Abstract
To determine whether it was feasible to perform a randomized controlled trial (RCT) comparing arthroscopic hip surgery to conservative care in patients with femoroacetabular impingement (FAI). This study had two phases: a pre-pilot and pilot RCT. In the pre-pilot, we conducted interviews with clinicians who treated FAI and with FAI patients to determine their views about an RCT. We developed protocols for operative and conservative care. In the pilot RCT, we determined the rates of patient eligibility, recruitment and retention, to investigate the feasibility of the protocol and we established methods to assess treatment fidelity. In the pre-pilot phase, 32 clinicians were interviewed, of which 26 reported theoretical equipoise, but in example scenarios 7 failed to show clinical equipoise. Eighteen patients treated for FAI were also interviewed, the majority of whom felt that surgery and conservative care were acceptable treatments. Surgery was viewed by patients as a ‘definitive solution’. Patients were motivated to participate in research but were uncomfortable about randomization. Randomization was more acceptable if the alternative was available at the end of the trial. In the pilot phase, 151 patients were assessed for eligibility. Sixty were eligible and invited to take part in the pilot RCT; 42 consented to randomization. Follow-up was 100% at 12 months. Assessments of treatment fidelity were satisfactory. An RCT to compare arthroscopic hip surgery with conservative care in patients with FAI is challenging but feasible. Recruitment has started for a full RCT.
INTRODUCTION
Arthroscopic hip surgery for femoroacetabular impingement (FAI) is a rapidly growing and evolving field with increasing number of patients undergoing surgical treatment [1, 2]. In case series, ∼80% of patients report improvement at 2 years after either surgery or a conservative care programme that includes physiotherapist-led treatments such as education and exercise [3, 4]. A recent Cochrane review highlighted the lack of evidence from randomised trials to assess the relative clinical and cost effectiveness of these two strategies [5]. There has been enthusiasm in the surgical community in several countries and among commissioners of healthcare for such a trial in the management of FAI [6].
However, conducting RCTs is challenging; clinician and patient equipoise, and the ability to recruit adequate numbers of patients are major obstacles to trials, especially in surgery [7–9]. This is made even more difficult if the comparator arm is conservative care [10, 11].
There was uncertainty as to whether surgeons or patients would be prepared to take part in an RCT comparing surgery with conservative care for FAI. We performed a feasibility study, including an internal pilot trial, to understanding clinician and patient equipoise, and to assess trial procedures, eligibility criteria, recruitment and retention rates in order to determine whether to proceed to a full trial.
METHODS
We conducted this mixed methods feasibility study in two phases: a pre-pilot qualitative research phase that aimed to explore surgeon and patient equipoise followed by a mixed methods internal pilot RCT that aimed to test out the planned trial procedures including eligibility assessment, participant recruitment and retention rates, as well as develop a method of assessing intervention fidelity. Ethical approval was obtained from the NHS Research Ethics Committee (11/WM/0389). A detailed report of this study is available in a Health Technology Assessment (HTA) Journal monograph [12].
Phase 1: Pre-pilot Phase
Clinician interviews
Semi-structured interviews were conducted with a convenience sample of clinicians with a specialist interest in treating hip pain including orthopaedic surgeons, sports physicians and physiotherapists, to explore the level of equipoise within the community about an RCT comparing surgery with conservative care. Clinicians were presented with a series of cases covering a spectrum of presentations of FAI and asked to ‘think aloud’ when considering the cases, with prompting questions used, to deconstruct the cognitive process when considering whether to recruit a patient into an RCT. Clinician interviews were recorded and analysed thematically [13].
Patient interviews
A panel of expert patients was established from patients who had been treated for FAI by D.G. The panel was selected by purposive sampling to be representative of a broad range of ages, pathology, socioeconomic class, activity levels and gender. The sample-included patients treated through surgery and conservative care. Invitations were sent to patients inviting them to participate in semi-structured, one-to-one interviews and to review proposed trial patient information sheets.
Interviews were audio recorded and fully transcribed. Transcriptions were thematically analysed to extract key messages about patients’ experiences and the acceptability of proposed trial treatments and of randomization.
Phase 2: Pilot RCT
A pragmatic, multicentre, two-parallel arm pilot RCT of arthroscopic hip surgery versus best conservative care for FAI was undertaken at 10 NHS centres across the UK to determine eligibility, recruitment and retention rates and to test trial procedures. The trial was designed as an internal pilot RCT such that, should the findings show that a full trial was feasible, the main trial could follow without significant delay [14]. The trial was overseen by a trial management group and independent trial steering and data monitoring committees.
Participants
Eligible patients were identified from among those presenting to young adult hip clinics in 10 NHS centres.
The following eligibility criteria for a RCT were agreed:
Subjects were included if they:
Were aged ≥ 16 years.
Had symptoms of hip pain—they may also have had symptoms of clicking, catching or giving way.
Showed radiographic evidence of pincer- or cam-type FAI on plain radiographs and cross-sectional imaging [15].
The treating surgeon believed that they would benefit from arthroscopic FAI surgery.
Were able to give written informed consent and to participate fully in the interventions.
Patients were excluded if there was:
Previous significant hip pathology such as Perthes’ disease, slipped upper femoral epiphysis or avascular necrosis.
Previous hip injury such as acetabular fracture, hip dislocation and femoral neck fracture.
Osteoarthritis, defined as Tönnis grade >1, or more than 2-mm loss of superior joint space width on AP pelvic radiograph [4, 16].
Evidence that the patient would be unable to participate fully in the interventions, adhere to trial procedures or complete questionnaires, such as cognitive impairment or intravenous drug abuse.
Previous shape changing surgery (open or arthroscopic) in the hip being considered for treatment.
Participating surgeons identified eligible patients in their routine hip clinics, and invited them to a trial information consultation with a trained research nurse. During this consultation, information was provided about FAI including an information sheet (see Supplementary data, Appendix S1), using methods described by Realpe et al. [17]. Patients who provided written informed consent were randomized in a 1:1 ratio, using a computer generated telephone randomization service minimized by study centre, to receive arthroscopic surgery or best conservative care.
Interventions
Arthroscopic surgery
The arthroscopic surgery within the trial was delivered by NHS orthopaedic consultant specialising in hip surgery and included:
Pre-operatively, routine pre-operative assessment.
Peri-operatively, patients underwent arthroscopic hip surgery under general anaesthesia. Shape abnormalities and consequent pathology were addressed. Adequacy of bony resection was assessed by intra-operative image intensifier radiographs and/or satisfactory impingement free range of movement of the hip.
Post-operatively, patients were discharged when they were ambulatory and safe with or without the aid of crutches. On discharge all patients were referred to outpatient-led physiotherapy services for the surgeons usual course of rehabilitation.
Best conservative care
During the feasibility study a protocol for best conservative care called ‘Personalised Hip Therapy’ (PHT) was developed through the Delphi method, nominal group technique and a consensus development conference [18–22]. Participants received the PHT protocol from an NHS physiotherapist specialising in musculoskeletal care who had attended a PHT workshop. The agreed protocol consisted of:
Pre-treatment—patients received an information leaflet that provided details about PHT.
-
Treatment consisted of:
A detailed assessment
Patient education
Help with pain relief
An exercise programme that was individualised, supervised in clinic and practiced at home and progressed over time
A minimum of 6 treatment contacts over at least 12 weeks.
After the 12-week treatment period, participants could be offered additional support or guidance in up to four contacts with the physiotherapist. Details of the development, and protocol for delivery, of PHT have been published elsewhere [12].
Outcomes
The aim of the pilot RCT was to assess eligibility, recruitment and retention rates and test study procedures including assessment of fidelity. Participants were followed up to test the protocol for a full RCT. Outcomes were collected by researchers blinded to the treatment allocation at baseline (before randomisation), and at 3, 6 and 12 months after randomisation, using postal questionnaires and, when participants failed to respond, phone calls and emails. The following patient-reported outcomes were collected:
Hip related quality of life measured by the International Hip Outcome tool 33 (iHOT33) [23].
Hip pain and function measured by the Non Arthritis Hip Score (NAHS) [24].
Activity level measured by the University of California Los Angeles (UCLA) activity score (baseline only) [25].
General health by the Short Form 12 (SF12) [26].
Health related quality of life by the EQ5D 5 L [27].
Adverse events (AE). At 6 weeks after treatment a complication log was collected by telephone. We recorded number and type of AE up to 12 months.
Resource utilisation Information on health care resource use was collected [28].
Treatment fidelity
The quality of the evidence produced by a full RCT depends on the fidelity with which the two treatments being compared are implemented. The following measures to determine treatment fidelity were developed and tested in the pilot RCT.
Arthroscopic hip surgery
For each participant, a vignette was prepared comprising; an operation note, two intra-operative photos and six reconstructed images from a post-operative three-dimensional (3D) single sequence magnetic resonance volume acquisition scan—see Supplementary data, Appendix S2.
Each vignette was then analysed by an international expert group comprising of M.P. (surgeon, USA), M.B. (surgeon, Switzerland), J.O. (surgeon, Australia) and C.H. (radiologist, UK), for adherence to the treatment protocol by assessing whether satisfactory surgery had been undertaken. Disagreements were resolved by discussion and group consensus.
Best conservative care: personalised hip therapy
For each trial participant, physiotherapists recorded full details of the advice and treatments, number and mode of treatment contacts, any non-attendance and any AE on specifically designed case report forms (CRFs)—see Supplementary data, Appendix S3. Following discharge from physiotherapy care, patients’ CRFs were assessed by the panel of experts who developed the PHT protocol (I.H. and D.R.; senior physiotherapists with interest in FAI, N.F.; academic musculoskeletal physiotherapist, P.W.; academic orthopaedic surgeon), to judge whether PHT was delivered per protocol. Disagreements were resolved by discussion and group consensus.
Sample size
The pilot RCT was not powered to estimate a between-group treatment effect, but rather to achieve a reasonable confidence interval around the estimate of the proportion of eligible patients who consent to participate. Previous studies in orthopaedic surgery of operative compared with non-operative treatments have achieved recruitment rates of ∼30%, so we modeled around this. Approaching 60 patients to invite them to take part in the trial would allow a 95% CI of 18–41% if the true recruitment rate was 30%. We judged this to be a good balance between cost and duration of the pilot RCT and the required precision of the estimate.
RESULTS
Phase 1: Pre-Pilot Phase
Clinician interviews
Twenty-eight clinicians agreed to be interviewed, including 14 hip surgeons, 12 physiotherapists and two sports physicians who attended an arthroscopic hip surgery conference (Sports Hip, Warwick 2012).
Equipoise in theoretical terms was stated by 26 (90%) clinicians. However, five surgeons and two physiotherapists did not express equipoise when presented with case scenarios of patients eligible for the RCT.
One surgeon fundamentally did not believe in FAI and felt it was over diagnosed and a trial lacked relevance. Two surgeons reported preferring a conservative treatment. The remaining two surgeons and two physiotherapists felt surgery was the optimal treatment.
Seven surgeons demonstrated active theoretical and clinical equipoise and were invited to participate in the pilot RCT.
When discussing the planned RCT, 10 surgeons were concerned about the duration of such a trial. They felt there was a need to balance length of follow-up, to measure the difference between treatments, with the potential for the deterioration with continuing with one treatment and failing to improve. They felt 12 months was acceptable allowing patients’ symptoms to stabilise after treatment. The majority of clinicians thought patients would prefer surgery and might respond negatively to randomisation.
The physiotherapists and sports physicians frequently mentioned need for clarity on eligibility criteria for a trial and the need for a unique conservative care since they felt that many FAI patients would report having tried a course of conservative care prior to their consultation with the surgeon. Full transcripts of the clinician interviews are published elsewhere [12].
Patient interviews
Eighteen patients were involved in the expert patient panel (surgery n = 14 conservative care n = 4); the majority was young and physically active. They reported that FAI had limited their everyday and recreational activities, and that it was this that led them to seek treatment.
The expert patients agreed that both treatments were acceptable. Many found that having a conservative care option was positive. However, the majority felt that surgery was a ‘solution’ to a condition caused by ‘abnormal bone’. Patients felt that conservative treatment was attractive as it was less invasive.
Patients were motivated to participate in research to help clinicians improve their knowledge but also to help future patients. The panel presumed that surgeons would know what was best for them and felt uncomfortable being randomised to receive a treatment as they perceived that the surgeon would not be looking after their best interest. The process of randomisation was more acceptable if the alternative treatment was available at the end of the trial should the patient still be symptomatic.
The patients’ felt that it was important that their treatment plan met their individual needs and the clinical team should continue to provide care during and after the trial, whichever arm they were allocated to. Full transcripts of the patient interviews are published elsewhere [12].
Phase 2: Pilot RCT
Recruitment
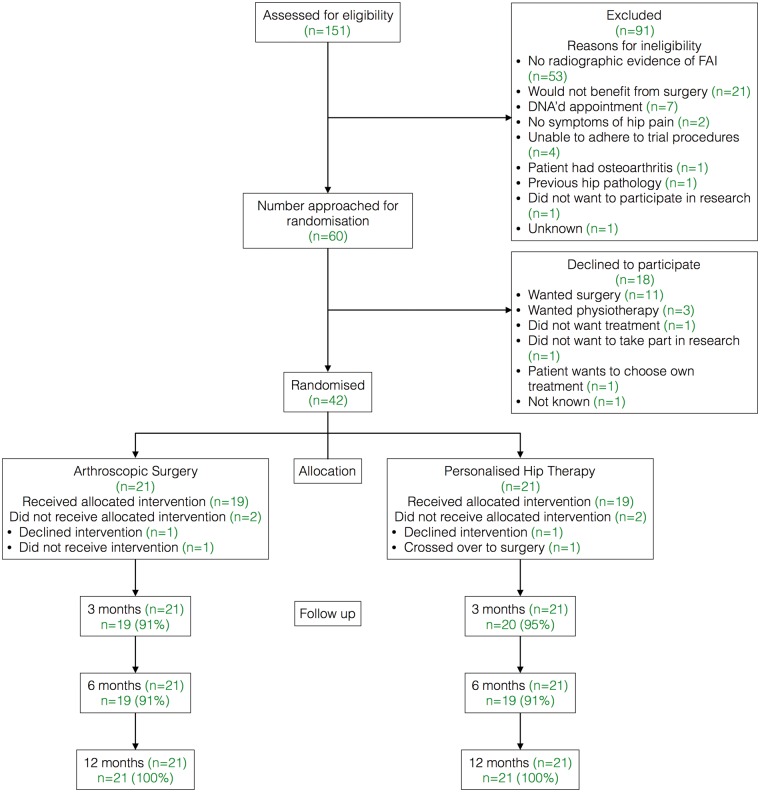
Details of the eligibility and recruitment of patients is displayed in Fig. 1. Trial set-up was delayed in one centre and it therefore did not participate in recruitment of the first 60 subjects. Sixty patients fulfilled the eligibility criteria and were approached. Forty-two patients (70%; 95% CI 58–81%) consented to participate in the trial. Eighteen patients declined to participate; 11 preferred surgery and 3 preferred PHT.
Fig. 1.
CONSORT diagram.
The baseline characteristics for the trial participants in each treatment arm are shown in Table I. Patients demographics were as expected; being young and active (50% had UCLA score of 1, 2 or 3 indicating regular participation in sport).
Table I.
Baseline participant data
| Characteristics | PHT (n = 21) | Arthroscopic hip surgery (n = 21) | All (n = 42) |
|---|---|---|---|
| Patient characteristics | |||
| Age (years)a | 33.4 (6.4) | 36.2 (9.8) | 34.8 (8.3) |
| Sex (female:male) | 6:15 | 10:11 | 16:26 |
| Side (left:right) | 11:10 | 5:16 | 16:26 |
| Duration of symptoms (months)a | 30.9 (24.4) | 30.9 (28.5) | 30.9 (26.2) |
| Scores | |||
| EQ5Da | 0.58 (0.23) | 0.57 (0.30) | 0.57 (0.27) |
| NAHSa | 57.9 (18.9) | 52.9 (18.5) | 55.4 (18.6) |
| iHOT-33a | 31.4 (15.2) | 31.0 (17.3) | 31.2 (16.0) |
| SF-12 MCSa | 46.4 (15.0) | 46.3 (11.8) | 46.3 (13.4) |
| SF-12 PCSa | 31.1 (14.8) | 28.6 (11.6) | 29.7 (13.3) |
MCS, mental component scale; PCS, physical component scale.
aMean (SD).
Follow up
Thirty-eight participants (91%) received the treatment to which they were randomised; one patient did not receive surgery within 12 months, two patients declined their treatment (PHT n = 1, surgery n = 1) and one patient crossed over from PHT to surgery before their 3 month follow-up. Follow-up rates were 93, 91 and 100% at 3, 6 and 12 months, respectively. There were no serious AE reported.
Treatment fidelity
Arthroscopic hip surgery
The first surgery review panel assessed seven patients who underwent surgery. The panel felt that the quantity and quality of the information presented was sufficient to make a decision on the adequacy of the surgery. Each operation was rated as either per protocol, borderline or not demonstrating protocol care. The panel rated six operations as per protocol care. One procedure was rated as not demonstrating per protocol care; in this case the surgeon did not proceed with shape changing surgery due to a large area of cartilage loss. The panel felt that although not per protocol care this was clinically appropriate.
Best conservative care—personalised hip therapy
The first PHT fidelity panel assessed PHT CRFs and the physiotherapy notes for the first eight patients to undergo treatment. The panel found that the CRF accurately reflected the physiotherapy notes and that the CRF could be used to judge treatment fidelity for future cases. Five of the eight cases reviewed were judged to be in line with the protocol. Protocol deviations were due to inadequate number of treatment contacts (n = 2) and no CRF completed (n = 1).
DISCUSSION
We have the feasibility of a pragmatic, multicentre RCT to compare the clinical and cost-effectiveness of arthroscopic hip surgery with best conservative care in patients with FAI. Clinicians are in sufficient equipoise and are willing to invite their patients to join an RCT. Our patient panel interviews demonstrated that patients are equally willing to participate in research although they want treatment to be individualised and are cautious about randomization. Having designed a package of best conservative care (PHT), we prospectively assessed patients’ willingness to participate in a pilot RCT. We found that 70% of eligible patients agreed to participate in an RCT.
The pre-pilot qualitative research offered an opportunity to detect key areas of concern for potential participants and respond to these challenges. Our expert patient panel expressed a reluctance to be randomised in a research trial; however, this retrospective view of patients already successfully treated was not apparent during the pilot RCT where 70% of eligible patients agreed to be randomised. We believe that this reflects the care that the research nurses, trained by the trial team, took to explain the trial in detail to eligible patients, and to the way in which the process of randomisation was presented to patients without negative connotations.
By designing a specific conservative care treatment, PHT, through consensus methodology, we were able to alleviate the fears of our clinician and patient panels that conservative treatment would need be ‘unique’ and ‘individualised’. Clinicians also thought that patients would prefer surgery. However only 30% eligible declined to participate; of those, 11 (61%) had a preference for surgery. When testing trial protocols, only one PHT patient crossed over to surgery during the 1-year follow up.
A strength of the study was that it took in the opinions of a broad group of clinicians treating FAI as well as patients with experience of surgery and conservative care. Strengths of the pilot RCT were that it was multicentre and pragmatic. A key strength was the design of the pilot RCT as an internal pilot trial, such that progression to the full trial could proceed without significant delay and that the 42 patients randomised in the pilot could be included in a full trial. It is for this reason that we cannot report the outcomes of the patients recruited in the pilot study.
Following this study, funding was received from the Health Technology Assessment programme (13/103/02) to progress to a full RCT (ISRCTN64081839). This trial is called FASHIoN: a Full randomised controlled trial of Arthroscopy Surgery for Hip Impingement versus best coNventional care. The full trial aims to recruit 344 patients (making this the largest RCT of FAI treatment planned worldwide) in 25 centres, and we anticipate reporting results in mid-2017. We have collaborated with colleagues in Australia to use the same protocol for a similar trial in five centres (ACTRN 12615001177549).
CONCLUSION
This feasibility and pilot study shows that it is feasible to conduct a full RCT comparing arthroscopic surgery to conservative care in patients with FAI. Supported by this evidence, the UK National Institute for Health Research HTA programme has now commissioned a full trial, which is underway.
ETHICS APPROVAL
Ethical approval was granted on the 12 February 2012 (11/WM0389) by the Edgbaston Research Ethics committee.
TRIAL SPONSOR
University of Warwick, Coventry, CV4 7AL, UK.
FUNDING
This work was supported by the Health Technology Assessment Programme of the National Institute of Health Research (grant number 10/41/02, awarded to D.G.). NEF, an NIHR Senior Investigator, is supported through an NIHR Research Professorship (NIHR-RP-011-015). J.L.D. is supported by the MRC ConDuCT Hub for Trials Methodology Research (MR/K025643/1), and the NIHR Collaboration for Leadership in Applied Health Research and Care (CLAHRC) West at University Hospitals Bristol NHS Foundation Trust, and is an NIHR Senior Investigator.
The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
SUPPLEMENTARY DATA
Supplementary data are available at Journal of Hip Preservation Surgery online.
Supplementary Material
ACKNOWLEDGMENTS
The authors wish to acknowledge support from hospital Trusts, Comprehensive Local Research Networks and collaborating surgeons, physiotherapists and research associates, and the work of Ceri Jones, R&D manager at the lead site, UHCW NHS Trust. Finally, we thank the patients who gave so generously of their time in the expert patient panel, and those who agreed to participate in the pilot RCT. The authors wish to thank the following clinicians who recruited into the pilot RCT: Prof. D.G. and Mr C. McBryde, University Hospitals Coventry and Warwickshire, Mr P. Latimer, Yeovil District Hospital; Mr M. Wilson, Royal Devon and Exeter Hospital, Mr E. Bache and Mr C. McBryde, Royal Orthopaedic Hospital Birmingham; Mr S. Sturridge, Frimley Park Hospital; Mr M. Norton and Mr G. Bartlett, Royal Cornwall Hospitals; Prof R. Field and Mr G. Stafford Epsom and St Helier University Hospitals, Mr M. Bankes and Mr M. George Guy’s and St Thomas’ Hospitals London, Mr M. Ramachandran St Bartholomew’s and Royal London Hospitals and Mr J. Witt University College Hospital London.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Reiman MP, Thorborg K. Femoroacetabular impingement surgery: are we moving too fast and too far beyond the evidence? Br J Sports Med 2015; bjsports-2014-093821. [DOI] [PubMed] [Google Scholar]
- 2. Montgomery SR, Ngo SS, Hobson T. et al. Trends and demographics in hip arthroscopy in the United States. Arthroscopy 2013; 29:661–5. [DOI] [PubMed] [Google Scholar]
- 3. Emara K, Samir W, Motasem E. et al. Conservative treatment for mild femoroacetabular impingement. J Orthop Surg (Hong Kong) 2011; 19:41–5. [DOI] [PubMed] [Google Scholar]
- 4. Philippon M, Briggs K, Yen Y-M. et al. Outcomes following hip arthroscopy for femoroacetabular impingement with associated chondrolabral dysfunction minimum two-year follow-up. J Bone Joint Surg Br Vol 2009; 91:16–23. [DOI] [PubMed] [Google Scholar]
- 5. Wall PD, Brown JS, Parsons N. et al. Surgery for treating hip impingement (femoroacetabular impingement). Cochrane Lib 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clohisy JC, Kim Y-J, Lurie J. et al. Clinical trials in orthopaedics and the future direction of clinical investigations for femoroacetabular impingement. J Am Acad Orthop Surg 2013; 21:S47–52. [DOI] [PubMed] [Google Scholar]
- 7. Ross S, Grant A, Counsell C. et al. Barriers to participation in randomised controlled trials: a systematic review. J Clin Epidemiol 1999; 52:1143–56. [DOI] [PubMed] [Google Scholar]
- 8. Mapstone J, Elbourne D, Roberts IG. Strategies to improve recruitment to research studies. Cochrane Lib 2007. [DOI] [PubMed] [Google Scholar]
- 9. Treweek S, Mitchell E, Pitkethly M. et al. Strategies to improve recruitment to randomised controlled trials. Cochrane Database Syst Rev 2010. [DOI] [PubMed] [Google Scholar]
- 10. McCulloch P, Taylor I, Sasako M. et al. Randomised trials in surgery: problems and possible solutions. BMJ 2002; 324:1448.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ergina PL, Cook JA, Blazeby JM. et al. Challenges in evaluating surgical innovation. Lancet 2009; 374:1097–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Griffin DR, Wall PD, Realpe A. et al. UK FASHIoN: Feasibility study of a randomised controlled trial of arthroscopic surgery for hip impingement compared with best conservative care. Health Technol Assess 2016; 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Buckingham CD, Adams A. Classifying clinical decision making: a unifying approach. J Adv Nurs 2000; 32:981–9. [PubMed] [Google Scholar]
- 14. Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract 2004; 10:307–12. [DOI] [PubMed] [Google Scholar]
- 15. Beall DP, Sweet CF, Martin HD. et al. Imaging findings of femoroacetabular impingement syndrome. Skeletal Radiol 2005; 34:691–701. [DOI] [PubMed] [Google Scholar]
- 16. Tönnis D, Heinecke A. Current concepts review-acetabular and femoral anteversion: relationship with osteoarthritis of the hip. J Bone Joint Surg 1999; 81:1747–70. [DOI] [PubMed] [Google Scholar]
- 17. Realpe A, Adams A, Wall P. et al. A new simple six-step model to promote recruitment to RCTs was developed and successfully implemented. J Clin Epidemiol 2016; doi: 10.1016/j.jclinepi.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murphy M, Black N, Lamping D. et al. Consensus development methods, and their use in clinical guideline development. Health Technol Assess (Winchester, England) 1998; 2:i. [PubMed] [Google Scholar]
- 19. Dalkey N, Helmer O. An experimental application of the Delphi method to the use of experts. Manage Sci 1963; 9:458–67. [Google Scholar]
- 20. Delbecq AL, Van de Ven AH. A group process model for problem identification and program planning. J Appl Behav Sci 1971; 7:466–92. [Google Scholar]
- 21. Van de Ven AH, Delbecq AL. The nominal group as a research instrument for exploratory health studies. Am J Public Health 1972; 62:337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fink A, Kosecoff J, Chassin M. et al. Consensus methods: characteristics and guidelines for use. Am J Public Health 1984; 74:979–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mohtadi NG, Griffin DR, Pedersen ME. et al. The development and validation of a self-administered quality-of-life outcome measure for young, active patients with symptomatic hip disease: the International Hip Outcome Tool (iHOT-33). Arthroscopy 2012; 28:595–610. [DOI] [PubMed] [Google Scholar]
- 24. Christensen CP, Althausen PL, Mittleman MA. et al. The nonarthritic hip score: reliable and validated. Clin Orthop Relat Res 2003; 406:75–83. [DOI] [PubMed] [Google Scholar]
- 25. Naal FD, Impellizzeri FM, Leunig M. Which is the best activity rating scale for patients undergoing total joint arthroplasty? Clin Orthop Relat Res 2009; 467:958–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ware J, Kosinski M, Turner-Bowker D. et al. SF-12v2: How to Score Version 2 of the SF-12 Health Survey. Lincoln, RI: Quality Metric Incorporated 2002; 29–38. [Google Scholar]
- 27. Janssen MF, Birnie E, Haagsma JA. et al. Comparing the Standard EQ‐5D Three‐Level System with a Five‐Level Version. Value Health 2008; 11:275–84. [DOI] [PubMed] [Google Scholar]
- 28. Van den Brink M, Van den Hout W, Stiggelbout A. et al. Self-reports of health care utilization: can a questionnaire replace a diary. The 16th Annual Meeting of the International Society for Technology Assessment in Health Care, The Hague, the Netherlands (ISTAHC)2000.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.