Abstract
Alzheimer’s disease (AD) is the most common form of dementia and lacks disease-altering treatments. Ginsenoside Rb1 (GsRb1), the key active compounds of ginsenoside found in ginseng. The present study aimed to determine whether GsRb1 could prevent cognitive deficit and take neuroprotective effects in Aβ1-40-induced rat model through apoptotic signaling pathway. Injection of soluble Aβ1-40 into the hippocampus caused impairment in learning and memory. Daily administration of Rb1 (12.5, 25, and 50 mg/kg, i.p.) for 14 consecutive days. All rats were tested for their capabilities of spatial navigation and memorization by Morris water maze. Apoptosis was tested using TUNEL staining in hippocampus neuronal cells. RT-PCR, immunohistochemical staining and western blotting were employed to confirm the expressions of Bcl-2, Bax and Cleaved Caspase-3. The results showed that Rb1 administration could prevent cognitive deficit, and significantly decreased the levels of Bax and Cleaved Caspase-3 meanwhile up regulation the level of Bcl-2 in the hippocampus. We suggest that GsRb1 may be effective for preventing or slowing the development of Alzheimer’s disease, which improving cognitive and memory functions by inhibiting the levels of pro-apoptosis mediators and improving the levels of anti-apoptosis mediators in the rat brain.
Keywords: Ginsenoside Rb1, Alzheimer’s disease, apoptosis, B-cell lymphoma-2 (Bcl-2), Bcl-2 associated X protein (Bax), cysteinyl aspartate specific proteinase-3 (Caspase-3)
Introduction
Being the most common form of senile dementia, Alzheimer’s disease (AD) affects more than 35 million individuals across the globe [1]. The manifestation of the degenerative neurological disorder include the presence of senile plaques (SP) around reactive microglia, abundant intraneuronal neurofibrillary tangles (NFT), and selective neuronal death in the brains of affected patients [2,3]. Fibrillar β-amyloid (Aβ) proteins such as Aβ1-40 and Aβ1-42 are the primary constituents of such plaque [2]. Research by Luo, D et al. suggested that the overproduction and accumulation of β-amyloid (Aβ) protein has been a crucial cornerstone in the pathogenesis of AD [4]. It is believed that the formation of neurofibrillary tangles, inflammation, axonal injury, synapse loss, and neuronal apoptosis, which leads to AD [5]. Therefore, it implies that a reduced Aβ level should render a neuroprotective effect against AD.
In addition, apoptosis also plays a pivotal role in the pathophysiology of AD [6]. Apoptosis is a sophisticated biological mechanism by which the body eliminate unwanted cells. The Bcl-2 family, which composed of a wide variety of anti-apoptosis protein such as Bcl-2 and pro-apoptotic protein such as Bax, is an important regulator of apoptosis, and it has expanded significantly with both pro- and anti-apoptotic molecules included. The presence of an anti-apoptotic molecule such as Bcl-2 or Bcl-xl can inhibit the activation of Bax following a death signal [7]. Caspase-3, the effector caspase and final executor of apoptosis, can cleave cytoskeletal and nuclear proteins [8].
In search of a curative formula, Panax ginseng, which had traditionally been utilized as a tonic remedy in the thousands of years’ history of Chinese medicine, caught the eyes of many researchers. Among the key active compounds of ginsenoside found in ginseng, Ginsenoside Rb1 (GRb1) has been demonstrated to have the ability of enhancing cholinergic function and increasing synaptophysin levels in the hippocampus, and thereby, it’s thought to have ameliorable effects on memory and learning [9]. Recent literatur indicated that GRb1 plays a significant neuronal protection role during brain’s response to ischemia [10-12]. In vivo evidences suggested that GRb1 may increase the expression of anti-apoptotic genes after the brain undergone transient cerebral ischemia. Previous studies have also shown that GRb1 may scavenger toxic species, such as glutamate and hydrogen peroxide [13,14]. Since oxidative stress significantly contributes to neuronal apoptosis in patients with AD, which prompts the researchers to speculate whether GRb1 plays a role in cellular defending oxidative stress and neuronal apoptosis induced by Aβ at cellular level. The authors of these studies suggested that the inhibition of the activation of caspase-3 maybe a result of enhanced Bcl-2 to Bax protein ratio which is attributed to the effect of Rb1.
In this study, we hypothesized that Rb1 may act as a positive modulator for neurons undergoing Aβ-induced injury. We aimed to provide new insights into the examination of the effects of Rb1 on Aβ-induced neurons apoptosis and to determine the potential apoptosis-related signaling mechanisms.
Materials and methods
Materials
Ginsenoside Rb1 was purchased from Boyun biotechnology co., Ltd. (Shanghai, China) in the form of white powder-like crystals, with a molecular weight of 1108, general formula C54H92O23. Rat Aβ1-40 were purchased from Sigma (St.Louis, MO, USA). Donepezil (Aricept) was purchased from the Eisai Pharmaceutical Cl., Ltd. (Batch number 100223A) and used as positive control. Polyclonal rabbit anti-rat Bcl-2, Bax and Caspase-3 antibodies were purchased from Wuhan Boster Biological engineering Co., Ltd (Wuhan, China). SP immunohistochemistry kit and DAB staining kit were purchased from Bioss (Beijing, China). Total protein extraction reagents and BCA protein assay kit were purchased from Pierce (USA). TRizol reagent was purchased from Shenggong biological engineering co., LTD (Shanghai).
Animals and animal preparation
Eighty-four male Sprague-Dawley (SD) rats (body weight 250 ± 20 g, 3 months old) were provided by Shanghai slack laboratory animal co., LTD (license no. SCXK [Lu] 2012-002). Animals were housed in a room maintained at 23°C with a 12-hour light-dark cycle, and were allowed free access to food and water. All animal experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals issued by National Academy of Sciences, Institute of Laboratory Animal Resources, Commission on Life Sciences, and National Research Council. All procedures used in this study were approved by the local ethical committee for animal research. Rats were randomly divided into 7 groups after 1 week adaption as follows: they are normal control group (0+NS), sham operation group (NS+NS), model group (Aβ+NS), Aricept group (Aricept), ginsenoside Rb1 low (L-Rb1), medium (M-Rb1), high dosage group (H-Rb1), with 12 animals in each group. The rat model of AD was prepared as described previously [15,16], Aβ1-40 was diluted in double distilled H2O (ddH2O) to a final concentration of 2.5 μg/ml. Rats were anesthetized with chloral hydrate (3-4 ml/kg, intraperitoneally) and fixed on a rat brain stereotaxic instrument. The scalp was incised, and the bregma and biparietal suture were exposed. Holes were drilled in the skull using a dental drill, 3.0 mm behind the bregma and 1.5 mm from the biparietal suture. A microsyringe was then advanced 3.0 mm under the dura mater for injection of both hippocampal CA1 regions. In addition to the control group, the rest of the use of various bilateral hippocampal were injected with 2 μl soluble Aβ1-40 at a rate of 0.5 μl/min, whereas the sham operation group received equal ddH2O. The syringe was removed 10 min after injection. After operation, the scalp was sutured, and iodophor was used on the wound to prevent infection.
Treatment
Two days after the operation, the intragastric administration started by the dosage of 10 ml/kg/d, lasting 14 consecutive days, the control, sham operation and model group were given a gavage of corresponding dosage of normal saline. Ginsenoside Rb1 high, medium, low dosage group were given by 50 mg/kg/d, 25 mg/kg/d, 12.5 mg/kg/d. Aricept dosage is 1.67 mg/kg/d. The treatment occurred once a day at 10:00 am.
The morris water maze detection
Spatial learning and memory of rats was assessed in Morris water maze as published previously [15] by two investigators completely blind to the treatment of the animals. The Morris water maze consisted of a circular pool (160 cm diameter and 50 cm deep) filled with warm water (23 ± 1°C) to a depth of 27 cm and an escape platform (10 cm diameter) submerged 1 cm below the surface of the water. Swimming activity of the rats was monitored via a video camera mounted overhead and automatically recorded via a video tracking system.
At the beginning of each trial, the rats were placed into the water facing the wall of the pool at one of the four quadrants. Each rat was allowed 60 s to find and mount the platform. When the rat found the platform, it was kept on the platform for 10 s. The rats were given four trials per day and the average time to find the platform of the 4 trials (escape latency) was recorded by video tracking software. The rats were then towel dried and placed in a cage with a heating pad underneath until totally dry and being replaced to their home cage. The training session was conducted for 4 consecutive days in which the platform was never moved. The shorter escape latency, the stronger spatial learning ability.
Memory retention was evaluated by the platform-cross number in a probe trial. On the 5th day, the platform was removed. In this probe trial, the rats were put into the pool and allowed to swim freely in the pool for 60 s. The times for rats crossing the location of the platform were recorded.
Working memory was evaluated by the Trail1/Trail4 index. On the 6th, 7th, and 8th days, we placed the platform on the opposite quadrants. The rats were put into the pool and the total distance traveled was recorded until the rats found the platform. The rats were given 4 consecutive trials every day, and the interval between 2 trials was less than 2 min. The distance was recorded as trail1, trail2, trail3, and trail4, and the ratio from trail1 to trail4 (Trail1/Trail4) indicates the ability of working memory.
Brain tissue fixed and cryopreserved
After the three days’ behavioral tests, rats were sacrificed and immediately perfused through the heart with 0.9% NaCl, which was followed with 4% PFA-0.1 M phosphate buffer (PH 7.2-7.4). Then the half of rats in each group were removed and placed into 4% paraformaldehyde fixed fluid, stored at 4°C. The rests of the group were directly isolated cortex and hippocampus, stored at -80°C.
Immunohistochemistry
The hippocampus were removed and processed for 4 μm paraffin sections for immunohistochemistry. Sections were dewaxed and subjected to 3% H2O2 for 10 min, several washes in distilled water were followed with 2 times. Then subjected heat-mediated antigen retrieval with 0.01 M citric acid buffer (pH 6.0). Following several washes in PBS, sections were blocked with 10% goat serum (30 min), and then incubated with rabbit anti-rat Bax antibody (1:1000), Bcl-2 antibody (1:1000) and Caspase-3 antibody (1:1000) at 4°C overnight. After incubation with the goat secondary antibody, at room temperature for 2 h. After detected with a DAB staining kit, the sections were counterstained with hematoxylin. 6 sections were selected from each group and randomly observed 5 horizons in each section, precisely selected all of the positive particles to the vision and the computer automatically integrated option density (IOD) and area. OD was used to quantify immunohistochemical positive reaction, by using IPP6.0 image analysis system.
Western blotting
A western blot analysis was performed as described previously [16]. The hippocampus from 12 individual rats in each group were stored in -80°C, homogenized and total proteins were quantified by the BCA protein assay kit. Proteins were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis, transferred onto a polyvinylidene fluoride membrane, which were washed in TBST before incubation in 5% skim milk (diluted in ddH2O) at 37°C for 2 h, and reacted with the primary antibodies, such as rabbit anti-Bcl-2 (1:1,000), rabbit anti-Bax (1:1,000) or rabbit anti-Caspase-3 (1:1000), overnight at 4°C. A horseradish peroxidase-conjugated goat-anti-mouse IgG (1:5,000) was used as a secondary antibody at 37°C for 2 h. Immunoreactive bands were inspected by enhanced chemiluminescence and exposed to x-ray film. Bands were analyzed by Quantity One software. Values obtained were normalized basing on density values of internal β-actin.
Real-time PCR
Quantitative gene expression was measured by real-time PCR. Total RNA was extracted from the hippocampus, using Trizol reagent, and cDNA was synthesized with reverse transcriptase (The Bioneer). Primers were designed by Primer Premier 5.0 according the mRNA sequences of Bax, Bcl-2 and caspase-3 genes retrieved from GenBank, and synthesized by the Shanghai Rui Jingsheng Biological Engineering Co.,Ltd. Primer sequences were as follows: Bax forward primer 5’CCCGAGAGGTCTTCTTCCG3’, reverse primer 5’ GAAGTCCAGTGTCCAGCCCA 3’, Bcl-2 forward primer 5’ GTGAACTGGGGGAGGATTGT 3’, reverse primer 5’ GCATCCCAGCCTCCGTTA3’, caspase-3 forward primer 5’ CGAAACTCTTCATCATTCAGGC3’, reverse primer 5’AGTAAGCATACAGGAAGTCGGC3’. The real-time PCR was performed by using SYBR I (20*) according to the manufacturer’s instructions. The amplification conditions were as follows: 4 min at 95°C for denaturation followed by 35 cycles of 20 s at 94°C, 30 s at 60°C and 30 s at 72°C. Then the signal was detected at 72°C.
Tunel assay
To detect cells undergoing apoptosis, TUNEL technique was performed according to the manufacturer’s protocol supplied within the TUNEL-pod kit (Roche, USA). At first, the brain sections of cortex were immersed in xylene and dehydrated with serial dilutions of alcohol followed by a wash in distilled water. After treatment with 3% H2O2 for 10 min at room temperature, the sections were incubated with proteinase K for 20 min at 37°C to enhance the permeability. Then, the sections were incubated for 60 min with TUNEL reaction mixture and for 30 min with converter-POD at 37°C. At last, after incubated for 10 min with DAB substrate solution (Zymed, USA), the sections were counterstained with haematoxylin, to be examined under a light microscope. Positive and negative controls were carried out on slides from the same block. Stained slides were randomly observed at a high-power field (×400 magnification) and the pathological changes near the injection needle were photographed. The image pictures were processed by the America IPP6.0 software and the apoptotic ratio was calculated according to the following formula: apoptotic ratio = the number of TUNEL-positive cells/the total number of cells.
Statistical analysis
All data were expressed as mean ± standard deviation and were analyzed by the SPSS16.0 software. One-way analysis of variance (ANOVA) was used to analyze the expression of Bax, Bcl-2 and Cleaved Caspase-3 in different groups. Pairwise comparisons were homogeneity using LSD test, it was not homogeneity using DunnettT3 test. p values less than 0.05 were considered statistically significant.
Results
Effects of Rb1 on learning and memory capability in AD rats
In the Morris water maze test, all animals were able to swim normally and find the hidden platform during the training trials. After being trained four times per day for four consecutive sessions, normal control and sham-operation rats were able to reach the hidden platform in a shorter time during the training. However, the learning and memory abilities of Aβ1-40-injected rats were significantly impaired compared with the sham-operation group (P<0.01) (Figure 1). A significant decrease in escape latency was observed in the Aricept or Rb1 group compared with the Aβ1-40-injected group (P<0.01) and at dose of 25 mg/kg/d showed the maximum decline (Figure 1). In the probe trial of the Morris water maze test, injection of Aβ1-40 had a significant effect on the platform-cross number compared with the sham-operation group (P<0.01). Compared with the Aβ1-40-injected group, Aricept or Rb1-treated rats displayed more platform-cross number (P<0.05) (Figure 2). In the Morris water maze test, injection of Aβ1-40 had a significantly lower effect on the Trail1/Trail4 index compared with the sham-operation group (P<0.01). Compared with the Aβ1-40-injected group, Aricept or Rb1-treated rats displayed higher Trail1/Trail4 index (P<0.05) (Figure 2).
Figure 1.
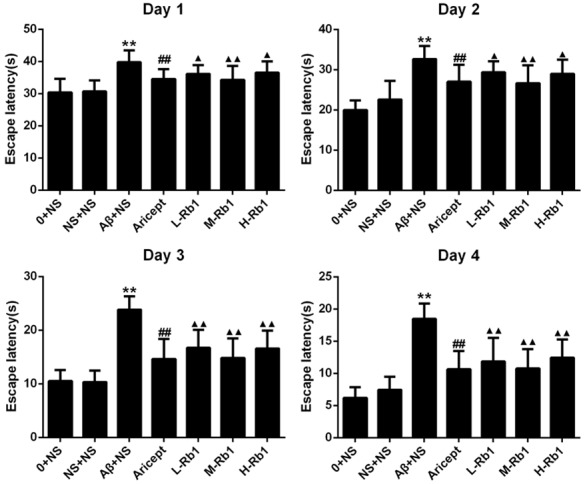
The comparison of escape latency in rats. In 4 d training trials, the rats escape latencies were measured to assess the rats memory ability, and the bar charts show the Aricept and Rb1 group were significant decreased in escape latency. **P<0.01, compared with sham-operation group; ▲▲P<0.01, compared with Aβ+NS group; ▲P<0.05, compared with Aβ+NS group; ##P<0.01, compared with Aβ+NS group. Data are presented as mean ± standard deviation (n = 6). NS: normal saline; Aβ: amyloid-β; L-Rb1: Ginsenoside Rb1 low dosage group; M-Rb1: Ginsenoside Rb1 medium dosage group; H-Rb1: Ginsenoside Rb1 high dosage group.
Figure 2.
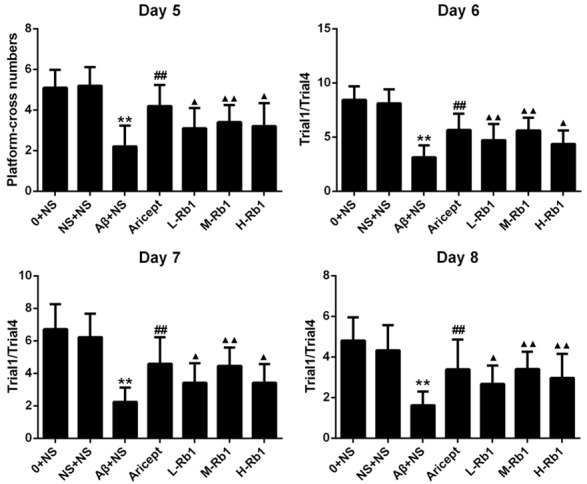
The comparison of platform-cross numbers and Trail1/Trail4 in rats. On Day 5, the platform-cross number in a probe trial was aim to evaluate the memory retention. On Day 6 to Day 8, the Trail1/Trail4 were indicated the ability of working memory for each group. **P<0.01, compared with sham-operation group; ▲▲P<0.01, compared with Aβ+NS group; ▲P<0.05, compared with Aβ+NS group; ##P<0.01, compared with Aβ+NS group. Data are presented as mean ± standard deviation (n = 6). NS: normal saline; Aβ: amyloid-β; L-Rb1: Ginsenoside Rb1 low dosage group; M-Rb1: Ginsenoside Rb1 medium dosage group; H-Rb1: Ginsenoside Rb1 high dosage group.
Effects of Rb1 on Bcl-2, Bax and Caspase-3 expression in immunohistochemistry
The effects of Rb1 to Bcl-2, Bax and Caspase-3 in the hippocampus were investigated by immunohistochemical staining. Distinct yellow stains for Bcl-2, Bax and Caspase-3 were observed in the cytoplasm and membranes of the cone-shaped cells of the hippocampus (arrows in Figure 3). Compared with the sham-operation group, injection of Aβ1-40 in the hippocampus highly increased the expression of Bax and Caspase-3 and decreased the expression of Bcl-2 (P<0.01). Compared with the injection of Aβ1-40 group, Aricept or Rb1 group significantly decreased the expression of Bax and Caspase-3 and increased the expression of Bcl-2 (P<0.01).
Figure 3.
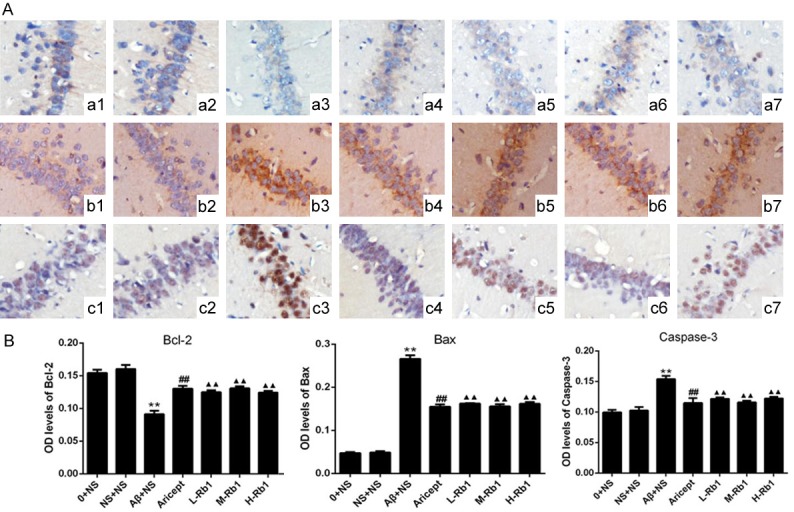
Immunohistochemical staining of the CA1 region of the hippocampus (×200). A: a, b, and c, immunohistochemical staining for Bcl-2, Bax, and Caspase-3 protein expression in hippocampus, respectively. 1, 2, 3, 4, 5, 6, 7: control, sham operation, normal saline, Aricept, ginsenoside Rb1 low, medium, high dosage groups, respectively. B: Comparison of Bcl-2, Bax and Caspase-3 optical density levels in the hippocampus for each group. **P<0.01, compared with sham-operation group; ▲▲P<0.01, compared with Aβ+NS group; ##P<0.01, compared with Aβ+NS group. Data are presented as mean ± standard deviation (n = 6). NS: normal saline; Aβ: amyloid-β; L-Rb1: Ginsenoside Rb1 low dosage group; M-Rb1: Ginsenoside Rb1 medium dosage group; H-Rb1: Ginsenoside Rb1 high dosage group.
Effects of Rb1 on Bcl-2, Bax and Caspase-3 expression in western blot
Western blot analysis for Bcl-2, Bax and Caspase-3 were shown in Figure 4. Compared with the sham-operation group, the protein levels of Bax and Cleaved Caspase-3 were highly increased and the protein levels of Bcl-2 were significantly decreased after injection of Aβ1-40 (P<0.01). After treatment with Aricept or Rb1, the protein levels of Bax and Cleaved Capase-3 were significantly decreased and the protein levels of Bcl-2 were highly increased at different degrees (P<0.01). Among the three doses of Rb1, the Rb1 25 mg/kg rats (M-Rb1 group) shown the better effects, which was comparable to Aricept positive control.
Figure 4.
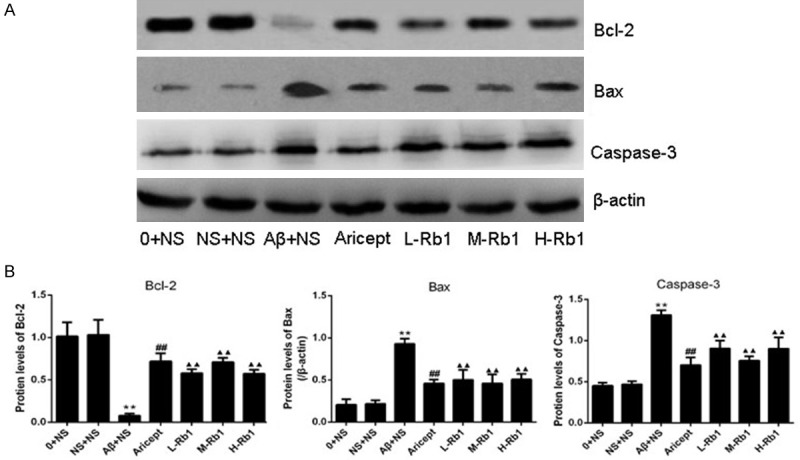
Protein expression of Bcl-2, Bax, and Caspase-3 in the hippocampus CA1 area. A: Western blotting for Bcl-2, Bax, and Caspase-3 in the hippocampus CA1 area in each group, the gels were run under the same experimental conditions. The Original images are available in Figure S1. B: Optical density values of Bcl-2, Bax, and Caspase-3 expressions are quantified and analyzed in each group. **P<0.01, compared with sham-operation group; ▲▲P<0.01, compared with Aβ+NS group; ##P<0.01, compared with Aβ+NS group. Data are presented as mean ± standard deviation (n = 6). NS: normal saline; Aβ: amyloid-β; L-Rb1: Ginsenoside Rb1 low dosage group; M-Rb1: Ginsenoside Rb1 medium dosage group; H-Rb1: Ginsenoside Rb1 high dosage group.
Effects of Rb1 on Aβ-induced expression of Bcl-2, Bax and Caspase-3 mRNAs in the hippocampus
The effects of Rb1 on the expression of Bcl-2, Bax and Caspase-3 mRNA were investigated by real-time PCR. Compared with the sham-operation group, injection of Aβ1-40 in the hippocampus highly increased the expression of Bax mRNA and Caspase-3 mRNA and decreased the expression of Bcl-2 mRNA (P<0.01). Compared with the injection of Aβ1-40 group, Aricept or Rb1 group significantly decreased the expression of Bax mRNA and Caspase-3 mRNA and increased the expression of Bcl-2 mRNA (P<0.01) (Figure 5).
Figure 5.
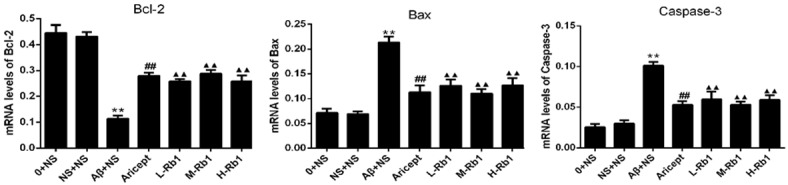
Hippocampus mRNA expression of Bcl-2, Bax and Caspase-3. The three panels show the quantification results of Bcl-2, Bax and Capase-3 gene expression for each group. The Aricept and Rb1 group were significantly decreased compare with Aβ+NS group. **P<0.01, compared with sham-operation group; ▲▲P<0.01, compared with Aβ+NS group; ##P<0.01, compared with Aβ+NS group. Data are presented as mean ± standard deviation (n = 6). NS: normal saline; Aβ: amyloid-β; L-Rb1: Ginsenoside Rb1 low dosage group; M-Rb1: Ginsenoside Rb1 medium dosage group; H-Rb1: Ginsenoside Rb1 high dosage group.
Tunel assay results
Microscopic inspection of the cortical sections from normal control and sham-operation rats revealed morphologically normal neurons with no TUNEL reaction. Compared with the control group, the number of apoptotic cells was significantly increased in the model group (P<0.01; Figure 6). After treatment with donepezil or Rb1, the number of TUNEL-positive cells were significantly reduced, compared with the model group (P<0.01). Of the Rb1 treatment, a dose of 25 mg/kg/per day produced the strongest effect, which was comparable to that 12.5 mg/kg/per day or 50 mg/kg/per day.
Figure 6.
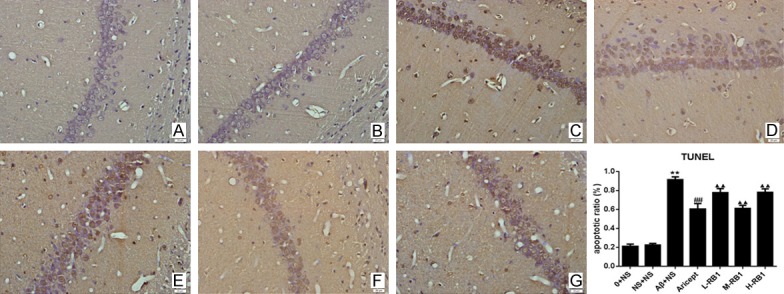
TUNEL staining showing cell apoptosis in the CA1 region of hippocampus (×400). A-G: Control, sham operation, control model, Aricept, Rb1 low, medium, high dosage groups, respectively. Apoptosis was expressed as the percentage of the number of TUNEL-positive cells to the total number of cells. The bar chart shows the apoptosis ratio of hippocampus neuron for each group. Data are presented as mean ± standard deviation (n = 6). **P<0.01, compared with sham-operation group in the same time point; ▲▲P<0.01, compared with Aβ+NS group in the same time point; ##P<0.01, compared with Aβ+NS group in the same time point. 0+NS: normal control group; NS+NS: sham-operated group; Aβ+NS: model control group; Aricept: treated with Aricept [1.67 mg/(kg·d)]; L-Rb1: treated with Rb1 (a low dose of 12.5 mg/(kg·d)); M-Rb1: treated with Rb1 (a medium dose of 25 mg/(kg·d)); H-Rb1: treated with RB1 (a high dose of 50 mg/(kg·d)).
Discussion
AD is a specific form of dementia in aged humans, affecting more than 35 million individuals worldwide [1]. The disease is characterized by cognitive impairments, gradual memory loss and deterioration of language skills [17]. Although environmental and age factors might increase the risk of the disorder, genetic background is significantly implicated in AD [18,19], the definite mechanisms of neuronal degeneration in AD are still unknown. Available data presents that in the amyloid precursor protein (APP) metabolic abnormalities, as a causative factor, which can lead to mitochondrial dysfunction and eventually cell death. Amyloid-beta (Aβ), derived from APP, is a crucial initial factor that triggers a complex pathological cascade [20]. The deposition of aggregated Aβ triggers a cellular stress response known as the unfolded protein response (UPR); the UPR signaling pathway is a cellular defense system for dealing with the accumulation of misfolded proteins, but switches to apoptosis when endoplasmic reticulum stress is prolonged [21] meanwhile the toxic effect of Aβ is manifested by ROS generation, induction of apoptosis and impaired memory [22]. In vivo and in vitro experiments have indicated that soluble Aβ, by reducing cytochrome oxidase activity and increasing hydrogen peroxide generation, impairs mitochondria metabolism [23]. As the overproduction and accumulation of β-amyloid (Aβ) protein has been proposed as a pivotal event in AD pathogenesis [4], the injection of Aβ1-40 into rat hippocampus provides an effective model to impair memory and to elicit the pathologic changes of AD is feasible [24-26]. In the present study, we found that Aβ1-40-injection can induce the impairment of spatial learning and memory and Rb1 treatment can also effectively ameliorate Aβ-induced memory deficits.
The accumulation of Aβ can induce neuronal apoptosis, which plays a pivotal role in AD pathogenesis [27]. The conditions of inducing apoptosis including serum starvation, reactive oxygen species (ROS), nitric oxide (NO), UV and γ-irradiation, glucocorticoid and Bax overexpression are known as major factors to cause redistribution of cytochrome c (cyt c) from the intermembrane space of mitochondria to the cytoplasm [28,29]. Bcl-2 family of proteins tightly regulate the Cyt c release [30]. The family composed of a wide variety of anti-apoptosis protein such as Bcl-2 and pro-apoptotic protein such as Bax. The general balance between anti- and pro-apoptotic proteins determines the activation of intrinsic apoptotic signals involving mitochondria [31]. Caspase-9 is known as an important factor in the development of mammalian nervous system and is the main initiator caspase in intrinsic apoptotic pathway involving mitochondria [32]. When these upstream caspases are activated, they activation triggers downstream effector caspases cleavage such as caspase-3, which can cleave cytoskeletal and nuclear proteins to induce apoptosis [33]. Caspase-3 is the effector caspase and final executor of apoptosis [7]. In the present study, we found that Rb1 can ameliorate Alzheimer’s disease through apoptotic signaling pathway, which could down regulate the expression of Bax and caspase-3 and increase the expression of Bcl-2 in the hippocampus of rats which with Aβ1-40-induced.
Ginseng is one of the most widely used herbal medicines in human. Ginsenosides are classified into three categories based on their structural differences. The panaxadiol group includes Rb1, Rb2, Rb3, Rc, Rd, Rg3, Rh2, and Rs1 and panaxatriol group includes Re, Rf, Rg1, Rg2, and Rh1. Ro is classified as oleanolic acid group [34]. With the deep study of AD in decades, many pausible targets to treat AD have been suggested as followings: 1) increase in the uptake of choline in central nervous system, 2) release of acetylcholine from hippocampus, 3) increased activity or expression of choline acetyltransferase, 4) protection against the Aβ or tau protein-induced neurotoxic effects by several mechanisms including inhibition of neuroinflammation, increased production of neurotrophic factor, and regulation of apoptotic processes, 5) repair of Aβ-damaged neuronal networks by increased neurogenesis and synaptic plasticity, and 6) reducing the level of Aβ by decreased production or increased elimination [35]. The results of the present study demonstrated that Rb1 can protect neurons against Aβ1-40-induced apoptotic insults. Thus, we demonstrated that in vivo treatment with G-Rb1 exerted protective effects against Aβ-induced injury in rat hippocampus.
In this study, we demonstrated that the treatment of Rb1 can effectively ameliorate the Alzheimer’s disease, reduce Bax and Caspase-3 expression and increase Bcl-2 expression in hippocampus of AD rats, especially the mid-ginsenoside Rb1 (25 mg/kg/d). Levels of Bax and Caspase-3 expression in hippocampus of AD normal saline group were remarkably increased, meanwhile the Bcl-2 expression in this group is reduced, compared to any other groups. Ginsenoside Rb1 may down-regulate Bax and Caspase-3 expression, and increase level of Bcl-2, then inhibit neuronal apoptosis process. Moreover, further studies are needed to explain other involved protective effects and mechanisms of ginsenoside Rb1 on AD according to clinical practice.
Acknowledgements
This project was supported by the Natural Science Foundation of China [No. 30973780] (to Haiyan Hu), [No. 81601705] (to Kailiang Zhou); Zhejiang Province Natural Science Foundation [No. Y2080273] (to Haiyan Hu); Zhejiang Provincial Medicine and Health Technology Project [No. 2017KY472] (to Kailiang Zhou); Project of Wenzhou Municipal Science and Technology Bureau in Zhejiang province [Y20080106] (to Haiyan Hu).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 2.Mattson MP. Cellular actions of beta-amyloid precursor protein and its soluble and fibrillogenic derivatives. Physiol Rev. 1997;77:1081–1132. doi: 10.1152/physrev.1997.77.4.1081. [DOI] [PubMed] [Google Scholar]
- 3.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 4.Luo D, Hou X, Hou L, Wang M, Xu S, Dong C, Liu X. Effect of pioglitazone on altered expression of Abeta metabolism-associated molecules in the brain of fructose-drinking rats, a rodent model of insulin resistance. Eur J Pharmacol. 2011;664:14–19. doi: 10.1016/j.ejphar.2011.04.045. [DOI] [PubMed] [Google Scholar]
- 5.Tanzi RE, Moir RD, Wagner SL. Clearance of Alzheimer’s Abeta peptide: the many roads to perdition. Neuron. 2004;43:605–608. doi: 10.1016/j.neuron.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 6.Pompl PN, Yemul S, Xiang Z, Ho L, Haroutunian V, Purohit D, Mohs R, Pasinetti GM. Caspase gene expression in the brain as a function of the clinical progression of Alzheimer disease. Arch Neurol. 2003;60:369–376. doi: 10.1001/archneur.60.3.369. [DOI] [PubMed] [Google Scholar]
- 7.Gross A, Jockel J, Wei MC, Korsmeyer SJ. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 1998;17:3878–3885. doi: 10.1093/emboj/17.14.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Amelio M, Sheng M, Cecconi F. Caspase-3 in the central nervous system: beyond apoptosis. Trends Neurosci. 2012;35:700–709. doi: 10.1016/j.tins.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Mook-Jung I, Hong HS, Boo JH, Lee KH, Yun SH, Cheong MY, Joo I, Huh K, Jung MW. Ginsenoside Rb1 and Rg1 improve spatial learning and increase hippocampal synaptophysin level in mice. J Neurosci Res. 2001;63:509–515. doi: 10.1002/jnr.1045. [DOI] [PubMed] [Google Scholar]
- 10.Fujita K, Hakuba N, Hata R, Morizane I, Yoshida T, Shudou M, Sakanaka M, Gyo K. Ginsenoside Rb1 protects against damage to the spiral ganglion cells after cochlear ischemia. Neurosci Lett. 2007;415:113–117. doi: 10.1016/j.neulet.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Sakanaka M, Zhu P, Zhang B, Wen TC, Cao F, Ma YJ, Samukawa K, Mitsuda N, Tanaka J, Kuramoto M, Uno H, Hata R. Intravenous infusion of dihydroginsenoside Rb1 prevents compressive spinal cord injury and ischemic brain damage through upregulation of VEGF and Bcl-XL. J Neurotrauma. 2007;24:1037–1054. doi: 10.1089/neu.2006.0182. [DOI] [PubMed] [Google Scholar]
- 12.Yuan QL, Yang CX, Xu P, Gao XQ, Deng L, Chen P, Sun ZL, Chen QY. Neuroprotective effects of ginsenoside Rb1 on transient cerebral ischemia in rats. Brain Res. 2007;1167:1–12. doi: 10.1016/j.brainres.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 13.Liu M, Zhang J. Effects of ginsenoside Rb1 and Rg1 on synaptosomal free calcium level, ATPase and calmodulin in rat hippocampus. Chin Med J (Engl) 1995;108:544–547. [PubMed] [Google Scholar]
- 14.Liao B, Newmark H, Zhou R. Neuroprotective effects of ginseng total saponin and ginsenosides Rb1 and Rg1 on spinal cord neurons in vitro. Exp Neurol. 2002;173:224–234. doi: 10.1006/exnr.2001.7841. [DOI] [PubMed] [Google Scholar]
- 15.Hu HY, Cui ZH, Li HQ, Wang YR, Chen X, Li JH, Xv DM, Zheng GQ. Fumanjian, a classic Chinese herbal formula, can ameliorate the impairment of spatial learning and memory through apoptotic signaling pathway in the hippocampus of rats with Abeta 1-40-induced Alzheimer’s disease. Evid Based Complement Alternat Med. 2014;2014:942917. doi: 10.1155/2014/942917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L, Yu S, Zhang R, Xing Y, Li Y, Li L. Tetrahydroxystilbene glucoside antagonizes age-related alpha-synuclein overexpression in the hippocampus of APP transgenic mouse model of Alzheimer’s disease. Restor Neurol Neurosci. 2013;31:41–52. doi: 10.3233/RNN-120260. [DOI] [PubMed] [Google Scholar]
- 17.Blennow K, de Leon MJ, Zetterberg H. Alzheimer’s disease. Lancet. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 18.Levy-Lahad E, Wasco W, Poorkaj P, Romano DM, Oshima J, Pettingell WH, Yu CE, Jondro PD, Schmidt SD, Wang K, et al. Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science. 1995;269:973–977. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- 19.Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K, Tsuda T, Mar L, Foncin JF, Bruni AC, Montesi MP, Sorbi S, Rainero I, Pinessi L, Nee L, Chumakov I, Pollen D, Brookes A, Sanseau P, Polinsky RJ, Wasco W, Da Silva HA, Haines JL, Perkicak-Vance MA, Tanzi RE, Roses AD, Fraser PE, Rommens JM, St George-Hyslop PH. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature. 1995;375:754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- 20.Golde TE, Dickson D, Hutton M. Filling the gaps in the abeta cascade hypothesis of Alzheimer’s disease. Curr Alzheimer Res. 2006;3:421–430. doi: 10.2174/156720506779025189. [DOI] [PubMed] [Google Scholar]
- 21.Kang EB, Kwon IS, Koo JH, Kim EJ, Kim CH, Lee J, Yang CH, Lee YI, Cho IH, Cho JY. Treadmill exercise represses neuronal cell death and inflammation during Abeta-induced ER stress by regulating unfolded protein response in aged presenilin 2 mutant mice. Apoptosis. 2013;18:1332–1347. doi: 10.1007/s10495-013-0884-9. [DOI] [PubMed] [Google Scholar]
- 22.Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, Caspersen C, Chen X, Pollak S, Chaney M, Trinchese F, Liu S, Gunn-Moore F, Lue LF, Walker DG, Kuppusamy P, Zewier ZL, Arancio O, Stern D, Yan SS, Wu H. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer’s disease. Science. 2004;304:448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- 23.Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of a beta accumulation in Alzheimer’s disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet. 2006;15:1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- 24.Shin RW. Interaction of aluminum with paired helical filament tau is involved in neurofibrillary pathology of Alzheimer’s disease. Gerontology. 1997;43(Suppl 1):16–23. doi: 10.1159/000213882. [DOI] [PubMed] [Google Scholar]
- 25.Yamaguchi Y, Miyashita H, Tsunekawa H, Mouri A, Kim HC, Saito K, Matsuno T, Kawashima S, Nabeshima T. Effects of a novel cognitive enhancer, spiro[imidazo-[1,2-a] pyridine-3,2-indan] -2(3H)-one (ZSET1446), on learning impairments induced by amyloid-beta1-40 in the rat. J Pharmacol Exp Ther. 2006;317:1079–1087. doi: 10.1124/jpet.105.098640. [DOI] [PubMed] [Google Scholar]
- 26.Zou K, Kim D, Kakio A, Byun K, Gong JS, Kim J, Kim M, Sawamura N, Nishimoto S, Matsuzaki K, Lee B, Yanagisawa K, Michikawa M. Amyloid beta-protein (Abeta)1-40 protects neurons from damage induced by Abeta1-42 in culture and in rat brain. J Neurochem. 2003;87:609–619. doi: 10.1046/j.1471-4159.2003.02018.x. [DOI] [PubMed] [Google Scholar]
- 27.Zhou ZD, Chan CH, Ma QH, Xu XH, Xiao ZC, Tan EK. The roles of amyloid precursor protein (APP) in neurogenesis: implications to pathogenesis and therapy of Alzheimer disease. Cell Adh Migr. 2011;5:280–292. doi: 10.4161/cam.5.4.16986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 29.Kroemer G, Blomgren K. Mitochondrial cell death control in familial Parkinson disease. PLoS Biol. 2007;5:e206. doi: 10.1371/journal.pbio.0050206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 31.Woo RS, Lee JH, Yu HN, Song DY, Baik TK. Expression of ErbB4 in the apoptotic neurons of Alzheimer’s disease brain. Anat Cell Biol. 2010;43:332–339. doi: 10.5115/acb.2010.43.4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Degterev A, Boyce M, Yuan J. A decade of caspases. Oncogene. 2003;22:8543–8567. doi: 10.1038/sj.onc.1207107. [DOI] [PubMed] [Google Scholar]
- 33.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 34.Tachikawa E, Kudo K, Harada K, Kashimoto T, Miyate Y, Kakizaki A, Takahashi E. Effects of ginseng saponins on responses induced by various receptor stimuli. Eur J Pharmacol. 1999;369:23–32. doi: 10.1016/s0014-2999(99)00043-6. [DOI] [PubMed] [Google Scholar]
- 35.Jesky R, Hailong C. Are herbal compounds the next frontier for alleviating learning and memory impairments? An integrative look at memory, dementia and the promising therapeutics of traditional chinese medicines. Phytother Res. 2011;25:1105–1118. doi: 10.1002/ptr.3388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


