Abstract
To investigate the correlation between femoroacetabular cartilage thickness and lateral acetabular coverage in patients undergoing hip arthroscopy for a variety of indications. Articular cartilage at the hip is hypothesized to undergo adaptive change secondary to unique patterns of pathomechanical loading which results in a direct relationship between acetabular coverage and femoroacetabular cartilage thickness. A cohort of 252 patients presenting to our dedicated hip preservation service between June 2013 and June 2015 were retrospectively analysed. Preoperative radiographs and MRI studies were obtained for all symptomatic hips and classified according to radiographic lateral center edge angle (LCEA) as follows: normal acetabular coverage (25–40°), acetabular overcoverage (≥40°), borderline dysplasia (20–24.9°) and frank dysplasia (<20°). Femoroacetabular cartilage thickness was measured on a preoperative MRI-scan at the fovea, middle sourcil, and lateral sourcil. In all groups, cartilage thickness was maximized at the lateral sourcil relative to the middle sourcil or fovea (P < 0.001). Furthermore, articular cartilage thickness was significantly increased when comparing one group to successive groups with diminished lateral acetabular coverage. Indeed, multivariate analyses confirmed LCEA to be the strongest determinant of femoroacetabular cartilage thickness compared with age, gender, body-mass index or presence of cam/pincer lesions. Patients with borderline and frank dysplasia exhibit increased values of femoroacetabular cartilage thickness in the weight-bearing zone, potentially indicating a compensatory reaction to the lack of bony coverage. Articular cartilage thickness may serve as an instability marker and inform clinical decision-making for patients with borderline dysplasia.
INTRODUCTION
Abnormal bony morphology in the hip is often accompanied by secondary soft-tissue changes. Hip dysplasia is characterized by reduced acetabular coverage resulting in increased shear stress within the joint, ultimately causing secondary labral hypertrophy [1–3]. Despite this compensatory enlargement, tears are frequently seen in the dysplastic labrum as it may be exposed to 10 times the normal loads placed on the hip during ambulation [1–6]. In contrast, pincer-type femoroacetabular impingement (FAI) and protrusio acetabuli represent conditions in which excessive lateral, anterior, or global acetabular coverage is associated with a hypoplastic labrum exhibiting partial or complete osseous metaplasia.
Articular cartilage thickness has been shown to exhibit adaptive changes similar to the labrum [5, 7]. However, existing studies investigating the relationship between lateral acetabular coverage and femoroacetabular cartilage thickness have utilized small groups of dysplastic patients with healthy volunteers serving as controls [6, 8]. As a result, some categories of acetabular morphology, such as patients with borderline dysplasia and pincer-FAI, have been excluded from analysis [6, 8–10].
The optimal treatment for borderline acetabular dysplasia has not yet been firmly established, in part due to a paucity of dedicated research studies investigating this unique class of dysplasia. This subgroup represents a true dilemma for the hip preservation surgeon, as options for treatment range from arthroscopic-only approaches to those involving pelvic and femoral realignment osteotomies [7, 11–13].
The purpose of this study was to evaluate the correlation between lateral acetabular coverage and femoroacetabular cartilage thickness for patients with widely varying degrees of coverage. We hypothesize that, like the labrum, the width of articular cartilage will exhibit adaptive changes forming a continuum with the frankly dysplastic patients exhibiting the largest values and the acetabular overcoverage patients exhibiting the smallest values. Additionally, we aim to resolve characteristics unique to the borderline dysplastic group to advance our understanding of this challenging clinical entity.
MATERIALS AND METHODS
Following Institutional Review Board approval, we retrospectively analysed a cohort of 252 patients presenting with hip pain to our dedicated hip preservation clinic between June 2013 and June 2015. Common indications for referral included FAI, hip instability, acetabular dysplasia and associated abnormalities of femoral torsion or acetabular version. To be included in this study, patients had to demonstrate the following factors: (i) persistent hip pain and mechanical symptoms unresponsive to non-operative treatment for >3 months, (ii) clinical exam findings suggestive of impingement and/or instability and (iii) joint-space width exceeding 2.5 mm on all views of plain radiography (including weight-bearing films) as well as cross-sectional imaging. Additionally, patients were excluded from the study if preoperative radiographic or MRI imaging studies were unavailable for review. Patients undergoing surgical treatment for diagnoses of slipped capital femoral epiphysis, Legg-Calves-Perthe’s disease, osteochondromatosis, or post-dislocation syndrome were also excluded from this study.
Demographic variables including age, clinical diagnosis, gender, height, weight, and body-mass index (BMI) were recorded for all patients. Patients were classified according to their BMI (kg/m2) as follows: normal weight, 18.00–24.99; overweight, 25.00–29.99; obese, 30.00 or greater.
Imaging protocol and measurements
After a comprehensive clinical evaluation by the senior author, patients underwent a standardized series of plain radiographs (including supine AP, cross-table lateral and AP pelvic views) as well as preoperative MRI and whole-pelvis computed tomography (CT) scans.
Standard AP pelvic films were obtained with the patient positioned supine with the lower extremities internally rotated 15° to maximize femoral neck length. The x-ray beam was directed midway between the anterior superior iliac spine (ASIS) and the pubic symphysis, with a focus-film-distance of 100 cm. Radiographs were determined to be adequate with symmetric obturator foramina and a distance of 1–3 cm between the coccyx and pubic symphysis [14, 15]. The lateral center edge angle (LCEA) was determined on AP pelvic radiography as described by Ogata et al. [16], as the angle subtended by: (i) a vertical line through center of the femoral head and orthogonal to the transverse line passing through the teardrops of both hips, and (ii) an oblique line drawn from the center of the femoral head to the lateral weight-bearing sclerotic zone (sourcil) of the acetabular rim. All patients underwent preoperative magnetic resonance imaging of the symptomatic hip. Fat-saturated proton density sequences were selected to obtain cartilage thickness measurements which maximize spatial resolution and anatomic detail. A single coronal image through the center of the femoral head, cross referenced with the sagittal and axial images, was utilized to measure femoral head and acetabular cartilage thickness, similar to other well-established MRI studies investigating hip cartilage [17]. With a slice thickness of 2 mm, this method allowed for optimal reproducibility. Joint space width was measured between the subchondral bone of the acetabulum and subchondral bone of the femoral head at the lateral sourcil (lateral margin of the sourcil), middle sourcil (apical transection of the weight-bearing surface by a vertical line through the center of the femoral head), and fovea (medial margin of the weight-bearing surface bordering on the fovea) (Fig. 1). The sourcil was chosen as this represents the weight-bearing portions of the acetabulum and would thus be most likely to vary with abnormal loading. Measurement of cartilage thickness as described earlier allows for precise localization and standardization among hips. Although the MRI sequences are performed with the patient supine, prior radiographic studies investigating the effect of weight bearing on joint spacing have determined the difference to be small (less than 0.5 mm) and thus beyond the resolution of MRI [18]. Furthermore, although this may introduce a small systematic bias to the absolute value of measured cartilage thickness, it is unlikely to affect the comparative analysis between the different groups.
Fig. 1.
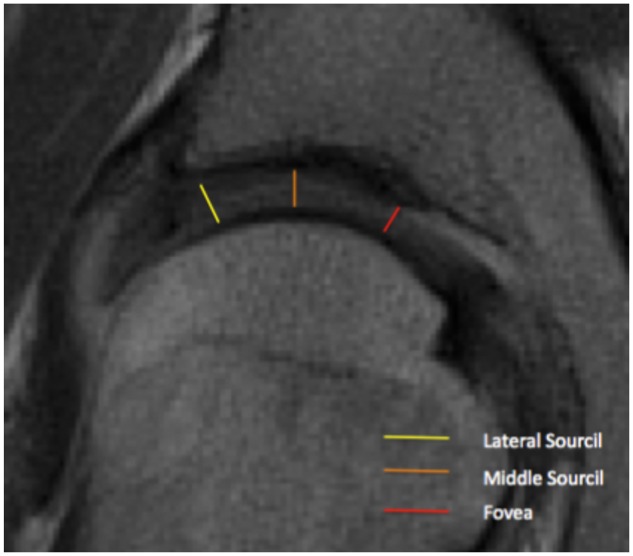
Representative locations of cartilage thickness measurements.
In addition to pertinent physical examination findings, the clinical diagnoses of bony impingement and/or acetabular dysplasia were determined according to accepted pathomorphologic measurements on radiographic and MRI imaging [19, 20]. Confirmative findings of pincer-FAI included features corresponding to focal acetabular overcoverage (crossover sign or ischial spine sign), an LCEA exceeding 40°, and/or acetabular inclination ≤0° for pincer-FAI; an alpha angle exceeding 50° on radial sequences of the head–neck junction and a femoral head-neck offset ratio <0.18 for cam-FAI; and an LCEA <20° for acetabular dysplasia. Patients were categorized according to LCEA measurement as follows: normal acetabular coverage (25–40°), acetabular overcoverage (≥40°), borderline dysplasia (20–24.9°), and frank dysplasia (<20°).
Examiners
Clinical examination and radiographic findings were determined by a senior hip preservation orthopedic surgeon. The method of measurement on the MRI was determined by a single fellowship trained musculoskeletal radiologist and the senior author. All measurements were performed by a musculoskeletal radiologist and a radiology resident trained to perform these measurements.
Interobserver reproducibility of radiographic measurement of LCEA and MRI measurements of the cartilage thickness were evaluated by the authors in a blinded random subset of 25 hips using a two-way, mixed, absolute agreement single-measures intraclass correlation coefficient (ICC). ICC values >0.80 indicate excellent reliability; 0.61–0.80, substantial reliability; 0.41–0.60, moderate reliability; 0.21–0.40, fair reliability; and <0.20, poor reliability [21]. Accordingly, the ICC demonstrated excellent reliability for measurements of the LCEA performed on plain radiography (ICC = 0.934; 95% CI, 0.850–0.971), measurements of cartilage thickness at the lateral sourcil on MRI (ICC = 0.992; 95% CI, 0.980–0.997), measurement of cartilage thickness at the middle aspect of the weight-bearing zone on MRI (ICC = 0.907; 95% CI, 0.728–0.966), and measurement of cartilage thickness at the fovea on MRI (ICC = 0.906; 95% CI, 0.765–0.963).
Statistical analysis
All variables were evaluated for distribution of normality using a combination of histograms, quantile–quantile (Q–Q) plots, and Shapiro-Wilk tests. Descriptive statistics were summarized as means and SDs for quantitative variables and as counts and frequencies for categorical variables. The significance of mean differences in cartilage thickness between independent groups, measurement location, and their interaction effect were evaluated using a 3 (measurement location) × 4 (LCEA group) mixed-model analysis of variance (ANOVA) with post hoc Tukey’s HSD tests and repeated-measures one-way ANOVAs with post hoc Bonferroni-corrected paired t-tests. Degrees of freedom of the F distribution were adjusted using the Greenhouse-Geisser correction for violations of sphericity. Stepwise multiple linear regression procedures were conducted to evaluate whether any significant (P < 0.05) or near-significant factors (P < 0.10) from univariate analyses served as independent predictors of cartilage thickness at each measurement location. Statistical significance for all comparisons was set at P < 0.05 (two-tailed). All analyses were conducted using IBM SPSS Statistics Version 22.0 (Statistical Package for the Social Sciences, Chicago, IL, USA).
Post hoc power analysis indicated that 152 hips would be required to achieve statistical significance given a 3 × 4 mixed-model ANOVA with an effect size of the primary outcome measure of 0.25, an alpha of 0.05, and a required power (1 – beta) of 0.90 [22].
RESULTS
Participants and descriptive data
The study cohort comprised 252 patients (252 hips, 68 males, 184 females) with a mean age of 34.2 years (SD, 11.7 years). Mean patient height was 168.9 cm (SD, 9.7 cm), mean patient weight was 69.5 kg (SD, 15.8 kg), and mean patient BMI was 24.3 kg/m2 (SD, 4.6 kg/m2). One hundred and fifty hips (59.5%) had normal acetabular coverage, 35 (13.9%) had acetabular overcoverage, 36 (14.3%) had borderline dysplasia and 31 (12.3%) had frank dysplasia (Table I). All demographic variables were statistically equivalent between these independent groups, with the exception of height, which exhibited a clinically insignificant difference (Table I).
Table I.
Patient demographics and baseline characteristics (n = 252)
| Patient variables | LCEA < 20° | LCEA 20–24.9° | LCEA 25–39.9° | LCEA > 40° | P-value |
|---|---|---|---|---|---|
| No. of Patients | 31 | 36 | 150 | 35 | |
| Age, mean (SD), y | 31.2 (8.0) | 34.6 (11.5) | 34.0 (12.1) | 37.2 (12.6) | 0.124 |
| Male Gender, n (%) | 9 (29.0) | 5 (13.9) | 41 (27.3) | 13 (37.1) | 0.171 |
| Height, mean (SD), cm | 169.4 (7.3) | 165.2 (6.8) | 169.4 (10.5) | 170.1 (10.2) | 0.018* |
| Weight, mean (SD), kg | 70.2 (14.7) | 65.9 (13.4) | 69.8 (16.4) | 71.7 (16.3) | 0.459 |
| BMI, mean (SD), kg/m2 | 24.4 (4.8) | 24.2 (4.9) | 24.2 (4.7) | 24.6 (4.2) | 0.971 |
Abbreviations: BMI, body-mass index; LCEA, lateral center-edge angle.
*Statistically significant, P ≤ 0.05, represented as bold.
Hip cartilage thickness according to degree of lateral acetabular coverage
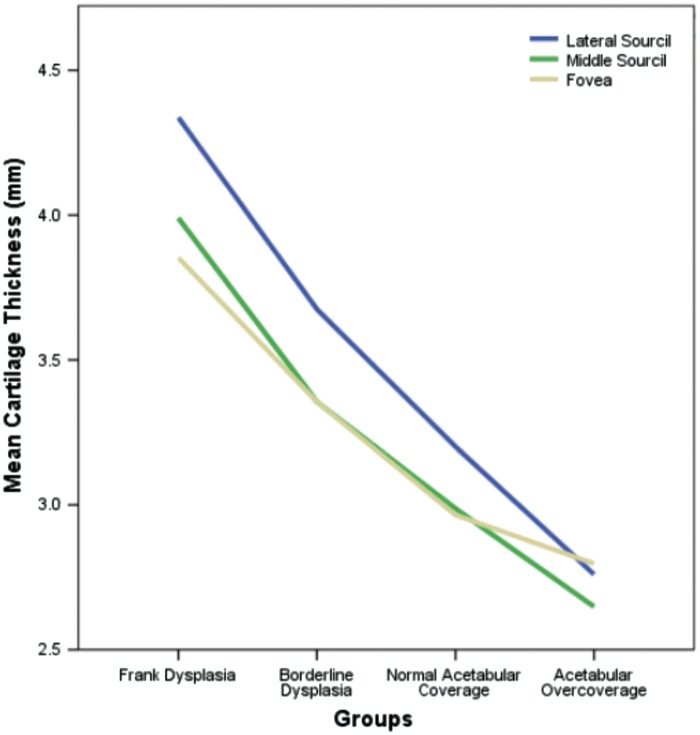
Femoroacetabular cartilage thickness was significantly correlated to the degree of lateral acetabular coverage [F(2,492) = 13.956, P < 0.001] and the location of measurement [F(3,246) = 29.332, P < 0.001]. Post hoc analyses demonstrated that cartilage thickness was significantly increased at the lateral sourcil as compared with the middle sourcil or fovea across all clinical subgroups (P < 0.001), with the latter two measurement locations being statistically equivalent (Figs. 2 and 3). Furthermore, mean cartilage thickness at all measurement locations was significantly decreased when comparing one group with successive clinical subgroups having increased lateral acetabular coverage (P = 0.43 for normal versus excessive acetabular coverage group, P < 0.001 for all other pairwise comparisons). Exact values of mean cartilage thickness within each measurement location and group are presented in Table II.
Fig. 2.
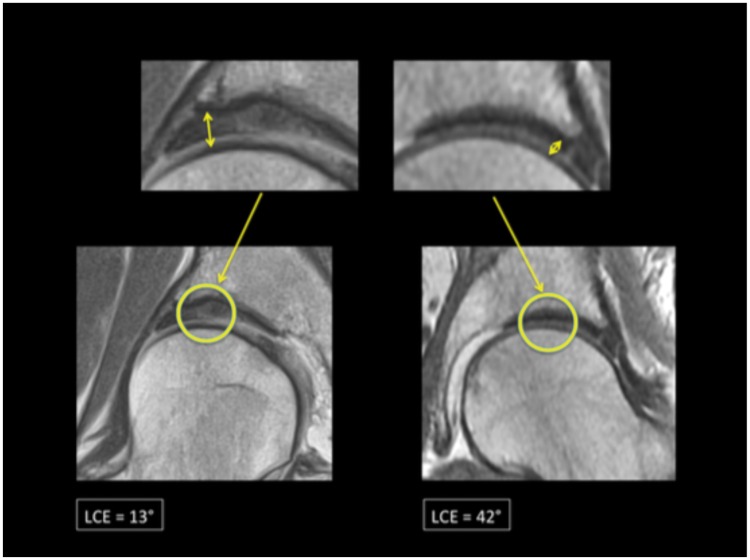
Differences in cartilage thickness in normal versus dysplastic hip as depicted on coronal MRI sequences.
Fig. 3.
Cartilage thickness at the lateral sourcil (blue) was significantly increased in all clinical subgroups relative to that at the middle sourcil (green) or fovea (yellow), with the latter two measurements being statistically equivalent. Cartilage thickness at all measurement locations was inversely proportional to the degree of lateral acetabular coverage.
Table II.
Hip cartilage thickness according to lateral acetabular coverage
| LCEA (deg) | Cartilage thickness (mm) | Within-subject effect | ||
|---|---|---|---|---|
| Lateral | Middle | Fovea | ||
| <20 | 4.33 (0.66) | 3.99 (0.65) | 3.85 (0.82) | |
| 20–25 | 3.68 (0.73) | 3.36 (0.70) | 3.36 (0.59) | |
| 25–40 | 3.20 (0.89) | 2.99 (0.72) | 2.96 (0.67) | F(2,492) = 13.956, P < 0.001 |
| >40 | 2.76 (0.86) | 2.65 (0.72) | 2.79 (0.83) | |
| Between-subject Effect | F(3,246) = 29.332, P < 0.001 | |||
aAll variables are represented as mean (SD).
Within-subject’s test for interaction of location and group (Greenhouse-Geisser): F(6,492) = 1.652, P = 0.144, partial η2 = 0.020.
Within-subject’s test for location only (Greenhouse-Geisser): F(2,492) = 13.956, P < 0.001, partial η2 = 0.054.
Pairwise comparisons show: lateral versus middle sourcil, P < 0.001; lateral versus fovea, P < 0.001; middle versus fovea, P = 1.000.
Between-subject’s test for LCEA group: F(3,246) = 29.332, P < 0.001, partial η2 = 0.263. Pairwise comparisons show: frank dysplasia versus all other groups, P < 0.001; borderline dysplasia versus all other groups, P < 0.001; normal acet coverage versus acet overcoverage (P = 0.043).
Independent determinants of femoroacetabular cartilage thickness
Stepwise multiple linear regression analysis was used to identify independent predictors of the measured cartilage thickness. The multiple regression model indicated that measurement at the lateral sourcil was significantly increased given decreasing lateral acetabular coverage (0.06 mm per degree reduction in LCEA) but was significantly reduced (by 0.45 mm) if the patient was female. These predictors outweighed the effect of all other demographic variables, including age and BMI (Table III). Similar trends were observed for cartilage thickness at the middle sourcil (Table IV) and fovea (Table V).
Table III.
Results of multiple linear regression analysis identifying independent predictors of cartilage thickness measured at lateral sourcil
| Unstandardized beta coefficient | 95% CI | P-value | |
|---|---|---|---|
| Intercept | 5.44 | 5.05 to 5.82 | < 0.001 |
| LCEA | −0.06 | −0.07 to −0.05 | < 0.001 |
| Gender (0 = male, 1 = female) | −0.45 | −0.67 to −0.23 | < 0.001 |
Abbreviations: CI, confidence interval. Adjusted R2 = 0.328. Overall multiple linear regression model: Cartilage Thickness (at lateral sourcil) = 5.44 − (LCEA × 0.06) – (Gender × 0.45)
Table IV.
Results of multiple linear regression analysis identifying independent predictors of cartilage thickness measured at middle sourcil
| Unstandardized beta coefficient | 95% CI | P-value | |
|---|---|---|---|
| Intercept | 4.45 | 4.07–4.84 | <0.001 |
| LCEA | −0.51 | −0.06 to 0.04 | <0.001 |
| Gender (0 = male, 1 = female) | −0.28 | −0.46 to 0.10 | 0.003 |
Abbreviations: CI = confidence interval. Adjusted R2 = 0.328. Overall multiple linear regression model: Cartilage Thickness (at middle sourcil) = 4.45 − (LCEA × 0.51) – (Gender × 0.28)
Table V.
Results of multiple linear regression analysis identifying independent predictors of cartilage thickness measured at the fovea
| Unstandardized Beta Coefficient | 95% CI | P-Value | |
|---|---|---|---|
| Intercept | 4.48 | 4.13–4.82 | <0.001 |
| LCEA | −0.04 | −0.05 to 0.03 | <0.001 |
| Gender (0 = male, 1 = female) | −0.31 | −0.50 to 0.11 | 0.002 |
Abbreviations: CI, confidence interval. Adjusted R2 = 0.212. Overall multiple linear regression model: Cartilage Thickness (at fovea) = 4.48 − (LCEA × 0.04) – (Gender × 0.31)
Discussion
The results of this study demonstrate a significant relationship between lateral acetabular coverage and femoroacetabular cartilage thickness, providing evidence for our hypothesis that articular cartilage undergoes adaptive change. Specifically, mean cartilage thickness at the lateral sourcil was 35% larger in frankly dysplastic hips and 14% smaller in hips with acetabular overcoverage relative to hips with normal coverage. This supports the notion that hips with deficient and excessive lateral acetabular coverage undergo unique patterns of pathomechanical loading, with compensatory hypertrophy seen only in the former.
Our research revealed a reproducible trend of increasing cartilage thickness from the middle to the lateral edge of the acetabulum within all groups of acetabular coverage. In fact, the cartilage thickness at the lateral margin of the dysplastic acetabula was found on average to measure almost twice that of the acetabula with overcoverage. This relationship was further accentuated if the patient was female. These findings suggest that the hip undergoes specific and asymmetric morphologic adaptations in the setting of abnormal joint loading. Lateral acetabular deficiency is often associated with increased shear stresses with joint loading due to the shallow articulation with the femoral head. The preferential increase in cartilage thickness at the lateral sourcil may serve a greater adaptive function as it reduces the effective upslope of the bony acetabular roof to a plane that is more horizontal, thereby mitigating the instability brought about by shear forces across the joint.
Our study revealed that borderline dysplastic hips (LCEA 20–25°) demonstrated similar, though less pronounced, adaptive cartilage thickening as frankly dysplastic hips (LCEA < 20°). More importantly, both borderline and frankly dysplastic hips exhibited a significant increase in cartilage thickness when compared with hips with normal coverage. These findings suggest that the applied loads and subsequent structural instability in the hip form a continuum from normal coverage to frank dysplasia. Currently, the majority of patients with borderline dysplasia are treated with hip arthroscopy and capsular plication, attempting to restore stability through a ‘soft-tissue only’ approach [23]. Given the degree of similarity in adaptive change between borderline and frank dysplasia, our study raises concern for the adequacy of this approach in restoring hip stability. Further work is necessary to determine the extent to which borderline dysplastic hips behave like frankly dysplastic hips, thereby warranting consideration for both soft-tissue and bony approaches to restoring stability via realignment osteotomy.
Prior studies focusing on the labrum and its association with hip dysplasia have proposed that labral hypertrophy is the body’s attempt to adapt to abnormal stress at the hip and redistribute the load over a broader area [1, 3, 5]. It stands to reason that additional components of the hip joint may also undergo similar adaptive changes induced by the abnormal bony anatomy, including the femoroacetabular cartilage as demonstrated in our study. Thus, the articular cartilage and labrum show compensatory changes that are in keeping with one another, reflecting exposure to a similar joint environment. One of the greatest strengths of our study was the inclusion of patients with all categories of acetabular morphology. Previously, only few studies have utilized MRI to evaluate the morphology and characteristics of hip cartilage in dysplastic patients. All of these studies were small in size and did not evaluate the full spectrum of disease associated with acetabular over or undercoverage. Tamura et al. [8] revealed a similar trend of increasing cartilage thickness in dysplastic patients, greatest at the anterolateral acetabular rim. Although this was indeed valuable, the study population was small and limited to a single ethnic group. Furthermore, their study focused solely on dysplastic patients, which significantly limits extrapolation to other degrees of acetabular coverage. Similarly, the study by Nishii also evaluated the distribution of acetabular cartilage thickness in dysplastic patients and discovered a general trend towards increasing cartilage thickness at the superolateral aspect of the hip joint [6]. Although this study did extend the population to include healthy controls, the sample size was again very small and lacked borderline dysplastic and overcoverage groups. By incorporating patients of all morphologic subtypes we were able to show not only a trend of increasing cartilage thickness proportional to the severity of dysplasia but we were able to evaluate relationships between subgroups, varying with location of measurement within the joint, in a manner not previously performed. Additionally, we provide normative values of cartilage thickness across all groups of lateral acetabular coverage. This standardization provides the foundation upon which other studies may be built, allowing for a frame of reference with which to compare future work.
Although we were able to successfully validate our hypotheses, there are some limitations that warrant discussion. Firstly, the study was limited by the lack of a healthy or asymptomatic control group for comparison. While we were unable to evaluate whether the trends observed in our sample population hold true in pain-free patients, the literature to date supports this hypothesis [6, 8, 24]. Furthermore, we refrain from drawing any conclusions regarding the pain-generating capacity of increased cartilage thickness, as this was not the aim of our study. It is likely that these morphologic features are present in asymptomatic hips, similar to cam lesions about the proximal femur [25]. In addition, many subjects in our study were symptomatic for less than 6 month while cartilage thickness adaptations would correspond to longstanding implications of ones morphology. Another potential limitation was that radiologists were not blinded to plain radiographs prior to measurement on MRI. Exact measurements of LCEA were not available at the time of MRI measurement but generalized visual inspection of the hip joint on plain radiography was not prohibited. We feel, however, that any potential biases that this may have introduced were ultimately marginal given the high degree of reproducibility demonstrated by our concordance studies.
We have shown a direct correlation between severity of dysplasia and cartilage thickness, demonstrating that even patients with borderline dysplasia have significantly increased values when compared with hips with normal coverage or overcoverage. Borderline dysplasia poses a unique treatment dilemma with options ranging from an arthroscopic-only approach to combined treatment with realignment osteotomie(s). Femoroacetabular cartilage thickness may represent one of many adaptive changes serving as a marker of instability to guide decision-making in patients with borderline dysplasia. Further studies are warranted to prospectively validate the use of cartilage thickness as a guide to treatment.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Gupta A, Chandrasekaran S, Redmond JM. et al. Does labral size correlate with degree of acetabular dysplasia?. Orthop J Sports Med 2015; 3:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hartig-Andreasen C, Soballe K, Troelsen A. The role of the acetabular labrum in hip dysplasia. Acta Orthopaedica 2013; 84: 60–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Toft F, Anliker E, Beck M. Is labral hypertrophy correlated with increased acetabular depth? J Hip Preserv Surg 2015; 0: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Crawford MJ, Dy CJ, Alexander JW. et al. The Biomechanics of the Hip Labrum and the Stability of the Hip. Clin Orthop Relat Res 2007; 465:16–22. [DOI] [PubMed] [Google Scholar]
- 5. Groth MM, Herrera J. A comprehensive review of hip labral tears. Curr Rev Musculoskelet Med 2009; 2:105–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nishii T, Sugano N, Sato Y. et al. Three-dimensional distribution of acetabular cartilage thickness in patients with hip dysplasia: a fully automated computational analysis of MR imaging. Osteoarthritis Cartilage 2004; 12:650–7. [DOI] [PubMed] [Google Scholar]
- 7. Hingsammer AM, Kalish LA, Stelzeneder D. et al. Does periacetabular osteotomy for hip dyspasia modulate cartilage biochemistry? J Bone Joint Surg Am 2015; 97:544–50. [DOI] [PubMed] [Google Scholar]
- 8. Tamura S, Nishii T, Shiomi T. et al. Three-dimensional patterns of early acetabular cartilage damage in hip dysplasia; a high-resolution CT arthrography study. Osteoarthr Cartil 2012; 20: 646–52. [DOI] [PubMed] [Google Scholar]
- 9. Daysal GA, Goker B, Gonen E. et al. The relationship between hip joint space width, center edge angle and acetabular depth. Osteoarthr Cartil 2007; 15:1446–51. [DOI] [PubMed] [Google Scholar]
- 10. Delaunay S, Dussault RG, Kaplan PA, Alford BA. Radiographic measurements of dysplastic adult hips. Skeletal Radiol 1997; 26:75–81. [DOI] [PubMed] [Google Scholar]
- 11. Clohisy JC, Barrett SE, Gordon E. et al. Periacetabular Osteotomy in the Treatment of Severe Acetabular Dysplasia. J Bone Joint Surg Am 2006; 88:65–88. [DOI] [PubMed] [Google Scholar]
- 12. Murphy SB, Ganz R, Muller ME. The prognosis in untreated dysplasia of the hip. A study of radiographic factors that predict the outcome. J Bone Joint Surg Am 1995; 77:985–9. [DOI] [PubMed] [Google Scholar]
- 13. Niknafs N, Murphy RJ, Armiger RS. et al. Biomechanical factors in planning of periacetabular osteotomy. Front Bioeng Biotechnol 2013; 1: Article 20: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bouttier R, Morvan J, Mazieres B. et al. Reproducibility of radiographic hip measurements in adults. Joint Bone Spine 2013; 80:52–6. [DOI] [PubMed] [Google Scholar]
- 15. Cadet ER, Babatunde OM, Gorroochurn P. et al. Inter- and intra-observer agreement of femoroacetabular impingement (FAI) parameters comparing plain radiographs and advanced, 3D computed tomographic (CT)-generated hip models in a surgical patient cohort. Knee Surg Sports Traumatol Arthrosc 2016; 24:2324. [DOI] [PubMed] [Google Scholar]
- 16. Ogata S, Moriya H, Tsuchiya K. et al. Acetabular cover in congenital dislocation of the hip. J Bone Joint Surg Br 1990; 72:190–6. [DOI] [PubMed] [Google Scholar]
- 17. Bittersohl B, Steppacher S, Haamberg T. et al. Cartilage damage in femoroacetabular impingement (FAI): preliminary results on comparison of standard diagnostic vs delayed gadolinium-enhanced magnetic resonance imaging of cartilage (dGEMRIC). Osteoarthr Cartil 2009; 17:1297–306. [DOI] [PubMed] [Google Scholar]
- 18. Fuchs-Winkelmann S, Peterlein CD, Tibesku CO, Weinstein SL. Comparison of pelvic radiographs in weight-bearing and supine positions. Clin Orthop Relat Res 2008; 466:809–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jesse MK, Petersen B, Strickland C, Mei-Dan O. Normal anatomy and imaging of the hip: emphasis on impingement assessment. Semin Musculoskelet Radiol 2013; 17:229–47. [DOI] [PubMed] [Google Scholar]
- 20. Tannast M, Siebenrock KA, Anderson SE. Femoroacetabular impingement: radiographic diagnosis–what the radiologist should know. AJR Am J Roentgenol 2007; 188:1540–52. [DOI] [PubMed] [Google Scholar]
- 21. Landis JR, Koch GG. “The measurement of observer agreement for categorical data.”. Biometrics 1977; 33:159–74. [PubMed] [Google Scholar]
- 22. Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007; 39:175–91. [DOI] [PubMed] [Google Scholar]
- 23. Domb BG, Stake CE, Lindner D. et al. Arthroscopic Capsular Plication and Labral Preservation in Borderline Hip Dysplasia. Am J Sports Med 2013; 41:2591–8. [DOI] [PubMed] [Google Scholar]
- 24. Hansen BJ, Harris MD, Anderson LA. et al. Correlation between radiographic measures of acetabular morphology with 3D femoral head coverage in patients with acetabular retroversion. Acta Orthop 2012; 83:233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jung KA, Restrepo C, Hellman M. et al. The prevalence of cam-type femoroacetabular deformity in asymptomatic adults. J Bone Joint Surg Br 2011. Oct; 93: 1303–7. [DOI] [PubMed] [Google Scholar]