Abstract
Low-intensity pulsed ultrasound (LIPUS) has been used widely in clinical therapy for bone fracture and soft tissue injury. However, whether LIPUS regulates primary preadipocyte function and adipogenesis remains unknown. In this study, we investigated the potential role of LIPUS in regulating visceral preadipocyte function. Resuspended rat visceral preadipocytes were treated with LIPUS (0.5 MHz, 109.44 mW/cm2) for 1 min and then cultured for an additional 48 hours. Cell proliferation was examined using the CCK-8 assay, and the early apoptosis rate was determined by flow cytometry. In addition, we evaluated the related signaling pathway via examination of proliferating cell nuclear antigen (PCNA), peroxisome proliferator-activated receptor gamma (PPARγ), Bcl2, Bax, cleaved caspase 3 (C-C3), and mitogen-activated protein kinase (MAPK) member protein levels using western blot or quantitative real-time PCR (qRT-PCR). LIPUS inhibited preadipocyte proliferation and induced cell apoptosis. The protein expression of proliferation markers decreased, while expression of the apoptosis-related modulators increased following LIPUS treatment. LIPUS treatment decreased extracellular signal-regulated kinase (ERK) phosphorylation and increased p38 MAPK phosphorylation. Inhibition of p38 MAPK rescued the LIPUS-induced proliferation inhibition and apoptosis induction. Thus, treatment of rat visceral preadipocytes with 0.5 MHz LIPUS suppresses proliferation and promotes apoptosis via activation of p38 MAPK signaling.
Keywords: LIPUS, proliferation, apoptosis, p38 MAPK, rat visceral preadipocytes
Introduction
Obesity, which is strongly associated with hypertension, diabetes, and atherosclerosis, has become a common public health problem around the world [1]. Obesity is characterized by the reconstruction and increasing volume of adipose tissue [2]. Epidemiological studies have demonstrated that visceral obesity rather than subcutaneous fat deposition plays a crucial role in cardiovascular diseases [3]. Fatty tissue is comprised of massive congregate adipocytes that provide a high capacity for fatty acid storage and are congenitally immune from lipotoxicity [4]. Preadipocytes are fibroblast-like cells that are able to differentiate into fat cells in response to the appropriate induction conditions [5,6]. Most previous studies on preadipocytes have focused on the mechanisms of adipogenesis stimulated by hormonal cocktails and on development of novel strategies to inhibit preadipocyte differentiation into mature adipocytes [6]. On the contrary, studies of preadipocyte differentiation, proliferation, and apoptosis induced by an external acoustic stress are much less common. Because the mechanism of preadipocyte apoptosis is very complicated, further studies are necessary to enable clinical manipulation of this process as a promising strategy for alleviating visceral obesity-related diseases.
Low-intensity pulsed ultrasound (LIPUS) is a low-frequency and -dosage ultrasound technique that plays a dominant role in mechanical and cavitation effects [7]. LIPUS has been suggested to exert multiple biological effects in studies by several investigators. Kusuyama et al. showed that LIPUS influences multilineage differentiation of mesenchymal stem cells by inhibition of PPARγ2 via the ROCK-Cot/Tpl2-MEK-ERK pathway [8]. LIPUS has also shown promise for promoting the biological effects of some reagents and drugs. Li et al. found that the combination of LIPUS and 5-aminolevulinic acid produces more potent pro-apoptotic effects than 5-aminolevulinic acid alone [9]. In addition, another study demonstrated that LIPUS accelerates soft-tissue regeneration by promoting cell proliferation in a morbid body [10]. These studies support the pleiotropic and complex bio-effects of LIPUS. While the known safety and efficacy profiles of this therapy make LIPUS a useful application for clinical treatment, the role of LIPUS in visceral preadipocyte biological function remains unknown. The identification of an appropriate LIPUS dose that would inhibit visceral preadipocyte proliferation and promote apoptosis of these cells will be very helpful for developing a novel strategy to combat visceral obesity.
In this study, we investigated the effects of different dose of LIPUS on visceral preadipocyte proliferation and apoptosis. We found that an average intensity of 109.44 mW/cm2 significantly decreased preadipocyte proliferation in a time-dependent manner. Flow cytometry showed that this same dose of LIPUS also promoted cell apoptosis. Moreover, LIPUS inhibited preadipocyte proliferation and promoted apoptosis via activation of the MAPK family member p38 and inhibition of ERK activation. Our data demonstrate that a certain dose of LIPUS specifically affects preadipocyte proliferation and apoptosis.
Materials and methods
Isolation and culture of rat visceral preadipocytes
Rat visceral preadipocytes were isolated and cultured as described previously [11]. Briefly, adult male Wistar rats, weighing 180-200 g, were sacrificed under anesthesia and submerged in 70% ethanol for 1 min. Visceral adipose tissue from epididymal fat was collected and incubated with 0.1% collagenase solution in a 37°C shaking water bath for 30 min. Following centrifugation, the pellets were resuspended in DMEM/F12 media with 10% fetal bovine serum, penicillin, and streptomycin (1%). The medium was changed after 24 h to remove nonadherent cells, and then, culture medium was changed every 3 days.
Pulsed low-intensity ultrasound stimulation
LIPUS irradiation was performed using a set of ultrasound devices, including a signal generator (Agilent Technologies, Santa Clara, CA, USA), wideband power amplifier (Electronics and Innovation Ltd, Rochester, NY, USA), and a planar transducer (Haifu, Chongqing, China). The planar transducer frequency was 0.5 MHz, the voltage applied to the transducer was 150 MVpp, and the number of cycles was 500-2000. In addition, the spatial temporal average sound pressure was 0.3 MPa. The transducer (diameter 6 cm) was placed in a water vat, and degassed water flowed over the transducer. Then, the bottom of the cell culture dish that was placed in the transducer filled with the degassed water (Supplementary Figure 1). The cell suspension was exposed to the LIPUS stimuli for 1 min (ultrasound group), while the control cell suspension was treated identically except for the absence of the LIPUS stimuli. The temperature of the cell culture media in the dishes did not exceed 37°C during the ultrasound procedures.
CCK-8 assay
Preadipocytes proliferation was evaluated with the Cell Counting Kit-8 (Dojindo, Japan). The cell suspension (5 × 103 cells/well) treated with or without the ultrasound stimulus was seeded in wells of a 96-well plate for 48 h. Then, CCK-8 reagent was added and incubated with the cells for 2 h at 37°C. The absorbance at 450 nm was measured using a microplate reader.
Flow cytometric analysis of cell apoptosis
The cell early apoptosis rate was analyzed by flow cytometry using Annexin-V-FITC staining. In brief, cells treated with or without LIPUS were collected and stained with propidium iodide and Annexin-V for 15 min in the dark at room temperature according to the manufacturer’s instructions and then analyzed by flow cytometry.
Western blot
The total proteins were extracted from preadipocytes and separated by SDS-PAGE (6-12%). Proteins were then transferred onto PVDF membranes and blocked with bovine serum albumin (BSA). Membranes were incubated with the following primary antibodies against p-38 (1:1000; Cat. 8690, Cell Signaling Technology, Danvers, MA, USA), p-p38 (1:1000; Cat. 4511, Cell Signaling Technology), ERK (1:1000; Cat. 4695, Cell Signaling Technology), p-ERK (1:1000; Cat. 4370, Cell Signaling Technology), Janus kinase (JNK, 1:1000; Cat. 9252, Cell Signaling Technology), p-JNK (1:1000; Cat. 4668, Cell Signaling Technology), PPARγ (1:500; Cat. 209350, Abcam, Cambridge, MA, USA), proliferating cell nuclear antigen (PCNA, 1:1000; Cat. 13110, Cell Signaling Technology), Bcl2 (1:1000; Cat. 2876, Cell Signaling Technology), Bax (1:1000; Cat. 2772, Cell Signaling Technology), and cleaved caspase 3, C-C3 (1:1000; Cat. 9661, Cell Signaling Technology) at 4°C overnight and then incubated with the appropriate secondary antibody, either peroxidase-labeled anti-rabbit or anti-mouse immunoglobulin, at room temperature. The target proteins were detected using an enhanced chemiluminescence (ECL) kit. Bands were normalized with β-tubulin, and protein levels were quantified by Image J.
qRT-PCR
Total RNA was isolated and transcribed into cDNA as previously described [12]. The cDNA products were used as the PCR template for the analysis of mRNA levels using the SYBR-Green Supermix Kit (Bio-Rad, Hercules, CA, USA). All samples were analyzed at least three times, and the relative expression levels were normalized to that of β-tubulin. The following specific primers for target genes were obtained from Invitrogen (Carlsbad, CA, USA): PPARα, forward 5’-ACGGAATTTGCCAAGGCTAT-3’, reverse 5’-ATCAAGGAGGACAGCATCGT-3’; PPARγ, forward 5’-TGACCACTCCCATTCCTTTG-3’, reverse 5’-CAACCATTGGGTCAGCTCTT-3’; PPARδ, forward 5’-CCCACTCACAACACTCCAGAC-3’, reverse 5’-AGCGGACTAATGGGCTCAC-3’; CCAAT/enhancer-binding protein (C/EBPα), forward 5’-CCATCCGCCTTGTGTGTACT-3’, reverse 5’-GTTTAGCATAGACGCGCACA-3’; C/EBPβ, forward 5’-CGGGTTTCGGGACTTGAT-3’, reverse 5’-CCCGCAGGAACATCTTTAAGT-3’; and C/EBPδ, forward 5’-ATCGACTTCAGCGCCTACAT-3’, reverse 5’-CCGCTTTGTGATTGCTGTT-3’.
Statistical analysis
All data were expressed as the mean ± SEM of three or more independent experiments and the normal distribution was tested. Statistical differences were determined by independent Student’s t-test for comparison between two groups. One-Way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparison test was used for comparison among four groups. P values smaller than 0.05 were considered significant. Statistical graphs were performed using GraphPad PRISM 6.01 statistical software (San Diego, California, USA).
Results
LIPUS inhibits proliferation and increases apoptosis of preadipocytes
To explore the effects of LIPUS on preadipocytes, we first treated preadipocytes with different doses of ultrasound (Supplementary Table 1). As showed by the CCK-8 assay (Figure 1A), the average sound intensity 109.44 mW/cm2 (cycle number reached 1700) led to an obvious reduction of cell proliferation compared to control treatment (P<0.05), and no significant change in proliferation was observed with cycles lower than 1700 cycles. We also assessed the effects of LIPUS on cells following different treatment durations (0.5, 1, and 1.5 min). We found a time-dependent decrease in preadipocyte proliferation, and the 1-min duration was determined to be the optimal duration for further experiments (Figure 1B). Furthermore, LIPUS treatment slightly increased the early apoptosis of preadipocytes as measured by flow cytometric analysis (Figure 1C and 1D).
Figure 1.
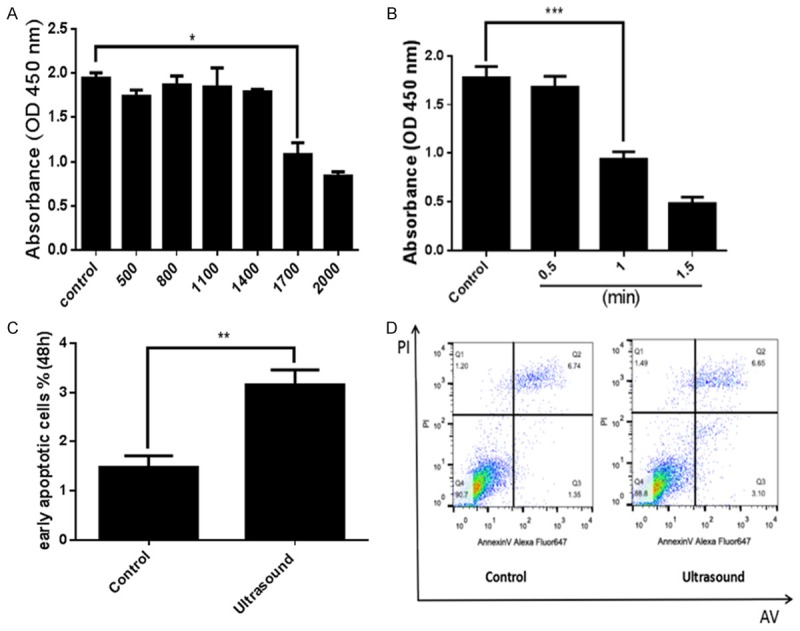
LIPUS inhibits preadipocyte proliferation and increases apoptosis. Primary cultured preadipocytes were treated with different doses of ultrasound (500, 800, 1100, 1400, 1700, and 2000 cycles) and different duration (0.5, 1, and 1.5 min). (A) Preadipocyte proliferation was evaluated with the CCK-8 assay. Ultrasound doses reaching 1700 cycles (average sound intensity 109.44 mW/cm2) led to a remarkable reduction of preadipocyte proliferation. (B) Effects of LIPUS on preadipocytes proliferation were evaluated at different points in time and the 1-min duration was determined to be the optimal duration. (C, D) The early apoptosis rate was analyzed by flow cytometry using Annexin-V-FITC staining. LIPUS treatment increased the number of early apoptotic preadipocytes compared to control treatment. All values are expressed as the mean ± SEM of three independent trials. For (A and B), data were analyzed with one-way ANOVA analysis, for (C), data were analyzed with independent t test. *P<0.05, **P<0.01, ***P<0.001.
LIPUS affects expression of proteins associated with proliferation and apoptosis
To further clarify the role of LIPUS on cellular proliferation and apoptosis, we measured the expression of key proliferation- and apoptosis-related molecules. We found that an obvious downregulation of the proliferation marker PCNA (P<0.01) and upregulation of PPARγ (P<0.05), which may be involved in cell proliferation inhibition or apoptosis induction, in the LIPUS-treated group compared to the control cells (Figure 2A and 2B). Furthermore, we examined the key intrinsic apoptosis signaling markers in preadipocytes following LIPUS treatment. The anti-apoptosis factor Bcl2 was markedly decreased in the cells treated with LIPUS (P<0.05). Moreover, the Bcl-2/Bax ratio was decreased, and the apoptosis maker C-C3 was increased in LIPUS-treated cells (P<0.05) (Figure 2C and 2D).
Figure 2.
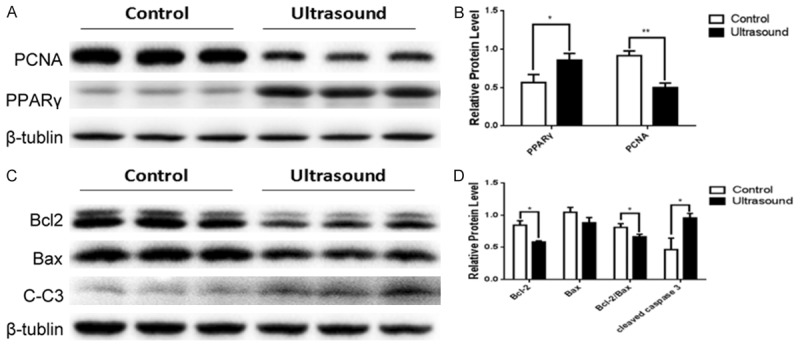
LIPUS affects expression of proteins associated with proliferation and apoptosis in preadipocytes. The expression levels of key proliferation- and apoptosis-related molecules (PCNA, PPARγ, Bcl2, Bax, and C-C3) were examined by western blotting in primary preadipocytes with or without LIPUS (1700 cycles, 1 min) treatment. A, B. The protein levels of PCNA protein decreased and the levels of PPARγ increased in LIPUS treated cells compared to untreated cells. C, D. LIPUS treatment downregulated the protein expression of Bcl2 and the Bcl2/Bax ratio, and upregulated the protein expression of C-C3. All values are expressed as the mean ± SEM of three independent trials. Data were analyzed with independent t test. *P<0.05, **P<0.01.
LIPUS does not promote transcription of PPAR members at the early stage
Due to the increase of the PPARγ protein level in cells following treatment with LIPUS, we speculated that LIPUS may affect PPAR members mRNA expression. Thus, the mRNA expression levels of PPARα, PPARγ, and PPARδ were analyzed by qRT-PCR. LIPUS treatment did not appear to affect mRNA levels of PPARα, PPARγ, or PPARδ (Figure 3A). In addition, we also measured the mRNA levels of the PPAR-related members of the C/EBP family of transcription factors. LIPUS treatment downregulated the C/EBPδ mRNA level (P<0.01) but did not affect the C/EBPα and C/EBPβ mRNA levels (Figure 3B).
Figure 3.
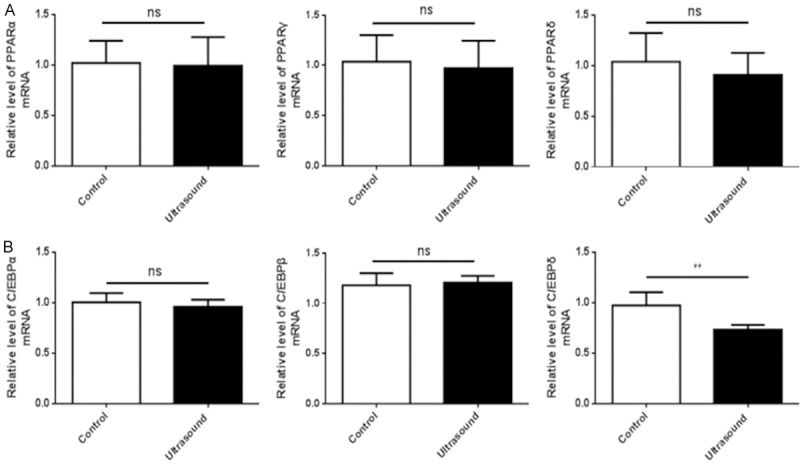
LIPUS does not promote transcription of PPAR family members during the early stage. mRNA levels of PPAR and C/EBP family members were assessed by qRT-PCR in LIPUS treated and untreated cells. β-tubulin was utilized as the internal standard for normalization. A. LIPUS did not affect the mRNA levels of PPARα, PPARγ, and PPARδ of rat visceral preadipocytes. B. LIPUS treatment downregulated the C/EBPδ mRNA level but did not affect the C/EBPα and C/EBPβ mRNA levels of rat visceral preadipocytes. All values are expressed as the mean ± SEM of three independent trials. Data were analyzed with independent t test. **P<0.01.
LIPUS activates p38 MAPK phosphorylation
The MAP Kinase pathway has been reported to be involved in stress-induced cell proliferation and apoptosis [13]. To elucidate the underlying molecular mechanism of LIPUS-induced proliferation inhibition and apoptosis in preadipocytes, we examined the expression and modification of MAPK members by western blot. Our data demonstrated that the phosphorylation level of p38 was significantly increased in the LIPUS-treated cells (P<0.05). In addition, LIPUS treatment inhibited the phosphorylation of ERK in these cells (P<0.05), while the phosphorylation level of JNK was comparable between the treated and untreated cells (Figure 4A and 4B).
Figure 4.
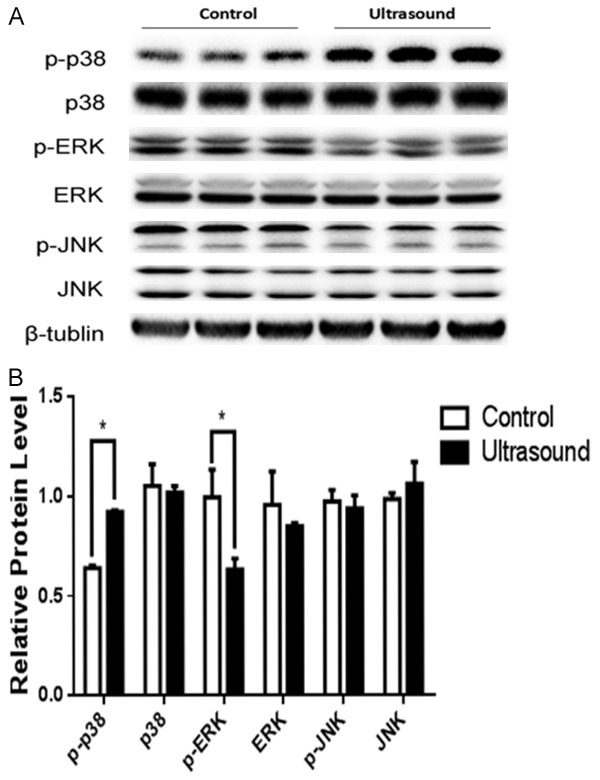
LIPUS induces phosphorylation of p38-MAPK. The expression and modification of MAPK members were assessed by western blotting in primary preadipocytes with or without LIPUS treatment. A. LIPUS treatment significantly promoted the phosphorylation level of p38 and inhibited the phosphorylation of ERK, and no change of the expression level of p-JNK were observed. B. Quantification of p-38, p-p38, ERK, p-ERK, JNK, and p-JNK protein levels were. Data were analyzed with independent t test. All values are expressed as the mean ± SEM of three independent trials. *P<0.05.
Inhibition of p38 rescues the effects of LIPUS
In order to determine whether the observed biological effects of LIPUS on preadipocytes are dependent on the increased phosphorylation of p38, preadipocytes were treated with the p38 signaling inhibitor SB203580. Flow cytometry and CCK-8 assays showed that p38 inhibition abrogated the induction of cell apoptosis (Figure 5A and 5B) and the inhibition of proliferation (Figure 5C) induced by LIPUS. Western blotting further showed that p38 inhibition reversed the changes of p-ERK, the apoptosis-related proteins Bcl2 and C-C3, and the proliferation-related marker PCNA induced by LIPUS (Figure 5D).
Figure 5.
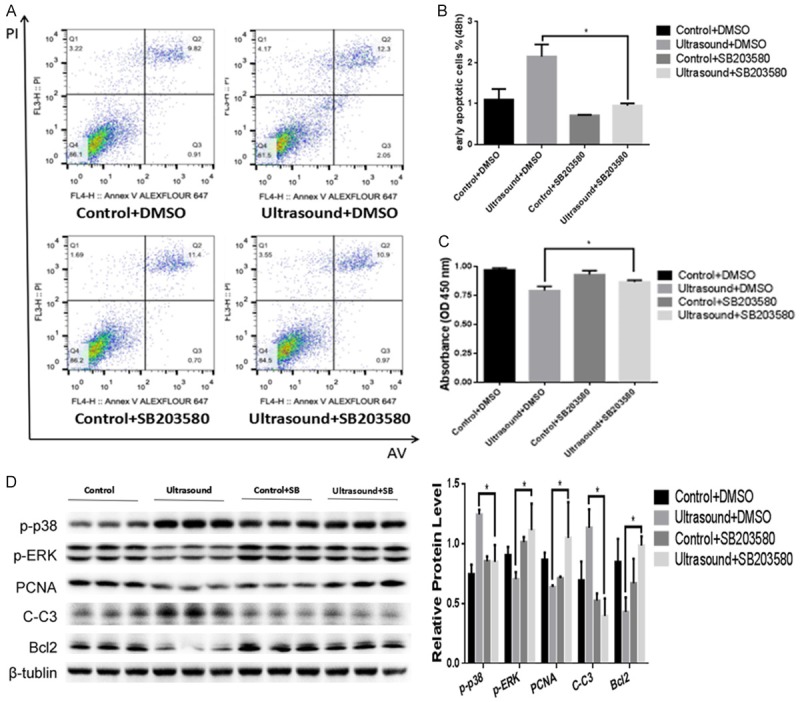
Inhibition of p38 phosphorylation rescues the effects of LIPUS. In order to explore whether the biological effects of LIPUS on preadipocytes are associated with p-p38, cell suspension exposed to LIPUS was treated with or without p38 MAPK inhibitor SB203580. A, B. p38 inhibition rescued the LIPUS-induced apoptosis as shown by flow cytometric analysis. The percentage of early apoptotic preadipocytes (%) was analyzed with one-way ANOVA analysis. C. CCK-8 assay showed that inhibition of p38 activation reversed the LIPUS-induced proliferation inhibition in primary preadipocytes. D. p-p38, p-ERK, C-C3, PCNA and Bcl2 protein levels were assessed by western blotting with or without p38 inhibitor treatment. Relative protein levels were analyzed with one-way ANOVA analysis. All values are expressed as the mean ± SEM of three independent trials. *P<0.05.
Discussion
The major findings of this study are that LIPUS (at an average dose of 109.44 mW/cm2) induces apoptosis and inhibits proliferation in rat primary visceral preadipocytes and that the increase of proliferation inhibition and apoptosis induced by LIPUS is mediated by enhanced p38 MAPK activation.
Fat, which serves as an important energy store in the body, plays a critical role in life activity; however, excessive visceral fat deposition can lead to various illnesses, including hypertension, diabetes, and atherosclerosis, and can impair health and quality of life [14]. The primary components of adipose tissue are adipocytes, which are characterized by the presence of lipid droplets and are derived from preadipocytes [15], which are fibroblast-like cells that are predetermined to differentiate into fat cells. Preadipocytes have already become one of the targets for obesity control, and as such, the inhibition of preadipocyte differentiation to adipocytes has been thoroughly studied for the purpose of alleviating obesity-related diseases [16,17]. Physical induction of inhibition of proliferation and induction of apoptosis in preadipocytes as a strategy for obesity control, by contrast, has not been studied extensively. In the current study, we demonstrated that clinical application of LIPUS induced inhibition of preadipocyte proliferation and promoted apoptosis, suggesting that such treatment may offer a promising strategy for obesity control.
Numerous studies have revealed the bio-effects of LIPUS on various cell types, including increasing cell membrane penetrability [18] and regulating cell apoptosis [19,20], proliferation [21], differentiation [22], and migration [23]. Other studies have shown that the abovementioned bio-effects of LIPUS may be associated with different doses of sound intensity. Kusuyama et al. found that LIPUS influenced multilineage cellular differentiation at an intensity of 30 mW/cm2 [8]. Jang et al. revealed that cell migration is promoted via the focal adhesion kinase pathway at the sound intensity of 27.5 mW/cm2 [23], and when the dose reached 200 mW/cm2, LIPUS increased cell proliferation and extracellular matrix production [21]. In the present study, we investigated the effects of LIPUS on preadipocyte proliferation and apoptosis and found that 1-min irradiation with an average dose of 109.44 mW/cm2 led to significant inhibition of proliferation and not cell necrosis of visceral preadipocytes. In addition, in contrast to the commonly used transducer frequency of 1 MHz [24] or 1.5 MHz [8], we used a 0.5-MHz planar transducer in order to avoid thermal effects. This type of fast irradiation may, therefore, be very useful for the clinical application of LIPUS for obesity-reduction therapy.
It is well known that apoptosis is primarily mediated through the mitochondria-dependent intrinsic pathway or the death receptor-dependent extrinsic pathway. Bcl-2 family proteins include anti- and pro-apoptotic family members that are vital regulators of the mitochondria-dependent signaling pathway [25]. Bcl-2, a crucial anti-apoptotic factor, localizes to intracellular mitochondria membranes and may restrain the release of apoptotic molecules [25,26]. Conversely, the pro-apoptotic factor Bax leads to cytochrome C release, resulting in cleavage of caspase 3 and induction of apoptosis [25,27]. In the present study, we demonstrated that LIPUS upregulates the expression of C-C3 and downregulates the expression of Bcl-2. Although no significant difference was observed in Bax levels between the LIPUS-treated cells and the control cells, the ratio of Bcl-2 to Bax decreased following treatment of cells with LIPUS. Taken together, these results suggest that LIPUS influences preadipocytes in a mitochondria-dependent manner.
The MAPK signaling pathways have been recognized as common signal transduction pathways for regulating cell proliferation and apoptosis [28]. In contrast to the activating role of a 30 mW/cm2 dose of LIPUS on p-ERK in the preadipocyte cell lines [8], we observed that a 109.4 mW/cm2 dose of LIPUS increased p-p38 and decreased p-ERK. To determine whether the underlying molecular mechanism of LIPUS-induced apoptosis is associated with p38 MAPK, we inhibited the expression of p-p38 using the p38 inhibitor SB250380 in preadipocyte culture media. We found that p38 inhibition rescued cell apoptosis, suggesting that LIPUS promotes apoptosis through p38 MAPK signaling in primary preadipocytes. We also found that p38 inhibition reverses the downregulation of p-ERK induced by LIPUS. This particular phenomenon is very interesting, because p38 MAPK signaling and ERK signaling comprise two parallel pathways that should not affect each other. We speculate that LIPUS may partly induce preadipocytes differentiation along with the PPARγ increase and p-ERK decrease as described above. Then, p-p38 inhibition could reverse the proliferation suppression induced by LIPUS, and this increase of cell proliferation capacity will offset the partial cell differentiation induction, resulting in upregulation of p-ERK. This hypothesis will be one of our top priorities for future investigation in order to determine whether LIPUS can induce preadipocyte differentiation.
In conclusion, our study demonstrates that LIPUS treatment effectively promotes apoptosis of rat primary preadipocytes and that this apoptotic effect may be mediated via the activation of the mitochondria-dependent apoptotic pathway. The effects of LIPUS on cell apoptosis are likely dependent on the p38 MAPK signaling pathway, and this research will ultimately pave the way to explore this promising strategy to mitigate obesity-related diseases. Importantly, there are some limitations that should be considered in this study. We did not determine whether other frequencies of transducer also exert similar pro-apoptotic effects, and thus, we need to determine the optimal treatment conditions in future work. In addition, our data suggests that the p38 MAPK pathway is involved in the effects of LIPUS on cell apoptosis. Further studies are needed to explore the upstream signaling and a more specific mechanism about LIPUS mediated biological effects.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81627802, No. 81570247), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD2014-2016). Dr. Wei Sun is an assistant Fellow at the Collaborative Innovation Center For Cardiovascular Disease Translational Medicine, Dr. Xiangqing Kong is a Fellow at the Collaborative Innovation Center For Cardiovascular Disease Translational Medicine.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Williams EP, Mesidor M, Winters K, Dubbert PM, Wyatt SB. Overweight and obesity: prevalence, consequences, and causes of a growing public health problem. Curr Obes Rep. 2015;4:363–370. doi: 10.1007/s13679-015-0169-4. [DOI] [PubMed] [Google Scholar]
- 2.Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nat Rev Cancer. 2011;11:886–895. doi: 10.1038/nrc3174. [DOI] [PubMed] [Google Scholar]
- 3.Abraham TM, Pedley A, Massaro JM, Hoffmann U, Fox CS. Association between visceral and subcutaneous adipose depots and incident cardiovascular disease risk factors. Circulation. 2015;132:1639–1647. doi: 10.1161/CIRCULATIONAHA.114.015000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Näslund E, Britton T, Concha H, Hassan M, Rydén M, Frisén J, Arner P. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 6.Fried SK, Lee MJ, Karastergiou K. Shaping fat distribution: New insights into the molecular determinants of depot- and sex-dependent adipose biology. Obesity. 2015;23:1345–1352. doi: 10.1002/oby.21133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Claes L, Willie B. The enhancement of bone regeneration by ultrasound. Prog Biophys Mol Biol. 2007;93:384–398. doi: 10.1016/j.pbiomolbio.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 8.Kusuyama J, Bandow K, Shamoto M, Kakimoto K, Ohnishi T, Matsuguchi T. Low intensity pulsed ultrasound (LIPUS) influences the multilineage differentiation of mesenchymal stem and progenitor cell lines through ROCK-Cot/Tpl2-MEK-ERK signaling pathway. J Biol Chem. 2014;289:10330–10344. doi: 10.1074/jbc.M113.546382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li YN, Zhou Q, Yang B, Hu Z, Wang JH, Li QS, Cao WW. Mechanism of rat osteosarcoma cell apoptosis induced by a combination of low-intensity ultrasound and 5-aminolevulinic acid in vitro. Genet Mol Res. 2015;14:9604–9613. doi: 10.4238/2015.August.14.23. [DOI] [PubMed] [Google Scholar]
- 10.Xin Z, Lin G, Lei H, Lue TF, Guo Y. Clinical applications of low-intensity pulsed ultrasound and its potential role in urology. Transl Androl Urol. 2016;5:255–266. doi: 10.21037/tau.2016.02.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez MJ, Spencer ND. In vitro adult rat adipose tissue-derived stromal cell isolation and differentiation. Methods Mol Biol. 2011;702:37–46. doi: 10.1007/978-1-61737-960-4_4. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Bei Y, Shen S, Huang P, Shi J, Zhang J, Sun Q, Chen Y, Yang Y, Xu T, Kong X, Xiao J. miR-21-3p controls sepsis-associated cardiac dysfunction via regulating SORBS2. J Mol Cell Cardiol. 2016;94:43–53. doi: 10.1016/j.yjmcc.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 13.Banerjee S, Bhattacharjee P, Chakraborty J, Panda AK, Bandyopadhyay A, Banik SK, Sa G. WITHDRAWN: curcumin shifts RAS-induced pro-proliferative MEK/ERK-signaling toward pro-apoptotic p38MAPK/JNK1-signaling, triggering p53 activation and apoptosis. J Biol Chem. 2017 doi: 10.1074/jbc.M117.784868. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Lee SG, Lee YJ, Jang MH, Kwon TR, Nam JO. Panax ginseng leaf extracts exert anti-obesity effects in high-fat diet-induced obese rats. Nutrients. 2017;9 doi: 10.3390/nu9090999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiao Y, Zhang J, Lu L, Xu J, Qin L. The Fto gene regulates the proliferation and differentiation of pre-adipocytes in vitro. Nutrients. 2016;8:102. doi: 10.3390/nu8020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Secco B, Camire E, Briere MA, Caron A, Billong A, Gelinas Y, Lemay AM, Tharp KM, Lee PL, Gobeil S, Guimond JV, Patey N, Guertin DA, Stahl A, Haddad E, Marsolais D, Bosse Y, Birsoy K, Laplante M. Amplification of adipogenic commitment by VSTM2A. Cell Rep. 2017;18:93–106. doi: 10.1016/j.celrep.2016.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Price NL, Holtrup B, Kwei SL, Wabitsch M, Rodeheffer M, Bianchini L, Suarez Y, Fernandez-Hernando C. SREBP-1c/MicroRNA 33b genomic loci control adipocyte differentiation. Mol Cell Biol. 2016;36:1180–1193. doi: 10.1128/MCB.00745-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo G, Ma Y, Guo Y, Zhang C, Guo X, Tu J, Yu ACH, Wu J, Zhang D. Enhanced porosity and permeability of three-dimensional alginate scaffolds via acoustic microstreaming induced by low-intensity pulsed ultrasound. Ultrason Sonochem. 2017;37:279–285. doi: 10.1016/j.ultsonch.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 19.Zhang B, Zhou HS, Cheng Q, Lei L, Hu B. Low-frequency ultrasound induces apoptosis of rat aortic smooth muscle cells (A7r5) via the intrinsic apoptotic pathway. Genet Mol Res. 2014;13:3143–3153. doi: 10.4238/2014.April.17.10. [DOI] [PubMed] [Google Scholar]
- 20.Feng Y, Tian ZM, Wan MX, Zheng ZB. Low intensity ultrasound-induced apoptosis in human gastric carcinoma cells. World J Gastroenterol. 2008;14:4873–9. doi: 10.3748/wjg.14.4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bohari SP, Grover LM, Hukins DW. Pulsed low-intensity ultrasound increases proliferation and extracelluar matrix production by human dermal fibroblasts in three-dimensional culture. J Tissue Eng. 2015;6:204173141561577. doi: 10.1177/2041731415615777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao Q, Walmsley AD, Cooper PR, Scheven BA. Ultrasound stimulation of different dental stem cell populations: role of mitogen-activated protein kinase signaling. J Endod. 2016;42:425–431. doi: 10.1016/j.joen.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 23.Jang KW, Ding L, Seol D, Lim TH, Buckwalter JA, Martin JA. Low-intensity pulsed ultrasound promotes chondrogenic progenitor cell migration via focal adhesion kinase pathway. Ultrasound Med Biol. 2014;40:1177–1186. doi: 10.1016/j.ultrasmedbio.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang JJ, Shi YQ, Li RL, Hu A, Lu ZY, Weng L, Wang SQ, Han YP, Zhang L, Li B, Hao CN, Duan JL. Angiogenesis effect of therapeutic ultrasound on HUVECs through activation of the PI3K-Akt-eNOS signal pathway. Am J Transl Res. 2015;7:1106–1115. [PMC free article] [PubMed] [Google Scholar]
- 25.Hardwick JM, Youle RJ. SnapShot: BCL-2 proteins. Cell. 2009;138:404, 404.e1. doi: 10.1016/j.cell.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yee C, Yang W, Hekimi S. The intrinsic apoptosis pathway mediates the pro-longevity response to mitochondrial ROS in C. elegans. Cell. 2014;157:897–909. doi: 10.1016/j.cell.2014.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chandra D, Maes ME, Schlamp CL, Nickells RW. Live-cell imaging to measure BAX recruitment kinetics to mitochondria during apoptosis. PLoS One. 2017;12:e0184434. doi: 10.1371/journal.pone.0184434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng Q, Deng Z, Pan H, Gu L, Liu O, Tang Z. Mitogen-activated protein kinase signaling pathway in oral cancer. Oncol Lett. 2018;15:1379–1388. doi: 10.3892/ol.2017.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


