Abstract
SurePath specimens from women referred to colposcopy were treated with Aptima Transfer Solution (ATS) before testing in Aptima HPV (AHPV) and Aptima HPV 16, 18/45 (AHPV-GT) assays. Untreated SurePath specimens were tested with the cobas HPV test. PreservCyt specimens were assessed for cytology and tested with AHPV. High-grade cervical intraepithelial neoplasia lesions served as the reference standard. Excellent agreement (95.5%; k=0.91) was observed for ATS-treated SurePath specimens between Tigris and Panther systems and between the PreservCyt and ATS-treated SurePath specimens (91.1%, k=0.81) with the AHPV assay on Tigris. Agreement between the AHPV and cobas assays with SurePath specimens was substantial (89.9%, k=0.80). AHPV sensitivity for CIN2+(n=147) was 91.2% for SurePath and PreservCyt. Cobas HPV sensitivity was 93.9% for SurePath specimens. AHPV testing of SurePath specimens was more specific (59.4%) than cobas (54.7%) (p<0.001). Detection and genotyping showed similar absolute and relative risks. ATS-treated SurePath specimens tested with AHPV and AHPV-GT assays showed similar performance with greater specificity than cobas HPV on SurePath specimens. Similar overall results were seen using a CIN3 disease endpoint.
Keywords: Human papillomavirus; SurePath; PreservCyt; Cervical intraepithelial neoplasia; CIN2+,; Aptima transfer solution (ATS)
1. Introduction
The United States cervical screening guidelines recommend high-risk human papillomavirus (HR-HPV) testing after abnormal cytological results and HR-HPV co-testing of women 30 years and older [1]. ThinPrep PreservCyt (Hologic Inc., Marlbourgh, MA) and SurePath (Becton Dickinson, Sparks, MD) liquid-based cytology media are used for HR-HPV testing. Most HPV assays have been validated with ThinPrep PreservCyt specimens [2] and have also been approved by the US Food and Drug Administration (FDA). Some HPV detection assays have used SurePath specimens [3], [4], [5], [6], [7], [8], [9], [10], [11], [12]. However, testing for HR-HPV out of the SurePath vial using nucleic acid assays has been considered a laboratory developed test and discouraged by the FDA [13], [14]. Only recently has the cobas HPV test been approved by the FDA for the use with SurePath specimens for co-testing of women 30 years or older and also for reflex testing for women 21 years or older with an abnormal Pap result (http://fda.gov/news).
The Aptima HPV (AHPV) and the Aptima HPV 16 18/45 genotype (AHPV-GT; Hologic) assays detect E6/E7 messenger RNA (mRNA). The AHPV assay detects 13 of the most common HPV types, 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68, as well as type 66 collectively. The AHPV-GT assay detects HR-HPV type 16 individually, and HR-HPV 18 and 45 together without differentiation. Both assays are approved by the FDA with PreservCyt specimens on Tigris DTS and Panther instruments (Hologic Inc). Both assays are also Conformite European (CE)-marked and approved by Health Canada for PreservCyt specimens and Aptima Cervical Specimen Collection and Transport (CSCT) samples.
The cobas HPV Test (Roche Molecular System, Pleasanton, CA) detects the same 14 oncogenic HPV genotypes as the AHPV assay but identifies types 16 and 18 individually from the other 12 h types and is approved by FDA and Health Canada and is CE marked for PreservCyt specimens [15].
Because SurePath specimens contain ethanol and formalin, HPV DNA, RNA, and proteins may become cross-linked during storage [16]. This appears to be less problematic for DNA than for RNA assays [17], [18]. Extraction of RNA from SurePath samples after Proteinase K digestion has shown optimal recovery [18], [19]. AHPV testing of fresh untreated SurePath specimens and stored SurePath specimens heat-treated with Fast Express reagent (Hologic) containing Proteinase K detected 95.2% (77.3–99.2) of CIN2+ cases compared to 95.5% (78.2–99.2) for paired PreservCyt specimens collected from the same patients [20]. Because of these findings, an Aptima Transfer Solution (ATS), containing proteinase K, was developed by Hologic to improve AHPV performance with SurePath specimens.
ATS-treated SurePath specimens were tested with AHPV and the AHPV-GT assays on Tigris DTS and Panther systems and compared to PreservCyt specimens collected from the same patients and cobas HPV testing of untreated SurePath. For the analysis, cervical intraepithelial neoplasia grade 2 or worse (CIN2+) was used as the disease endpoint. A subanalysis using a CIN3 endpoint was also conducted.
2. Materials and methods
2.1. Study population
Women 21 years of age or older referred for a colposcopic examination due to an abnormal Pap test, a previously positive HPV test, or other gynecologic reasons were invited to participate in the study. The study procedures were explained and only patients who provided a signed informed consent were enrolled. Enrollment was from the Hamilton Health Sciences Juravinski Hospital, Hamilton and the Thunder Bay Regional Health Sciences Centre, Thunder Bay, Ontario. The study was approved by the respective research ethics boards at both sites.
2.2. Specimen collection and processing
Cervical specimens were collected first into PreservCyt then into SurePath in accordance with the manufacturer's instructions using a spatula and cytobrush or a Cervex broom. A colposcopic exam was conducted and cervical biopsies were obtained per the sites’ standard practices.
SurePath specimens were processed for HPV testing within 1–5 days of collection. For cobas HPV, two ml of each SurePath specimen were transferred into a 13 ml screw cap tube (Sarstedt, Ref# 60.540.500) in the Infections Research Laboratory (IRL) at St. Joseph's Healthcare in Hamilton, ON, and shipped on ice packs to the Newfoundland & Labrador Public Health Laboratory (NLPHL), St. John's, Newfoundland and stored refrigerated at 2–8 °C until tested. For AHPV testing, 0.5 ml of each SurePath specimen was transferred into an Aptima specimen transfer tube containing 2.9 ml of specimen transport medium (STM, Hologic, Inc.). The Aptima samples were treated with ATS within 7 days of collection as per the manufacturer's instructions, and then tested with the AHPV and APHV-GT assays. PreservCyt samples were processed for cytology and tested with the AHPV assay according to the manufacturer's instructions.
2.3. HPV testing and genotyping
The cobas HPV test was performed per the manufacturer's instructions using untreated SurePath specimens. Testing was completed within 2 weeks of collection. AHPV and AHPV-GT assays were carried out at the IRL on the Tigris and Panther systems using ATS-treated SurePath specimens within 2 weeks of collection. Samples were tested with the AHPV assay first and AHPV positives were tested with the AHPV-GT assay. PreservCyt specimens were tested with AHPV within 2 weeks of collection.
2.4. Cytology and histology
The PreservCyt sample was examined and scored for cytology per the Bethesda system in the St. Joseph's Hospital Pathology Laboratory as follows: within normal limits (WNL), atypical squamous cells of undetermined significance (ASCUS), low-grade squamous cell intraepithelial lesions (LSIL), high-grade squamous cell intraepithelial lesions (HSIL), squamous cell carcinoma (SCC), or carcinoma in situ (CIS). Biopsies were stained with H&E and read by the institutes’ pathologists. The histology slides were categorized negative, cervical intraepithelial neoplasia (CIN) grade 1, 2 or 3, and cancer. CIN 2+ was categorized as disease positive and a subanalysis of CIN3 was conducted: <CIN 2 was categorized as disease negative. Subjects with no visible lesions upon colposcopic exam, from whom a biopsy was not obtained, were considered disease negative.
2.5. Data analysis
Data were independently audited by a study monitor. Overall, positive and negative agreement between the AHPV results obtained with ATS-treated SurePath specimens were compared between the Tigris and Panther instruments, as well as AHPV assay results between ATS-treated SurePath and PreservCyt specimens. Positive and negative agreement was also determined between the AHPV and cobas HPV assays with SurePath specimens. The kappa statistic was calculated for each pairwise comparison. The clinical sensitivity, specificity, positive and negative predictive values for the AHPV assay and the cobas HPV test were calculated using McNemar's test to determine the comparative clinical performance using CIN as the reference standard. The absolute and relative risk of disease for various HPV result categorization for the AHPV and AHPV-GT assay were compared with those obtained with the cobas HPV test. Statistical analysis was performed using SAS 9.1.
3. Results
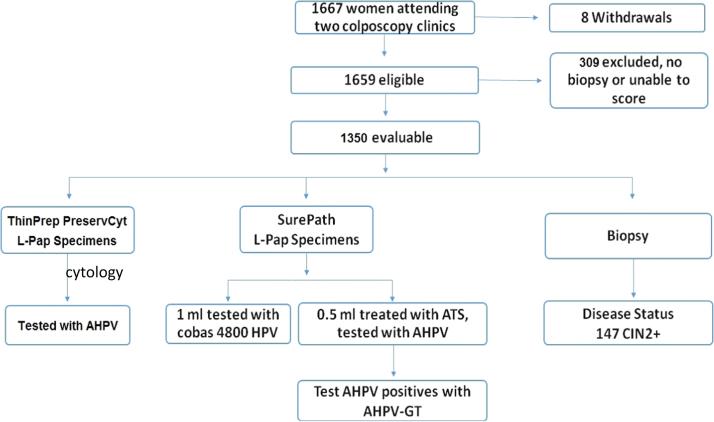
Enrollment was conducted over an 11 month period in 2014 with a total of 1667 women enrolled (Fig. 1). Eight were withdrawn and 309 excluded since no biopsy was taken or the biopsy could not be scored. This resulted in 1350 evaluable subjects for the study analysis. The mean age of the participants was 36.1 years (standard deviation 12.5 years, range 21–80 years, median 32 years). The proportion of women ≤30 years of age was 43.8%.
Fig. 1.
Study scheme.
A total of 1345 paired samples were evaluated to compare ATS-treated SurePath to PreservCyt specimens tested with the AHPV assay. A total of 1325 paired ATS-treated SurePath AHPV results were available for the comparison of the Tigris and Panther systems. Among the 1350 evaluable cases, there were 147 histology-confirmed CIN2+ and 71 cases of CIN3.
Cytology of the PreservCyt specimens from 1350 patients yielded 202 WNL (15.0%), 430 ASCUS (31.9%), 453 LSIL (33.6%), 258 HSIL (19.1%), and 8 SCC/CIS (0.59%). Colposcopy and histological evaluation of the 1350 evaluable subjects yielded 1064 negative (78.8%), 138 CIN 1 (10.2%), and 147 CIN 2+(10.9%; 71 CIN2, 71 CIN3, and 5 cancer) cases.
Excellent overall agreement was shown for paired SurePath samples in Table 1 that were tested by AHPV on the Tigris and Panther instruments (95.5%, k=0.91). ATS-treated SurePath and PreservCyt samples tested with the AHPV assay demonstrated substantial overall agreement (91.1%, k=0.81). Of the 120 (51 plus 69) discordant samples between PreservCyt and SurePath specimens, most (93.3%, 112/120) were from disease negative subjects and only 8 were from subjects with CIN2+(4 SurePath+/PreservCyt- and 4 SurePath-/PreservCyt+). Substantial agreement was observed between the AHPV assay with ATS treated SurePath on Tigris and the cobas HPV test (89.9%, k=0.80) (Table 1). The cobas HPV test had 99 discordant extra positives compared to 38 by AHPV and almost all were disease negative cases.
Table 1.
Agreement between assays, instruments and sample types.
| Assay sample type | AHPV Assay Tigris ATS-treated SurePath |
Agreement % (x/n) |
Kappa statistic | |||||
|---|---|---|---|---|---|---|---|---|
| + | - | Total | Positive | Negative | Overall | |||
| AHPV Assay Panther ATS-treated SurePath | + | 577 | 25 | 602 | 94.4% | 96.5% | 95.5% | 0.91 |
| – | 34 | 689 | 723 | |||||
| Total | 611 | 714 | 1325 | (577/611) | (689/714) | (1266/1325) | ||
| AHPV Assay Tigris PreservCyt | + | 552 | 51 | 603 | 88.9% | 93.0% | 91.1% | 0.81 |
| – | 69 | 673 | 742 | |||||
| Total | 621 | 724 | 1345 | (552/621) | (673/724) | 1225/1345) | ||
| Cobas HPV Test SurePath | + | 585 | 99 | 684 | 93.9% | 86.4% | 89.9% | 0.80 |
| – | 38 | 628 | 666 | |||||
| Total | 623 | 727 | 1350 | (585/623) | (628/727) | (1213/1350) | ||
Table 2 shows that the clinical sensitivity for a CIN2+ disease endpoint of the AHPV assay with both ATS-treated SurePath and PreservCyt specimens was 91.2% compared to 93.9% for the cobas HPV test (89.6% compared to 94.8% for CIN3). The specificities for the AHPV assay were 59.4% for ATS-treated SurePath and 60.9% for PreservCyt compared with 54.7% for cobas HPV (p<0.001). Similar statistical differences were observed for CIN3.
Table 2.
Sensitivity, specificity and predictive values of the Aptima HPV assay on the Tigris system and cobas HPV test for detection of CIN 2+(n=147) and CIN3 (n=71).
| HPV assay | Sample Type | % Sensitivity (95% CI) |
% Specificity (95% CI) |
% PPV (95% CI) |
% NPV (95% CI) |
||||
|---|---|---|---|---|---|---|---|---|---|
| CIN2+ | CIN3 | CIN2+ | CIN3 | CIN2+ | CIN3 | CIN2+ | CIN3 | ||
| Aptima | PreservCyt | 91.2 | 93.5 | 60.9 | 58.2 | 22.4 | 11.9 | 98.3 | 99.3 |
| (85.3–94.9) | (85.7–97.2) | (58.1–63.7) | (55.4–60.8) | (20.8–23.9) | (10.9–12.8) | (97.0–99.0) | (98.6–99.8) | ||
| ATS-treated SurePath | 91.2 | 89.6 | 59.4 | 56.5 | 21.7 | 11.1 | 98.2 | 98.9 | |
| (85.5–94.8) | (80.8–94.6) | (56.6–62.2) | (53.8–59.2) | (20.2–23.1) | (10.0–12.0) | (97.1–99.0) | (98.0–99.5) | ||
| Cobas | SurePath | 93.9 | 94.8 | 54.7 | 52.0 | 20.3 | 10.7 | 98.7 | 99.4 |
| (88.8–96.7) | (87.4–98.0) | (51.9–57.5) | (49.3–54.8) | (19.1–21.5) | (9.8–11.4) | (97.6–99.3) | (98.6–99.8) | ||
| p-Value | SurePath between AHPV and cobas | 0.2188 | 0.1250 | <0.001 | <0.001 | N/A | N/A | N/A | N/A |
CIN 2+=High grade cervical intraepithelial neoplasia grade 2 or worse.
Table 3 shows that the absolute risk of CIN2+ among those testing positive with the AHPV assay on Tigris using the ATS-treated SurePath specimens was 21.7% (18.6–25.1) compared to 20.3% (17.5–23.5) for those testing positive with the cobas HPV test performed on untreated SurePath specimens. Among those testing assay negative, the corresponding absolute risk for CIN2+ were 1.8% (1.0–2.9) for Aptima and 1.3% (0.7–2.4) for cobas. The relative risk of disease in test-positive versus test-negative cases was estimated at 12.1 (6.9–21.1) for the AHPV assay and 15.0 (7.7–29.1) for the cobas HPV test. Similar differences were observed by the CIN3 subanalysis (supplemental tables).
Table 3.
Comparison of absolute and relative risk of CIN2+ based on Aptima HPV on Tigris and cobas HPV on SurePath specimens.
| HPV assay | CIN2+/Test positive | % Absolute risk of CIN2+ in test positive (95% CI) | CIN2+/ test negative | % Absolute risk of CIN2+ in test negative (95% CI) | Relative risk of CIN2+(95% CI) |
|---|---|---|---|---|---|
| Aptima | 135/623 | 21.7 (18.6–25.1) | 13/728 | 1.8 (1.0–2.9) | 12.1 (6.9–21.1) |
| Cobas HPV | 139/684 | 20.3 (17.5–23.5) | 9/667 | 1.3 (0.7–2.4) | 15.0 (7.7–29.1) |
CIN 2+=High grade cervical intraepithelial neoplasia grade 2 or worse.
Genotype-specific absolute risk of CIN2+ was 32.1% (26.9–37.8) for those testing positive for types 16 and/or 18/45 by the AHPV-GT assay compared with 31.0% (26.1–36.4) testing positive for types 16 and/or 18 by the cobas HPV assay when testing SurePath samples (Table 4). For other high risk types, the absolute risks for Aptima and cobas assays were 13.1% (9.8–17.2) and 11.9% (9.3–14.7), respectively for those testing positive and 1.7% (1.0–2.9) and 1.3% (0.7–2.5) respectively for those testing negative. Table 4 also compares the genotype-specific relative risk of CIN2+ for the AHPV-GT and cobas assays on SurePath specimens. The relative risk of being positive for the designated genotypes and negative for the other oncogenic types was 19.3 (10.7–34.7) for the AHPV-GT assay and 23.0 (11.8–44.9) for the cobas test. The values for each assay were relatively similar for being positive for the designated types and positive for the other oncogenic types. Being positive versus negative for the other oncogenic types yielded similar values between the two assays. Similar results for absolute risks based on HPV genotypes were seen using a CIN3 endpoint (supplementary data).
Table 4.
Comparison of absolute risk of CIN2+ based on HPV genotypes detected by the Aptima HPV-GT and cobas HPV assays when testing SurePath specimens.
| HPV result | %Absolute risk of CIN2+(95% CI) | Relative Risk (95% CI) | |
|---|---|---|---|
| Positive for 16,18/45 or 16,18 | Aptima assays | 32.1 (26.9–37.8) | vs HPV negative: 19.3 (10.7–34.7) |
| vs other HR HPV: 2.5 (1.8–3.4) | |||
| Cobas HPV Test | 31.0 (26.1–36.4) | vs HPV negative: 23.0 (11.8–44.9) | |
| vs other HR HPV: 2.6 (1.9–3.6) | |||
| Positive for other high risk typesa,b | Aptima assays | 13.1 (9.8–17.2) | vs HPV negative: 7.9 (4.2–14.7) |
| Cobas HPV Test | 11.8 (8.9–15.4) | Vs HPV negative: 8.8 (4.3–17.7) | |
| Negative for oncogenic types | AHPV assay | 1.7 1.0–2.9) | N/A |
| Cobas HPV Test | 1.3 0.7–2.5) | N/A |
CIN 2+=High grade cervical intraepithelial neoplasia grade 2 or worse.
Other high risk HPV types detected by Aptima HPV assay: 31, 33, 35, 39, 51, 52, 56, 58, 59, 66, and 68.
Other high risk HPV types detected by cobas HPV test: 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68.
4. Discussion
The results from this study which evaluated Aptima assay performance in a referral population with both SurePath and PreservCyt specimens yielded sensitivity estimates of 91.2% for both ATS-treated SurePath and PreservCyt specimens for 147 women with a CIN2+ disease designation, with similar estimates for CIN3 (Table 2). These values are similar to estimates reported in previous studies with the Aptima assay [2], [21], [22], [23], [24]. This is the first study comparing Aptima with ATS-treated SurePath specimens to the cobas HPV test performance with SurePath specimens. The sensitivity of the cobas HPV test using SurePath specimens was 93.9%, which was not statistically different than the AHPV results (p=0.488). In contrast, the specificity estimates of the AHPV assay with PreservCyt specimens (60.9%) and with ATS-treated SurePath specimens (59.4%) were statistically higher than the 54.7% observed for cobas HPV (p<0.001). Similar trends were observed using a disease endpoint of CIN3 (Table 2). Using a disease endpoint of CIN 2+, the Predictors 4 study by Cuzick et al. [12] reported the sensitivity and specificity of the AHPV assay with PreservCyt specimens as 97.7% and 30.4%, respectively, as compared to SurePath samples treated with Fast Express Proteinase K (sensitivity, 93.1%; and specificity, 35.4%). The ATS-treatment of SurePath specimens described in this study also utilizes treatment with a proteinase K solution, however the procedure and workflow for ATS treatment is improved as compared to the Fast Express treatment described previously [12], [20].
Significant absolute and relative risks for disease were observed for the two assays with SurePath specimens (Table 3). An analysis of genotyping results from the two systems (Tables 4) showed similar, and significant absolute and relative risks of disease based on the categorization of high-risk genotypes from each assay. These results are consistent with observations with PreservCyt specimens [25] (Eaton et al., 2012 poster 1825; International Papillomavirus Conference, San Juan, Puerto Rico).
When tested with the AHPV assay, PreservCyt and ATS treated SurePath samples showed substantial agreement overall (91.1%; k=0.81) (Table 1). The overall agreement between AHPV and cobas HPV testing of SurePath specimens was substantial (89.9%; k=0.80) and similar to previously reported results with PreservCyt specimens (90.9%, k=0.81) [26].
On July 7, 2016, the FDA approved cobas HPV testing of SurePath collected cervical samples for women 21 years and older with ASCUS cytology and for women 30 years and older undergoing adjunctive testing with cytology (co-testing). (http://www.fda.gov/NewsEvents/Newsroom/Press/Announcements/ucm510215.htm). Approval was based on 952 eligible women 21 years and older with abnormal Pap results by showing agreement between the SurePath and PreservCyt samples (95.4 positives and 93.2 negatives). Similar agreements were observed in our study between ATS-treated SurePath samples and PreservCyt specimens tested by AHPV (Table 1).
The results from this study demonstrated excellent overall agreement for the AHPV assay with ATS-treated SurePath samples between the Tigris and Panther instruments (Table 1, 95.5% agreement; k=0.91) and the results are comparable to the cobas platform. The Tigris is a high volume batch-mode instrument whereas the Panther is a continuous load, random access platform. Both are able to process ATS-treated SurePath samples with the AHPV and AHPV-GT assays efficiently with high agreement.
A limitation of our study was that p16 staining of the tissue samples was not performed and histology was not reviewed by a panel of pathologists to provide consensus of scoring. Another limitation was that most of the women with a normal colposcopy did not have a biopsy taken and were assumed to be disease negative. It is possible that some percentage of disease would have been present in those women. SurePath specimens in the study were not processed for cytology before HPV testing, but the processing step does not create variable volumes for testing and would not likely have impacted the similarity of performance of the different tests.
The study was robust with a substantial enrollment and a high number of women with disease. We were able to collect both SurePath and PreservCyt specimens from each woman which allowed appropriate cytology and HPV evaluation using PreservCyt [15] and provided the ability to compare AHPV and AHPV-GT testing of ATS-treated SurePath with cobas HPV testing of SurePath. This careful comparison showed that AHPV testing of ATS-treated SurePath samples provided similar results to AHPV testing of PreservCyt specimens and cobas HPV testing of SurePath samples, with better specificity. The absolute and relative risks for CIN2+ were similar for each assay and their high risk genotyping components.
5. Conclusion
ATS-treated SurePath specimens and PreservCyt specimens tested with AHPV and AHPV-GT assays showed similar performance for each specimen type. Results from Tigris and Panther instruments showed excellent agreement. Percent absolute and relative risk for HR-HPV detection and genotyping were comparable for the 2 assays. For treated SurePath specimens, the AHPV assay had similar sensitivity to the cobas HPV test but was more specific.
Conflict of interest statement
M. Chernesky has received travel expenses to present data related to the study. D. Getman, B. Weinbaum and B. Kirkconnell are employees of Hologic. J Dockter was an employee of Hologic at the time of the study but presently an employee of illumina (San Diego, CA).
Acknowledgements
We would like to thank the participants in the study and the clinic staff at both the Juravinski Hospital and Thunder Bay Regional Health Sciences Centre Colposcopy Clinic. The study was funded by Hologic Inc. (San Diego, CA).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.pvr.2017.04.005.
Appendix A. Supplementary material
Supplementary material
References
- 1.Saslow D., Solomon D., Lawson H.W., Killackey M., Kulasingam S.L., Cain J. ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J. Clin. 2012;62(3):147–172. doi: 10.3322/caac.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szarewski A., Mesher D., Cadman L., Austin J., Ashdown-Barr L., Ho L. Comparison of seven tests for high-grade cervical intraepithelial neoplasia in women with abnormal smears: the Predictors 2 Study. J. Clin. Microbiol. 2012;50(6):1867–1873. doi: 10.1128/JCM.00181-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark-Gero M., Pitlik D., Barcelo M. Human papillomavirus testing using Roche Cobas HPV test with surepath preparation in gynecologic cytology. Am. J. Clin. Pathol. 2015;144(2):A237. [Google Scholar]
- 4.Gilbert L., Oates E., Ratnam S. Stability of cervical specimens in SurePath medium for human papillomavirus testing with the Roche cobas 4800 assay. J. Clin. Microbiol. 2013;51(10):3412–3414. doi: 10.1128/JCM.01391-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis-Devine S., Day S.J., Freund G.G. Test performance comparison of inform HPV and hybrid capture 2 high-risk HPV DNA tests using the SurePath liquid-based Pap test as the collection method. Am. J. Clin. Pathol. 2005;124(1):24–30. doi: 10.1309/BFVVU29HCC5RCKY5. [DOI] [PubMed] [Google Scholar]
- 6.Ko V., Tambouret R.H., Kuebler D.L., Black-Schaffer W.S., Wilbur D.C. Human papillomavirus testing using Hybrid Capture II with SurePath collection: initial evaluation and longitudinal data provide clinical validation for this method. Cancer. 2006;108(6):468–474. doi: 10.1002/cncr.22285. [DOI] [PubMed] [Google Scholar]
- 7.Kapala J., Jang D., Patel J., Biers K., Smieja M., Chernesky M. Pap cytopathology and the presence of high-risk human papillomavirus in SurePath liquid preservative and Digene cervical sampler specimens. J. Virol. Methods. 2007;142(1–2):223–225. doi: 10.1016/j.jviromet.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 8.Kuebler D., Illingworth A., Blenc A., Wilbur D. A peer comparison program for the quality assurance of human papillomavirus DNA detection using the Digene Hybrid Capture II/SurePath method shows excellent analytic interlaboratory correlation. Cancer. 2007;111(5):339–343. doi: 10.1002/cncr.22951. [DOI] [PubMed] [Google Scholar]
- 9.Siddiqi A., Spataro M., McIntire H., Akhtar I., Baliga M., Flowers R. Hybrid Capture 2 human papillomavirus DNA testing for women with atypical squamous cells of undetermined significance Papanicolaou results in SurePath and ThinPrep specimens. Cancer Cytopathol. 2009;117(5):318–325. doi: 10.1002/cncy.20043. [DOI] [PubMed] [Google Scholar]
- 10.Hardie A., Moore C., Patnick J., Cuschieri K., Graham C., Beadling C. High risk HPV detection in specimens collected in SurePath preservative fluid: comparison of ambient and refrigerated storage. Cytopathology. 2009;20(4):235–241. doi: 10.1111/j.1365-2303.2009.00661.x. [DOI] [PubMed] [Google Scholar]
- 11.Preisler S., Rebolj M., Untermann A., Ejegod D.M., Lynge E., Rygaard C. Prevalence of human papillomavirus in 5,072 consecutive cervical SurePath samples evaluated with the Roche Cobas HPV real-time PCR assay. PLoS One. 2013;8(3):e59765. doi: 10.1371/journal.pone.0059765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuzick J., Ahmad A.S., Austin J., Cadman L., Ho L., Terry G. A comparison of different human papillomavirus tests in PreservCyt versus SurePath in a referral population– Predictors 4. J. Clin. Virol. 2016;82:145–151. doi: 10.1016/j.jcv.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.TriPath Imaging. Comments on the FDA review of Digene’s PMA application [press release] Burlington NC: TriPath Imaging; July 10, 2002.
- 14.Naryshkin S., Austin R.M. Limitations of widely used high-risk human papillomavirus laboratory-developed testing in cervical cancer screening. Drug, Healthc. Patient Saf. 2012;4:167–172. doi: 10.2147/DHPS.S37273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roche Molecular Diagnostics, Roche Announces Expanded CE Mark Indication for cobas® 4800 HPV Test Supporting Its Use as a Primary Screen for Cervical Cancer [Press Release], 2012. Retrieved from: 〈http://molecular.roche.com/News/LocalNews/Pages/CEmarkcobas4800HPVTestPrimaryScreeningCervicalCancer.aspx〉.
- 16.Moelans C.B., Oostenrijk D., Moons M.J., van Diest P.J. Formaldehyde substitute fixatives: effects on nucleic acid preservation. J. Clin. Pathol. 2011;64(11):960–967. doi: 10.1136/jclinpath-2011-200152. [DOI] [PubMed] [Google Scholar]
- 17.Powell N., Smith K., Fiander A. Recovery of human papillomavirus nucleic acids from liquid-based cytology media. J. Virol. Methods. 2006;137(1):58–62. doi: 10.1016/j.jviromet.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 18.Dixon E.P., King L., Adams M.D., Gronn P., Murphy P.G., Brown C.A. Isolation of RNA from residual BD SurePath™ liquid-based cytology specimens and detection of HPV E6/E7 mRNA using the PreTect™ HPV-proofer assay. J. Virol. Methods. 2008;154(1–2):220–222. doi: 10.1016/j.jviromet.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Murphy P.G., Henderson D.T., Adams M.D., Horlick E.A., Dixon E.P., King L.M. Isolation of RNA from cell lines and cervical cytology specimens stored in BD SurePath™ preservative fluid and downstream detection of housekeeping gene and HPV E6 expression using real time RT-PCR. J. Virol. Methods. 2009;156(1–2):138–144. doi: 10.1016/j.jviromet.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 20.Chernesky M., Gilchrist J., Jang D., Toor R., Schroder A., Dockter J. EUROGIN; Prague, Czech Republic: 2012. Effect of Proteinase K Treatment of SurePath L-Pap Samples on detection of HPV mRNA. [Google Scholar]
- 21.Dockter J., Schroder A., Hill C., Guzenski L., Monsonego J., Giachetti C. Clinical Performance of the APTIMA HPV Assay for the Detection of High-Risk HPV and High-Grade Cervical Lesions. J. Clin. Virol. 2009;45(Suppl. 1):S55–S61. doi: 10.1016/S1386-6532(09)70009-5. [DOI] [PubMed] [Google Scholar]
- 22.Clad A., Reuschenbach M., Weinschenk J., Grote R., Rahmsdorf J., Freudenburg N. Performance of the aptima high-risk human papillomavirus mRNA assay in a referral population in comparison with hybrid capture 2 and cytology. J. Clin. Microbiol. 2011;49(3):1071–1076. doi: 10.1128/JCM.01674-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ratnam S., Coutlee F., Fontaine D., Bentley J., Escott N., Ghatage P. Aptima HPV E6/E7 mRNA test is as sensitive as Hybrid Capture 2 assay but more specific at detecting cervical precancer and cancer. J. Clin. Microbiol. 2011;49(2):557–564. doi: 10.1128/JCM.02147-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arbyn M., Roelens J., Cuschieri K., Cuzick J., Szarewski A., Ratnam S. The Aptima HPV assay versus the hybrid capture 2 test in triage of women with ASC-US or LSIL cervical cytology: a meta-analysis of the diagnostic accuracy. Int. J. Cancer. 2013;132(1):101–108. doi: 10.1002/ijc.27636. [DOI] [PubMed] [Google Scholar]
- 25.B. Eaton, M. Telebrico, D. Getman, J. Reid, J. Dockter, Risk of disease on an ASC-US population evaluated with the Aptima HPV 16, 18/45 assay and the cobas HPV test, in Proceedings of the 28th International Papillomavirus (IPV) Conference; Nov. 30-Dec. 06, 2012, San Juan, PR, 2012.
- 26.Castle P.E., Eaton B., Reid J., Getman D., Dockter J. Comparison of human papillomavirus detection by Aptima HPV and cobas HPV tests in a population of women referred for colposcopy following detection of atypical squamous cells of undetermined significance by Pap cytology. J. Clin. Microbiol. 2015;53(4):1277–1281. doi: 10.1128/JCM.03558-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material