Abstract
IgA nephropathy is the most common form of primary glomerulonephritis and an important cause of kidney failure. Cordyceps sinensis (CS) is a parasitic fungus that has a long history of use in Chinese medicine for the treatment of nephritis. Interleukin (IL)-22-producing helper T cells (Th22 cells) have been reported to be involved in lgA nephropathy. Th22 cells link the immune response to tissue inflammation. To elucidate the possible efficacy and mechanisms by which CS counteracts nephritis, we established an IgA nephropathy model in 6-week-old female BALB/c mice. The mice were randomly separated into 3 groups, the normal control, IgA nephropathy and CS (5 mg/kg/d) treatment groups. The Th22 cell frequencies and the relative pathological and cytokine changes were measured with flow cytometry, whereas the serum chemokine ligand 27 (CCL27) and IL-22 concentrations were detected with ELISA. The Th22 cell frequency decreased after 1 month of CS therapy. Additionally, mesangial cell proliferation decreased. Moreover, the chemokine receptor type 10 (CCR10), CCL27 and IL-22 expression levels were significantly reduced. In conclusion, CS may modulate the chemotaxis of Th22 cells to suppress inflammatory responses in IgA nephropathy.
Keywords: Cordyceps sinensis, IgA nephropathy, Th22 cells, CCR10, CCL27
Introduction
IgA nephropathy (IgAN) represents the leading cause of kidney failure among East Asian populations and is the most frequent form of primary glomerulonephritis among Asians, Europeans and some regions of the USA [1-3]. Increasing evidence has shown that inflammatory immune responses play an important role in the development and progression of IgAN.
Native CD4+ T cells can develop into various helper T subsets with different cytokine profiles that play discriminative roles in translating antigen-specific immune responses into tissue functions or immunopathologic changes. Cells that produce IL-22 are part of a new helper T (Th) cell subgroup that may be important for skin pathology [2]. Interleukin (IL)-22 is a member of the IL-10 cytokine family and is involv-ed in inflammatory and wound healing processes. Th22 cells are characterized by particularly high IL-22 production and specifically express the CCR10 [4]. Th22 cells are distinct from Th17 and Th1 cells and play important roles in skin homeostasis and pathological changes. Accumulating evidence suggests that IL-22 is important in epithelial cell homeostasis, infections, tissue repair and wound healing [5-7]. Th22 cells are positively correlated with the plasma IL-22 level in IgAN patients. Chemokines, which are members of a protein superfamily of structurally related, small, secreted proteins, are key mediators of leukocyte recruitment to inflammatory lesions where damage may have occurred. Chemokines have been shown to regulate trafficking of distinct leukocyte subsets into peripheral tissues [8]. CCL27 selectively attracts cutaneous lymphocyte-associated antigen (CLA)+ memory T cells by interacting with the CCR10 expressed by lymphocytes. The CCL27-CCR10 interaction plays a pivotal role in T cell-mediated skin inflammation [9]. Moreover, a significant positive correlation has been observed between Th22 and Th17 cells in IgAN patients. Furthermore, IgAN patients with proteinuria have a higher percentage of Th22 cells than IgAN patients without proteinuria [10]. In our previous study, we found an overrepresentation of Th22 cells in lgAN patients, which might be attributable to the actions of kidney chemokines and cytokines. Additionally, we found that Losartan and Dexamethasone might suppress inflammatory responses by inhibiting the chemotaxis of Th22 cells in IgA nephropathy patients [11,12].
Cordyceps sinensis (CS), which is a well-known traditional Chinese medicine, is a fungus that develops stroma and is found on the larvae of lepidopteran caterpillars. Numerous studies have demonstrated multiple pharmacologic actions of CS [13-15], such as reducing damage to renal tubules and protecting the Na+-K+-ATPase on cellular membranes [16]. CS can decrease chronic renal insufficiency, reduce resistance and pressure in the arteries and promote platelet formation [17]. Thus, CS may help prevent hypoxia by acting as a monoamine oxidase inhibitor [18]. In our previous study, we found that the proportion of Th22 cells was increased in IgA nephropathy [11,19]. Because the role for CS in the regulation of Th22 cells is unknown, we aim to investigate whether CS blocks Th22 cell infiltration and to demonstrate the possible mechanisms of action. We established an lgAN mouse model to elucidate how CS modulated Th22 cells.
Materials and methods
Ethics statement
The mice were housed under controlled humidity, temperature and lighting conditions in facilities accredited by the Experimental Animal Center of Central South University (Changsha, Hunan, China). All animals had free access to standard mouse chow and drinking water. Our study was conducted in accordance with the recommendations from the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health. The study protocol was approved by the Animal Experimental Ethics Committee of Hunan Province (Permit Number: 20150003). All efforts were made to minimize animal suffering.
Experimental animals
Female BALB/c mice (19±2 g, n=20, 5 mice/treatment group) were obtained from the Experimental Animal Center of Central South University (Changsha, Hunan, China) at 6 weeks of age. The IgAN model was induced by administering bovine serum albumin (BSA) (Roche, USA) in acidified water, CCL4, and castor oil combined with LPS (Sigma, USA) (50 µg) at different time points for two months after one week of adaptive feeding [20].
Drug treatment
The mice were randomly divided into 3 groups: the control mice (control), IgAN mice (IgAN) and CS-treated IgAN mice (CS-IgAN). For the CS-treated IgAN mouse group, IgAN mice were sensitized by intragastric gavage with CS (5 mg/kg/d) [21]. The control and IgAN groups received an equal amount of distilled water. At 4 weeks, all mice were anesthetized with 10% chloral hydrate, blood was collected by orbital bleeding, and the mice were sacrificed via cervical dislocation. Then, the tissues were collected.
Leukocyte isolation
The mice were exsanguinated via the retro-orbital plexus. The blood was placed in a heparin anticoagulant tube and diluted with PBS in a 1:2 ratio. The diluted blood was layered onto 3 ml of Ficoll lymphocyte separation liquid (GE, USA) in a 15-ml centrifuge tube and centrifuged for 30 min at 450 × g. Following centrifugation, the layer containing the peripheral mononuclear cells (PBMCs) was transferred to another test tube and washed twice with 10 ml of PBS at 450 × g. The PBMCs were resuspended in 1 ml of RPMI 1640 medium and counted.
Flow cytometry
The isolated murine leukocytes were equally distributed into tubes. The expression of T cell markers in the blood was determined via flow cytometry after the cells were stained for surface or intracellular markers with anti-mouse-specific antibodies conjugated to APC/Cy7, FITC, or PE. These antibodies included anti-CD3, anti-CD4 and anti-IL-22 and were purchased from BD Biosciences (Franklin Lakes, NJ, USA) or R&D Systems (Minneapolis, MN, USA). Appropriate species-matched antibodies served as the isotype controls. After stimulation with Leukocyte Activation Cocktail (BD, USA) in an incubator for 6 h at 37°C with 5% CO2, the cells were stained with fluorochrome-labeled antibodies specific for CD3 (APC-Cy7; BD, USA) and CD4 (FITC; BD) for 30 min at 4°C. After membrane permeabilization and fixation, intracellular staining for IL-22 (PE, BioLegend, USA) was performed. Flow cytometry was performed using the FACS Can to II flow cytometer (BD Biosciences), and the data were analyzed using the BD FACS and FlowJo software.
Histological analysis
The renal tissues were fixed in 4% paraformaldehyde, embedded in paraffin and serially cut. Tissue sections (1.5-mm thick) were stained with a periodic acid-Schiff reagent. The stained kidney sections were examined by a renal pathologist under a light microscope. For the immunofluorescence analysis, the renal tissues were cut into frozen slices and fixed in acetone for 1 min. After fixation, 5% normal goat serum in PBS (pH 7.4) was used to block non-specific protein binding sites. IgA in renal tissues was detected with fluorescein-labeled goat anti-mouse IgA (Abcam, USA). The renal tissues were incubated at 37°C for 1 h, washed 3 times in PBS for 3 min, mounted with glycerol, and visualized under a fluorescence microscope. Then, electron microscopic examination procedures were performed. The samples were examined using the H-7700 transmission electron microscope (Hitachi, Japan).
Enzyme-linked immunosorbent assay (ELISA)
The CCL27 and IL-22 levels in the renal tissue homogenates and sera were quantified using ELISA kits (eBiosciences, USA) according to the manufacturer’s instructions. All samples were assayed in duplicate.
Statistical analysis
The 2-tailed Mann-Whitney non-parametric U test was used to determine differences between two groups. A P-value < 0.05 was considered significant. The statistical analyses were performed using the SPSS 19.0 software (SPSS Inc., Chicago, IL, USA).
Results
The lgAN model and the basic pathological manifestations of the kidney were verified
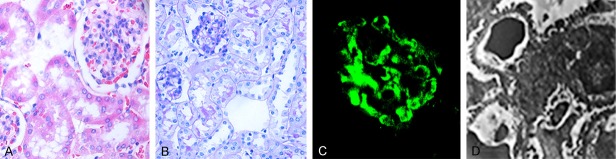
Two BALB/c mice were randomly selected in the 9th week. Twenty-four hour urine specimens were collected in metabolic cages. The kidneys were removed for pathological examination. Microscopic hematuria was observed, and the qualitative proteinuria results were positive. Glomerular mesangial cell proliferation was demonstrated with hematoxylin and eosin (HE) and periodic acid-Schiff (PAS) staining. Specifically, lgA was deposited in the glomeruli, and electron microscopy showed electron-dense deposits in the mesangial area (Figure 1).
Figure 1.
Basic pathological characteristics of the IgA nephropathy model mice. A: HE staining (× 400); B: PAS staining (× 400); C: Immunofluorescence for IgA (× 400); D: Electron microscopy (× 2000).
Th22 cells were decreased after CS treatment
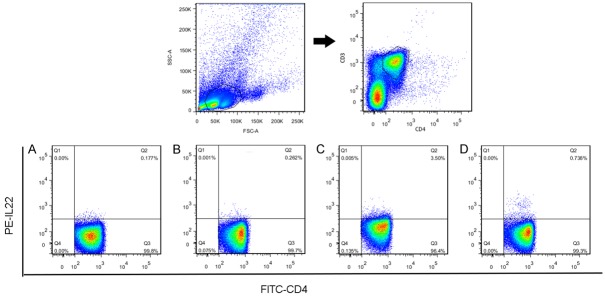
After we confirmed that the model was successfully generated, the lymphocytes were separated according to the treatment group and examined using flow cytometry to detect Th22 cells in the 13th week. In the first step, we analyzed the expression of Th22 cells in the normal BALB/c, lgAN and CS-treated lgAN mice. The isolated blood leukocytes were stained and analyzed to evaluate intracellular expression of the cytokine IL-22. Th22 cells were significantly elevated in the IgAN mice compared with the normal BABL/c mice (0.22±0.07% vs 4.71±0.56%, P < 0.01). However, the frequency of the Th22 cells was significantly reduced after CS treatment (4.71±0.56% vs 0.53±0.40%, P < 0.01) (Figure 2).
Figure 2.
Representative flow chart for the percentages of Th22 cells in homotype IgG (A), normal (B), IgAN (C), and CS-treated IgAN (D). The Th22 cell population within CD4+ T cells was identified based on CD3+ and CD4+ expression (n=5 mice/group). The percentages of Th22 cells were determined by flow cytometry.
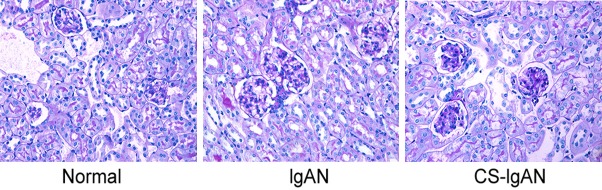
Mesangial cell proliferation decreased after CS treatment
Next, we determined whether changes occurred in renal pathological features that would contribute to the decrease in Th22 cells. We analyzed the PAS-stained images for each group and compared them to the normal group. Mesangial cells proliferated in the lgAN group. After 1 month of CS treatment, mesangial cell proliferation decreased significantly. These results suggest that the onset of lgAN in mice may be due to a decrease in effector Th22 cells during disease progression, as shown in Figure 3.
Figure 3.
Representative photographs of kidney PAS staining for each group. The IgAN group demonstrates pronounced proliferation of the mesangium compared with the normal group, whereas proliferation was reduced in the CS-IgAN group.
Reduced CCR10 and CCL27 expression in the CS-treated groups
We tested the possible mechanism by which CS decreased the ratio of Th22 cells and investigated whether inhibition of chemotaxis was associated with CS treatment. We investigated the role of the chemokine CCL27 and its receptor CCR10. We examined whether CS inhibited the chemotaxis mediated by CCR10 and CCL27. The CCR10 and CCL27 expression levels were examined using immunohistochemistry. Based on the immunohistochemistry analysis, the normal BALB/c mice did not express CCR10 or CCL27, whereas the expression of these molecules was significantly increased in the lgAN mice. However, after treatment with CS for one month, both CCL27 and CCR10 expression was reduced significantly (Figure 4).
Figure 4.
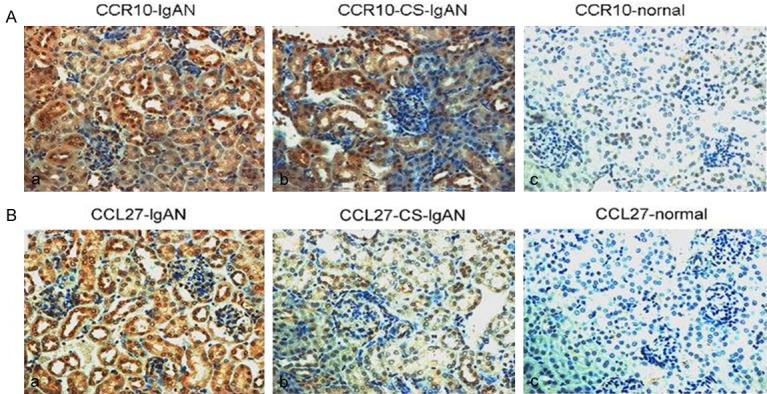
CCR10 and CCL27 expression in the biopsies of the control group, the IgAN group and the CS-IgAN group after one month of CS treatment. Immunohistochemistry was performed on renal biopsies from BALB/c mice (× 400). A prominent accumulation of CCR10-positive or CCL27-positive cells was found around the glomerular and tubular membranes.
Inhibition of chemotaxis reduced Th22 cell infiltration
Recruitment from peripheral blood could contribute to the increased number of Th22 cells in the kidney. Lymphocyte migration is tightly regulated by chemokine/CCR interactions. The plasma IL-22 and CCL27 concentrations were significantly higher in the IgAN group than in the normal group. After the CS treatments, the concentrations of both IL-22 (154.0±12.0 pg/ml, 634.2±29.8 pg/ml and 383.5±7.9 pg/ml, P < 0.01) and CCL27 (327.9±7.5 pg/ml, 465.0±6.9 pg/ml and 373.0±11.5 pg/ml, P < 0.01) were significantly decreased, which was consistent with the immunohistochemistry results (Figure 5).
Figure 5.
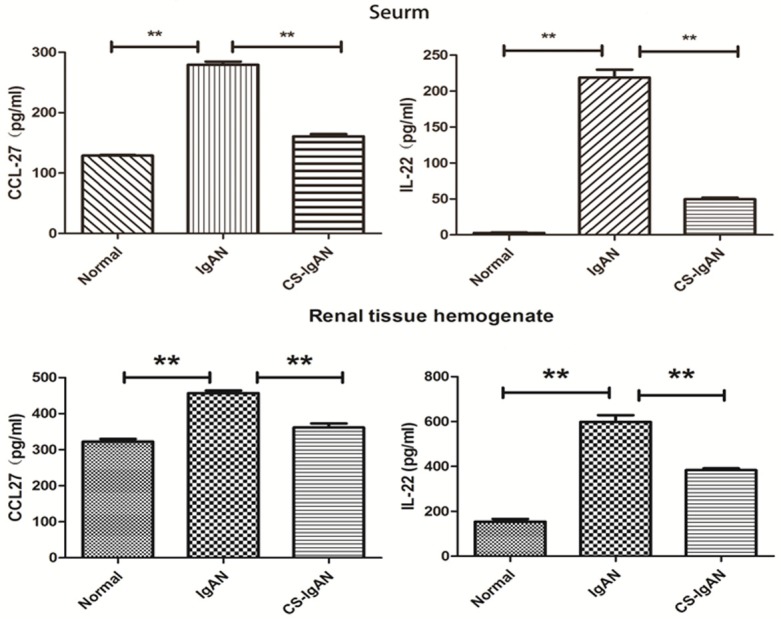
Plasma concentrations of the chemokines CCL27 and IL-22. Th22 cells produce IL-22, The CCL27 and IL-22 concentrations were measured using ELISA. The bars indicate the means; the data are reported as the means ± SEMs from 5 independent experiments. Differences between groups were compared using paired-sample T tests. **P < 0.01 compared with the control.
Discussion
To the best of our knowledge, the mechanism described herein by which CS may prevent the kidney damage induced by Th22 cells is novel. In this study, we compared the Th22 cell frequencies and the relative changes in chemokines, chemokine receptors and renal pathology before and after drug intervention. The Th22 cell frequency was increased in the IgAN model compared to the normal controls but decreased after CS treatment. This finding was consistent with the renal pathological changes and ELISA results.
IgAN has been recognized as an autoimmune disease characterized by deposition of IgA in the mesangial area. Up to 40% of patients with lgAN progress to end-stage renal disease [22]. Therefore, preventing, halting and decreasing the rate of progression of lgAN is a very worthwhile goal [23]. Disease progression is determined mainly by the balance between the microorganism and the host defense system [24,25]. IL-22 is a T cell mediator that directly promotes innate, non-specific immunity in tissues [5,24], which may be produced by skin-infiltrating lymphocytes that are potentially involved in the initiation and/or maintenance of the pathogenesis of psoriasis [6]. The possible mechanisms by which Th22 cells infiltrate the kidney have been elucidated in our early stage research.
CS is a precious herbal medicine that has been used to treat diseases for many years in China. Its application areas include the nervous system, respiratory system and renal system, and its extracts have been suggested to have a protective effect in renal tubular cells [26-28]. Many bioactive components have been extracted from CS, including nucleosides, polysaccharides, sterols, proteins, amino acids and polypeptides. These constituents correspond to demonstrated pharmacologic actions, such as anti-inflammatory, antioxidant, anti-tumor, anti-apoptotic, and immunomodulatory actions [29-33]. CS ameliorated albumin-induced EMT of HK2 cells by decreasing NADPH oxidase activity and inhibiting ROS production [34]. Additionally, CS prevented the recurrence of lupus nephritis, protected kidney function [35] and inhibited mesangial proliferation [36]. Inhibition of activated human mesangial cell proliferation by the natural product of CS may have implications for the treatment of IgA mesangial nephropathy [37]. Furthermore, CS has a mitogenic effect on spleen lymphocytes and is capable of increasing IL-2 production by splenocytes from CRF rats [31,38]. The IL-2 absorbency of splenocytes is promoted by CS and exhibits therapeutic effects on CRF rats, such as decreasing the BUN and serum creatinine levels and increasing the hemoglobin level. These results indicate that CS has a regulatory effect on cellular immunity in CRF rats [39]. CS can modulate the Treg-to-Th17 cell ratio in vivo and thus contributes to the inhibition of diabetes [40], possibly through chemokine activity. In contrast, cordycepin may induce CCL27 chemokine expression to counter inflammation. CS may contribute to the prevention mesangial cell proliferation. These features have been further validated by our study.
CS has been proven to be a powerful immunomodulatory compound [28,41]. An important immunomodulatory finding from our study is the new immune effect of CS. Our study showed that Th22 cells were increased significantly in lgAN mice, whereas CS reduced the number of Th22 cells. After CS treatment, mesangial proliferation was also decreased. The kidney immunohistochemistry analysis showed that CCL27 and CCR10 expression was also decreased. We speculated that CS might inhibit the chemotaxis action of Th22 cells, resulting in improved kidney function. We confirmed a significant increase in the frequency of Th22 cells, which was inhibited by CS. In light of this new evidence for the use of CS in IgAN, our study calls for further investigation of CS and future clinical studies.
In conclusion, this study shed light on the mechanism by which CS may improve kidney functions by inhibiting the chemotaxis of Th22 cells in lgAN.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Nos. 81173401 and 81470933) and the National Natural Science Foundation for Youth (81401024). The authors would like to thank Dr. Li Xuezhang whose criticisms improved this paper.
Disclosure of conflict of interest
None.
References
- 1.Kiryluk K, Novak J. The genetics and immunobiology of IgA nephropathy. J Clin Invest. 2014;124:2325–2332. doi: 10.1172/JCI74475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nair R, Walker PD. Is IgA nephropathy the commonest primary glomerulopathy among young adults in the USA? Kidney Int. 2006;69:1455–1458. doi: 10.1038/sj.ki.5000292. [DOI] [PubMed] [Google Scholar]
- 3.Pesce F, Schena FP. Worldwide distribution of glomerular diseases: the role of renal biopsy registries. Nephrol Dial Transplant. 2010;25:334–336. doi: 10.1093/ndt/gfp620. [DOI] [PubMed] [Google Scholar]
- 4.Akdis M, Palomares O, van de Veen W, van Splunter M, Akdis CA. TH17 and TH22 cells: a confusion of antimicrobial response with tissue inflammation versus protection. J Allergy Clin Immunol. 2012;129:1438–49. doi: 10.1016/j.jaci.2012.05.003. quiz 1450-1. [DOI] [PubMed] [Google Scholar]
- 5.Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–254. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Boniface K, Guignouard E, Pedretti N, Garcia M, Delwail A, Bernard FX, Nau F, Guillet G, Dagregorio G, Yssel H, Lecron JC, Morel F. A role for T cell-derived interleukin 22 in psoriatic skin inflammation. Clin Exp Immunol. 2007;150:407–415. doi: 10.1111/j.1365-2249.2007.03511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 9.Homey B, Alenius H, Muller A, Soto H, Bowman EP, Yuan W, McEvoy L, Lauerma AI, Assmann T, Bunemann E, Lehto M, Wolff H, Yen D, Marxhausen H, To W, Sedgwick J, Ruzicka T, Lehmann P, Zlotnik A. CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nat Med. 2002;8:157–165. doi: 10.1038/nm0202-157. [DOI] [PubMed] [Google Scholar]
- 10.Peng Z, Tian J, Cui X, Xian W, Sun H, Li E, Geng L, Zhang L, Zhao P. Increased number of Th22 cells and correlation with Th17 cells in peripheral blood of patients with IgA nephropathy. Hum Immunol. 2013;74:1586–1591. doi: 10.1016/j.humimm.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Xiao C, Zhou Q, Li X, Li H, Meng T, Zhong Y, Pu J, Zhu M, Xu Y, Gan L, Sun H, Xiao P. Differentiation and recruitment of IL-22-producing helper T cells in lgA nephropathy. Am J Transl Res. 2016;8:3872–3882. [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao C, Zhou Q, Li X, Li H, Zhong Y, Meng T, Zhu M, Sun H, Liu S, Tang R, Pu J, Xu Y, Xiao P. Losartan and Dexamethasone may inhibit chemotaxis to reduce the infiltration of Th22 cells in IgA nephropathy. Int Immunopharmacol. 2017;42:203–208. doi: 10.1016/j.intimp.2016.11.025. [DOI] [PubMed] [Google Scholar]
- 13.Huang R, Zhou Q, Veeraragoo P, Yu H, Xiao Z. Notch2/Hes-1 pathway plays an important role in renal ischemia and reperfusion injury-associated inflammation and apoptosis and the gamma-secretase inhibitor DAPT has a nephroprotective effect. Ren Fail. 2011;33:207–216. doi: 10.3109/0886022X.2011.553979. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Ao X, Li H, Deng S, Xiao Z, Peng W, Xiang J, Zhou Q. Cordyceps sinensis protects HK2 cells from ischemia-reperfusion injury through Sirt1 pathway. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2017;42:1263–1269. doi: 10.11817/j.issn.1672-7347.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Tu S, Zhou Q, Tang R, Tang T, Hu S, Ao X. [Proapoptotic effect of angiotensin II on renal tubular epithelial cells and protective effect of Cordyceps sinensis] . Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2012;37:67–72. doi: 10.3969/j.issn.1672-7347.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Tian J, Chen XM, Li LS. Effects of Cordyceps sinensis, rhubarb and serum renotropin on tubular epithelial cell growth. Zhong Xi Yi Jie He Za Zhi. 1991;11:547–549. 518. [PubMed] [Google Scholar]
- 17.Chen DM. [Platelet hemopoiesis and ultrastructure observations in mice treated with natural Cordyceps sinensis and its cultured mycelia] . Zhong Yao Tong Bao. 1987;12:47–49. [PubMed] [Google Scholar]
- 18.Wang Q, Zhao Y. Comparison of some pharmacological effects between Cordyceps sinensis (Berk). Sacc. and Cephalosporium sinensis Chen sp. nov. Zhong Yao Tong Bao. 1987;12:42–44. 64. [PubMed] [Google Scholar]
- 19.Gan L, Zhou Q, Li X, Chen C, Meng T, Pu J, Zhu M, Xiao C. Intrinsic renal cells induce lymphocytosis of Th22 cells from IgA nephropathy patients through B7-CTLA-4 and CCL-CCR pathways. Mol Cell Biochem. 2018;441:191–199. doi: 10.1007/s11010-017-3185-8. [DOI] [PubMed] [Google Scholar]
- 20.Meng T, Li X, Ao X, Zhong Y, Tang R, Peng W, Yang J, Zou M, Zhou Q. Hemolytic Streptococcus may exacerbate kidney damage in IgA nephropathy through CCL20 response to the effect of Th17 cells. PLoS One. 2014;9:e108723. doi: 10.1371/journal.pone.0108723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng YJ, Cheng SM, Teng YH, Shyu WC, Chen HL, Lee SD. Cordyceps sinensis prevents apoptosis in mouse liver with D-galactosamine/lipopolysaccharide-induced fulminant hepatic failure. Am J Chin Med. 2014;42:427–441. doi: 10.1142/S0192415X14500281. [DOI] [PubMed] [Google Scholar]
- 22.Donadio JJ, Bergstralh EJ, Offord KP, Spencer DC, Holley KE. A controlled trial of fish oil in IgA nephropathy. Mayo nephrology collaborative group. N Engl J Med. 1994;331:1194–1199. doi: 10.1056/NEJM199411033311804. [DOI] [PubMed] [Google Scholar]
- 23.Krochmal M, Cisek K, Filip S, Markoska K, Orange C, Zoidakis J, Gakiopoulou C, Spasovski G, Mischak H, Delles C, Vlahou A, Jankowski J. Identification of novel molecular signatures of IgA nephropathy through an integrative -omics analysis. Sci Rep. 2017;7:9091. doi: 10.1038/s41598-017-09393-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Da SM, Tiburcio MG, Machado JR, Silva DA, Rodrigues DB, Rodrigues V, Oliveira CJ. Complexity and controversies over the cytokine profiles of t helper cell subpopulations in tuberculosis. J Immunol Res. 2015;2015:639107. doi: 10.1155/2015/639107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H, Tang Y, Wen L, Kong X, Chen X, Liu P, Zhou Z, Chen W, Xiao C, Xiao P, Xiao X. Neferine reduces cisplatin-induced nephrotoxicity by enhancing autophagy via the AMPK/mTOR signaling pathway. Biochem Biophys Res Commun. 2017;484:694–701. doi: 10.1016/j.bbrc.2017.01.180. [DOI] [PubMed] [Google Scholar]
- 26.Cheng Z, He W, Zhou X, Lv Q, Xu X, Yang S, Zhao C, Guo L. Cordycepin protects against cerebral ischemia/reperfusion injury in vivo and in vitro. Eur J Pharmacol. 2011;664:20–28. doi: 10.1016/j.ejphar.2011.04.052. [DOI] [PubMed] [Google Scholar]
- 27.Xiao L, Ge Y, Sun L, Xu X, Xie P, Zhan M, Wang M, Dong Z, Li J, Duan S, Liu F, Xiao P. Cordycepin inhibits albumin-induced epithelial-mesenchymal transition of renal tubular epithelial cells by reducing reactive oxygen species production. Free Radic Res. 2012;46:174–183. doi: 10.3109/10715762.2011.647688. [DOI] [PubMed] [Google Scholar]
- 28.Yuan M, Tang R, Zhou Q, Liu K, Xiao Z, Pouranan V. Effect of Cordyceps sinensis on expressions of HIF-1alpha and VEGF in the kidney of rats with diabetic nephropathy. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2013;38:448–457. doi: 10.3969/j.issn.1672-7347.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Wang J, Wang W, Zhang H, Zhang X, Han C. The chemical constituents and pharmacological actions of cordyceps sinensis. Evid Based Complement Alternat Med. 2015;2015:575063. doi: 10.1155/2015/575063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang TT, Lai HC, Ko YF, Ojcius DM, Lan YW, Martel J, Young JD, Chong KY. Hirsutella sinensis mycelium attenuates bleomycin-induced pulmonary inflammation and fibrosis in vivo. Sci Rep. 2015;5:15282. doi: 10.1038/srep15282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng Y, Huang K, Shen L, Tao YY, Liu CH. Cultured mycelium cordyceps sinensis allevi notates CCl4-induced liver inflammation and fibrosis in mice by activating hepatic natural killer cells. Acta Pharmacol Sin. 2016;37:204–216. doi: 10.1038/aps.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zou YX, Liu YX, Ruan MH, Zhou Y, Wang JC, Chu ZY. Cordyceps sinensis oral liquid inhibits damage induced by oxygen and glucose deprivation in SH-SY5Y cells. Altern Ther Health Med. 2016;22:37–42. [PubMed] [Google Scholar]
- 33.Li DG, Ren ZX. Cordyceps sinensis promotes immune regulation and enhances bacteriostatic activity of PA-824 via IL-10 in Mycobacterium tuberculosis disease. Braz J Med Biol Res. 2017;50:e6188. doi: 10.1590/1414-431X20176188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao L, Ge Y, Sun L, Xu X, Xie P, Zhan M, Wang M, Dong Z, Li J, Duan S, Liu F, Xiao P. Cordycepin inhibits albumin-induced epithelial-mesenchymal transition of renal tubular epithelial cells by reducing reactive oxygen species production. Free Radic Res. 2012;46:174–183. doi: 10.3109/10715762.2011.647688. [DOI] [PubMed] [Google Scholar]
- 35.Lu L. Study on effect of Cordyceps sinensis and artemisinin in preventing recurrence of lupus nephritis. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2002;22:169–171. [PubMed] [Google Scholar]
- 36.Yang LY, Chen A, Kuo YC, Lin CY. Efficacy of a pure compound H1-A extracted from Cordyceps sinensis on autoimmune disease of MRL lpr/lpr mice. J Lab Clin Med. 1999;134:492–500. doi: 10.1016/s0022-2143(99)90171-3. [DOI] [PubMed] [Google Scholar]
- 37.Lin CY, Ku FM, Kuo YC, Chen CF, Chen WP, Chen A, Shiao MS. Inhibition of activated human mesangial cell proliferation by the natural product of Cordyceps sinensis (H1-A): an implication for treatment of IgA mesangial nephropathy. J Lab Clin Med. 1999;133:55–63. doi: 10.1053/lc.1999.v133.a94239. [DOI] [PubMed] [Google Scholar]
- 38.Wang D, Zhang Y, Lu J, Wang Y, Wang J, Meng Q, Lee RJ, Wang D, Teng L. Cordycepin, a natural antineoplastic agent, induces apoptosis of breast cancer cells via caspase-dependent pathways. Nat Prod Commun. 2016;11:63–68. [PubMed] [Google Scholar]
- 39.Cheng Q. Effect of cordyceps sinensis on cellular immunity in rats with chronic renal insufficiency. Zhonghua Yi Xue Za Zhi. 1992;72:27–29. 63. [PubMed] [Google Scholar]
- 40.Shi B, Wang Z, Jin H, Chen YW, Wang Q, Qian Y. Immunoregulatory cordyceps sinensis increases regulatory T cells to Th17 cell ratio and delays diabetes in NOD mice. Int Immunopharmacol. 2009;9:582–586. doi: 10.1016/j.intimp.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 41.Li DG, Ren ZX. Cordyceps sinensis promotes immune regulation and enhances bacteriostatic activity of PA-824 via IL-10 in Mycobacterium tuberculosis disease. Braz J Med Biol Res. 2017;50:e6188. doi: 10.1590/1414-431X20176188. [DOI] [PMC free article] [PubMed] [Google Scholar]