Abstract
This study reports a case of a 4-year-old boy patient with abnormalities of muscle tone, movement and motor skills, as well as unstable gait leading to frequent falls. The results of the electroencephalogram (EEG) indicate moderately abnormal EEG, accompanied by irregular seizures. Based on these clinical characteristics, the patient was diagnosed with cerebral palsy (CP) in our hospital. In this study, the patient was treated with umbilical cord mesenchymal stem cell (UC-MSC) transplantation therapy. This patient received UC-MSC transplantation 3 times (5.3*107) in total. After three successive cell transplantations, the patient recovered well and showed obvious improvements in EEG and limb strength, motor function, and language expression. However, the improvement in intelligence quotient (IQ) was less obvious. These results indicate that UC-MSC transplantation is a promising treatment for cerebral palsy.
Keywords: Cerebral palsy, umbilical cord, mesenchymal stem cell, transplantation
Introduction
Human umbilical cord-derived mesenchymal stem cells (UC-MSCs) are a class of cells with significant self-renewal and multi-lineage differentiation properties [1,2]. Human umbilical cord Wharton’s jelly contains abundant mesenchymal stem cells that are immature compared to bone marrow MSCs [2]. Traditionally, the study of the therapeutic potential of these cells has focused on tissue repair and regeneration, due to the ability of the cells to differentiate into many cell types [3]. UC-MSCs have strong biological activity and have the ability to differentiate. In other words, repeatedly passaged and amplified UC-MSCs still maintain a strong function, which provides a sufficient source of MSCs for experimental and clinical use [4]. Notably, chemical and neurotrophic factors [5,9] can induce UC-MSCs to differentiate into neural stem cells. UC-MSCs can also differentiate into oligodendrocyte precursor cells, secrete a variety of nerve growth factors (e.g., vascular endothelial growth factor(VEGF), Glial cell line-Derived Neurotrophic Factor (GDNF), and brain-derived neurotrophic factor (BDNF), and promote axonal growth [6]. UC-MSC-differentiated cells not only exhibit the morphology and phenotype of oligodendrocyte precursor cells but also perform their corresponding function [7,8]. Here, we report the case of a 4-year-old boy with cerebral palsy (CP) who was treated with UC-MSCs. The patient received transplantation 3 times (5.3*107) and recovered very well. Thus, we suggest that UC-MSCs transplantation may be an important and feasible method to treat CP.
Case report
In 2011, a 4-year-old boy twitched for a year and a half. The patient was born full term with a birth weight of 3 kg, and he stayed in the incubator for 11 days due to lack of oxygen. When this patient was 18 months old, he experienced a convulsion after respiratory infections, and the following symptoms appeared: staring out of both eyes, clenched teeth, pale face, did not react when his name was called, and urinary incontinence, as well as twitching of the left limbs, corners of the mouth, and eyes. These symptoms abated after 20 minutes. The patient then began to exhibit somnolence and fatigue with rapid and shallow breathing. The patient was treated with Nidazepam (1/3 tablet in the morning and 1/2 tablet at night, 5 mg/tablet) and lystrine tablets (twice a day), but the convulsions continued to occur approximately once every 5 months. An electroencephalogram (EEG) examination indicated a moderately abnormal electroencephalogram with abnormal discharge (Figure 1A and 1B). An enhanced magnetic resonance imaging exam showed white matter degeneration and softening. The patient received 3 successive administrations of UC-MSCs that were infused via intravenous injection and intrathecal injection. In the first session, the patient received 7.0*106/intrathecal injection and 5.6*106/intravenous injection. In the second session, the patient received 1.625*107/intrathecal injection and 3.6*106/intravenous injection. In the third and final session, the patient received 2.05*107/intrathecal injection. Informed consent was obtained from the patient’s family. In 2014, the Institutional Review Board of the Jiaxing Hospital approved the present study. After treatment, the patient showed obvious improvement in electromyography, motor function, and language expression (Supplementary Videos 1, 2, 3, 4, 5, Figure 1C).
Figure 1.
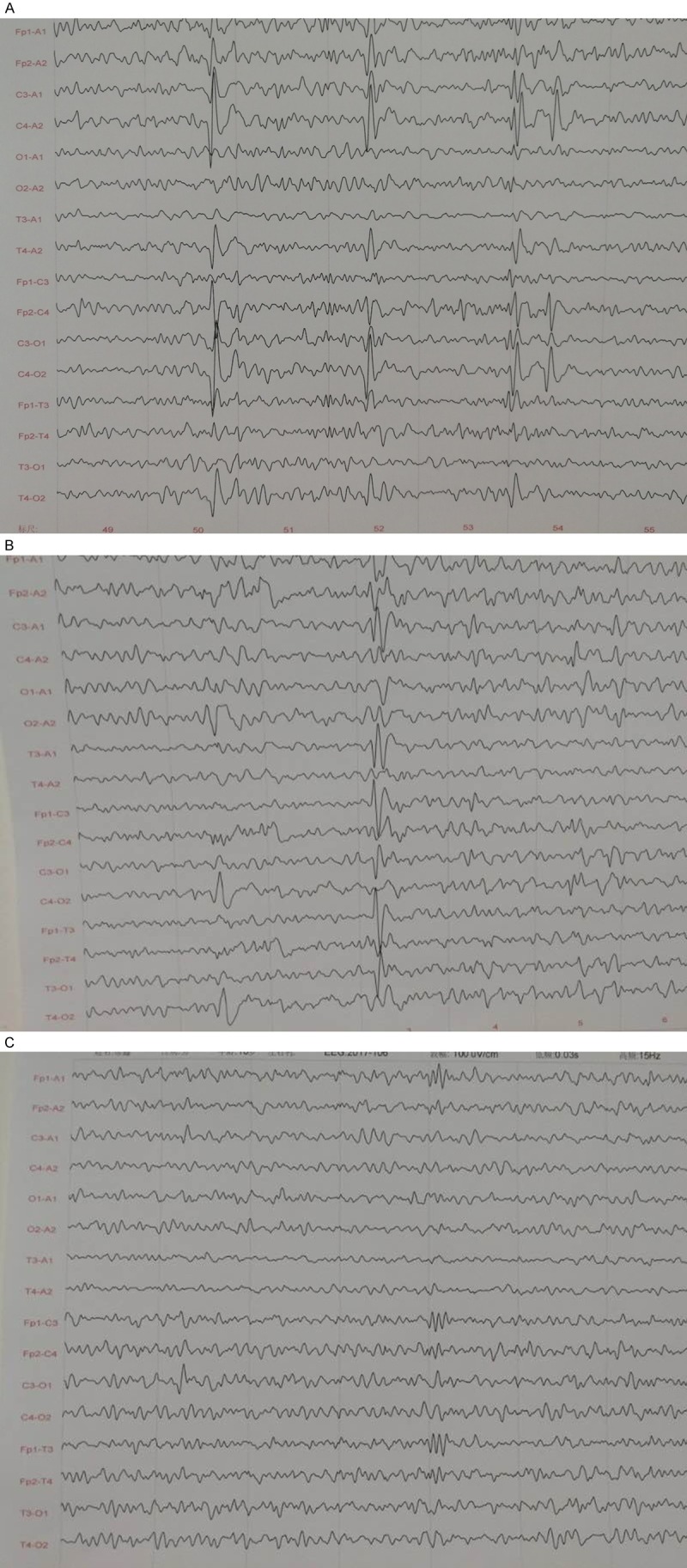
The EEG of the patient before and after the treatment with UC-MSC transplantation. A. An electroencephalogram (EEG) examination indicated a moderately abnormal electroencephalogram with abnormal discharge pre-treatment of the UC-MSCs transplantation. B. After the first transplantation of UC-MSCs, the EEG showed less abnormality than it did before treatment. C. After three sequential UC-MSC transplantations, the EEG was significantly improved and was generally normal.
Discussion
Human umbilical cord-derived mesenchymal stem cells (UC-MSCs) are a class of cells with significant self-renewal and multi-lineage differentiation properties [3,6]. UC-MSCs show strong biological activity and differentiation ability [1,9,10]. Several reports indicate that chemical and neurotrophic factors can induce UC-MSCs to differentiate into neural stem cells and oligodendrocyte precursor cells, and UC-MSCs can secrete a variety of nerve growth factors (e.g., VEGF, GDNF and BDNF) and promote axonal growth [4,11]. These UC-MSC-differentiated cells not only have the morphology and phenotype of oligodendrocyte precursor cells but also exhibit the corresponding function [12,13]. UC-MSCs can also be induced to differentiate into mesodermal-originated bone cells, cartilage cells [14], fat cells, liver cells [15,16], myocardial cells and nerve cells [8,17].
The low immune inhibition ability and low immunogenicity of UC-MSCs allows their use as allografts and xenografts. Recent studies suggest that stem cell-based approaches are promising therapies for the treatment of demyelinating diseases, such as multiple sclerosis (MS) and that UC-MSC transplantation combined with minimally invasive hematoma aspiration could significantly reduce p53 expression and damage of the nerve cells, and could be more beneficial to the recovery of neural function [18]. Mesenchymal stem cells (MSCs) have emerged as an attractive candidate for the treatment of neurological pathologies based on their effectiveness [5], relative accessibility, ease of expansion and expression of trophic factors [19]. UC-MSCs and some of the biologically active substances they secrete, such as hepatocyte growth factor (HGF) interact with each other in both the central nervous system (CNS) and immune system; in the CNS, HGF is expressed both during development and in adulthood, where it may act as a neurotrophic factor [19]. Researchers previously believed that MSCs ameliorated neurological disease by differentiating into, and thus replacing, abnormal neurons or oligodendrocytes. However, researchers now believe that MSCs treat disease through immunomodulation [4,11,20]. Research has verified the therapeutic effects of MSCs after transplantation. It has long been known that MSCs produced abundant growth factors and bio-active cytokines, many of which modulate the immune system, limiting inflammation, aiding healing; the field then adopted the revisionist viewpoint that MSCs affect tissue repair largely via paracrine factors and stimulation of host cells, which suggests that MSCs repair damaged tissue through the ‘bystander effect’ or cell-to-cell communication using exosomes, metabolites and cytokines [1-3,21,22]. At present, we cannot specify whether cell replacement or the ‘bystander effect’ is responsible for these therapeutic effects, but we believe that MSCs could treat nervous system diseases.
Cerebral palsy (CP) is a neurodevelopmental condition that affects muscle tone, movement and motor skills. This is not a single disease but rather a heterogeneous clinical syndrome resulting from injury to the developing brain [23]. There is no specific cure for cerebral palsy worldwide. In this report, the patient received UC-MSC transplantation and recovered very well. There was no discomfort in the process of UC-MSCs therapy, and no obvious side effects were observed. Following UC-MSC treatment, our patient showed obvious improvements in EEG, limb strength, motor function, and language expression, although the improvement in intelligence quotient (IQ) was less obvious. The results of this study indicate that UC-MSCs transplantation could significantly improve the symptoms of CP.
In summary, UC-MSCs have immunomodulatory properties that allow them to be used in allograft transplantation. Moreover, UC-MSCs have immunosuppressive properties that make them useful for cell therapy. After UC-MSCs transplantation, this cerebral palsy patient recovered well, which shows that UC-MSC transplantation is a promising therapy for cerebral palsy. These results provide valuable information on the investigation of new therapy methods for CP. Through years of clinical practice, we concluded that in cases of cerebral palsy, earlier treatment with UC-MSCs resulted in better patient outcomes.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81401295, 81401067, 81771352), the Tianjin Research Program of Application Foundation and Advanced Technology (15JCQNJC45200 and 16JCYBJC27600), the PUMC Youth Fund and the Fundamental Research Funds for the Central Universities (3332015126).
Disclosure of conflict of interest
None.
Supplementary Video 1. Patient gait prior to UC-MSC transplantation. The patient sways when he walks, the heel does not make contact with the ground, and he falls easily
Supplementary Video 2. Patient gait after the first transplantation of UC-MSCs. The gait of the patient is still unstable, and he falls easily. There is no obvious improvement over his condition prior to treatment
Supplementary Video 3. Patient gait after the second transplantation of UC-MSCs. The walking posture of this patient is significantly improved, and the heel is able to make contact with the ground. The gait is more stable than it was prior to treatment and after the first transplantation of UC-MSCs
Supplementary Video 4. Patient gait after the third transplantation of UC-MSCs. After the third UC-MSC transplantation, the patient’s walking posture and gait are further improved, and the walking is stable
Supplementary Video 5. Patient gait after three sequential UC-MSC transplantation treatments. After three sequential UC-MSC transplantation treatments, the patient’s walking posture and gait are roughly normal
References
- 1.Li G, Yang Y, Dong HJ, Lin L. The research progress of mesenchymal stem cells in the treatment of Traumatic brain injury. Turk Neurosurg. 2017 doi: 10.5137/1019-5149.JTN.20829-17.1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Dong H, Li G, Ding HJ, Luo Y, Zhao M, Lin L. The potential value of Adipose Tissue-Derived((rAT)) Mesenchymal Stem Cells (MSCs) Turk Neurosurg. 2017 doi: 10.5137/1019-5149.JTN.21566-17.0. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Jia Y, Shi X, Xie Y, Xie X, Wang Y, Li S. Human umbilical cord stem cell conditioned medium versus serum-free culture medium in the treatment of cryopreserved human ovarian tissues in in-vitro culture: a randomized controlled trial. Stem Cell Res Ther. 2017;8:152. doi: 10.1186/s13287-017-0604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galindo LT, Filippo TR, Semedo P, Ariza CB, Moreira CM, Camara NO, Porcionatto MA. Mesenchymal stem cell therapy modulates the inflammatory response in experimental traumatic brain injury. Neurol Res Int. 2011;2011:564089. doi: 10.1155/2011/564089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lo Furno D, Mannino G, Giuffrida R. Functional role of mesenchymal stem cells in the treatment of chronic neurodegenerative diseases. J Cell Physiol. 2018;233:3982–3999. doi: 10.1002/jcp.26192. [DOI] [PubMed] [Google Scholar]
- 6.Dong HJ, Shang CZ, Li G, Niu Q, Luo YC, Yang Y, Meng HP, Yin HJ, Zhang HX, Zhao ML, Lin L. The distribution of transplanted umbilical cord mesenchymal stem cells in large blood vessel of experimental design with traumatic brain injury. J Craniofac Surg. 2017;28:1615–1619. doi: 10.1097/SCS.0000000000003563. [DOI] [PubMed] [Google Scholar]
- 7.Oh SH, Choi C, Chang DJ, Shin DA, Lee N, Jeon I, Sung JH, Lee H, Hong KS, Ko JJ, Song J. Early neuroprotective effect with lack of long-term cell replacement effect on experimental stroke after intra-arterial transplantation of adipose-derived mesenchymal stromal cells. Cytotherapy. 2015;17:1090–1103. doi: 10.1016/j.jcyt.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Phinney DG, Pittenger MF. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells. 2017;35:851–858. doi: 10.1002/stem.2575. [DOI] [PubMed] [Google Scholar]
- 9.Ding DC, Chang YH, Shyu WC, Lin SZ. Human umbilical cord mesenchymal stem cells: a new era for stem cell therapy. Cell Transplant. 2015;24:339–347. doi: 10.3727/096368915X686841. [DOI] [PubMed] [Google Scholar]
- 10.El Omar R, Beroud J, Stoltz JF, Menu P, Velot E, Decot V. Umbilical cord mesenchymal stem cells: the new gold standard for mesenchymal stem cell-based therapies? Tissue Eng Part B Rev. 2014;20:523–544. doi: 10.1089/ten.TEB.2013.0664. [DOI] [PubMed] [Google Scholar]
- 11.Borger V, Bremer M, Ferrer-Tur R, Gockeln L, Stambouli O, Becic A, Giebel B. Mesenchymal stem/stromal cell-derived extracellular vesicles and their potential as novel immunomodulatory therapeutic agents. Int J Mol Sci. 2017:18. doi: 10.3390/ijms18071450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z, Luo Y, Chen L, Liang W. Safety of neural stem cell transplantation in patients with severe traumatic brain injury. Exp Ther Med. 2017;13:3613–3618. doi: 10.3892/etm.2017.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Chopp M, Meng Y, Katakowski M, Xin H, Mahmood A, Xiong Y. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg. 2015;122:856–867. doi: 10.3171/2014.11.JNS14770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta PK, Das AK, Chullikana A, Majumdar AS. Mesenchymal stem cells for cartilage repair in osteoarthritis. Stem Cell Res Ther. 2012;3:325. doi: 10.1186/scrt116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berardis S, Dwisthi Sattwika P, Najimi M, Sokal EM. Use of mesenchymal stem cells to treat liver fibrosis: current situation and future prospects. World J Gastroenterol. 2015;21:742–758. doi: 10.3748/wjg.v21.i3.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25:1384–1392. doi: 10.1634/stemcells.2006-0709. [DOI] [PubMed] [Google Scholar]
- 17.Harting MT, Jimenez F, Xue H, Fischer UM, Baumgartner J, Dash PK, Cox CS. Intravenous mesenchymal stem cell therapy for traumatic brain injury. J Neurosurg. 2009;110:1189–1197. doi: 10.3171/2008.9.JNS08158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Q, Shang X, Hao M, Zheng M, Li Y, Liang Z, Cui Y, Liu Z. Effects of human umbilical cord mesenchymal stem cell transplantation combined with minimally invasive hematoma aspiration on intracerebral hemorrhage in rats. Am J Transl Res. 2015;7:2176–2186. [PMC free article] [PubMed] [Google Scholar]
- 19.Bai L, Lennon DP, Caplan AI, DeChant A, Hecker J, Kranso J, Zaremba A, Miller RH. Hepatocyte growth factor mediates mesenchymal stem cell-induced recovery in multiple sclerosis models. Nat Neurosci. 2012;15:862–870. doi: 10.1038/nn.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savukinas UB, Enes SR, Sjoland AA, Westergren-Thorsson G. Concise review: the bystander effect: mesenchymal stem cell-mediated lung repair. Stem Cells. 2016;34:1437–1444. doi: 10.1002/stem.2357. [DOI] [PubMed] [Google Scholar]
- 21.Komaki M, Numata Y, Morioka C, Honda I, Tooi M, Yokoyama N, Ayame H, Iwasaki K, Taki A, Oshima N, Morita I. Exosomes of human placenta-derived mesenchymal stem cells stimulate angiogenesis. Stem Cell Res Ther. 2017;8:219. doi: 10.1186/s13287-017-0660-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu K, Li HY, Yang K, Wu JL, Cai XW, Zhou Y, Li CQ. Exosomes as potential alternatives to stem cell therapy for intervertebral disc degeneration: in-vitro study on exosomes in interaction of nucleus pulposus cells and bone marrow mesenchymal stem cells. Stem Cell Res Ther. 2017;8:108. doi: 10.1186/s13287-017-0563-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gulati S, Sondhi V. Cerebral palsy: an overview. Indian J Pediatr. 2017 doi: 10.1007/s12098-017-2475-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


