Abstract
Aim: This study aimed to investigate the protective effects of paeoniflorin (PAE) on radiation-induced hepatic fibrosis in a rat model. Methods: Fifty healthy male Sprague-Dawley rats were randomly assigned to normal control group, hepatic fibrosis group, and PAE treatment groups. X-ray exposure was employed to establish radiation-induced hepatic fibrosis model. PAE was administered once daily, and rats were sacrificed at week 26 after irradiation. The liver histopathology was evaluated under a light microscope after HE staining and Masson staining. Meanwhile, the protein expression of transforming growth factor-beta 1 (TGF-β1), Smad3/4 and Smad7 was detected by immunohistochemistry. Results: Radiation-induced liver damage and collagen deposition were observed in the model group as compared to normal control group, but PAE treatment significantly attenuated the liver injury and reduce collagen deposition (P<0.05 or 0.01). The hepatic hydroxyproline content and serum levels of TGF-β1, hyaluronic acid, ro-collagen type III and laminin markedly increased in model group as compared to control group (P<0.01), but they decreased dramatically after PAE treatment. The expression of TGF-β1, Smad3/4 and Smad7 in the liver increased significantly in model group as compared to control group (P<0.01), and PAE could down-regulate the expression of Smad3/4 and up-regulate Smad7 expression (P<0.05 or 0.01). The activities of serum amino-transferase and aspartate aminotransferase were significantly higher in hepatic fibrosis group than in normal control group, but PAE treatment markedly reduced them (P<0.05). Conclusion: PAE can inhibit the radiation induced hepatic fibrosis via regulating TGF-β1/Smads signaling pathway.
Keywords: Paeoniflorin, radiation-induced hepatic fibrosis, transforming growth factor-beta 1, Smads
Introduction
Radiotherapy is an important strategy for the treatment of malignant abdominal diseases, such as malignant lymphoma, hepatocellular carcinoma, cholangiocarcinoma, and stomach cancer. However, it may inevitably cause damage to organs (such as radiation induced liver fibrosis, RILF) and sometimes it will be lethal [1], which significantly limits the total radiation dose that can be safely administered to patients. Radiation related liver injury is a potential sequela of radiotherapy and radiation accidents. Late sequelae of radiation induced damage to the liver include severe depletion of parenchymal cells and a marked increase in fibrotic tissues in the central vein and periductal regions [2]. The pronounced radiosensitivity of hepatic tissue sometimes limits the use of radiotherapy. With the increasing introduction of radioactive resources to our daily life and medical interventions, there is a great demand for understanding the detailed mechanism of radiation-induced injury and developing possible preventive strategies. Recent studies revealed that hepatic fibrosis was reversible. Interrupting and reversing liver fibrosis may be another way to treat hepatic fibrosis or prevent against hepatocellular carcinoma.
Hepatic fibrosis is a common characteristic of some chronic liver diseases and its pathogenesis has involvement of excess accumulation of extracellular matrix (ECM). Hepatic stellate cells (HSCs) are major targets of transforming growth factor-beta (TGF-β), a profibrogenic agent. TGF-β is well known as an inducer of apoptosis, and it can inhibit hepatocyte regeneration and activate HSCs, which are the principal cellular source of excess ECM, during hepatic fibrosis though TGF-β/Smad signaling pathway [3-5]. TGF-β signaling pathway has become a new target in the prevention and treatment of hepatic fibrosis. Studies have confirmed that fibrosis is a dynamic process and may be bidirectional. However, no effective therapies or medicines are available yet for the treatment of hepatic fibrosis. Therefore, it is imperative to develop effective strategies for the prevention and treatment of hepatic fibrosis.
Traditional Chinese medicine is effective in the treatment of some chronic diseases and some Chinese herbs are proven to possess the ability to prevent fibrogenesis [6]. Paeoniflorin (PAE, C23H28O11), a monoterpene glucoside, is one of the main bioactive components of total glucosides of paeony extracted from the root of Paeonia lactiflora [7]. Many studies have suggested that PAE contributes to the main bioactivity of Moutan cortex and has anti-inflammatory, sedation, analgesic, anti-allergic, immunoregulatory and hepatoprotective effects [8]. PAE may protect cells from radiation-induced damage and suppresses the production of inflammatory mediators [9,10]. Our previous studies demonstrated that PAE significantly ameliorated hepatic fibrosis through regulating TGF-β1 signaling pathway in HSCs in a rat model [11]. In this study, a rat model of radiation-induced hepatic fibrosis (RIHF) was established, the effects of PAE on the RIHF was investigated and of the role of TGF-β/Smad signaling pathway was further explored, which may provide theoretical evidence for the treatment of RIHF with PAE.
Materials and methods
Animals
This study was approved by the Anhui Medical University Animal Care Committee and all the procedures were conducted according to The Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health (NIH publication No.85-23, revised 1996). Rats were purchased from the Experimental Animal Center of Anhui Medical University. Fifty specific pathogen free Sprague-Dawley (SD) adult male rats were used in the present study. Animals were housed in a temperature-controlled (20-24°C) room on a 12 h light/dark cycle.
Drugs and reagents
Paeoniflorin was purchased from Xuancheng Baicao Plants Industry and Trade Co., Ltd. Commercial kits used for the detection of aspartate aminotransferase (AST), alanine amino-transferase (ALT) and hydroxyproline (HYP) were from Nanjing Jiancheng Institute of Biotechnology (China). The hyaluronic acid (HA), TGF-β1, laminin (LN) and pro-collagen type III (PC III) ELASA kits were purchased from BPB, USA. Masson staining and brilliant green staining Kits used for immunohistochemistry for TGF-β1 and Smad3/4/7 II were from Beijing Boisynthesis Biotechnology Co., Ltd. Primary antibodies to Smad3/4 and Smad7 were obtained from Abcam Co., Ltd..
Animal treatments
Rats were randomly divided into five groups (n=10 per group): normal control group, hepatic fibrosis group, and PAE treatment groups (20 mg/kg, 40 mg/kg and 80 mg/kg). Rats in all groups, except the normal control group were intraperitoneally anesthetized with 10% chloral hydrate at 3 ml/kg, and the right lobe of the liver (2.5×2.5 cm) was exposed to a single-dose of 30 Gy X-ray irradiation (600 cGy/min) [12]. The remaining part of the body was protected using customized lead shielding.
During one week before exposure, PAE treatment groups (20, 40, 80 mg/kg) were administered by intragastrically once daily until the experiment was over. Rats in the control group and the hepatic fibrosis group were administered equal volume (0.5 ml/100 g body weight) of normal saline by intragastrically.
Sample collection
After 26-week treatment, blood was harvested for analysis. The right lobe of liver were rapidly collected, fixed immediately in 10% formalin for 24 h and processed for histological and immunohistochemical examinations.
Biochemical examinations
Serum contents of TGF-β1, HA, PC-III and LN were assessed by using ELASA kits (BPB, USA). Serum activities of ALT and AST were measured by spectrophotometry using commercially available kits (Nanjing Jiancheng Institute of Biotechnology, China). The content of HYP in the liver was assayed by using commercially available kit (Nanjing Jiancheng Institute of Biotechnology, China).
Histopathological examination
The liver tissues were collected, fixed in 10% formalin and embedded in paraffin. Hematoxylin and Eosin (HE) staining was performed for the evaluation of liver injury and Masson staining was done to assess the collagen deposition according to the standard procedure. The degree of liver fibrosis was categorized according to a scoring system [13] (Table 1).
Table 1.
Pathological staging of hepatic fibrosis
| Stage of fibrosis | Changes of Histopatholgy |
|---|---|
| 0 | No fibrosis, normal liver and absence of fibrosis; |
| 1 | Confined to portal tracts |
| 2 | Portal tracts plus spurs radiating into parenchyma |
| 3 | Linkage of some portal tracts but intact architecture |
| 4 | Linkage of most portal tracts with architechural distortion |
| 5 | Cirrhosis |
Immunohistochemistry
Immunohistochemistry was performed using avidin-biotin-complex method. Briefly, 4-mm sections were deparaffinized and rehydrated. Sections were then incubated with 0.01 M phosphate-buffered saline (PBS; pH=6.0), followed by antigen retrieval in a microwave oven. After blocking with 3% hydrogen peroxide, sections were washed with 0.01 M PBS, and blocked with normal goat serum. Then, sections were incubated overnight with primary antibody (50 μl, 1:200). After rinsing in PBS, they were incubated with biotinylated goat anti-rabbit IgG. Finally, the sections were incubated with avidin-biotin complex kit and visualization was done with the diaminobenzidine (DAB) reagent for 5-10 minutes, followed by counterstaining with hematoxylin. Sections were examined under a light microscope at a magnification of 400. In negative control, primary antibody was replaced with PBS. The expression of TGF-β1, Smad3/4, and Smad7 was graded according to the extent and intensity of staining [14]. The integral optical density (OD) of each image was determined, and the relative protein expression was quantified densitometrically and presented in the mean optical density (MOD) units.
Western blotting
The liver tissues were harvested, washed with ice-cold normal saline (NS) (4°C), cut into pieces, followed by homogenization in lysis buffer containing: [Hepes 20 (pH 7.7), MgCl2 2.5, dithiothreitol (DTT) 0.5, EDTA 0.1, (-glycerophosphate 20, Sodium orthovanadate 0.1, NaCl 75 and leupeptin 4 (g/ml, phenyl-methylsulfonyl fluoride (PMSF) 20 (g/ml, TritonX-100 0.05% (v/v)] on ice for 30 minutes. After centrifugation at 12,000 rpm for 5 min at 4°C, the supernatant (200 μl) was collected. The protein concentration of each sample was determined using a BCA protein assay kit. For the detection of protein expression of detect p-Smad3/Smad3, Smad4 and Smad7, proteins of equal amount (40 μg) were loaded and separated on 12% SDS-PAGE. Then, proteins were transferred onto polyvinylidene difluoride membranes for 120 min at 100 mA. The membranes were blocked overnight at 4°C in 5% non-fat milk to block nonspecific binding. After incubation for 2 h at room temperature with primary antibodies at 1:1,000, the membranes were then washed and treated with secondary antibodies (goat anti-mouse or goat anti-rabbit immunoglobulin G) conjugated with horseradish peroxidase for 2 h. Proteins were visualized using enhanced chemiluminescence ECL kit.
Statistical analysis
All data are presented as mean ± standard deviation. Differences among groups were assessed using unpaired Student’s t test and one-way analysis of variance (ANOVA). A value of P less than 0.05 was considered statistically significant. Statistical analysis was performed with SPSS version 17.0.
Results
Effect of PAE on the activities of ALT and AST
Compared with normal control group, the serum activities of ALT and AST markedly increased in model group (P<0.01). However, PAE treatment significantly decreased the ALT and AST activities of the serum as compared to model group (P<0.01 or P<0.05). These suggest that PAE may inhibit the elevation of transaminases in response to liver injury (Figure 1).
Figure 1.
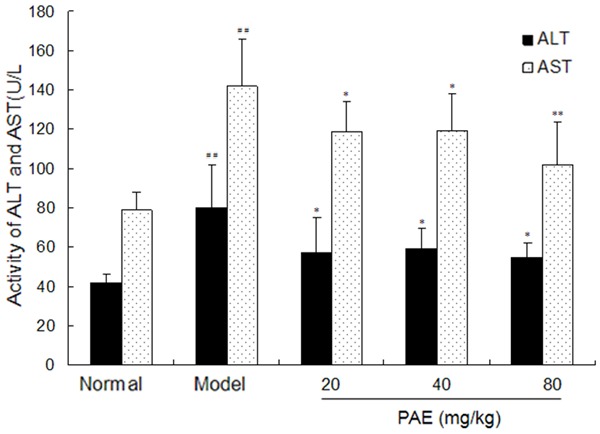
Effect of PAE on the activities of AST and ALT in RIHF Rats. Note: PAE: paeoniflorin. Data are presented as mean ± standard deviation. ##P<0.01 vs normal group. *P<0.05 vs model group. **P<0.01 vs model group.
Effect of PAE on the serum contents of TGF-β1, TNF-α and IL-6
There were significant increases in the serum contents of TGF-β1, TNF-α and IL-6 in model group as compared to normal control group (P<0.01). However, PAE treatment significantly lowered the serum contents of TGF-β1, TNF-α and IL-6 as compared to model group (P<0.05). These indicate that PAE is able to decrease the serum contents of TGF-β1, TNF-α and IL-6 in RIHF rats (Figure 2).
Figure 2.
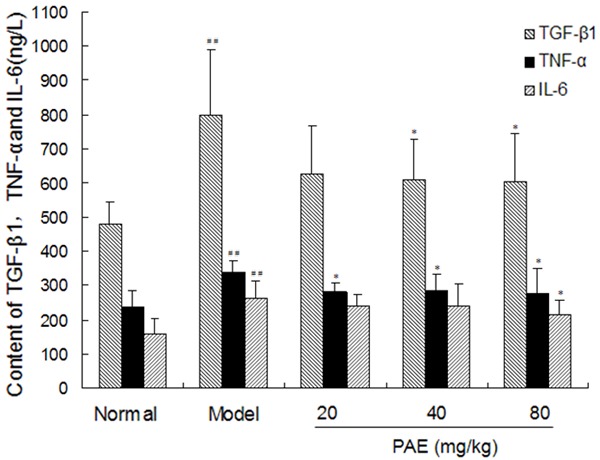
Effect of PAE on the contents of TGF-β1, TNF-α and IL-6 in RIHF Rats. Note: PAE: paeoniflorin. Data are presented as the mean ± standard deviation. ##P<0.01 vs normal group. *P<0.05 vs model group. **P<0.01 vs model group.
Effect of PAE on the Serum levels of HA, LN and PC III and the liver content of Hyp
As shown in Table 2, the serum levels of HA, LN and PC III, three markers of hepatic fibrosis, increased markedly in model group (P<0.01). Administration with PAE effectively and dramatically decreased the serum levels of HA, LN and PC III (P<0.05 or P<0.01) as compared to model group. These suggest that PAE is able to decrease the serum levels of HA, PC-III and LN, which contributes to its anti-fibrotic effects on RIHF. And radiation markedly increased the liver content of hydroxyproline as compared to normal control group, but treatment with PAE significantly reduced the content of hydroxyproline in liver tissues as compared to model group (P<0.05 or P<0.01) (Table 3).
Table 2.
Effects of PAE on collagen deposition in RIHF rats
| Group | n | - | + | ++ | +++ | |
|---|---|---|---|---|---|---|
| Normal | 10 | 10 | 0 | 0 | 0 | |
| Model | 8 | 0 | 1 | 2 | 5** | |
| PAE | 20 mg/kg | 9 | 1 | 2 | 4 | 2 |
| 40 mg/kg | 10 | 1 | 4 | 3 | 2# | |
| 80 mg/kg | 10 | 1 | 6 | 2 | 1## | |
Note: Mean ± standard deviation
P<0.01 vs normal group;
P<0.05 vs model group;
P<0.01 vs model group.
Table 3.
Effects of PAE on the serum HA, LN, PCIII and Hyp levels of RIHF rats
| Group | n | HA (pg/ml) | PC-III (ng/ml) | LN (ng/ml) | Hyp (ug/mg) | |
|---|---|---|---|---|---|---|
| Normal | 10 | 902.2±165.9 | 9.7±2.0 | 53.0±18.4 | 0.48±0.14 | |
| Model | 8 | 1784.5±214.9** | 18.1±2.1** | 103.1±22.0** | 1.36±0.29** | |
| PAE | 20 mg/kg | 9 | 1483.0±271.1# | 15.0±3.1# | 69.2 ±18.0## | 1.05±0.35# |
| 40 mg/kg | 10 | 1691.5±320.5 | 15.5±4.2# | 79.3±15.5# | 0.96±0.24# | |
| 80 mg/kg | 10 | 1304.4±209.7# | 14.5±3.1# | 61.6±7.6## | 0.77±0.23## | |
Note: Mean ± standard deviation;
P<0.01 vs normal group;
P<0.05 vs model group;
P<0.01 vs model group.
Effect of PAE on the liver histology of RIHF rat (HE staining)
HE staining (Figure 3) indicated that the radiation exposure caused significant damage to the liver morphology, which was characterized by marked fatty degeneration, necrosis, ballooning degeneration of hepatocytes and infiltration of inflammatory cells in the interstitium while the liver in normal control group showed normal lobular architecture with clear central veins and radial hepatic cords (P<0.01). PAE treatment markedly alleviated the degree of liver injury and inflammation (P<0.05 or P<0.01) (Table 4).
Figure 3.
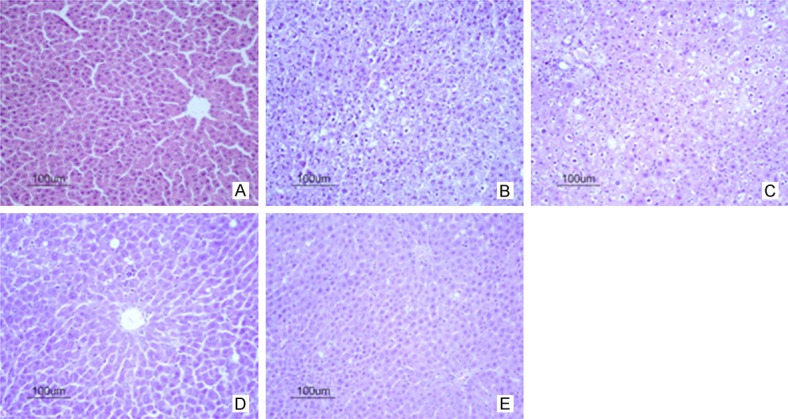
Effect of PAE on the liver histology of RIHF rats (HE staining, 200×). A. Normal control group; B. Model group; C. 20 mg/kg PAE group; D. 40 mg/kg PAE group; E. 80 mg/kg PAE group. Note: Pae: paeoniflorin.
Table 4.
Effect of PAE on the fibrosis score of RIHF rats
| Group | n | Fibrosis score | |
|---|---|---|---|
| Normal | 10 | 0.13±0.35 | |
| Model | 8 | 3.88±1.13** | |
| PAE | 20 mg/kg | 9 | 2.50±1.07# |
| 40 mg/kg | 10 | 2.43±1.03# | |
| 80 mg/kg | 10 | 2.13±0.99## | |
Note: Mean ± standard deviation
P<0.01 vs normal group;
P<0.05 vs model group;
P<0.01 vs model group.
Effect of PAE on collagen deposition in RIHF Rats by Masson staining
As shown in Figure 4, as compared to normal group, the normal hepatic lobules in model group disappeared, collagen deposition increased significantly and the pseudolobule formed. However, PAE treatment markedly alleviated the degree of hepatic fibrosis and improved the histological scores (Table 2).
Figure 4.
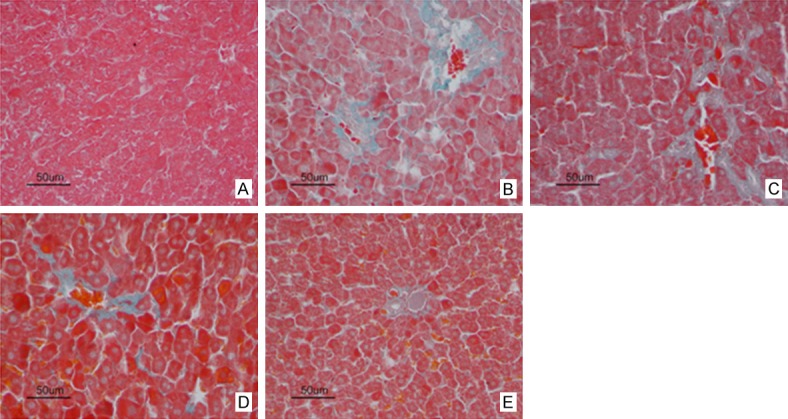
Effect of PAE on the liver collagen deposition of RIHF rats (Masson staining, 400×). A. Normal control group; B. Model group; C. 20 mg/kg PAE group; D. 40 mg/kg PAE group; E. 80 mg/kg PAE group. Note: Pae: paeoniflorin.
Effect of PAE on TGF-β1 production, and expression of Smad3/4 and Smad7 in RIHF rats
As shown in Figure 5, the expression of TGF-β1, Smad3/4 and Smad7 was observed in the cytoplasm of hepatocytes in all groups. In normal control livers, Smad3/4 expression was rarely observed, but Smad7 expression was widely distributed in hepatocytes. In the liver of model group, Smad7 expression reduced significantly in hepatocytes (P<0.01). After PAE treatment, the expression of Smad3/4 decreased markedly and the smad7 expression increased dramatically as compared to model group (P<0.05 or P<0.01).
Figure 5.
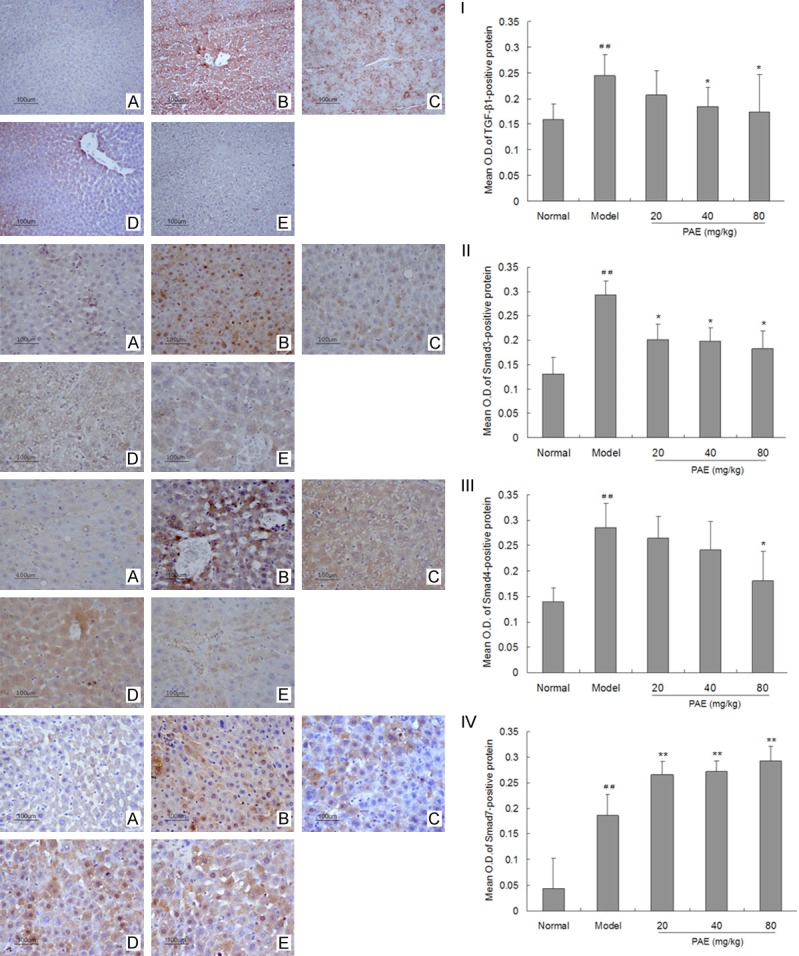
Effect of PAE on the expression of TGF-β1 (I), Smad3 (II), Smad4 (III) and Smad7 (IV) in the liver of RIHF Rats. (A) Normal control group; (B) Model group; (C) 20 mg/kg PAE group; (D) 40 mg/kg PAE group; (E) 80 mg/kg PAE group. Note: PAE: paeoniflorin. Data are presented as the mean ± standard deviation. ##P<0.01 vs normal group. *P<0.05 vs model group.
As shown in Figure 6, in model group, the protein expression of phosphorylated Smad3 and Smad4 increased in hepatic fibrosis rats, but PAE significantly suppressed the phosphorylation of Smad3, and decreased the expression of Smad4 in hepatic fibrosis rats as compared to model group. Moreover, PAE treatment significantly elevated the expression of smad7 in the liver as compared to model group (P<0.05 or P<0.01).
Figure 6.
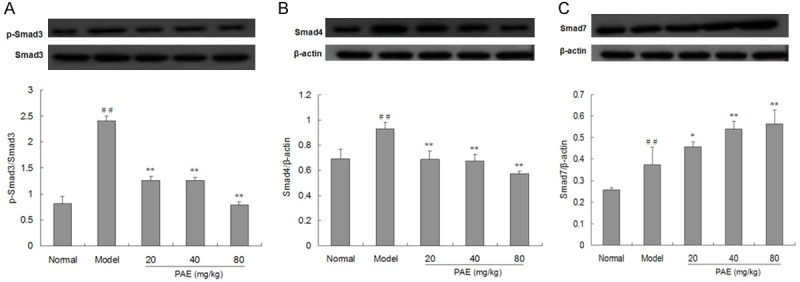
Effects of PAE on the protein expression of p-Smad3/Smad3, Smad4 and Smad7 in the liver of RIHF Rats (Western blotting). Protein expression of p-Smad3/Smad3 (A), Smad4 (B) and Smad7 (C) in the liver was determined by Western blotting. β-actin served as a loading control. The protein expression of p-Smad3/Smad3, Smad4 and Smad7 was normalized to that of β-actin. Protein bands were quantified densitometrically and expressed as MOD. Data are presented as mean ± standard deviation ##P<0.01 vs normal group. **P<0.01 vs model group.
Discussion
Radiation of normal tissues may result in their fibrosis, which is perhaps the most common sequelae of radiation. In the liver, radiation-induced late injury is histologically characterized by the loss of parenchymal hepatocytes, the distortion of lobular architecture and the pericentral and periportal fibrosis [15]. Hepatic fibrosis is present in various chronic hepatic diseases. It is well known that persistent hepatic fibrosis can lead to the development of hepatocellular carcinoma [16]. Thus, interrupting and/or reversing hepatic fibrosis may be a new approach to block its progression to hepatocellular carcinoma [17]. However, the pathogenesis of RIHF remains unclear and no effective strategies have been developed for the prevention and treatment of hepatic fibrosis so far. Recently, studies on the development of new drugs have refocused on natural products. Traditional Chinese medicine has been practiced widely in China for thousands of years and is a potential source of pharmaceutical remedies [18]. In this study, the protective effects of PAE were investigated in the rat model of RIHF, and the potential mechanism was further explored.
Hepatocyte necrosis may lead to the elevation of some serum enzymes due to their release from the liver. The increases in ALT and AST are the most common indicators of liver injury [19]. Our study demonstrated that the serum activities of ALT and AST in model group were significantly higher than in normal control group, but treatment with PAE (20, 40 or 80 mg/kg) could significantly reduce the activities of ALT and AST, implying the hepatoprotective effect of PAE.
The products and related enzymes of ECM can serve as markers of hepatic fibrosis after they are released to the serum, and thus the circulating ECM components can indirectly reflect the degree of hepatic fibrosis. It has been confirmed that HA, LN and PC III are important biomarkers of hepatic fibrogenesis [20,21]. HA is a glycosaminoglycan, mainly synthesized in HSCs and degraded by sinusoidal endothelial cells. HA has been an important biomarker of hepatic fibrogenesis in the liver [22], and there is a significant correlation between serum HA level and degree of hepatic fibrosis. LN is one of the main glycoproteins of the basement membrane and mainly synthesized by hepatocytes and sinusoidal cells. Castera et al report that serum LN content increased in early stages of chronic liver diseases and the highest serum LN content was observed in active cirrhosis and chronic active hepatitis [23]. In addition, serum levels of HA, LN and PC-III showed positive correlations with the degree of hepatic fibrosis. HYP in liver is an important factor related to the degree of hepatic fibrosis, and hepatic fibrosis can be quantified by measuring HYP content of the liver [13]. In this study, the serum levels of TGF-β1, HA, PC-III and LN, and the liver content of HYP were significantly higher in model group than in normal control group, suggesting the liver injury after radiation exposure. However, after treatment with PAE (20, 40 or 80 mg/kg), the serum levels of HA, PCIII and LN and liver content of HYP reduced dramatically in RIHF rats, suggesting the attenuation of hepatic fibrosis.
Currently, histopathological examination is a gold standard for the evaluation of hepatic fibrosis. In the present study, histological examination showed that the normal structure of lobules was significantly destroyed, and pseudolobule formed in the liver. HE and Masson staining showed that PAE (20, 40 or 80 mg/kg) could significantly reduce the contents of collagens in the liver of hepatic fibrosis rats. After PAE treatment, the hepatic pathology was significantly improved, and the collagen content was also reduced in the liver of RIHF rats. These also suggest the hepatoprotective effect of PAE in RIHF rats.
Cytokines have been demonstrated to play vital roles in the development of RIHF. TGF-β1 is one of the most important cytokines involved in hepatic fibrosis. At different stages of hepatic fibrosis, TGF-β1 may stimulate and activate HSCs to proliferate, and the activated HSCs may synthesize and secrete ECM constantly by autocrine and paracrine mechanisms, which subsequently activate adjacent HSCs, leading to a sustained fibrosis [24]. Numerous studies have revealed that TGF-β1/Smads signaling pathway plays a central role in the pathogenesis of hepatic fibrosis. TGF-β1 mainly activates HSCs through TGFβ1/Smad signaling pathway, causing hepatic fibrosis [25-27]. After binding to its receptors on cell membrane, TGF-β1 may activate transmembrane receptor serine/threonine kinases Smad proteins, which modulate the transcription of target genes, exerting biological effects [5,28]. It has been reported that the activation of TGF-β1 receptors (TβR) I and II can increase the expression of phosphorylated Smad3. Then, phosphorylated Smad3 binds to Smad4, forming a complex which then translocates into the nucleus [29], resulting in the increased expression of intracellular and extracellular fibrogenic proteins. Smad7 may antagonize the TGF-β1/Smads signaling pathway by interacting with activated TβRI, preventing Smad3 phosphorylation. Recent studies show that Smad7 expression is significantly reduced in liver fibroblasts [30,31]. Latella et al also reported that the worse the hepatic fibrosis and the higher the expression of TGF-β1 and Smad3, the lower the Smad7 protein expression is [32]. Smad7 can inhibit the signal transduction of TGFβ1 through combining with TβRI [5,33,34].
Our results indicated that the serum TGF-β1 level and the liver TGF-β1 expression significantly increased in model group (P<0.01) as compared to normal control group, but PAE treatment decreased them markedly (P<0.05 or P<0.01). In addition, the expression of Smad3/4 increased significantly in model group when compared with normal control group (P<0.01), and PAE down-regulated their expression. The Smad7 expression was markedly decreased in model group when compared with control group (P<0.01), but PAE treatment significantly increased Smad7 expression. These findings suggest that PAE (20, 40 or 80 mg/kg) is effective to prevent against RIHF in rats, which may be ascribed to the suppression of TGF-β1 expression and regulation of TGF-β1/Smads signaling pathway.
In conclusion, PAE has protective effects on liver injury and can inhibit the progression of hepatic fibrosis induced by radiation, which may be associated with its regulation of TGF-β1/Smads signaling pathway. Additional studies are needed to investigate the pathogenesis of hepatic fibrosis and the mechanism underlying the hepatoprotective effects of PAE in detail, which may provide evidence on the treatment of RIHF.
Acknowledgements
We thank Mr. De-Wu Huang for the experimental assistance. This study was supported by the Major Programs of the 105th Hospital of Chinese People’s Liberation Army (2013YZ12).
Disclosure of conflict of interest
None.
References
- 1.Feng J, Chen SB, Wu SJ, Sun P, Xin TY, Chen YZ. Quantitative analysis of contrast-enhanced ultrasonography in acute radiation-induced liver injury: an animal model. Exp Ther Med. 2015;10:1807–1811. doi: 10.3892/etm.2015.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang HZ, Liang SX, Li YF. Progress in study on the dose of radiation tolerance of the liver. Guangxi Med J. 2009;31:1018–1020. [Google Scholar]
- 3.Fidler IJ. Blockade of the TGF-beta superfamily by Smad7: breaking a link in the metastatic chain. J Natl Cancer Inst. 2005;97:1714–1715. doi: 10.1093/jnci/dji437. [DOI] [PubMed] [Google Scholar]
- 4.Stolfi C, Marafini I, De Simone V, Pallone F, Monteleone G. The dual role of Smad7 in the control of cancer growth and metastasis. Int J Mol Sci. 2013;14:23774–23790. doi: 10.3390/ijms141223774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu F, Liu C, Zhou D, Zhang L. TGF-beta/SMAD pathway and its regulation in hepatic fibrosis. J Histochem Cytochem. 2016;64:157–167. doi: 10.1369/0022155415627681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luk JM, Wang X, Liu P, Wong KF, Chan KL, Tong Y, Hui CK, Lau GK, Fan ST. Traditional Chinese herbal medicines for treatment of liver fibrosis and cancer: from laboratory discovery to clinical evaluation. Liver Int. 2007;27:879–890. doi: 10.1111/j.1478-3231.2007.01527.x. [DOI] [PubMed] [Google Scholar]
- 7.Sun LR, Cao X, Hou FQ, Zhu XH, Gao TM. [Progressive studies of paeoniflorin] . Zhongguo Zhong Yao Za Zhi. 2008;33:2028–2032. [PubMed] [Google Scholar]
- 8.Jiang B, Qiao J, Yang Y, Lu Y. Inhibitory effect of paeoniflorin on the inflammatory vicious cycle between adipocytes and macrophages. J Cell Biochem. 2012;113:2560–2566. doi: 10.1002/jcb.22173. [DOI] [PubMed] [Google Scholar]
- 9.Lee S, Lim JM, Jin MH, Park HK, Lee EJ, Kang S, Kim YS, Cho WG. Partially purified paeoniflorin exerts protective effects on UV-induced DNA damage and reduces facial wrinkles in human skin. J Cosmet Sci. 2006;57:57–64. [PubMed] [Google Scholar]
- 10.Zhang LL, Wei W, Wang NP, Wang QT, Chen JY, Chen Y, Wu H, Hu XY. Paeoniflorin suppresses inflammatory mediator production and regulates G protein-coupled signaling in fibroblast-like synoviocytes of collagen induced arthritic rats. Inflamm Res. 2008;57:388–395. doi: 10.1007/s00011-007-7240-x. [DOI] [PubMed] [Google Scholar]
- 11.Hu ZT, Gao SL, Qin F, Zhu J, Zhen YL, Dong LY. Effect and mechanisms of paeoniflorin on radiation-induced hepatic fibrosis in rats. Pharm J Chin PLA. 2012;28:283–288. [Google Scholar]
- 12.Hu ZT, Gao SL, Qin F, Zhu J, Huang DW, Dong LY. Establishment and evaluation on the model of radiation-induced liver injury of rat caused by once dose radiation. Anhui Med Pharm J. 2013;17:58–62. [Google Scholar]
- 13.Yan B, Chen FH, Wu FR, Hu W, Yuan LP, Li J. Therapeutic effects of total flavones of Bidens bipinnata L (TFB) on liver fibrosis in rats and its mechanisms. Chin Pharmac Bulletin. 2008;24:1640–1645. [Google Scholar]
- 14.Dong L, Fan Y, Shao X, Chen Z. Vitexin protects against myocardial ischemia/reperfusion injury in Langendorff-perfused rat hearts by attenuating inflammatory response and apoptosis. Food Chem Toxicol. 2011;49:3211–3216. doi: 10.1016/j.fct.2011.09.040. [DOI] [PubMed] [Google Scholar]
- 15.Anscher MS. Targeting the TGF-beta1 pathway to prevent normal tissue injury after cancer therapy. Oncologist. 2010;15:350–359. doi: 10.1634/theoncologist.2009-S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Della Corte C, Aghemo A, Colombo M. Individualized hepatocellular carcinoma risk: the challenges for designing successful chemoprevention strategies. World J Gastroenterol. 2013;19:1359–1371. doi: 10.3748/wjg.v19.i9.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fowell AJ, Iredale JP. Emerging therapies for liver fibrosis. Dig Dis. 2006;24:174–183. doi: 10.1159/000090320. [DOI] [PubMed] [Google Scholar]
- 19.Achliya GS, Wadodkar SG, Dorle AK. Evaluation of hepatoprotective effect of Amalkadi Ghrita against carbon tetrachloride-induced hepatic damage in rats. J Ethnopharmacol. 2004;90:229–232. doi: 10.1016/j.jep.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 20.Kaneda H, Hashimoto E, Yatsuji S, Tokushige K, Shiratori K. Hyaluronic acid levels can predict severe fibrosis and platelet counts can predict cirrhosis in patients with nonalcoholic fatty liver disease. J Gastroenterol Hepatol. 2006;21:1459–1465. doi: 10.1111/j.1440-1746.2006.04447.x. [DOI] [PubMed] [Google Scholar]
- 21.Li CH, Pan LH, Yang ZW, Li CY, Xu WX. Preventive effect of Qianggan-Rongxian decoction on rat liver fibrosis. World J Gastroenterol. 2008;14:3569–3573. doi: 10.3748/wjg.14.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui DL, Yao XX. Serological detection of hepatic fibrosis. World Chin J Digest. 2000;8:683–684. [Google Scholar]
- 23.Castera L, Hartmann DJ, Chapel F, Guettier C, Mall F, Lons T, Richardet JP, Grimbert S, Morassi O, Beaugrand M, Trinchet JC. Serum laminin and type IV collagen are accurate markers of histologically severe alcoholic hepatitis in patients with cirrhosis. J Hepatol. 2000;32:412–418. doi: 10.1016/s0168-8278(00)80391-8. [DOI] [PubMed] [Google Scholar]
- 24.Liu LX, Zhang HY, Zhang QQ, Guo XH. Effects of insulin-like growth factor binding protein-related protein 1 in mice with hepatic fibrosis induced by thioacetamide. Chin Med J (Engl) 2010;123:2521–2526. [PubMed] [Google Scholar]
- 25.Gressner AM, Weiskirchen R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-beta as major players and therapeutic targets. J Cell Mol Med. 2006;10:76–99. doi: 10.1111/j.1582-4934.2006.tb00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inagaki Y, Okazaki I. Emerging insights into Transforming growth factor beta Smad signal in hepatic fibrogenesis. Gut. 2007;56:284–292. doi: 10.1136/gut.2005.088690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu XL, Zeng WZ, Jiang MD, Qin JP, Xu H. Effect of oxymatrine on the TGFbeta-Smad signaling pathway in rats with CCl4-induced hepatic fibrosis. World J Gastroenterol. 2008;14:2100–2105. doi: 10.3748/wjg.14.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flanders KC. Smad3 as a mediator of the fibrotic response. Int J Exp Pathol. 2004;85:47–64. doi: 10.1111/j.0959-9673.2004.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 30.Kopp J, Preis E, Said H, Hafemann B, Wickert L, Gressner AM, Pallua N, Dooley S. Abrogation of transforming growth factor-beta signaling by SMAD7 inhibits collagen gel contraction of human dermal fibroblasts. J Biol Chem. 2005;280:21570–21576. doi: 10.1074/jbc.M502071200. [DOI] [PubMed] [Google Scholar]
- 31.Yu H, Bock O, Bayat A, Ferguson MW, Mrowietz U. Decreased expression of inhibitory SMAD6 and SMAD7 in keloid scarring. J Plast Reconstr Aesthet Surg. 2006;59:221–229. doi: 10.1016/j.bjps.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 32.Latella G, Vetuschi A, Sferra R, Catitti V, D’Angelo A, Zanninelli G, Flanders KC, Gaudio E. Targeted disruption of Smad3 confers resistance to the development of dimethylnitrosamine-induced hepatic fibrosis in mice. Liver Int. 2009;29:997–1009. doi: 10.1111/j.1478-3231.2009.02011.x. [DOI] [PubMed] [Google Scholar]
- 33.Wrighton KH, Lin X, Feng XH. Phospho-control of TGF-beta superfamily signaling. Cell Res. 2009;19:8–20. doi: 10.1038/cr.2008.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan X, Liu Z, Chen Y. Regulation of TGF-beta signaling by Smad7. Acta Biochim Biophys Sin (Shanghai) 2009;41:263–272. doi: 10.1093/abbs/gmp018. [DOI] [PMC free article] [PubMed] [Google Scholar]


