Abstract
Objectives: Excess estrogen states, such as those generated by obesity, have long been associated with the development of type I endometrial cancers. Epidemiological studies have linked consumption of isoflavones with a decreased incidence of endometrial malignancy. Thus, our goal was to assess the effect of the isoflavones, novasoy and genistein, on cell proliferation, cell cycle, apoptosis, progesterone receptor (PR) and estrogen receptor-alpha (ERα) expression and the AKT/mTOR and MAPK pathways in endometrial cancer cells. Methods: The endometrial cancer cell lines ECC-1 and RL-95-2 were used. Cell proliferation was assessed with MTT assay after exposure to novasoy and genistein at varying concentrations. Cell cycle progression was analyzed by flow cytometry. Apoptosis was assessed by flow cytometery for annexin V expression and ELISA for caspase-3 activity. Expression of ERα, PR and hTERT mRNA were evaluated using real time RT-PCR. Western immunoblotting was performed to evaluate the effects of novasoy and genistein on the AKT/mTOR and MAPK signaling pathways. Results: Novasoy and genistein inhibited cell growth in a dose-dependent manner in both cell lines through induction of cell cycle G2 arrest and apoptosis. Treatment with novasoy and genistein decreased hTERT expression in a dose-dependent manner. Genistein decreased ERα mRNA expression while increasing PR expression. Genistein induced phosphorylation of p42/44 in a dose dependent manner in both cell lines but reduced phosphorylation of S6 in only the RL-95-2 cells. Conclusions: Novasoy and genistein inhibited cell proliferation through varying pathways in different cell lines but included decreased ERα expression and subsequent alteration in the expression of proteins upstream and downstream of the AKT/mTOR and MAPK pathways. Thus, isoflavones may be a promising therapeutic agent in the treatment and prevention of endometrial cancer.
Keywords: Endometrial cancer, genistein, novasoy, mTOR pathway, MAPK pathway, estrogen receptor
Introduction
Endometrial cancer is the fourth most common cancer among women, with an estimated 61,380 new cases diagnosed in the United States in 2017 [1]. The incidence of this disease is on the rise, with diabetes and obesity being major contributing factors [2,3]. Among all cancers, increasing body mass index is most strongly associated with endometrial cancer risk, with >50% of all endometrial cancers attributable to obesity [4]. Furthermore, endometrial cancer patients who are obese have a poorer prognosis and an increased risk of death [3,4]. The mechanism by which obesity induces endometrial carcinogenesis is thought to result from the induction of high levels of circulating insulin and estrogen, leading to activation of multiple cell signaling pathways including the AKT/mTOR and MAPK pathways in the endometrial epithelium. Elevated estrogen levels, when not opposed by sufficient progesterone, increase a woman’s risk of developing type I (endometriod) endometrial cancer, the most prevalent histological subtype of this disease [5,6]. Although women diagnosed with early stage endometrial cancers do well when treated with surgery +/- radiation, the management of advanced stage and recurrent endometrial cancer remains controversial, as current treatments have yielded little improvements in long-term survival rates [7,8]. In an effort to improve outcomes, it is important to explore new adjuvant therapies for this highly obesity-driven cancer.
Phytoestrogens are plant-derived compounds that resemble the human reproductive hormone estrogen chemically and have been shown to bind ER in vitro [9]. Soybeans and soy products are rich in several phytoestrogens, with the isoflavone genistein being the major component in these products [10]. In recent years, epidemiologic evidence has shown that populations consuming diets rich in soy products have lower incidence rates of several estrogen-related cancers, including breast and endometrial cancer [11,12]. In vitro studies have demonstrated chemotherapeutic activity of genistein in ovarian, breast, colorectal and prostate cancers through various mechanisms including induction of apoptosis, G2/M cell cycle arrest and down-regulation of the Akt/mTOR pathway [13-19]. Furthermore, early phase 1 clinical trials evaluating genistein in the treatment of prostate cancer have been promising, demonstrating patterns of decreased metastasis [20]. However, the biological activity of soy products in endometrial cancer cells remains unclear, as genistein has been shown to exert diverse biological effects. At low concentrations, genistein has been reported to interact with the ER, while at high concentrations genistein has been shown to act as a tyrosine kinase inhibitor [21,22]. Similarly confounding results have been reported for studies involving endometrial cancer cells in vitro, with some studies reporting that genistein acts as a survival factor contributing to tumor progression, while others suggesting that genistein inhibits cellular proliferation and induces apoptosis [23-25]. Clearly, further research investigating the effects of genistein in endometrial cancer cells is warranted. Thus, the aim of this study was to investigate the effects of the isoflavones novasoy and genistein on endometrial cancer cells and to understand their potential underlying molecular mechanisms of action.
Materials and methods
Cell culture and reagents
Two endometrial cancer cell lines, ECC-1 and RL-95-2, were used for all experiments. The ECC-1 cells were grown in RPMI 1640 medium supplemented with 5% fetal bovine serum, 100 units/ml penicillin and 100 ug/ml streptomycin under 5% CO2. The RL-95-2 cells were grown in RPMI 1640 supplemented with 10% fetal bovine serum, 300 mM l-glutamine, 10,000 U/ml penicillin and 10,000 μg/ml streptomycin under 5% CO2. Novasoy was purchased from ADM (Vista, CA). Genistein, RNase A and RIPA buffer were purchased from Sigma (St. Louis, MO). Anti-phosphorylated p-Akt (Ser473), p-Akt (Thr308), p-S6 (Ser 235/236), eIf4E and the Caspase-3 Activity Assay Kit were obtained from Cell Signaling Technology (Beverly, MA). The Annexin V FITC Kit was purchased from BioVision (Mountain View, CA). Enhanced chemiluminescence western blotting detection reagents were purchased from Amersham (Arlington Heights, IL). All other chemicals were purchased from Sigma.
Cell proliferation assay
The ECC-1 and RL-95-2 cells were plated and grown in 96-well plates at a concentration of 5000 cells/well for 24 hours. These cells were then treated with various concentrations of novasoy and genistein for a period of 72 hours. After the addition of MTT dye (5 mg/mL), the 96-well plates were incubated for 1-2 hours at 37°C. 100 uL of DMSO was then added to the plates in order to terminate the MTT reaction, and the plates were subsequently read by measuring absorption at 595 nm. The effect of novasoy and genistein was calculated as a percentage of control cell growth obtained from PBS (1%) treated cells grown in the same 96-well plates. Each experiment was repeated three times to assess for consistency of results.
Flow cytometry
The two endometrial cancer cell lines were plated at 2.5-3.5×105 cells/well in 6 well plates in the appropriate media for 24 hours. The cells were then treated with novasoy and genistein at varying concentrations for 24 hours. After treatment, the cells were washed twice with PBS and fixed in 90% methanol solution and then stored in a -20°C freezer until analysis. Cold PBS was used to wash the cells twice. Following this, the cells were centrifuged, suspended in 100 uL PBS and 10 uL of RNase A solution (250 ug/mL) and incubated for 30 minutes at 37°C. After incubation, 110 uL of propidium iodide (100 ug/mL) stain was added to each tube and incubated for 30 minutes at 4°C. Flow cytometric analysis was conducted using a CyAn machine (Beckman Coulter, Miami, Fl). ModFit (Verity Software House, Topsham, ME) was utilized for the analysis to control for dead cells and debris. The experiments were performed in triplicate and repeated twice to assess for consistency of response.
Annexin V assay
Annexin V was assessed using the Annexin V-FITC Apoptosis Detection Kit. The ECC-1 and RL-95-2 cells (2.5×105 per well) were plated in 6 well plates for 24 hours and then treated with varying concentrations of novasoy and genistein. The cells were then collected, washed with PBS and re-suspended in the binding buffer. 5 μL of annexin V-FITC and 5 μL of propidium iodide (50 μg/mL) were added in the binding buffer for 5 minutes in the dark. The samples were immediately measured by the CyAn flow cytometer, and the results were analyzed by Cellquest software. Apoptotic cells are expressed as a percentage of the total number of cells stained. All experiments were performed in triplicate and repeated twice to assess for consistency of response.
Real-time RT-PCR for hTERT
Analysis of the effect of novasoy and genistein on expression of the hTERT gene was conducted using real-time RT-PCR. Total RNA was extracted from both cell lines using the RNAquoes Kit (Ambion, Austin, TX) and further purified by the DNA-free Kit (Ambion). The reverse transcription and PCR reactions were performed using the TaqMan Gold One Step RT-PCR Kit in the ABI Prism 7700 Sequence Detection System (Applied Biosystems, Foster City, CA). Reverse transcription was carried out at 48°C for 30 minutes. The PCR conditions consisted of a 10 minute step at 95°C, 40 cycles at 95°C for 15 seconds each and 1 minute at 65°C. A housekeeping control gene, acidic ribosomal phosphoprotein P0 (RPLP0), was used as an internal control to correct for differences in the amount of RNA in each sample. The standard curve for hTERT was generated by using dilutions of a known amount of cRNA synthesized by in vitro transcription of a cloned fragment. The normalized level of hTERT in each sample was estimated by a ratio of the hTERT level to the RPLP0 level. Each experiment was performed in triplicate and repeated twice to assess for consistency of results.
Real-tine RT-PCR for ERα and PR
Total RNA was extracted using RNeasy Mini Kit (Qiagen, GmBH, Germany) according to the manufacturer’s instructions. Complementary DNA was synthesized from RNA using the First-Strand cDNA Synthesis Roche Kit (Indianapolis, IN). The primers and probes for ERα, PR and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were designed and synthesized by AB Applied Biosystems (Foster City, CA). The expression of ERα and PR and GAPDH level was evaluated using TaqMan Gene Expression Assays according to the manufacturer’s instructions. To quantify mRNA levels, Ct values were defined as ΔCt target gene/ΔCt GAPDH. Target gene levels relative to GAPDH levels were defined as 2-ΔCt. Each experiment was performed in triplicate and repeated 3 times to assess for consistency of results.
Caspase 3 activity assay
To evaluate the mechanism of growth inhibition by novasoy and genistein, the induction of cleaved caspase 3 was analyzed after exposure to both of these compounds. Both cell lines were cultured in 6 well plates at concentrations of 2-4×105 cells/well for 24 hours and then treated with novasoy and genistein at the indicated doses for an additional 24 hours. ELISA analysis with a Caspase-3 Activity Assay Kit was performed according to the manufacturer’s instructions. Briefly, the cells were lysed and protein concentrations measured to confirm equal loading onto the ELISA plate. Reagents were added as described by the manufacturer, and the ELISA plate was read by measuring absorption at 450 nm. All experiments were performed in triplicate and repeated twice to assess for consistency of response.
Western immunoblotting
The ECC-1 and RL-95-2 cells were plated at 2-4×105 cells/well in 6 well plates in their appropriate media and were treated for 24 hours with genistein in 0.5% stripped serum. Cell lysates were prepared in RIPA buffer (1% NP40, 0.5% sodium deoxycholate and 0.1% SDS) plus PhosStop. Equal amounts of protein were separated by gel electrophoresis and transferred onto a PVDF membrane. The membrane was blocked with 5% nonfat dry milk and then incubated with a 1:1000 dilution of primary antibody overnight at 4°C. The membrane was then washed and incubated with a secondary peroxidase conjugated antibody for 1 hour after washing. Antibody binding was detected using an enhanced chemiluminescence detection buffer by Alpha Innotech imaging system (San Leandro, CA). Each experiment was repeated three times to assess for consistency of results.
Statistical analysis
All experiments were repeated a minimum of three times. Data were presented as mean ± S.E.M. Statistical analyses of the differences between the groups were determined with the two-sided unpaired student’s t-test using GraphPad software (La Jolla, CA USA), and a value of P<0.05 was considered as significant.
Results
Novasoy and genistein inhibit cell growth in endometrial cancer cell lines
The effect of novasoy and genistein on proliferation was assessed in the endometrial cancer cell lines, ECC-1 and RL-95. Both cell lines were exposed to varying doses of novasoy and genistein for 72 hours. As shown in Figure 1A and 1B, both novasoy and genistein inhibited growth in a dose-dependent manner in both endometrial cancer cell lines. The mean IC50 value for novasoy in the ECC-1 cells was 200 mg/ml and in the RL-95-2 cells was 310 mg/ml, at 72 hours treatment. For the cells treated with genistein, the mean IC50 value was approximately 41 and 50 uM for RL-95-2 and ECC-1 cells at 72 hours, respectively. The results suggest that both novasoy and genistein effectively inhibit proliferation in endometrial cancer cells.
Figure 1.
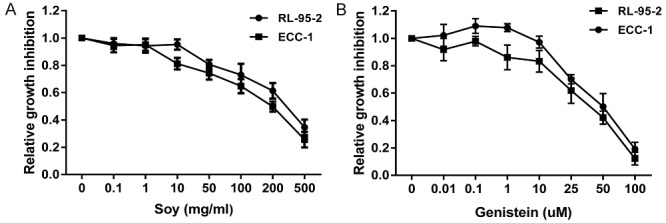
Novasoy and genistein inhibited cell proliferation in endometrial cancer cells. The ECC-1 and RL-95-2 cells were cultured for 24 hours and then treated with novasoy (A) and genistein (B) at the indicated doses for 72 hours. Cell proliferation was assessed by MTT assay. Data are presented as the mean of three independent experiments.
Novasoy and genistein induce cell cycle arrest in G2
To evaluate the underlying mechanism of growth inhibition by novasoy and genistein, the cell cycle profile was analyzed after treating the ECC-1 and RL-95-2 cells with varying doses of novasoy and genistein for 24 hours. As illustrated in Figure 2A-D, novasoy and genistein treatment resulted in G2 cell cycle arrest and reduced G1 phase in a dose-dependent manner in both the ECC-1 and RL-95-2 endometrial cancer cell lines. In the ECC-1 cells, G2 phase arrest increased from 18.9% in control to 49.56% in the cells treated with genistein at 100 uM. In the RL-95-2 cells, treatment with genistein increasd G2 phase arrest from 9.7% in control to 20.04% at a dose of 50 uM and 36.44% at a dose of 100 uM. Thus, genistein proved to be a more potent in arresting cells in G2 as compared to novasoy.
Figure 2.
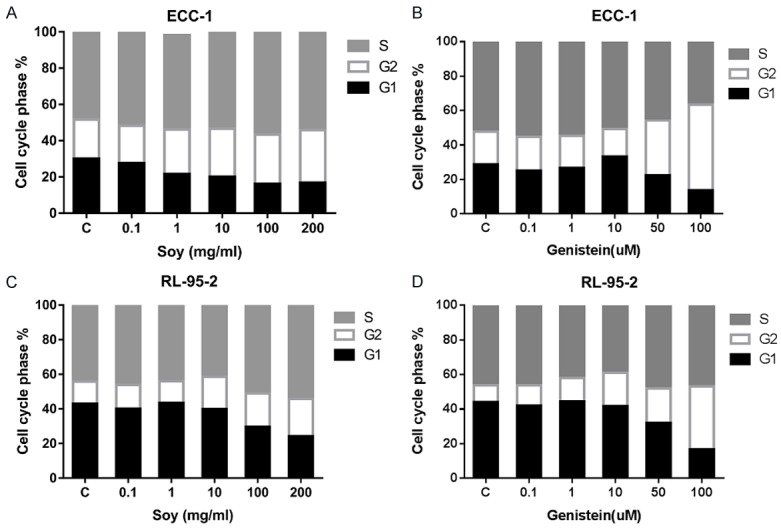
Novasoy and genistein induced cell cycle G2 arrest in endometrial cancer cells. The ECC-1 (A and B) and RL-95-2 (C and D) cells were treated with novasoy and genistein at the indicated doses for 24 hours and then analyzed for cell cycle distributions by flow cytometry.
Novasoy and genistein induce apoptosis
To determine whether the growth inhibition of endometrial cancer cells by novasoy and genistein was related to apoptosis, we evaluated the apoptotic effect of both compounds on the ECC-1 and RL-95-2 cells by Annexin-V FITC stain analysis. This assay detects the phospholipid phosphatidylserine (PS) translocated from the inner (cytoplasmic) leaflet of the cell membrane to the external surface in very early apoptotic cells. As shown in Figure 3A and 3B, after treatment of the cells with novasoy and genistein at the indicated concentrations for 24 hours, the percentage of apoptotic cells increased in a dose-dependent manner in both cell lines (P<0.01).
Figure 3.
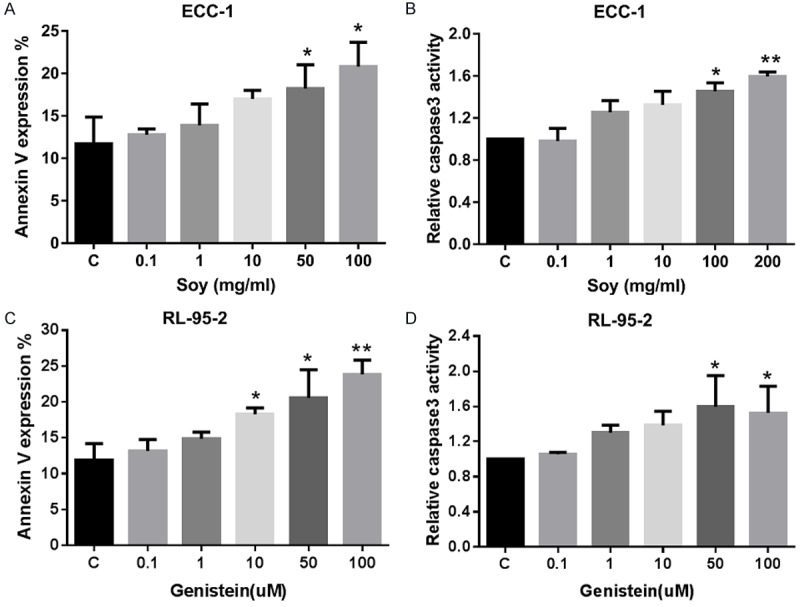
Novasoy and genistein induced apoptosis in endometrial cancer cells. The ECC-1 (A and B) and RL-95-2 (C and D) cells were treated with novasoy and genistein at the indicated doses for 24 hours and then analyzed for annexin V and PI staining by flow cytometery and caspase-3 activity by ELISA. *P<0.05 and **P<0.01.
To further analyze the effect of both compounds on the apoptotic pathway, an ELISA assay was used to detect the activity of caspase-3 in the ECC-1 and RL-95-2 cell lines treated with novasoy and genistein. Caspase-3 is a member of the caspase family, which is a cysteine protease that acts in a cascade manner to trigger apoptosis and is considered to be one of the effector caspases involved in cell disassembly [26]. We found that both novasoy and genistein increased cleaved caspase-3 activity in a dose-dependent manner after 24 hours of treatment (Figure 3C and 3D). These data suggest that both novasoy and genistein reduce proliferation, in part, through induction of apoptosis in endometrial cancer cells.
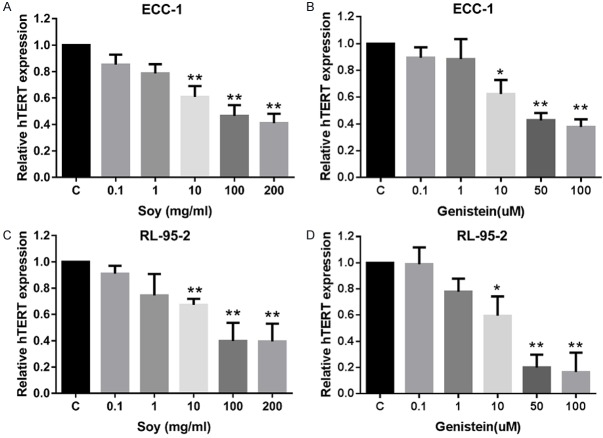
Effect of novasoy and genistein on hTERT mRNA Level
The maintenance of telomere length via the expression of telomerase is vital to the ability of cancer cells to remain proliferative [27]. hTERT expression is the rate-limiting determinant of the enzymatic activity of human telomerase [28]; and thus, real time RT-PCR was used to quantify hTERT mRNA expression in the endometrial cancer cell lines. Treatment with novasoy and genistein decreased hTERT mRNA expression in a dose-dependent manner in both cell lines within 24 hours of exposure (P<0.01). Increasing doses of both compounds produced dramatic reductions in hTERT expression for the RL-95-2 and ECC-1 cell lines (P<0.01, Figure 4A-D). This data suggest that both novasoy and genistein may inhibit telomerase activity, a marker of cell proliferation, by rapidly decreasing hTERT mRNA levels.
Figure 4.
Novasoy and genistein reduced hTERT mRNA expression in endometrial cancer cells. The ECC-1 (A and B) and RL-95-2 (C and D) cells were treated with novasoy and genistein at the indicated doses for 24 hours. hTERT nRNA expression was determined by real-time RT-PCR. Data are presented as the mean of two independent experiments. *P<0.05 and **P<0.01.
Effect of novasoy and genistein on ER and PR mRNA expression
Genistein has been established as a potential stimulant of cancer growth at lower concentrations while exhibiting anti-cancer effects at higher concentrations [29]. Furthermore, genistein has also been shown to augment progresterone receptor (PR) expression, antagonizing the activity of the estrogen receptor [30,31]. In order to evaluate the effect of novasoy and genistein on ER/PR expression, we examined the effect of both compounds on ERα and PR mRNA expression. Both novasoy and genistein were found to decrease ERα mRNA expression in a dose-dependent manner in the ECC-1 and RL-95-2 endometrial cancer cell lines (P<0.01, Figure 5A, 5B). In addition, novasoy and genistein were found to increase PR mRNA expression in a dose dependent manner in both endometrial cancer cell lines (P<0.01, Figure 5C, 5D).
Figure 5.
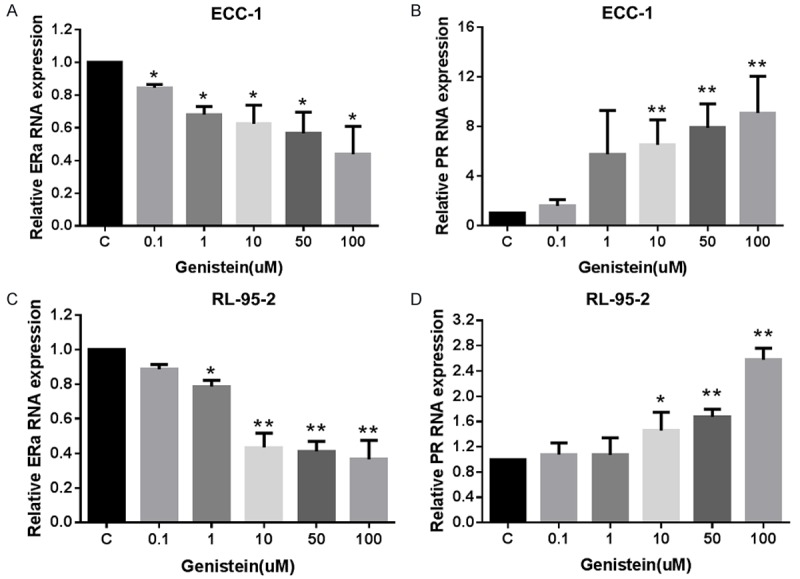
Effect of novasoy and genistein on ERα and PR RNA expression in endometrial cancer cells. The ECC-1 (A and B) and RL-95-2 (C and D) cells were treated with genistein at the indicated doses for 24 hours. ERα and PR mRNA expression was determined by real-time RT-PCR. Genistein significantly inhibited ERα mRNA expression (A and C) and increased PR mRNA expression (B and D) in both cell lines after 24 hours treatment. Data are presented as the mean of two independent experiments. *P<0.05 and **P<0.01.
Effect of genistein on the AKT/mTOR and MAPK pathways
It is well known that the activation of the extracellular signal regulated kinase (ERK) and the phosphatidylinositol 3 kinase/mammalian target of rapamycin (PI3K/AKT/mTOR) pathways play a crucial role in control of cell growth and survival in endometrial cancer and inhibition of these pathways leads to the halting of endometrial cancer growth [10]. To investigate the mechanisms underlying the inhibition of cell growth by genistein, we characterized the effect of genistein on these signaling pathways by western immunoblotting. Genistein decreased phosphorylation of Akt and increased phosphorylation of p42/44 (ERK) in both endometrial cancer cell lines within 48 hours of exposure (Figure 6). We then evaluated the effect of genistein on the PI3K/AKT/mTOR pathway. Our results revealed that genistein decreased phosphorylation of ribosomal protein S6 in the RL-95-2 cells but had no effect on the phosphorylation of S6 in the ECC-1 cells. These data suggest that genistein may exert its anti-tumorigenic effects via activation or inhibition of different cell signaling pathways, depending on the endometrial cancer cell line being examined.
Figure 6.
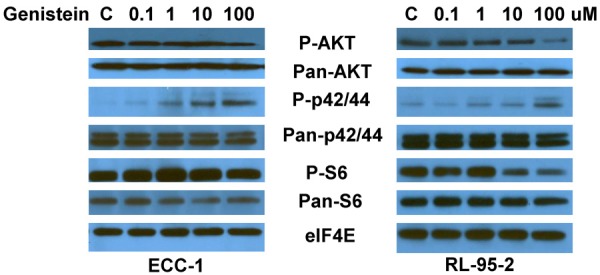
Effect of genistein on the Akt/mTOR and MAPK pathways in endometrial cancer cells. The ECC-1 and RL-95-2 cells were treated with genistein at the indicated doses in for 24 hours. Phosphorylated-Akt, phosphorylated-S6 and phosphorylated-p42/44 protein expression was determined by western immunoblotting.
Discussion
Isoflavones, including the phytoestrogen genistein, have attracted considerable interest for their anti-cancer properties. Novasoy and genistein have been investigated in cancer therapeutics and prevention over the past ten years. Genistein has been found to completely inhibit estradiol-induced mitoses in uterine luminal epithelium, endometrial stroma and myometrium in the prepubertal rat uterus, block the proliferative effects of estrogen on endometrial cancer cells and inhibit the proliferation of endometrial cancer cells and endometrial glandular epithelium simultaneously in vitro [32]. Furthermore, long-term consumption of genistein is negatively associated with subsequent endometrial cancer risk in patients with tamoxifen-treated female breast cancer, suggesting an ability of these compounds to generate anti-proliferative effects in vivo [25,32,33]. In this study, we investigated the impact of isoflavones on the growth of endometrial cancer cells and found that novasoy and genistein strongly inhibited endometrial cancer cell proliferation in a dose-dependent manner. Growth inhibition was accompanied by induction of apoptosis, an accumulation of the endometrial cancer cells in G2/M phase and decreased hTERT mRNA expression. In addition, novasoy and genistein treatment increased PR mRNA expression with a concomitant decrease in ERα mRNA expression. These results suggest that isoflavones may have therapeutic potential in the prevention and treatment of endometrial cancer.
The mechanism of anti-proliferation induced by novasoy and genistein is thought to be related to multiple molecular targets in cancer cells, including inhibition of protein tyrosine kinases (EGFR/VEGFR/Her2) and modulation of ERα/β [29]. Apoptosis and cell cycle arrest induced by genistein in cancer cells may be a result of targeting these signaling pathways [29,34]. Our results showed that the anti-proliferative effects exerted by novasoy and genistein can be attributed to both the induction of G2/M cell cycle arrest and apoptosis in the ECC-1 and RL-95-2 cells (Figure 2). Similar results have been found in prostate, breast, brain, ovarian and colon cancer cell lines with genistein treatment [35-37]. We also found that both novasoy and genistein significantly increased annexin V expression and cleaved caspase-3 activity in the RL-95-2 cells but not the ECC-1 cells. These results suggest that novasoy and genistein induce G2 cell cycle arrest as the predominant mechanism in the inhibition of proliferation while induction of apoptosis may be unique to certain endometrial cancer cells.
Genistein and novasoy are thought to exhibit anti-cancer effects via down-regulation of critical signaling pathways in human cancers, including the NF-κB, AKT/mTOR, MAPK, JNK, EGF and IGF pathways [38], and thus, both may be attractive agents for cancer prevention or treatment [10,38]. Genistein has been found to significantly activate phosphorylation of ERK in a GRP30-dependent manner in endometrial cancer cells [39]. However, little is currently known about genistein’s effects on the AKT/mTOR pathways in cancer cells. In order to determine the effects of genistein on the AKT/mTOR and MAPK pathways in endometrial cancer cells, we detected changes in the phosphorylation of AKT/ribosomal protein S6 and p42/44, key downstream targets of the AKT/mTOR and MAPK signaling, respectively. Decreased phosphorylation of AKT and increased phosphorylation of p42/44 were observed in both cell lines after treatment with genistein for 48 hours. Interestingly, genistein was shown to reduce phosphorylation of S6 in the RL-95-2 endometrial cell line but not in the ECC-1 cell line (Figure 6). Thus, inhibition of cell proliferation by novasoy and genistein may involve different cell signaling pathways, in addition to G2 cell cycle arrest and apoptosis.
Nearly all human cancers have been shown to be telomerase positive whereas most benign, premalignant tumors are characterized by the absence of telomerase [40]. Human telomerase reverse transcriptase, hTERT, is the rate limiting enzyme responsible for the activation of telomerase [41]. Recent data has suggested that geinstein induces cell growth inhibition through suppression of telomerase activity and hTERT mRNA expression, thereby suggesting that hTERT may be a potential therapeutic target in cancer [41,42]. In this study, novasoy and genistein decreased hTERT expression in the endometrial cancer cell lines. More than a 50% decrease in hTERT expression was seen with the higher doses of genistein (50 uM and 100 uM) and novasoy (100 and 200 mg/ml). To our knowledge, this is the first study to evaluate the role of genistein and novasoy on telomerase activity in endometrial cancer cell lines. Furthermore, the level of hTERT mRNA expression may serve as an indicator of the potential sensitivity of endometrial cancer cells to isoflavones.
The hyperestrogen state generated by obesity has a well-known association with the carcinogenesis and progression of endometrial cancer due to its unopposed stimulatory effects on the endometrium, acting through two types of ERs: ERα and ERβ, encoded by different genes and with different tissue distributions and ligand specificities [43,44]. Genistein is structurally similar to 17β-estradiol and has a high affinity for binding to ERs, particularly ERβ, which is involved in the suppression of ERα-stimulated estrogenic signal mechanisms [43]. The potential beneficial effects of genistein in modulation of estrogen-regulated gene expression and signaling pathways has been shown to be dependent upon dose, tissues analyzed and relative proportions of ER isoforms α/β [29]. Nutritionally relevant concentrations (nM) of genistein have been found to significantly inhibit the proliferative effects of estrogen on endometrial adenocarcinoma cells, presumably through activation of stromal cell ERβ [33]. Pre-treatment with genistein (0.5 mg/kg) completely inhibited estradiol-induced mitoses in the uterine luminal epithelium, endometrial stroma and myometrium and partially inhibited estradiol-induced uterine eosinophilia and endometrial edema the prepubertal rat uterus, indicating that genistein protects against estrogen-induced cell proliferation in the uterus [32]. Furthermore, a single subcutaneous administration of genistein significantly decreased the levels of 17β-estradiol (5 ppm in diet)-induced expression of c-jun, interleukin-1α (IL-1α) and tumor necrosis factor-α (TNF-α) mRNAs in the uteri of ovariectomized mice, and long term treatment of genistein significantly reduced the incidences of endometrial hyperplasia and adenocarainoma induced by 17β-estradiol in ICR mice [45]. In this study, our results demonstrated a decrease in relative ERα mRNA expression in endometrial cancer lines treated with genistein or novasoy along with a concomitant increase in relative progesterone receptor mRNA expression.
Given that genistein has anti-proliferative activity in several cancers as demonstrated in cell and animal models, the anti-cancer effects of this compound has also been investigated in clinical trials. Long-term genistein treatment did not change mammographic breast density, endometrial thickness or BRCA1 and BRCA2 expression, whereas it reduced sister chromatid exchange, high frequency cell counts and chromosomal aberration frequency and exhibited a positive effect on bone formation in postmenopausal women, supporting the protective effects of genistein maintaining genomic integrity by preventing DNA damage [46,47]. Serum concentrations of genistein after oral administration at high doses has been found to result in micromolar levels that have been associated with anti-tumor activity in vitro [48]. Similarly, treatment of pancreatic cancer patients with AXP107-11 (genistein) in combination with gemcitabine resulted in a favorable pharmacokinetics profile with high serum levels of genistein, without signs of either hematological or non-hematological toxicity [49]. A phase II clinical trial in prostate cancer demonstrated that soy isoflavone supplementation inhibited the linear rise in PSA in both androgen-sensitive and androgen-insensitive patient populations without any toxicity [48]. Moreover, the combination of soy isoflavones with lycopene had activity in prostate cancer patients with PSA relapse disease and thus, may potentially delay progression of both hormone-refractory and hormone-sensitive prostate cancer [50]. Currently, there are no clinical trials that have evaluated the benefits of isoflavones in the treatment of gynecological cancers, including endometrial cancer. In order to further the investigation of the anti-cancer properties of genistein in endometrial cancer patients, future studies conducted in our laboratory will focus on evaluating the effects of genistein on tumor growth in our genetically engineered mouse model of endometrial cancer. Based on the preliminary data generated by these studies, we believe that genistein might prove to be a novel and well-tolerated therapeutic agent in the treatment of obesity- and hormonally-driven endometrial cancer.
Acknowledgements
This work was generously supported by NIH/NCI 1K23CA143154-01A1, Steelman fund and National Natural Science Foundation of China (11672192).
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Shaw E, Farris M, McNeil J, Friedenreich C. Obesity and endometrial cancer. Recent Results Cancer Res. 2016;208:107–136. doi: 10.1007/978-3-319-42542-9_7. [DOI] [PubMed] [Google Scholar]
- 3.Wise MR, Jordan V, Lagas A, Showell M, Wong N, Lensen S, Farquhar CM. Obesity and endometrial hyperplasia and cancer in premenopausal women: a systematic review. Am J Obstet Gynecol. 2016;214:689.e1–689.e17. doi: 10.1016/j.ajog.2016.01.175. [DOI] [PubMed] [Google Scholar]
- 4.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 5.Gambacciani M, Monteleone P, Sacco A, Genazzani AR. Hormone replacement therapy and endometrial, ovarian and colorectal cancer. Best Pract Res Clin Endocrinol Metab. 2003;17:139–147. doi: 10.1016/s1521-690x(02)00086-6. [DOI] [PubMed] [Google Scholar]
- 6.Weiderpass E, Adami HO, Baron JA, Magnusson C, Bergstrom R, Lindgren A, Correia N, Persson I. Risk of endometrial cancer following estrogen replacement with and without progestins. J Natl Cancer Inst. 1999;91:1131–1137. doi: 10.1093/jnci/91.13.1131. [DOI] [PubMed] [Google Scholar]
- 7.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U. S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 8.Steiner E, Plata K, Interthal C, Schmidt M, Faldum A, Hengstler JG, Sakuragi N, Watari H, Yamamoto R, Kolbl H. Diabetes mellitus is a multivariate independent prognostic factor in endometrial carcinoma: a clinicopathologic study on 313 patients. Eur J Gynaecol Oncol. 2007;28:95–97. [PubMed] [Google Scholar]
- 9.Gertz J, Reddy TE, Varley KE, Garabedian MJ, Myers RM. Genistein and bisphenol A exposure cause estrogen receptor 1 to bind thousands of sites in a cell type-specific manner. Genome Res. 2012;22:2153–2162. doi: 10.1101/gr.135681.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spagnuolo C, Russo GL, Orhan IE, Habtemariam S, Daglia M, Sureda A, Nabavi SF, Devi KP, Loizzo MR, Tundis R, Nabavi SM. Genistein and cancer: current status, challenges, and future directions. Adv Nutr. 2015;6:408–419. doi: 10.3945/an.114.008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwasaki M, Tsugane S. Risk factors for breast cancer: epidemiological evidence from Japanese studies. Cancer Sci. 2011;102:1607–1614. doi: 10.1111/j.1349-7006.2011.01996.x. [DOI] [PubMed] [Google Scholar]
- 12.Rozman KK, Bhatia J, Calafat AM, Chambers C, Culty M, Etzel RA, Flaws JA, Hansen DK, Hoyer PB, Jeffery EH, Kesner JS, Marty S, Thomas JA, Umbach D. NTP-CERHR expert panel report on the reproductive and developmental toxicity of genistein. Birth Defects Res B Dev Reprod Toxicol. 2006;77:485–638. doi: 10.1002/bdrb.20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang KA, Kang NH, Yi BR, Lee HR, Park MA, Choi KC. Genistein, a soy phytoestrogen, prevents the growth of BG-1 ovarian cancer cells induced by 17beta-estradiol or bisphenol A via the inhibition of cell cycle progression. Int J Oncol. 2013;42:733–740. doi: 10.3892/ijo.2012.1719. [DOI] [PubMed] [Google Scholar]
- 14.Ouyang G, Yao L, Ruan K, Song G, Mao Y, Bao S. Genistein induces G2/M cell cycle arrest and apoptosis of human ovarian cancer cells via activation of DNA damage checkpoint pathways. Cell Biol Int. 2009;33:1237–1244. doi: 10.1016/j.cellbi.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Luo H, Jiang BH, King SM, Chen YC. Inhibition of cell growth and VEGF expression in ovarian cancer cells by flavonoids. Nutr Cancer. 2008;60:800–809. doi: 10.1080/01635580802100851. [DOI] [PubMed] [Google Scholar]
- 16.Chen FP, Chien MH. Phytoestrogens induce apoptosis through a mitochondria/caspase pathway in human breast cancer cells. Climacteric. 2014;17:385–392. doi: 10.3109/13697137.2013.869671. [DOI] [PubMed] [Google Scholar]
- 17.Liu X, Sun C, Jin X, Li P, Ye F, Zhao T, Gong L, Li Q. Genistein enhances the radiosensitivity of breast cancer cells via G(2)/M cell cycle arrest and apoptosis. Molecules. 2013;18:13200–13217. doi: 10.3390/molecules181113200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Z, Wang CZ, Du GJ, Qi LW, Calway T, He TC, Du W, Yuan CS. Genistein induces G2/M cell cycle arrest and apoptosis via ATM/p53-dependent pathway in human colon cancer cells. Int J Oncol. 2013;43:289–296. doi: 10.3892/ijo.2013.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmad A, Biersack B, Li Y, Bao B, Kong D, Ali S, Banerjee S, Sarkar FH. Perspectives on the role of isoflavones in prostate cancer. AAPS J. 2013;15:991–1000. doi: 10.1208/s12248-013-9507-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takimoto CH, Glover K, Huang X, Hayes SA, Gallot L, Quinn M, Jovanovic BD, Shapiro A, Hernandez L, Goetz A, Llorens V, Lieberman R, Crowell JA, Poisson BA, Bergan RC. Phase I pharmacokinetic and pharmacodynamic analysis of unconjugated soy isoflavones administered to individuals with cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:1213–1221. [PubMed] [Google Scholar]
- 21.Peterson G. Evaluation of the biochemical targets of genistein in tumor cells. J Nutr. 1995;125:784S–789S. doi: 10.1093/jn/125.suppl_3.784S. [DOI] [PubMed] [Google Scholar]
- 22.Wang TT, Sathyamoorthy N, Phang JM. Molecular effects of genistein on estrogen receptor mediated pathways. Carcinogenesis. 1996;17:271–275. doi: 10.1093/carcin/17.2.271. [DOI] [PubMed] [Google Scholar]
- 23.Poonyachoti S, Deachapunya C. Modulatory effects of phytoestrogens on the expression of Fas ligand and the release of cytochrome C in normal and cancerous endometrial cells. J Med Assoc Thai. 2012;95(Suppl 12):S105–112. [PubMed] [Google Scholar]
- 24.Parajuli B, Shin SJ, Kwon SH, Cha SD, Lee HG, Bae I, Cho CH. The synergistic apoptotic interaction of Indole-3-Carbinol and Genistein with TRAIL on endometrial cancer cells. J Korean Med Sci. 2013;28:527–533. doi: 10.3346/jkms.2013.28.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sha GH, Lin SQ. Genistein inhibits proliferation of human endometrial endothelial cell in vitro. Chin Med Sci J. 2008;23:49–53. doi: 10.1016/s1001-9294(09)60010-9. [DOI] [PubMed] [Google Scholar]
- 26.Biava PM, Nicolini A, Ferrari P, Carpi A, Sell S. A systemic approach to cancer treatment: tumor cell reprogramming focused on endocrine-related cancers. Curr Med Chem. 2014;21:1072–1081. doi: 10.2174/0929867321666131201143124. [DOI] [PubMed] [Google Scholar]
- 27.Nachajova M, Brany D, Dvorska D. Telomerase and the process of cervical carcinogenesis. Tumour Biol. 2015;36:7335–7338. doi: 10.1007/s13277-015-3976-z. [DOI] [PubMed] [Google Scholar]
- 28.Zhou C, Steplowski TA, Dickens HK, Malloy KM, Gehrig PA, Boggess JF, Bae-Jump VL. Estrogen induction of telomerase activity through regulation of the mitogen-activated protein kinase (MAPK) dependent pathway in human endometrial cancer cells. PLoS One. 2013;8:e55730. doi: 10.1371/journal.pone.0055730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russo M, Russo GL, Daglia M, Kasi PD, Ravi S, Nabavi SF, Nabavi SM. Understanding genistein in cancer: the “good” and the “bad” effects: a review. Food Chem. 2016;196:589–600. doi: 10.1016/j.foodchem.2015.09.085. [DOI] [PubMed] [Google Scholar]
- 30.Norrby M, Madej A, Ekstedt E, Holm L. Effects of genistein on oestrogen and progesterone receptor, proliferative marker Ki-67 and carbonic anhydrase localisation in the uterus and cervix of gilts after insemination. Anim Reprod Sci. 2013;138:90–101. doi: 10.1016/j.anireprosci.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 31.Zhang M, Liu X, Holman CD. Effect of dietary intake of isoflavones on the estrogen and progesterone receptor status of breast cancer. Nutr Cancer. 2010;62:765–773. doi: 10.1080/01635581003605979. [DOI] [PubMed] [Google Scholar]
- 32.Gaete L, Tchernitchin AN, Bustamante R, Villena J, Lemus I, Gidekel M, Cabrera G, Carrillo O. Genistein selectively inhibits estrogen-induced cell proliferation and other responses to hormone stimulation in the prepubertal rat uterus. J Med Food. 2011;14:1597–1603. doi: 10.1089/jmf.2010.0349. [DOI] [PubMed] [Google Scholar]
- 33.Sampey BP, Lewis TD, Barbier CS, Makowski L, Kaufman DG. Genistein effects on stromal cells determines epithelial proliferation in endometrial co-cultures. Exp Mol Pathol. 2011;90:257–263. doi: 10.1016/j.yexmp.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ravindranath MH, Muthugounder S, Presser N, Viswanathan S. Anticancer therapeutic potential of soy isoflavone, genistein. Adv Exp Med Biol. 2004;546:121–165. doi: 10.1007/978-1-4757-4820-8_11. [DOI] [PubMed] [Google Scholar]
- 35.Khaw AK, Yong JW, Kalthur G, Hande MP. Genistein induces growth arrest and suppresses telomerase activity in brain tumor cells. Genes Chromosomes Cancer. 2012;51:961–974. doi: 10.1002/gcc.21979. [DOI] [PubMed] [Google Scholar]
- 36.Mizushina Y, Shiomi K, Kuriyama I, Takahashi Y, Yoshida H. Inhibitory effects of a major soy isoflavone, genistein, on human DNA topoisomerase II activity and cancer cell proliferation. Int J Oncol. 2013;43:1117–1124. doi: 10.3892/ijo.2013.2032. [DOI] [PubMed] [Google Scholar]
- 37.Choi EJ, Kim T, Lee MS. Pro-apoptotic effect and cytotoxicity of genistein and genistin in human ovarian cancer SK-OV-3 cells. Life Sci. 2007;80:1403–1408. doi: 10.1016/j.lfs.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 38.Sahin K, Tuzcu M, Basak N, Caglayan B, Kilic U, Sahin F, Kucuk O. Sensitization of cervical cancer cells to cisplatin by genistein: the role of NFkappaB and Akt/mTOR signaling pathways. J Oncol. 2012;2012:461562. doi: 10.1155/2012/461562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petrie WK, Dennis MK, Hu C, Dai D, Arterburn JB, Smith HO, Hathaway HJ, Prossnitz ER. G protein-coupled estrogen receptor-selective ligands modulate endometrial tumor growth. Obstet Gynecol Int. 2013;2013:472720. doi: 10.1155/2013/472720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shay JW. Role of telomeres and telomerase in aging and cancer. Cancer Discov. 2016;6:584–593. doi: 10.1158/2159-8290.CD-16-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roy Choudhury S, Karmakar S, Banik NL, Ray SK. Synergistic efficacy of sorafenib and genistein in growth inhibition by down regulating angiogenic and survival factors and increasing apoptosis through upregulation of p53 and p21 in malignant neuroblastoma cells having N-Myc amplification or non-amplification. Invest New Drugs. 2010;28:812–824. doi: 10.1007/s10637-009-9324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ouchi H, Ishiguro H, Ikeda N, Hori M, Kubota Y, Uemura H. Genistein induces cell growth inhibition in prostate cancer through the suppression of telomerase activity. Int J Urol. 2005;12:73–80. doi: 10.1111/j.1442-2042.2004.00973.x. [DOI] [PubMed] [Google Scholar]
- 43.Lee JY, Kim HS, Song YS. Genistein as a potential anticancer agent against ovarian cancer. J Tradit Complement Med. 2012;2:96–104. doi: 10.1016/s2225-4110(16)30082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gibson DA, Saunders PT. Endocrine disruption of oestrogen action and female reproductive tract cancers. Endocr Relat Cancer. 2014;21:T13–31. doi: 10.1530/ERC-13-0342. [DOI] [PubMed] [Google Scholar]
- 45.Lian Z, Niwa K, Tagami K, Hashimoto M, Gao J, Yokoyama Y, Mori H, Tamaya T. Preventive effects of isoflavones, genistein and daidzein, on estradiol-17beta-related endometrial carcinogenesis in mice. Jpn J Cancer Res. 2001;92:726–734. doi: 10.1111/j.1349-7006.2001.tb01154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Atteritano M, Pernice F, Mazzaferro S, Mantuano S, Frisina A, D’Anna R, Cannata ML, Bitto A, Squadrito F, Frisina N, Buemi M. Effects of phytoestrogen genistein on cytogenetic biomarkers in postmenopausal women: 1 year randomized, placebo-controlled study. Eur J Pharmacol. 2008;589:22–26. doi: 10.1016/j.ejphar.2008.04.049. [DOI] [PubMed] [Google Scholar]
- 47.Marini H, Bitto A, Altavilla D, Burnett BP, Polito F, Di Stefano V, Minutoli L, Atteritano M, Levy RM, D’Anna R, Frisina N, Mazzaferro S, Cancellieri F, Cannata ML, Corrado F, Frisina A, Adamo V, Lubrano C, Sansotta C, Marini R, Adamo EB, Squadrito F. Breast safety and efficacy of genistein aglycone for postmenopausal bone loss: a follow-up study. J Clin Endocrinol Metab. 2008;93:4787–4796. doi: 10.1210/jc.2008-1087. [DOI] [PubMed] [Google Scholar]
- 48.Banerjee S, Li Y, Wang Z, Sarkar FH. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008;269:226–242. doi: 10.1016/j.canlet.2008.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lohr JM, Karimi M, Omazic B, Kartalis N, Verbeke CS, Berkenstam A, Frodin JE. A phase I dose escalation trial of AXP107-11, a novel multi-component crystalline form of genistein, in combination with gemcitabine in chemotherapy-naive patients with unresectable pancreatic cancer. Pancreatology. 2016;16:640–645. doi: 10.1016/j.pan.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 50.Vaishampayan U, Hussain M, Banerjee M, Seren S, Sarkar FH, Fontana J, Forman JD, Cher ML, Powell I, Pontes JE, Kucuk O. Lycopene and soy isoflavones in the treatment of prostate cancer. Nutr Cancer. 2007;59:1–7. doi: 10.1080/01635580701413934. [DOI] [PubMed] [Google Scholar]