the mechansim of aerobic exercise in relief of VCI has practical significance. Recently, many researchers have focused on physical exercise to evaluate the effects of exercise on cognitive function [26-28], but few studies have examined the exact mechanism of physical activity in improving cognitive function. In 2017, Assis and Almondes [29] found that exercise-dependent BDNF regulated cognitive impairment and improved executive functions in individuals with dementia. Goel et al [25] reported that memory impairment could be attenuated by regulating NF-κB/BDNF/CREB. In this study, we observed that in vascular dementia rat model, NF-κB level was remarkably increased and BDNF was downregulated, which were consistent with previously reports.
miRNAs can modulate the process of degrading RNA and inhibiting protein translation by the combination of target genes. In previous reports, miR-503 played critical roles in inhibiting cell proliferation, migration, and invasion of cancers [30], regulating inflammation mediated angiogenesis [31], and promoting bone formation in distraction osteogenesis [32]. Moreover, miR-503 participated in cell proliferation and invasion in glioma [33]. Andrea et al [34] found that p75(NTR)-dependent activation of NF-κB modulated miR-503 transcription in diabetic patients after limb ischaemia. In this study, we observed that miR-503 expression increased in vascular dementia rat model and OGD-treated hippocampal neuron cells. When treated with PDTC, miR-503 expression was remarkably decreased, indicating that NF-κB could positively regulate miR-503, which was consistent with previous report [16]. Furthermore, we confirmed BDNF was a direct target of miR-503, verifying that NF-κB participated in VCI via miR-503/BDNF.
In conclusion, these results suggest that aerobic exercise relieved VCI via NF-κB/miR-503/BDNF pathway. This research first reveals the role of miR-503 in the progress of VCI, and confirmed there were combination sites between miR-503 and BDNF, which provide a scientific basis for VCI treatment.
Disclosure of conflict of interest
None.
References
- 1.Langdon KD, Granter-Button S, Harley CW, Moody-Corbett F, Peeling J, Corbett D. Cognitive rehabilitation reduces cognitive impairment and normalizes hippocampal CA1 architecture in a rat model of vascular dementia. J Cereb Blood Flow Metab. 2013;33:872–879. doi: 10.1038/jcbfm.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pena-Altamira E, Petralla S, Massenzio F, Virgili M, Bolognesi ML, Monti B. Nutritional and pharmacological strategies to regulate microglial polarization in cognitive aging and Alzheimer’s disease. Front Aging Neurosci. 2017;9:175. doi: 10.3389/fnagi.2017.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arino H, Armangue T, Petit-Pedrol M, Sabater L, Martinez-Hernandez E, Hara M, Lancaster E, Saiz A, Dalmau J, Graus F. Anti-LGI1-associated cognitive impairment: presentation and long-term outcome. Neurology. 2016;87:759–765. doi: 10.1212/WNL.0000000000003009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stradecki-Cohan HM, Youbi M, Cohan CH, Saul I, Garvin AA, Perez E, Dave KR, Wright CB, Sacco RL, Perez-Pinzon MA. Physical exercise improves cognitive outcomes in 2 models of transient cerebral ischemia. Stroke. 2017;48:2306–2309. doi: 10.1161/STROKEAHA.117.017296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chupel MU, Direito F, Furtado GE, Minuzzi LG, Pedrosa FM, Colado JC, Ferreira JP, Filaire E, Teixeira AM. Strength training decreases inflammation and increases cognition and physical fitness in older women with cognitive impairment. Front Physiol. 2017;8:377. doi: 10.3389/fphys.2017.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wouters H, Aalbers T, Maessen M, Verbeek A, Olde Rikkert M, Kessels R, Hopman M, Eijsvogels T. Physical activity and cognitive function of long-distance walkers: studying four days marches participants. Rejuvenation Res. 2017;20:367–374. doi: 10.1089/rej.2016.1876. [DOI] [PubMed] [Google Scholar]
- 7.Hess NC, Smart NA. Isometric exercise training for managing vascular risk factors in mild cognitive impairment and Alzheimer’s disease. Front Aging Neurosci. 2017;9:48. doi: 10.3389/fnagi.2017.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 2013;12:86–86. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeon WK, Ma J, Choi BR, Han SH, Jin Q, Hwang BY, Han JS. Effects of fructus mume Extract on MAPK and NF-kappaB signaling and the resultant improvement in the cognitive deficits induced by chronic cerebral hypoperfusion. Evid Based Complement Alternat Med. 2012;2012:450838. doi: 10.1155/2012/450838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koo JH, Jang YC, Hwang DJ, Um HS, Lee NH, Jung JH, Cho JY. Treadmill exercise produces neuroprotective effects in a murine model of Parkinson’s disease by regulating the TLR2/MyD88/NF-kappaB signaling pathway. Neuroscience. 2017;356:102–113. doi: 10.1016/j.neuroscience.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 11.Pandey R, Velasquez S, Durrani S, Jiang M, Neiman M, Crocker JS, Benoit JB, Rubinstein J, Paul A, Ahmed RP. MicroRNA-1825 induces proliferation of adult cardiomyocytes and promotes cardiac regeneration post ischemic injury. Am J Transl Res. 2017;9:3120–3137. [PMC free article] [PubMed] [Google Scholar]
- 12.Lu X, Lv S, Mi Y, Wang L, Wang G. Neuroprotective effect of miR-665 against sevoflurane anesthesia-induced cognitive dysfunction in rats through PI3K/Akt signaling pathway by targeting insulin-like growth factor 2. Am J Transl Res. 2017;9:1344–1356. [PMC free article] [PubMed] [Google Scholar]
- 13.Huang W, Li Z, Zhao L, Zhao W. Simvastatin ameliorate memory deficits and inflammation in clinical and mouse model of Alzheimer’s disease via modulating the expression of miR-106b. Biomed Pharmacother. 2017;92:46–57. doi: 10.1016/j.biopha.2017.05.060. [DOI] [PubMed] [Google Scholar]
- 14.Cui M, Xiao H, Li Y, Dong J, Luo D, Li H, Feng G, Wang H, Fan S. Total abdominal irradiation exposure impairs cognitive function involving miR-34a-5p/BDNF axis. Biochim Biophys Acta. 2017;1863:2333–2341. doi: 10.1016/j.bbadis.2017.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pati S, Supeno NE, Muthuraju S, Abdul Hadi R, Ghani ARI, Idris FM, Maletic-Savatic M, Abdullah JM, Jaafar H. MicroRNA profiling reveals unique miRNA signatures in IGF-1 treated embryonic striatal stem cell fate decisions in striatal neurogenesis in vitro. Biomed Res Int. 2014;2014:503162. doi: 10.1155/2014/503162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie Z, Xiao Z, Wang F. Hepatitis C virus nonstructural 5A protein (HCV-NS5A) inhibits hepatocyte apoptosis through the NF-kappab/miR-503/bcl-2 pathway. Mol Cells. 2017;40:202–210. doi: 10.14348/molcells.2017.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adachi N, Numakawa T, Richards M, Nakajima S, Kunugi H. New insight in expression, transport, and secretion of brain-derived neurotrophic factor: Implications in brain-related diseases. World J Biol Chem. 2014;5:409–428. doi: 10.4331/wjbc.v5.i4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suzuki H, Matsumoto Y, Ota H, Sugimura K, Takahashi J, Ito K, Miyata S, Arai H, Taki Y, Furukawa K, Fukumoto Y, Shimokawa H. Reduced brain-derived neurotrophic factor is associated with cognitive dysfunction in patients with chronic heart failure. Geriatr Gerontol Int. 2017;17:852–854. doi: 10.1111/ggi.12959. [DOI] [PubMed] [Google Scholar]
- 19.Zhang C, Fang X, Yao P, Mao Y, Cai J, Zhang Y, Chen M, Fan W, Tang W, Song L. Metabolic adverse effects of olanzapine on cognitive dysfunction: a possible relationship between BDNF and TNF-alpha. Psychoneuroendocrinology. 2017;81:138–143. doi: 10.1016/j.psyneuen.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 20.Sepramaniam S, Armugam A, Lim KY, Karolina DS, Swaminathan P, Tan JR, Jeyaseelan K. MicroRNA 320a functions as a novel endogenous modulator of aquaporins 1 and 4 as well as a potential therapeutic target in cerebral ischemia. J Biol Chem. 2010;285:29223–29230. doi: 10.1074/jbc.M110.144576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cardoso FDS, França EF, Serra FT, Victorino AB, de Almeida AA, Fernandes J, Cabral FR, Venancio DP, Arida RM, Gomes da Silva S. Aerobic exercise reduces hippocampal ERK and p38 activation and improves memory of middle-aged rats. Hippocampus. 2017;27:899–905. doi: 10.1002/hipo.22740. [DOI] [PubMed] [Google Scholar]
- 22.Trigiani LJ, Hamel E. An endothelial link between the benefits of physical exercise in dementia. J Cereb Blood Flow Metab. 2017;37:2649–2664. doi: 10.1177/0271678X17714655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Husain I, Akhtar M, Vohora D, Abdin MZ, Islamuddin M, Akhtar MJ, Najmi AK. Rosuvastatin attenuates high-salt and cholesterol diet induced neuroinflammation and cognitive impairment via preventing nuclear factor kappaB pathway. Neurochem Res. 2017;42:2404–2416. doi: 10.1007/s11064-017-2264-2. [DOI] [PubMed] [Google Scholar]
- 24.Liu Q, Chen Y, Shen C, Xiao Y, Wang Y, Liu Z, Liu X. Chicoric acid supplementation prevents systemic inflammation-induced memory impairment and amyloidogenesis via inhibition of NF-kappaB. FASEB J. 2017;31:1494–1507. doi: 10.1096/fj.201601071R. [DOI] [PubMed] [Google Scholar]
- 25.Goel R, Bhat SA, Hanif K, Nath C, Shukla R. Angiotensin II receptor blockers attenuate lipopolysaccharide-induced memory impairment by modulation of NF-kappaB-mediated BDNF/CREB expression and apoptosis in spontaneously hypertensive rats. Mol Neurobiol. 2017:1–15. doi: 10.1007/s12035-017-0450-5. [DOI] [PubMed] [Google Scholar]
- 26.Teixeira RB, Marins JCB, Amorim PRS, Teoldo I, Cupeiro R, Andrade MOC, Martins YLX, Castilho PR, Magalhães DD, Palotás A, Lima LM. Evaluating the effects of exercise on cognitive function in hypertensive and diabetic patients using the mental test and training system. World J Biol Psychiatry. 2017:1–10. doi: 10.1080/15622975.2017.1337222. [DOI] [PubMed] [Google Scholar]
- 27.Lipardo DS, Aseron AMC, Kwan MM, Tsang WW. Effect of exercise and cognitive training on falls and fall-related factors in older adults with mild cognitive impairment: a systematic review. Arch Phys Med Rehabil. 2017;98:2079–2096. doi: 10.1016/j.apmr.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 28.Devenney KE, Sanders ML, Lawlor B, Olde Rikkert MGM, Schneider S. Erratum to: the effects of an extensive exercise programme on the progression of mild cognitive impairment (MCI): study protocol for a randomised controlled trial. BMC Geriatr. 2017;17:112. doi: 10.1186/s12877-017-0500-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Assis GG, de Almondes KM. Exercise-dependent BDNF as a modulatory factor for the executive processing of individuals in course of cognitive decline. A systematic review. Front Psychol. 2017;8:584. doi: 10.3389/fpsyg.2017.00584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang L, Zhao Z, Zheng L, Xue L, Zhan Q, Song Y. Downregulation of miR-503 Promotes ESCC cell proliferation, migration, and invasion by targeting cyclin D1. Genomics Proteomics Bioinformatics. 2017;15:208–217. doi: 10.1016/j.gpb.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee A, Papangeli I, Park Y, Jeong HN, Choi J, Kang H, Jo HN, Kim J, Chun HJ. A PPARgamma-dependent miR-424/503-CD40 axis regulates inflammation mediated angiogenesis. Sci Rep. 2017;7:2528. doi: 10.1038/s41598-017-02852-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun Y, Xu J, Xu L, Zhang J, Chan K, Pan X, Li G. MiR-503 promotes bone formation in distraction osteogenesis through suppressing Smurf1 expression. Sci Rep. 2017;7:409. doi: 10.1038/s41598-017-00466-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu H, Song Z, Liao D, Zhang T, Liu F, Zheng W, Luo K, Yang L. miR-503 inhibits cell proliferation and invasion in glioma by targeting L1CAM. Int J Clin Exp Med. 2015;8:18441–18447. [PMC free article] [PubMed] [Google Scholar]
- 34.Caporali A, Meloni M, Nailor A, Mitić T, Shantikumar S, Riu F, Sala-Newby GB, Rose L, Besnier M, Katare R, Voellenkle C, Verkade P, Martelli F, Madeddu P, Emanueli C. p75(NTR)-dependent activation of NF-κB regulates microRNA-503 transcription and pericyte-endothelial crosstalk in diabetes after limb ischaemia. Nat Commun. 2015;6:8024. doi: 10.1038/ncomms9024. [DOI] [PMC free article] [PubMed] [Google Scholar]