Abstract
As the major reason for limb dysfunction, osteoarthritis (OA) is closely correlated with the level of cellular autophagy. PI3K/Akt is a classical signaling pathway which regulates autophagy, but with unclear roles in OA related cartilage injury. Studying PI3K/Akt induced autophagy in rat knee joint cartilage injury and possible functional mechanism is of critical importance for clinical treatment. This study established a rat knee joint cartilage injury model, in which Akt agonist IGF-1 or autophagy inducer Rapamycin was administrated. Western blot was used to detect the expression level of AKT, phosphorylated AKT, Beclin-1 and LC3-II/I. Formation of autophagosome and lysosome was assessed by transmission electron microscopy. HE, safranin O and toluidine blue staining was used to evaluate cartilage injury. TUNEL staining was performed to measure cell apoptosis. Real-time quantitative PCR measured the expression of cartilage injury indexes such as Aggrecan, Collagen II and MMP13. Compared with normal group, iodacetic acid treatment group showed cartilage injury, whereas AKT activation and autophagy induction groups had significant improvement. mRNA analysis showed enhanced degradation of Aggrecan and Collagen II in AKT activation and autophagy groups with decreased MMP13 mRNA level (P<0.05). Western blot results showed that after AKT activation and autophagy induction, protein levels of Beclin-1 and LC3-II/I were remarkably elevated (P<0.05). TUNEL assay showed significant inhibition of cell apoptosis. In conclusion, activation of PI3K/Akt signal pathway can improve iodacetic acid induced rat knee joint cartilage injury through inducing cell autophagy.
Keywords: Osteoarthritis cartilage injury, PI3K/Akt signaling pathway, cell autophagy
Introduction
Osteoarthritis (OA) is the most common joint disease, and can cause irreversible cartilage damage [1]. OA is mainly manifested as joint pain or dysfunction, and is an important factor contributing to limb dysfunction, with 53% rate causing immobility. It is now estimated that about 1 million people are affected by OA worldwide [2]. Various risk factors such as genetics, aging, obesity and joint deformation may be related with OA occurrence and progression [3,4]. Knee joint replacement surgery may relieve pains, and the improvement of joint motility is the most effective way for OA treatment [5].
Autophagy is a highly conserved cellular behavior and mainly participates in the clearance of damaged organelles to re-cycle or re-use large biological molecules within cells to maintain their homeostasis as well as facilitate cell survival [6]. Recent study demonstrated the existence of autophagy in various body tissues, and its critical protective role in the progression of various diseases [7,8]. Cartilage is a specialized mesenchymal tissue, and study showed the co-current occurrence of suppressed cartilage autophagy level in the course of neurodegenerative disease. Meanwhile, cell apoptosis also plays a crucial role in cartilage degeneration, and elevated autophagy level can inhibit cell apoptosis [9,10].
Apoptosis of chondrocytes is an initiating and driving factor for various diseases such as intervertebral disc herniation or OA. Therefore, inhibition of cell apoptosis may facilitate cartilage repair and relive symptoms. The elevation of autophagy level is an important mechanism in inhibiting the initiation of cell apoptosis. Current study showed the high correlation between cellular autophagy level and OA condition. Akt is a serine/threonine protein kinase and is recruited to plasma membrane after activation by PI3K, and plays important roles in regulating cell growth and apoptosis. Recent study has shown its important roles in mediating cell autophagy. PI3K/Akt signal pathway is a classical signaling pathway to mediate cell autophagy [11], although its role in OA related cartilage injury is still inconclusive. Therefore, investigation of the role of PI3K/Akt signal pathway induced cell autophagy in rat knee joint cartilage injury along with related mechanisms are critical importance for clinical treatment.
Therefore, we generated an iodacetic acid induced OA cartilage injury model, in which autophagy inducer Rapamycin or Akt agonist IGF-1 was administrated to up-regulate autophagy level or to activate PI3K/Akt singaling pathway, respectively. Cellular autophagy level and apoptosis, along with cartilage injury condition were examined, to investigate the protective role of PI3K/Akt induced cell autophagy against OA related cartilage injury.
Materials and methods
Major materials and reagents
Iodacetic acid, Rapamycin and IGF-1 were purchased from Sigma (US). Real-time quantitative PCR kit was purchased from Quanshijin Biotech (China). Antibody against Akt/phospho-Akt, Beclin-1 and LC3-II/I were purchased from Biovision (US). HRP conjugated goat anti-rabbit secondary antibody was purchased from Zhongshan Jinqiao (China). Eosin, hematoxylin, safranin O and toluidine blue staining dyes were purchased from Qiwu Biotech (China). PCR specific primers were provided by Toyobo (China).
Experimental animals
Male SD rats (2 month age) were provided by Laboratory Animal Center, Guangzhou Medical University (Guangdong China). All experimental procedures involving animals were approved by the ethic committee of the Guangzhou Medical University.
Animal grouping and generation of knee joint OA
All experimental animals were divided into four groups: normal control group, iodacetic acid treatment group, iodacetic acid + IGF-1 group, and iodacetic acid + Rapamycin group. All rats were fasted 12 h before surgery. After weighting, rats were anesthetized with 3% pentobarbital sodium (at 30 mg/kg) and fixed in supine position. Knee OA model was prepared as previously described [12]. In brief, 100 μL (amg) iodacetic acid solution was injected into knee joint cavity of right hind-limb, and normal control group received equal volume of saline. After recovery, rats were housed in the home cage. IGF-1 (10 mg/kg) or Rapamycin (10 mg/kg) was administrated via tail venous injection since second day post-surgery. Equal volume of saline was applied in the model group.
Sample collection
2 weeks after drug delivery, all rats were sacrificed and knee joint cartilage was immediately removed for imaging under stereoscope. After frozen in liquid nitrogen, tissues were kept in -80°C for further analysis.
Western blot
100 mg cartilage tissues were homogenized in liquid nitrogen, and mixed with 1 mL RIPA lysis buffer (Beyotime, China) for 15 min incubation. The lysate was centrifuged at 12000 g for 10 min at 4°C, and the supernatant was collected followed by quantification of the protein concentration by BCA method. Protein samples across groups were adjusted to equal concentrations by adding saline, and then mixed with 2X loading buffer for 5 min boiling to denature the protein. 20 μL samples was loaded and separated by SDS-PAGE, and transferred to PVDF membrane, which was then blocked with 5% defatted milk powder for 2 h at room temperature. The membrane was incubated with rabbit anti-Akt or phosphorylated Akt (1:1000), or Beclin-1 and LC3-II/I (1:1000) for 4°C overnight incubation. On the second day, the membrane was rinsed for three times in TTBS (10 min each), HRP-conjugated goat anti-rabbit secondary antibody (1:1000) was added for 2 h room temperature incubation. After 3 times of TTBS rinsing (10 min each), ECL approach was employed for development. Statistical analysis was performed for fluorescent intensity.
Real-time quantitative PCR
Cartilage tissues from all groups were homogenized in liquid nitrogen, and 100 mg cartilage tissues were mixed with 1 mL lysis buffer for iced incubation for 5 min. All lysates were transferred into new tubes followed by addition of 200 μL chloroform, 15 s vigorous shaking and 3 min room temperature incubation. The mixture was then centrifuged at 4°C for 15 min at 12000 g. The upper aqueous phase was carefully collected and mixed with 500 μL isopropanol for 10 min room temperature incubation. The mixture was again centrifuged at 12000 g for 10 min at 4°C. The supernatant was discarded and the precipitation was rinsed with 1 mL ethanol. The supernatant was carefully removed and 20 μL DEPC water was added to dissolve the RNA.
Primers for Aggrecan, Collagen II and MMP-13 and β-actin were synthesized as previously described [13-15] by Toyobo Bio (China) as shown in Table 1. PCR was performed in a 50 μL system following the manual instructions. Reaction protocols were: 50°C pre-heating for 30 min and 95°C pre-denature for 5min, followed by 40 cycles each containing 95°C 30 s, 55°C 30 s and 72°C 50 s, and ended with 72°C for 5 min. After reaction, real-time PCR was used to confirm the amplification curve and resolving curve to compare relative expression level of target genes by comparing their Ct values with internal reference gene using 2-ΔΔCt method, with normal group defined as 1.
Table 1.
Primer sequence for Aggrecan, Collagen II, MMP13 and β-actin
| Gene name | Primer sequence |
|---|---|
| Aggrecan | Forward: 5’-CTAGAGATCAGTGGACTGCCT-3’ |
| Reverse: 5’-TCTGGAGCTGTGCAGTCTAGTGG-3’ | |
| Collagen II | Forward: 5’-CAACATCACCTATTGGATCC-3’ |
| Reverse: 5’-TGGGTGTAGAGTCTCTCGCT-3’ | |
| MMP13 | Forward: 5’-CAACATCACCTATTGGATCC-3’ |
| Reverse: 5’-TGGGTGTAGAGTCTCTCGCT-3’ | |
| β-actin | Forward: 5’-CGCGAGAAGATGACCCAGAT-3’ |
| Reverse: 5’-GCACTGTGTTGGCGTACAGG-3’ |
TUNEL staining
Rat cartilage tissues were fixed in paraformaldehyde and prepared in paraffin sections. 3% H2O2-methanol solution was used for 10 min rinsing. The slices were then incubated with 0.2% Triton for 5 min, followed by two times of PBS rinsing. Following the manual instructions, 50 μL TUNEL reaction mixture was added into each tissue slice, followed by 37°C dark incubation. After three times of PBS rinsing, images were taken under a fluorescent microscope.
HE, safranin O and toluidine blue staining
Freshly prepared cartilage tissues were fixed in 4% paraformaldehyde for 24 h, and embedded in paraffin for sectioning. Before staining, slices were de-waxed and treated with HE, safranin O or toluidine blue. After PBS rinsing, dehydration in gradient ethanol and xylene treatment was performed. After that, slices were mounted for observation.
Transmission electron microscopy
Freshly prepared cartilage tissues were fixed in 1.6% glutaraldehyde for 2 h, and rinsed in PBS for three times. Tissues were then re-fixed in 2% OsO4, and were embedded into Epon resin. Under transmission electron microscope, ultrathin slices (80 nm thickness) stained with 0.1% citrate lead and 10% uranium acetate.
Statistical methods
SPSS19.0 was used for statistical analysis. Each data was obtained from at least three independent replicates. Measurement data were presented as mean ± standard deviation (SD), and between-group comparison was performed by student t-test. Multi-group comparison was performed by one-way analysis of variance (ANOVA), and paired comparison was carried out in Dunneet test. A statistical significance was defined when P<0.05.
Results
AKT and ATK phosphorylation level
Western blot was employed to test tissue Akt and phosphorylated Akt levels. As shown in Figure 1, compared with normal group, iodacetic acid group rats showed significantly decreased phosphorylated Akt level in cartilage. After IGF-1 or Rapamycin treatment, phosphor-Akt expression level was significantly elevated (P<0.05 compared with model group). These results showed the inhibition of PI3K/Akt signal pathway in iodacetic acid model group, which can be activated by IGF-1 or Rapamycin.
Figure 1.
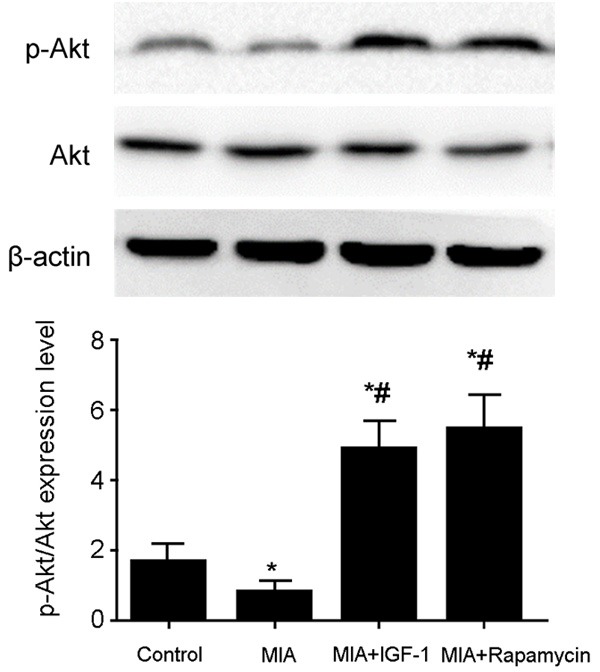
Akt expression level in cartilage tissues. Total protein was extracted from cartilage tissues followed by measuring Akt expression level by western blot. The phosphorylation level of Akt was quantified as a ratio of its phosphorylation relative to total level. Compared with normal group, MIA rats showed significantly decreased phosphorylated Akt level, which was significantly elevated after IGF-1 or Rapamycin treatment. *, P<0.05 compared to normal group; #, P<0.05 compared to model group.
Expression of autophagy related proteins Beclin-1 and LC3-II/I
Beclin-1 and LC3-II/I are representative proteins for mediating cell autophagy. Western blot was used to test tissue expression of Beclin-1 and LC3-II/I. As shown in Figure 2, compared with normal group, Beclin-1 and LC3-II/I expression levels were significantly lower in iodiacetic model. After IGF-1 or Rapamycin treatment, expression of these proteins was significantly increased with statistical difference (P<0.05). These results indicated that IGF-1 or Rapamycin treatment significantly up-regulated autophagy level in cartilage tissues.
Figure 2.
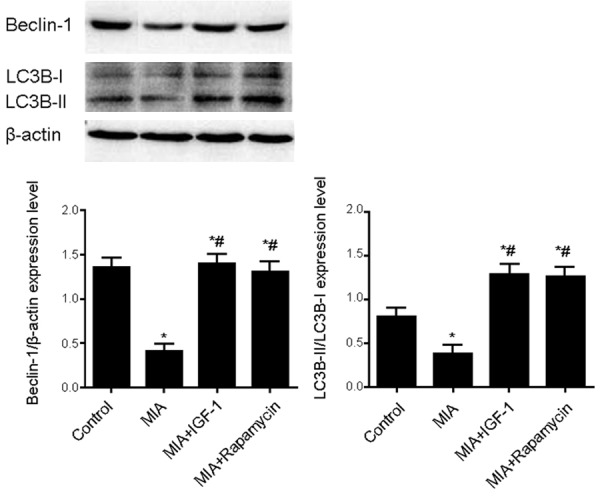
Beclin-1 and LC3-II/I expression level in cartilage tissues. Total protein was extracted from cartilage tissues for western blot analysis of the expression of Beclin-1 and LC3-II/I. The expression level was quantified as a ratio relative to β-actin. Compared with normal group, MIA group displayed significantly lower Beclin-1 and LC3-II/I expression, which were significantly increased after IGF-1 or Rapamycin treatment. *, P<0.05 compared to normal group; #, P<0.05 compared to model group.
General observation of cartilage tissues
Cartilage samples were extracted and observed for surface features of joint tissues under stereoscope. As shown in Figure 3, iodacetic acid group showed granule on knee joint cartilage with severe damage. IGF-1 or Rapamycin treatment improved cartilage injury to certain extents. These data showed that initiation of cell autophagy or activation of Akt signal pathway has protective effects on cartilage injury.
Figure 3.
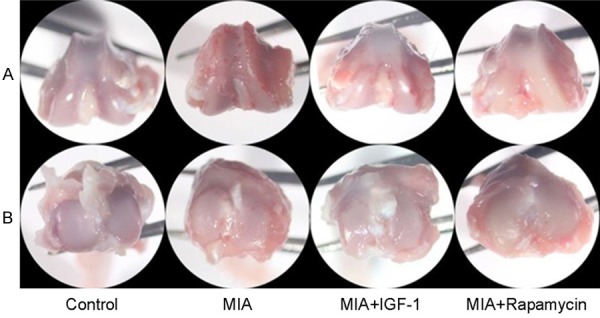
General observation of rat cartilage tissues from all groups. A. Lower section of femur; B. Upper section of tibia. MIA group showed granule on knee joint cartilage with severe damage. IGF-1 or Rapamycin treatment improved cartilage injury to certain extents.
HE, safranin O and toluidine blue staining for cartilage injury
Severity of chondrocyte lesion was observed by staining approach. As shown in Figure 4, control group had smooth and intact cartilage tissues, with good status in extracellular matrix (ECM). Chondrocytes were well-distributed in ECM. In iodacetic acid induced OA group, cartilage showed granules and irregularity on surface, with decreased chondrocyte number and chromosome loss. Administration of IGF-1 or Rapamycin alleviated such abnormalities in cartilage and improved these pathological conditions. These results indicated that cell autophagy and Akt signal pathway are involved in the pathological changes of cartilage morphology.
Figure 4.
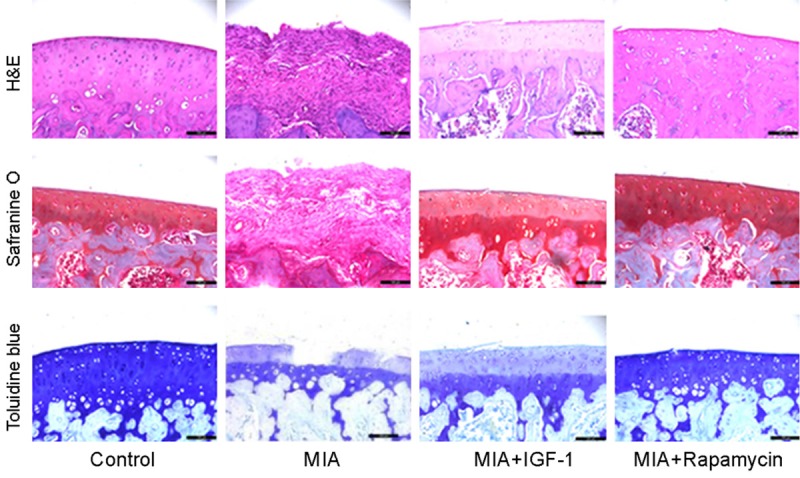
HE, safranin O and toluidine blue staining. Freshly prepared cartilage tissues were fixed and embedded in paraffin for sectioning followed by being de-waxed and treated with HE, safranin O or toluidine blue. Control group had smooth and intact cartilage tissues, with good status in ECM. In MIA group, cartilage showed granules and irregularity on surface, with decreased chondrocyte number and chromosome loss. Administration of IGF-1 or Rapamycin alleviated such abnormalities in cartilage and improved these pathological conditions.
Apoptosis of cartilage tissues
TUNEL staining was performed to observe apoptosis of chondrocytes. As shown in Figure 5, compared with normal group, iodacetic acid treated rats had significantly elevated number of TUNEL-positive (green fluorescent) cells. However, adminstration of IGF-1 or Rapamycin decreased TUNEL-positive cell number, suggesting that IGF-1 or Rapamycin could decrease iodacetic acid induced chondrocyte apoptosis.
Figure 5.
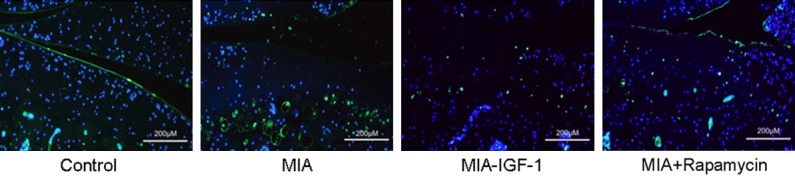
TUNEL assay for cartilage tissues. Cartilage tissues were fixed and prepared in paraffin sections followed by incubation with 0.2% Triton for 5 min. After that, 50 μL TUNEL reaction mixture was added and incubated at 37°C under dark followed by taking images under a fluorescent microscope. Blue, TUNEL-positive signal; Blue, DAPI. Compared with normal group, MIA rats had significantly elevated number of TUNEL-positive (green fluorescent) cells. However, adminstration of IGF-1 or Rapamycin decreased TUNEL-positive cell number.
Expression of cartilage injury indexes Aggrecan, Collagen II and MMP13
Real-time quantitative PCR was used to measure mRNA expression level of MMP13, Aggrecan and collagen II in cartilage tissues. As shown in Figure 6, compared with normal control group, iodacetic acid treated rats had significantly higher MMP-13, and lower Aggrecan or collagen II levels. However, opposite changes of these markers were observed after IGF-1 or Rapamycin treatment, indicating that facilitation of cell autophagy or activation of Akt signal pathway could protect cartilage injury.
Figure 6.
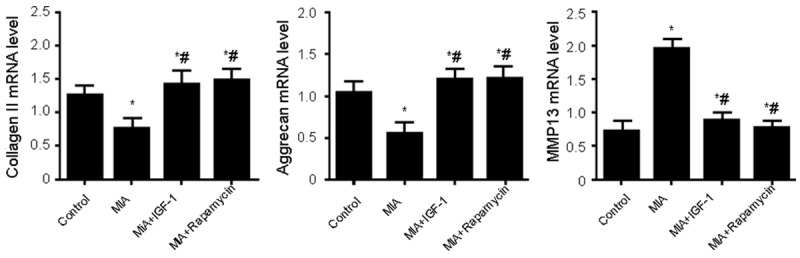
mRNA expression of cartilage injury index proteins Aggrecan, Collagen II and MMP13. Total RNA was extracted from cartilage tissues for measuring the mRNA expression of Aggrecan, Collagen II and MMP13 by real-time quantitative PCR. *, P<0.05 compared to normal group; #, P<0.05 compared to model group. Compared with normal control group, MIA rats had significantly higher MMP-13, and lower Aggrecan or collagen II levels. However, opposite changes of these markers were observed after IGF-1 or Rapamycin treatment.
Autophagy level of cartilage
Transmission electron microscopy observed autophagy levels in chondrocytes. As shown in Figure 7, asterisk indicated nucleus and black arrowhead presented autophagosome or autophago-lysosome. We observed relatively fewer autophagosome in normal control group, whereas, barely any autophagosome could be observed in normal iodacetic acid group. IGF-1 or Rapamycin treatment increased the number of autophagosome and autophagy, indicating possible correlation between IGF-1 or Rapamycin-induced cartilage injury suppression and up-regulated autophagy levels.
Figure 7.
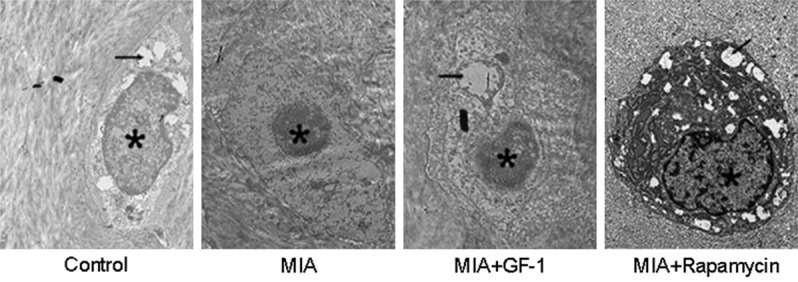
Autophagy level of cartilage tissues. Freshly prepared cartilage tissues were fixed and rinsed in PBS for three times. Tissues were then re-fixed in 2% OsO4, and embedded into Epon resin. Under transmission electron microscope, ultrathin slices (80 nm thickness) stained with 0.1% citrate lead and 10% uranium acetate. Asterisk indicated nucleus and black arrowhead presented autophagosome or autophago-lysosome.
Discussion
OA is the most common joint disorder and lacks effective treatment approaches except joint replacement, which, however, cannot resolve the issue of joint motility but does not improve cartilage destruction or related inflammation. Therefore during the inhibition of OA progression, the protection of cartilage injury is critical [16]. Various studies showed that cartilage destruction was mainly caused by chondrocyte apoptosis and ECM degradation [17].
Autophagy is a highly conserved cellular activity, and plays important roles in OA induced cartilage injury [18]. Early study showed inhibited autophagy level in OA injury cartilage, which was consistent with our results [19]. More evidences were consistent with our study, indicating that autophagy induction in rat chondrocytes with late stage glycosylation end-products could suppress MMP-3 and MMP-13 expression and eventually inhibit chondrocyte apoptosis [20]. Currently it is believed that acceleration of cellular autophagy had protective roles against OA with unknown mechanisms. PI3K/Akt signal pathway is a classical pathway for mediating cell autophagy [21]. Therefore in this study, we aimed to investigate the protective role of PI3K/Akt signal pathway in OA-related cartilage injury.
In this study, we used intra-joint cavity injection of iodacetic to generate OA cartilage injury model. Autophagy inducer Rapamycin or Akt agonist were administrated [22,23] to observe the protective effects of PI3K/Akt signal pathway induced cell autophagy on OA cartilage injury. Our study also showed suppressed Akt signal pathway in cartilage injury model. After treated with autophagy inducor Rapamycin or Akt agonist, chondrocyte autophagy level was elevated, accompanied with lower expression of inflammation and collagen. General observation and pathological staining showed decreased cartilage injury level. All these results showed that activation of PI3K/Akt signal pathway could protect against OA induced cartilage injury via up-regulating autophagy level of chondrocytes.
This study provided the first piece of evidence showing protective function of PI3K/Akt signal pathway against cartilage injury. However, as only recombinant protein agonist was used, effectiveness or specific function cannot be completely guaranteed. Therefore cell culture was introduced for further study using siRNA or shRNA targeting autophagy targets. In addition, this study only investigated the protective role after PI3K/Akt signal pathway activation. Moreover, whether other autophagy inhibitory signal pathways such as mTOR participate in the regulation of autophagy is unclear.
In a word, autophagy may form one self-protection mechanism of chondrocytes. In iodacetic induced rat knee joint cartilage injury model, autophagy was negatively correlated with the degree of cartilage injury. In cartilage with injury, elevation of autophagy level may work as a novel approach for cartilage protection.
Conclusion
Activation of Akt can promote cell autophagy, improve cartilage injury and related index. The activation of PI3K/Akt signal pathway can induce autophagy to improve knee joint cartilage injury which is caused by iodiacetic acid.
Acknowledgements
This work was supported by Department of Science and Technology of Guangdong Province (2017ZC0267); Ministry of Science and Technology in Guangzhou, Liwan District (NO. 201704048); Department of Science and Technology of Guangdong Province (2016ZC0147); Bureau of science and technology and information technology, Liwan district, Guangzhou city (20151217094); Department of Science and Technology of Guangdong Province (201511013).
Disclosure of conflict of interest
None.
References
- 1.Sacks JJ, Luo YH, Helmick CG. Prevalence of specific types of arthritis and other rheumatic conditions in the ambulatory health care system in the United States, 2001-2005. Arthritis Care Res (Hoboken) 2010;62:460–464. doi: 10.1002/acr.20041. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia D, Bejarano T, Novo M. Current interventions in the management of knee osteoarthritis. J Pharm Bioallied Sci. 2013;5:30–38. doi: 10.4103/0975-7406.106561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li X, Feng K, Li J, Yu D, Fan Q, Tang T, Yao X, Wang X. Curcumin inhibits apoptosis of chondrocytes through activation ERK1/2 signaling pathways induced autophagy. Nutrients. 2017;9 doi: 10.3390/nu9040414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao Z, Wang S, Wang J. Peroxiredoxin 4 inhibits IL-1beta-induced chondrocyte apoptosis via PI3K/AKT signaling. Biomed Pharmacother. 2017;90:414–420. doi: 10.1016/j.biopha.2017.03.075. [DOI] [PubMed] [Google Scholar]
- 5.Zhai X, Meng R, Li H, Li J, Jing L, Qin L, Gao Y. miR-181a modulates chondrocyte apoptosis by targeting glycerol-3-phosphate dehydrogenase 1-like protein (GPD1L) in osteoarthritis. Med Sci Monit. 2017;23:1224–1231. doi: 10.12659/MSM.899228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Y, Zhuang J, Zhang X, Yue C, Zhu N, Yang L, Wang Y, Chen T, Wang Y, Zhang LW. Autophagy associated cytotoxicity and cellular uptake mechanisms of bismuth nanoparticles in human kidney cells. Toxicol Lett. 2017;275:39–48. doi: 10.1016/j.toxlet.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Cong L, Zhu Y, Tu G. A bioinformatic analysis of microRNAs role in osteoarthritis. Osteoarthritis Cartilage. 2017;25:1362–1371. doi: 10.1016/j.joca.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Kim EK, Kwon JE, Lee SY, Lee EJ, Kim DS, Moon SJ, Lee J, Kwok SK, Park SH, Cho ML. IL-17-mediated mitochondrial dysfunction impairs apoptosis in rheumatoid arthritis synovial fibroblasts through activation of autophagy. Cell Death Dis. 2017;8:e2565. doi: 10.1038/cddis.2016.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baudi P, Catani F, Rebuzzi M, Ferretti M, Smargiassi A, Campochiaro G, Serafini F, Palumbo C. Morphological study: ultrastructural aspects of articular cartilage and subchondral bone in patients affected by post-traumatic shoulder instability. Anat Rec (Hoboken) 2017;300:1208–1218. doi: 10.1002/ar.23529. [DOI] [PubMed] [Google Scholar]
- 10.Shen T, Alvarez-Garcia O, Li Y, Olmer M, Lotz MK. Suppression of Sestrins in aging and osteoarthritic cartilage: dysfunction of an important stress defense mechanism. Osteoarthritis Cartilage. 2017;25:287–296. doi: 10.1016/j.joca.2016.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhong JT, Yu J, Wang HJ, Shi Y, Zhao TS, He BX, Qiao B, Feng ZW. Effects of endoplasmic reticulum stress on the autophagy, apoptosis, and chemotherapy resistance of human breast cancer cells by regulating the PI3K/AKT/mTOR signaling pathway. Tumour Biol. 2017;39:1010428317697562. doi: 10.1177/1010428317697562. [DOI] [PubMed] [Google Scholar]
- 12.Choi HS, Im S, Park JW, Suh HJ. Protective effect of deer bone oil on cartilage destruction in rats with monosodium iodoacetate (MIA)-induced osteoarthritis. Biol Pharm Bull. 2016;39:2042–2051. doi: 10.1248/bpb.b16-00565. [DOI] [PubMed] [Google Scholar]
- 13.Lauing KL, Cortes M, Domowicz MS, Henry JG, Baria AT, Schwartz NB. Aggrecan is required for growth plate cytoarchitecture and differentiation. Dev Biol. 2014;396:224–236. doi: 10.1016/j.ydbio.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hass V, de Paula AM, Parreiras S, Gutierrez MF, Luque-Martinez I, de Paris Matos T, Bandeca MC, Loguercio AD, Yao X, Wang Y, Reis A. Degradation of dentin-bonded interfaces treated with collagen cross-linking agents in a cariogenic oral environment: an in situ study. J Dent. 2016;49:60–67. doi: 10.1016/j.jdent.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 15.Chen D, Li Y, Dai X, Zhou X, Tian W, Zhou Y, Zou X, Zhang C. 1,25-Dihydroxyvitamin D3 activates MMP13 gene expression in chondrocytes through p38 MARK pathway. Int J Biol Sci. 2013;9:649–655. doi: 10.7150/ijbs.6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith TO, Dainty JR, MacGregor AJ. Changes in social isolation and loneliness following total hip and knee arthroplasty: longitudinal analysis of the English longitudinal study of ageing (ELSA) cohort. Osteoarthritis Cartilage. 2017;25:1414–1419. doi: 10.1016/j.joca.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Hua B, Olsen EHN, Sun S, Gudme CN, Wang L, Vandahl B, Roepstorff K, Kjelgaard-Hansen M, Sorensen BB, Zhao Y, Karsdal MA, Manon-Jensen T. Serological biomarkers detect active joint destruction and inflammation in patients with haemophilic arthropathy. Haemophilia. 2017;23:e294–e300. doi: 10.1111/hae.13196. [DOI] [PubMed] [Google Scholar]
- 18.Alvarez-Garcia O, Matsuzaki T, Olmer M, Plate L, Kelly JW, Lotz MK. Regulated in Development and DNA damage response 1 deficiency impairs autophagy and mitochondrial biogenesis in articular cartilage and increases the severity of experimental osteoarthritis. Arthritis Rheumatol. 2017;69:1418–1428. doi: 10.1002/art.40104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xue JF, Shi ZM, Zou J, Li XL. Inhibition of PI3K/AKT/mTOR signaling pathway promotes autophagy of articular chondrocytes and attenuates inflammatory response in rats with osteoarthritis. Biomed Pharmacother. 2017;89:1252–1261. doi: 10.1016/j.biopha.2017.01.130. [DOI] [PubMed] [Google Scholar]
- 20.Huang W, Ao P, Li J, Wu T, Xu L, Deng Z, Chen W, Yin C, Cheng X. Autophagy protects advanced glycation end product-induced apoptosis and expression of MMP-3 and MMP-13 in rat chondrocytes. Biomed Res Int. 2017;2017:6341919. doi: 10.1155/2017/6341919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao T, Guo W, Chen M, Huang J, Yuan Z, Zhang Y, Wang M, Li P, Peng J, Wang A, Wang Y, Sui X, Shang L, Xu W, Lu S, Zahng X, Liu S, Guo Q. Extracellular vesicles and autophagy in osteoarthritis. Biomed Res Int. 2016;2016:2428915. doi: 10.1155/2016/2428915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwata T, Kuribayashi K, Nakasono M, Saito-Tarashima N, Minakawa N, Mizusawa N, Kido R, Yoshimoto K. The AMPK/mTOR pathway is involved in D-dopachrome tautomerase gene transcription in adipocytes differentiated from SGBS cells, a human preadipocyte cell line. Cytokine. 2017;96:195–202. doi: 10.1016/j.cyto.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 23.Bell TM, Espina V, Senina S, Woodson C, Brahms A, Carey B, Lin SC, Lundberg L, Pinkham C, Baer A, Mueller C, Chlipala EA, Sharman F, de la Fuente C, Liotta L, Kehn-Hall K. Rapamycin modulation of p70 S6 kinase signaling inhibits rift valley fever virus pathogenesis. Antiviral Res. 2017;143:162–175. doi: 10.1016/j.antiviral.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]


