Abstract
Podocyte apoptosis is a typical early feature of diabetic nephropathy (DN), with loss of nephrin integrity contributing to increased proteinuria in patients with DN. Emerging evidence shows that microRNAs (miRNAs) play vital roles in the pathogenesis of DN. Thus, we aimed to further elucidate the role of miRNAs in podocyte apoptosis in DN. We used db/db and db/m mice maintained under a continuous feeding regime for 12 weeks. Using microarray analysis, we found several miRNAs potentially related to podocyte apoptosis. In addition, we cultured a conditionally immortalized human podocyte cell line in 30 mM D-glucose and found that miR-134-5p was upregulated in both db/db mice and high-glucose (HG)-treated podocytes. Upregulation of miR-134-5p was accompanied by podocyte apoptosis and downregulation of nephrin. Inhibition of miR-134-5p produced the opposite effect. Dual-luciferase reporter assays showed that miR-134-5p directly targeted the 3’-untranslated region of the B-cell lymphoma-2 gene (BCL2), and further study confirmed an increase in bcl-2 protein level in HG-treated podocytes transfected with anti-miR-134-5p. Knockdown of BCL2 impeded the antiapoptotic effect of anti-miR-134-5p. Finally, we found that miR-134-5p might regulate apoptosis in db/db mice and podocytes by targeting BCL2. Taken together, our findings suggest that miR-134-5p promotes podocyte apoptosis under HG conditions by targeting BCL2. Our study provides a meaningful approach to interpret the mechanisms of action of miRNAs involved in DN.
Keywords: Diabetic nephropathy, apoptosis, podocyte, high glucose, microRNA, BCL2
Introduction
Diabetic nephropathy (DN) is a major debilitating complication of both type 1 and type 2 diabetes that progresses to end-stage renal disease [1]. Persistent microalbuminuria is widely used as a biomarker of early DN, which indicates a progressive decline in renal function [2]. Podocytes are terminally differentiated cells that are unable to proliferate [3], and which form the glomerular filtration barrier, together with endothelial cells and the glomerular basement membrane [4]. Previous research has shown that podocyte apoptosis is associated with decreased expression of podocin, nephrin, and slit-associated proteins [5], resulting in massive proteinuria in DN [6]. Therefore, it is critical to further explore podocyte-based therapies that can prevent or cure DN in the early stages.
MicroRNAs (miRNAs) are single-stranded, small, noncoding RNAs (21-25 nucleotides) [7] that regulate gene expression by binding to the mRNAs of protein-coding genes to inhibit their translation [8]. Cumulative studies suggest that miRNAs are involved in the pathogenesis of various diseases, including kidney disease [9]. MiR-26a [10], miR-192 [11], miR-200 [12], and miR-215 [13] have been shown to be involved in DN in a transforming growth factor (TGF)-β-dependent manner. Emerging evidence suggests that miRNAs also play an important role in podocytes. MiR-29c has been determined to be involved in podocyte apoptosis by targeting Sprouty homolog 1 in diabetic mice [14]. Knockdown of miR-34c in podocytes has been shown to reduce apoptosis by blocking upregulation of proapoptotic factors induced by high-glucose (HG) treatment [15]. MiR-195 has been found to promote podocyte apoptosis by targeting BCL2 in cultured podocytes [16]. Our previous studies have found that several miRNAs were differentially expressed in both db/db mice and HG-treated podocytes. Among these, miR-383-5p, miR-205-5p, and miR-134-5p were upregulated. In addition, overexpression of miR-383-5p has been demonstrated to block the increase in autophagy and attenuation of HG-induced apoptosis induced by resveratrol [17]. However, the mechanisms by which miRNAs modulate disease are complex and further investigations are required to clarify the role of these miRNAs in DN.
The BCL2 gene family and its related protein bcl-2 were the first apoptosis-related genes to be studied [18]. Using two miRNA target analyzing databases (TargetScan and miRNAWalk 2.0), we found that the well-known anti-apoptotic gene BCL2 might be a direct target of miR-134-5p. This led us to hypothesize that miR-134-5p might be involved in the pathological process of DN by regulating bcl-2 expression. This study aimed to gain insight into the biological roles of miR-134-5p. We found that miR-134-5p accelerates HG-induced podocyte apoptosis by suppressing its direct target gene, BCL2.
Materials and methods
Animal model of DN
Twenty male mice (eight weeks of age), including ten C57BL/KsJ db/db mice as the experimental group and ten C57BL/KsJ db/m mice as the control group, were purchased from the Experimental Animal Center of Nanjing Medical University (Nanjing, China). Db/db mice are a genetic model of the early-stage type 2 DN, with features of hyperglycemia and urinary albumin excretion enhancement [19]. Animal experiments were conducted in accordance with Nanjing Medical University guidelines and ethical norms for animal care. The mice were adaptively fed for a week and then housed in well-ventilated plastic cages with stainless steel grid tops at 22 ± 2°C with a 12-h light/dark cycle. After 20 weeks of sustained hyperglycemia, all mice were weighed and placed in individual metabolic cages. Urine samples were collected after 24 h to test the urine albumin excretion rate (UAER) using the microalbuminuria ELISA Kit (SenBeiJia Biological Technology Company, Nanjing, China). Blood samples from the orbital vein of mice were used for detection of blood urea nitrogen, creatinine, and other biochemical parameters. Mice were sacrificed by cervical dislocation, and all renal tissues were isolated immediately. Half of the kidney samples from each mouse were sent at low temperature in formaldehyde to Google Biotechnology Co., Ltd. (Wuhan, China) for staining and immunohistochemistry. Samples of the harvested kidneys were also sent to the kidney laboratory of The Second Affiliated Hospital of Nanjing Medical University for immunofluorescence analysis. The remaining samples were stored at -80°C.
Podocyte culture and treatment
Conditionally immortalized human podocytes were kindly provided by Dr. Junwei Yang (Center for Kidney Disease, Second Affiliated Hospital of Nanjing Medical University, Nanjing, China). Podocytes were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (Invitrogen, Grand Island, NY, USA) at 33°C in a humidified atmosphere of 5% CO2. After culturing to a confluence of 80%-90%, the podocytes were subcultured under similar conditions for 10-14 days to induce cell differentiation. After serum starvation for 12 h, the cells were exposed to the indicated high-glucose (HG, 30 mM D-glucose) or normal glucose (NG, 5 mM D-glucose) conditions for 24, 48, or 72 h.
qRT-PCR
Total RNA was extracted with TRIzol reagent (Invitrogen, Shanghai, China). Gene expression was detected by qRT-PCR using the Mir-X miRNA First-Strand Synthesis and SYBR qRT-PCR Kits (Takara, Nanjing, China). The primers used were purchased from GeneCopoeia (Rockville, MD, USA). U6 was used as an internal reference for quantification of the relative expression of mRNA and miRNA using the 2-ΔΔCt method.
Cell transfection
Hsa-miR-134-5p mimic, hsa-miR-134-5p inhibitor, and hsa-miR-ctrl were chemically synthesized by GenePharma (Shanghai, China). Human bcl-2 and bcl-2 shRNA plasmids were constructed and supplied by GenePharma. Human BCL2 cDNA was cloned into the pcDNA-vector to generate pcDNA-bcl2 or pcDNA-sh-bcl2 recombinant plasmids. All oligonucleotides and plasmids were transfected into cells using Lipofectamine 2000 Transfection Reagent (Invitrogen, Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions.
Western blot analysis
Proteins from podocytes were extracted using RIPA lysis buffer (Beyotime Biotechnology, Shanghai, China) and the protein concentration was determined by the BCA Protein Assay Kit (Beyotime Biotechnology). Proteins were separated by electrophoresis on 10% SDS-polyacrylamide denaturing gels and transferred to nitrocellulose membranes, which were then blocked in 5% non-fat dry milk for 2 h, followed by overnight incubation at 4°C with primary antibodies against nephrin (0.5-1 µg/ml; ab58968 Abcam, Cambridge, MA, USA), Bcl-2 (1:1000; #2872 Cell Signaling Technology, Danvers, MA, USA), cleaved caspase-3 (1:1000, #29034, SAB), and GAPDH (1:1000, #21612, SAB). After washing in TBS-Tween buffer, the membranes were incubated with peroxidase-conjugated goat anti-rabbit IgG (1:3000) for 1 h at room temperature. Protein bands were visualized using an imager. The intensities of the identified bands were quantified.
Analysis of apoptosis by flow cytometry
Apoptosis of the podocytes was evaluated 48 h after their exposure to the various treatments. Briefly, podocytes were trypsinized and centrifuged at 1000 rpm for 5 min at room temperature. Next, 1× binding buffer was added to the precipitate followed by Annexin V Fluorescein isothiocyanate (FITC) stock solution (Annexin V-FITC Apoptosis Detection Kit, Sigma). Cells were incubated for 10 min at 4°C. Propidium iodide (PI) was added immediately before flow cytometric analysis. A total of 20,000 cells per sample were employed for flow cytometry analysis. Flow cytometric data were used to determine the percentage of cells undergoing apoptosis.
Dual luciferase reporter assay
To validate whether miR-134-5p directly targets the bcl-2 3’-untranslated region (3’-UTR), we performed a firefly luciferase reporter assay. Wildtype (WT) and mutated (mut) putative miR-134-5p seed-matching sites in bcl-2 3’-UTRs were amplified from human cDNA by PCR and inserted into the Sac I and Hind III restriction enzyme sites of the pmiRNA-Report vector (Genechem, Shanghai, China). Podocytes were seeded in a 24-well plate and co-transfected with WT or mut reporter plasmid, Renilla luciferase (pRL) plasmids, or miR-134-5p mimic or miR-ctrl. After transfection for 24 h, the cells were harvested and luciferase activity was analyzed with the Dual Luciferase Reporter Assay System (Promega, Madison, WI, USA).
Statistical analysis
All data are presented as the means ± S.E.M. of at least three independent assays, each performed in duplicate. Means and standard error of the mean or standard deviation were subjected to the Student’s t-test for pairwise comparison or ANOVA for multivariate analysis using Graphpad Prism 5 software. The level of statistical significance was set at P < 0.05.
Results
Establishment of the DN mouse model
Hematoxylin-eosin (HE) and Periodic acid-Schiff (PAS) tissue staining were used for morphological analysis of all mice at 20 weeks of age (Figure 1A). All ten db/db mice had early symptoms of DN, including glomerular hypertrophy, mesangial matrix expansion, and capillary basement membrane thickening. In contrast, the renal function of db/m mice was normal. The expression of nephrin and bcl-2 in glomeruli was significantly decreased in db/db mice compared with that in db/m mice.
Figure 1.
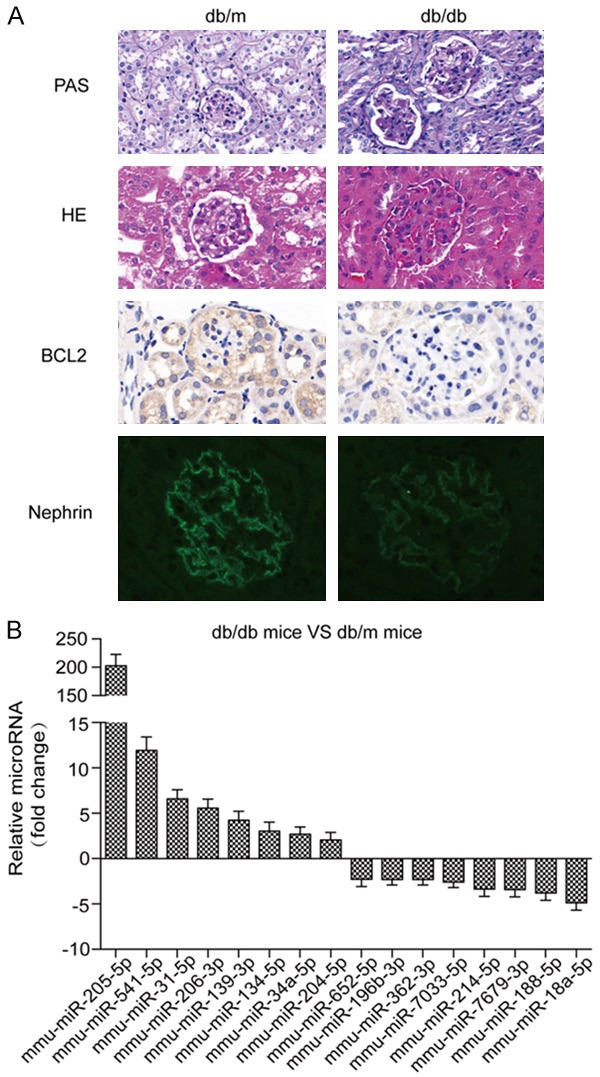
Characteristics of db/db and db/m mouse kidney samples used for miRNA microarray analysis. A. Compared with db/m mice, glomerular hypertrophy, mesangial matrix expansion, and capillary basement membrane thickening changes were apparent in HE-stained and PAS-stained tissue sections from kidney specimens from db/db mice. Immunohistochemistry and immunofluorescence were used to detect bcl-2 and nephrin protein expression in renal tissue. B. Differentially expressed miRNAs are shown from microarray miRNA profiling.
Microarray miRNA profiling
To investigate the potential function of miRNAs during DN, we conducted a microarray analysis of the renal tissues from the two groups of mice. Compared with the db/m group, 43 miRNAs were differentially expressed in the db/db group, of which 28 were significantly upregulated and 15 were significantly downregulated. The top 16 up- or down-regulated miRNAs are presented in Figure 1B.
HG induces higher expression of miR-134-5p
To explore the potential function of miR-134-5p in HG-treated podocytes, we used qRT-PCR to examine the expression of miR-134-5p at different timepoints (0, 24, 48, and 72 h) following HG (30 mM) stimulation. Notably, miR-134-5p levels in podocytes were upregulated after HG treatment in a time-dependent manner (Figure 2A), indicating that miR-134-5p might play a critical role in HG-treated podocytes.
Figure 2.
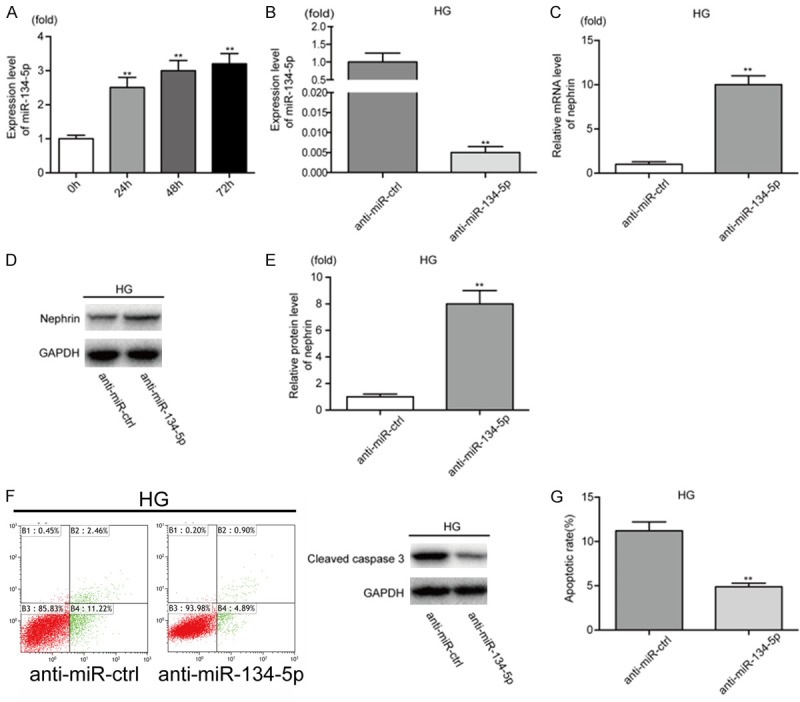
MiRNA-134-5p is upregulated in HG-treated podocytes and promotes podocyte apoptosis. A. The expression of miR-134-5p in podocytes treated with HG (30 mM) at different time points (0-72 h) was analyzed by qRT-PCR. B. The expression of miR-134-5p was analyzed by qRT-PCR after anti-miR-134-5p transfection in HG-treated podocytes. C. The relative mRNA level of nephrin was analyzed by qRT-PCR in HG-treated podocytes after transfection with anti-miR-ctrl or anti-miR-134-5p. D, E. The expression level of nephrin in HG-treated podocytes was analyzed by western blotting. F, G. Apoptosis in HG-treated podocytes was measured by western blot analysis and flow cytometry analysis of Annexin V and PI double staining. **P < 0.01. Data are presented as the mean ± SE.
MiR-134-5p promotes apoptosis of podocytes
Podocytes are thought to be injured at an early stage in DN; therefore, we investigated the functional role of miR-134-5p in podocyte apoptosis. First, anti-miR-ctrl or anti-miR-134-5p was transfected into HG-treated podocytes prior to analysis by qRT-PCR. Importantly, the relative expression of miR-134-5p in HG-treated podocytes transfected with anti-miR-134-5p was significantly reduced compared with that in cells transfected with anti-miR-ctrl (Figure 2B). Next, we used qRT-PCR and western blotting to examine the effect of miR-134-5p on the expression of nephrin, a key marker of podocytes in the filtration slits. Notably, mRNA and protein levels of nephrin were significantly increased in HG-treated podocytes transfected with anti-miR-134-5p compared with that in cells transfected with anti-miR-ctrl (Figure 2C-E). In addition, we performed flow cytometry and western blotting to detect apoptosis in podocytes. We found that the apoptosis rate and levels of cleaved caspase 3 decreased in HG-treated podocytes transfected with anti-miR-134-5p, compared with that in cells transfected with anti-miR-ctrl (Figure 2F, 2G). In contrast, NG-treated podocytes transfected with miR-ctrl or miR-134-5p showed the opposite results (Figure S1). These results indicate that miR-134-5p acts as a promoter of podocyte apoptosis.
Luciferase assay validates bcl-2 as a direct target of miR-134-5p
To explore the mechanism by which miR-134-5p promotes HG-induced apoptosis of podocytes, we searched two miRNA target analyzing databases (TargetScan and miRNAWalk 2.0) and found that bcl-2 is a latent target of miR-134-5p. We then performed luciferase reporter assays. The sequence of the 3’-UTR of bcl-2 mRNA matched the seed sequence of miR-134-5p. To test the functional significance of this finding, the 3’-UTR sequences, containing putative binding sites of the WT or mut for the seed matching sites, were introduced into a luciferase reporter vector (Figure 3A) and each was co-transfected into podocytes with the miR-134-5p mimic or miR-ctrl (Figure 3B). The results demonstrated that bcl-2 is a direct target of miR-134-5p. To further examine the effect of miR-134-5p on bcl-2, we transfected HG-treated podocytes with anti-miR-134-5p or anti-miR-ctrl. Western blotting was performed to assess bcl-2 protein levels. The results showed that bcl-2 protein levels were increased in HG-treated podocytes transfected with anti-miR-134-5p compared with that in cells transfected with anti-miR-ctrl (Figure 3C, 3D). Overexpression of miR-134-5p by miR-134-5p mimic transfection in podocytes reduced the expression of bcl-2 under NG conditions (Figure S2). Together, these results indicate that bcl-2 is a direct target of miR-134-5p.
Figure 3.
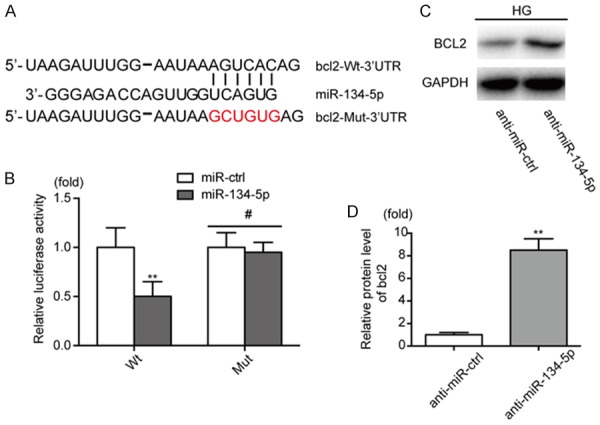
BCL2 is a direct target of miR-134-5p. A. Alignment of miR-134-5p with Bcl-2 3’-UTR sequences. B. Relative luciferase activity of reporters containing wild-type or mutated type with miR-134-5p target sites in NG-treated podocytes. C, D. The expression level of bcl-2 was analyzed by western blotting after co-transfection with anti-miR-ctrl or anti-miR-134-5p in HG-treated podocytes. **P < 0.01. Data are presented as the mean ± SE.
Inhibition of bcl-2 enhances the proapoptotic effect of miR-134-5p, while overexpression of bcl-2 attenuates it
To investigate the contribution of Bcl-2 to the proapoptotic effect of miR-134-5p in podocytes, we co-transfected human sh-bcl2 plasmids and anti-miR-134-5p into HG-treated podocytes. The effects of miR-134-5p on apoptosis were enhanced following reduction in bcl-2 levels in HG-treated podocytes (Figure 4). In addition, downregulation of bcl-2 alone markedly enhanced the effects of miR-134-5p on HG-treated podocytes. We found that the effects of miR-134-5p on apoptosis were inhibited by bcl-2 overexpression in NG-treated podocytes (Figure S3). Our results suggest that miR-134-5p functionally promotes podocyte apoptosis in a bcl-2-dependent manner.
Figure 4.
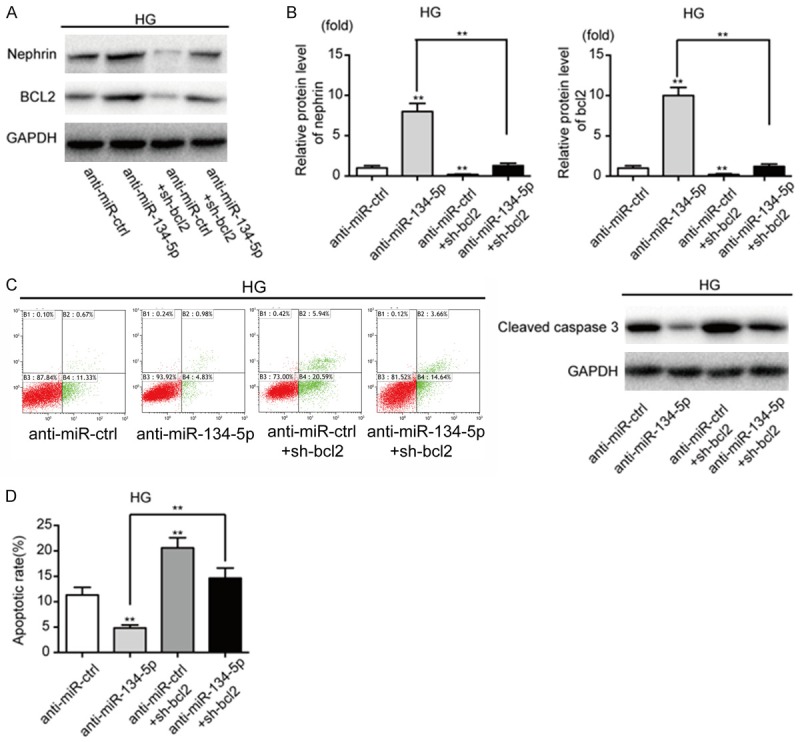
Inhibition of bcl-2 attenuates the anti-apoptotic effect of anti-miR-134-5p and decreases nephrin expression. A, B. The expression levels of nephrin and bcl-2 were measured by western blotting after co-transfection with anti-miR-ctrl or anti-miR-134-5p, and sh-Bcl2 expression plasmid in HG-treated podocytes, together or separately. C, D. Apoptosis was measured by western blotting and flow cytometry analysis of Annexin V and PI double staining. **P < 0.01. Data are presented as the mean ± SE.
Discussion
In this study, we successfully established a DN model in C57BL/KsJ db/db mice and found that glomerular miR-134-5p is differentially expressed in the mouse kidney in diabetes, with or without proteinuria. To further explore the roles of miRNAs, we cultured cells from a conditionally immortalized human podocyte cell line in medium supplemented with 30 mM D-glucose to mimic conditions in diabetes. Our data showed that miR-134-5p expression is higher in high-glucose than in normal (non-diabetic) glucose conditions. We found that miR-134-5p mimics can reduce bcl-2 protein levels when transfected into podocytes. As a result, the podocyte-specific biomarker nephrin was downregulated and the podocytes became apoptotic. The reduced nephrin levels could lead to the impairment of podocyte function and structure. In contrast, miR-134-5p inhibitors conferred protection from apoptosis, even under hyperglycemic conditions.
MiRNAs are a class of non-coding small RNA molecules consisting of 21-25 nucleotides, which not only cause the degradation of target mRNAs, but also inhibit translation through target mRNA-specific base pairing [20,21]. Recent studies have shown that miRNAs are essential regulators of expression and important therapeutic targets in DN [22-24]. Studies have also demonstrated that miRNAs negatively regulate protein-coding genes at the posttranscriptional level, primarily by binding to their 3’-UTR [20,21,25]. Therefore, identification of the key roles of miRNAs in apoptosis, via modulation of various targets, has promoted research into miRNAs as biomarkers and therapeutic targets for the treatment of DN. Using miRNA target gene prediction tools and bioinformatics-based analyses, we found that miR-134-5p, which is expressed in cultured podocytes dependent on the extracellular glucose concentration, might complement a single site in the 3’-UTR of transcripts of BCL2, FUT2, ESRRG, TMEM184A, DRP2, PUSL1, and PXMP4. Among these genes, we validated that BCL2 was a direct target of miR-134-5p and intrinsically linked to apoptosis.
Apoptosis is a defined set of molecular cascades that result in lethal changes to the cell, including membrane blebbing, mitochondrial breakdown, and DNA fragmentation [26,27]. Podocyte apoptosis has been reported to play a key role in the pathophysiology of DN, both dependent and independent of miRNAs [28-34]. In addition, podocyte apoptosis not only causes podocytopenia but also affects the glomerular slit diaphragm by downregulating podocyte biomarkers such as nephrin, which is expressed in mature renal podocytes [35-37]. All of these changes cause a critical impairment of podocytes and subsequently the function of the filtration barrier, which would be at least partially responsible for proteinuria in DN. BCL2 is a well-known prosurvival gene that plays a critical role in a variety of cell systems, including podocytes [38]. Bcl-2 regulates cell death primarily by controlling mitochondrial membrane permeability and functions together with caspases and other proteins in a feedback loop system [16,39]. Hundreds of discrete miRNA sequences have the potential to inhibit BCL2 [40-42]. Among these miRNAs, miR-134-5p may inhibit bcl-2 expression and increase apoptosis in HG-cultured podocytes. miR-134-5p regulates BCL2 at the post-transcriptional level and induces apoptosis in podocytes under HG conditions by inhibiting bcl-2. Apoptotic podocytes fail to synthesize sufficient levels of specific functional proteins and subsequently undergo actin rearrangement, thus resulting in functional and structural defects that are similar to those observed in humans and animal models with DN. BCL2 shRNA plasmids can also induce podocyte apoptosis, although not at the post-transcriptional level, by targeting BCL2 expression.
Taken together, our findings demonstrate that miR-134-5p promotes podocyte apoptosis under HG conditions by reducing the expression level of bcl-2. This study provides a useful approach for deciphering the mechanisms used by miRNAs in the pathogenesis of DN.
Acknowledgements
We thank the entire staff at the Biotech Treatment Center, The Second Affiliated Hospital of Nanjing Medical University, Nanjing, China for their valuable technical assistance with this research. This work was supported by grants from the National Natural Science Foundation of China (Grant nos. 81270896, 81100577) and Six talent peaks project in Jiangsu Province (Grant nos. 2013-WSN-049, 2015-WSN-016).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Rao PV, Lu X, Standley M, Pattee P, Neelima G, Girisesh G, Dakshinamurthy KV, Roberts CT Jr, Nagalla SR. Proteomic identification of urinary biomarkers of diabetic nephropathy. Diabetes Care. 2007;30:629–637. doi: 10.2337/dc06-2056. [DOI] [PubMed] [Google Scholar]
- 2.Jesic M, Jesic M, Sajic S, Bogicevic D, Buljugic S, Maglajlic S. The effect of metabolic and hormonal parameters on microalbuminuria in adolescents with type 1 diabetes mellitus. Srp Arh Celok Lek. 2013;141:315–319. doi: 10.2298/sarh1306315j. [DOI] [PubMed] [Google Scholar]
- 3.Kume S, Koya D. Autophagy: a novel therapeutic target for diabetic nephropathy. Diabetes Metab J. 2015;39:451–460. doi: 10.4093/dmj.2015.39.6.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Visweswaran GR, Gholizadeh S, Ruiters MH, Molema G, Kok RJ, Kamps JA. Targeting rapamycin to podocytes using a vascular cell adhesion molecule-1 (VCAM-1)-harnessed SAINT-based lipid carrier system. PLoS One. 2015;10:e0138870. doi: 10.1371/journal.pone.0138870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ying Q, Wu G. Molecular mechanisms involved in podocyte EMT and concomitant diabetic kidney diseases: an update. Ren Fail. 2017;39:474–483. doi: 10.1080/0886022X.2017.1313164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J, Chen JK, Harris RC. EGF receptor deletion in podocytes attenuates diabetic nephropathy. J Am Soc Nephrol. 2015;26:1115–1125. doi: 10.1681/ASN.2014020192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang C, Zhang X, Wang HM, Liu XM, Zhang XJ, Zheng B, Qian GR, Ma ZL. MicroRNA-18a-5p functions as an oncogene by directly targeting IRF2 in lung cancer. Cell Death Dis. 2017;8:e2764. doi: 10.1038/cddis.2017.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ambros V, Lee RC, Lavanway A, Williams PT, Jewell D. MicroRNAs and other tiny endogenous RNAs in C. elegans. Curr Biol. 2003;13:807–818. doi: 10.1016/s0960-9822(03)00287-2. [DOI] [PubMed] [Google Scholar]
- 9.Harvey SJ, Jarad G, Cunningham J, Goldberg S, Schermer B, Harfe BD, McManus MT, Benzing T, Miner JH. Podocyte-specific deletion of dicer alters cytoskeletal dynamics and causes glomerular disease. J Am Soc Nephrol. 2008;19:2150–2158. doi: 10.1681/ASN.2008020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koga K, Yokoi H, Mori K, Kasahara M, Kuwabara T, Imamaki H, Ishii A, Mori KP, Kato Y, Ohno S, Toda N, Saleem MA, Sugawara A, Nakao K, Yanagita M, Mukoyama M. MicroRNA-26a inhibits TGF-beta-induced extracellular matrix protein expression in podocytes by targeting CTGF and is downregulated in diabetic nephropathy. Diabetologia. 2015;58:2169–2180. doi: 10.1007/s00125-015-3642-4. [DOI] [PubMed] [Google Scholar]
- 11.Kato M, Zhang J, Wang M, Lanting L, Yuan H, Rossi JJ, Natarajan R. MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-beta-induced collagen expression via inhibition of E-box repressors. Proc Natl Acad Sci U S A. 2007;104:3432–3437. doi: 10.1073/pnas.0611192104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kato M, Arce L, Wang M, Putta S, Lanting L, Natarajan R. A microRNA circuit mediates transforming growth factor-beta1 autoregulation in renal glomerular mesangial cells. Kidney Int. 2011;80:358–368. doi: 10.1038/ki.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mu J, Pang Q, Guo YH, Chen JG, Zeng W, Huang YJ, Zhang J, Feng B. Functional implications of microRNA-215 in TGF-beta1-induced phenotypic transition of mesangial cells by targeting CTNNBIP1. PLoS One. 2013;8:e58622. doi: 10.1371/journal.pone.0058622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Long J, Wang Y, Wang W, Chang BH, Danesh FR. MicroRNA-29c is a signature microRNA under high glucose conditions that targets Sprouty homolog 1, and its in vivo knockdown prevents progression of diabetic nephropathy. J Biol Chem. 2011;286:11837–11848. doi: 10.1074/jbc.M110.194969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu XD, Zhang LY, Zhu TC, Zhang RF, Wang SL, Bao Y. Overexpression of miR-34c inhibits high glucose-induced apoptosis in podocytes by targeting Notch signaling pathways. Int J Clin Exp Pathol. 2015;8:4525–4534. [PMC free article] [PubMed] [Google Scholar]
- 16.Chen YQ, Wang XX, Yao XM, Zhang DL, Yang XF, Tian SF, Wang NS. MicroRNA-195 promotes apoptosis in mouse podocytes via enhanced caspase activity driven by BCL2 insufficiency. Am J Nephrol. 2011;34:549–559. doi: 10.1159/000333809. [DOI] [PubMed] [Google Scholar]
- 17.Huang SS, Ding DF, Chen S, Dong CL, Ye XL, Yuan YG, Feng YM, You N, Xu JR, Miao H, You Q, Lu X, Lu YB. Resveratrol protects podocytes against apoptosis via stimulation of autophagy in a mouse model of diabetic nephropathy. Sci Rep. 2017;7:45692. doi: 10.1038/srep45692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomadaki H, Scorilas A. BCL2 family of apoptosis-related genes: functions and clinical implications in cancer. Crit Rev Clin Lab Sci. 2006;43:1–67. doi: 10.1080/10408360500295626. [DOI] [PubMed] [Google Scholar]
- 19.Wang M, Wang S, Yao D, Yan Q, Lu W. A novel long non-coding RNA CYP4B1-PS1-001 regulates proliferation and fibrosis in diabetic nephropathy. Mol Cell Endocrinol. 2016;426:136–145. doi: 10.1016/j.mce.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 20.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 21.Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 22.Zhao B, Li H, Liu J, Han P, Zhang C, Bai H, Yuan X, Wang X, Li L, Ma H, Jin X, Chu Y. MicroRNA-23b targets Ras GTPase-activating protein SH3 domain-binding protein 2 to alleviate fibrosis and albuminuria in diabetic nephropathy. J Am Soc Nephrol. 2016;27:2597–2608. doi: 10.1681/ASN.2015030300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rudnicki M, Beckers A, Neuwirt H, Vandesompele J. RNA expression signatures and posttranscriptional regulation in diabetic nephropathy. Nephrol Dial Transplant. 2015;30(Suppl 4):iv35–42. doi: 10.1093/ndt/gfv079. [DOI] [PubMed] [Google Scholar]
- 24.Kato M, Arce L, Natarajan R. MicroRNAs and their role in progressive kidney diseases. Clin J Am Soc Nephrol. 2009;4:1255–1266. doi: 10.2215/CJN.00520109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang B, Pan X, Anderson TA. MicroRNA: a new player in stem cells. J Cell Physiol. 2006;209:266–269. doi: 10.1002/jcp.20713. [DOI] [PubMed] [Google Scholar]
- 26.Tang HL, Tang HM, Hardwick JM, Fung MC. Strategies for tracking anastasis, a cell survival phenomenon that reverses apoptosis. J Vis Exp. 2015 doi: 10.3791/51964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang HM, Talbot CC Jr, Fung MC, Tang HL. Molecular signature of anastasis for reversal of apoptosis. F1000Res. 2017;6:43. doi: 10.12688/f1000research.10568.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun J, Li ZP, Zhang RQ, Zhang HM. Repression of miR-217 protects against high glucose-induced podocyte injury and insulin resistance by restoring PTEN-mediated autophagy pathway. Biochem Biophys Res Commun. 2017;483:318–324. doi: 10.1016/j.bbrc.2016.12.145. [DOI] [PubMed] [Google Scholar]
- 29.Stitt-Cavanagh E, MacLeod L, Kennedy C. The podocyte in diabetic kidney disease. ScientificWorldJournal. 2009;9:1127–1139. doi: 10.1100/tsw.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanchez-Nino MD, Benito-Martin A, Ortiz A. New paradigms in cell death in human diabetic nephropathy. Kidney Int. 2010;78:737–744. doi: 10.1038/ki.2010.270. [DOI] [PubMed] [Google Scholar]
- 31.Durvasula RV, Shankland SJ. Podocyte injury and targeting therapy: an update. Curr Opin Nephrol Hypertens. 2006;15:1–7. doi: 10.1097/01.mnh.0000199012.79670.0b. [DOI] [PubMed] [Google Scholar]
- 32.Ho J, Ng KH, Rosen S, Dostal A, Gregory RI, Kreidberg JA. Podocyte-specific loss of functional microRNAs leads to rapid glomerular and tubular injury. J Am Soc Nephrol. 2008;19:2069–2075. doi: 10.1681/ASN.2008020162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mundel P, Shankland SJ. Podocyte biology and response to injury. J Am Soc Nephrol. 2002;13:3005–3015. doi: 10.1097/01.asn.0000039661.06947.fd. [DOI] [PubMed] [Google Scholar]
- 34.Allen DA, Yaqoob MM, Harwood SM. Mechanisms of high glucose-induced apoptosis and its relationship to diabetic complications. J Nutr Biochem. 2005;16:705–713. doi: 10.1016/j.jnutbio.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 35.Kim JJ, Li JJ, Jung DS, Kwak SJ, Ryu DR, Yoo TH, Han SH, Choi HY, Kim HJ, Han DS, Kang SW. Differential expression of nephrin according to glomerular size in early diabetic kidney disease. J Am Soc Nephrol. 2007;18:2303–2310. doi: 10.1681/ASN.2006101145. [DOI] [PubMed] [Google Scholar]
- 36.Liu G, Kaw B, Kurfis J, Rahmanuddin S, Kanwar YS, Chugh SS. Neph1 and nephrin interaction in the slit diaphragm is an important determinant of glomerular permeability. J Clin Invest. 2003;112:209–221. doi: 10.1172/JCI18242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ilatovskaya DV, Levchenko V, Lowing A, Shuyskiy LS, Palygin O, Staruschenko A. Podocyte injury in diabetic nephropathy: implications of angiotensin II-dependent activation of TRPC channels. Sci Rep. 2015;5:17637. doi: 10.1038/srep17637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M, Wojcik SE, Aqeilan RI, Zupo S, Dono M, Rassenti L, Alder H, Volinia S, Liu CG, Kipps TJ, Negrini M, Croce CM. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci U S A. 2005;102:13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nehra R, Riggins RB, Shajahan AN, Zwart A, Crawford AC, Clarke R. BCL2 and CASP8 regulation by NF-kappaB differentially affect mitochondrial function and cell fate in antiestrogen-sensitive and -resistant breast cancer cells. FASEB J. 2010;24:2040–2055. doi: 10.1096/fj.09-138305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Huang F, Wang J, Peng L, Luo H. MiR-15b mediates liver cancer cells proliferation through targeting BCL-2. Int J Clin Exp Pathol. 2015;8:15677–15683. [PMC free article] [PubMed] [Google Scholar]
- 41.Li Q, Ren P, Shi P, Chen Y, Xiang F, Zhang L, Wang J, Lv Q, Xie M. MicroRNA-148a promotes apoptosis and suppresses growth of breast cancer cells by targeting B-cell lymphoma 2. Anticancer Drugs. 2017;28:588–595. doi: 10.1097/CAD.0000000000000498. [DOI] [PubMed] [Google Scholar]
- 42.Li WH, Wu HJ, Li YX, Pan HG, Meng T, Wang X. MicroRNA-143 promotes apoptosis of osteosarcoma cells by caspase-3 activation via targeting Bcl-2. Biomed Pharmacother. 2016;80:8–15. doi: 10.1016/j.biopha.2016.03.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


