Abstract
Background
In Finland a vaccination programme against human papillomavirus (HPV) was introduced in November 2013 for girls aged 11–12 years with a catchup for girls 13–15 years. Allegations that HPV vaccine is causing Guillain Barré syndrome (GBS) and non-specific diagnostic entities, such as chronic fatigue syndrome/systemic exertion intolerance disease (CFS/SEID) and postural orthostatic tachycardia syndrome (POTS), continue to surface. We examined population register-based incidence rates of CFS/SEID, GBS and POTS to provide baseline data for future HPV vaccine safety evaluations.
Methods
First diagnosis of CFS/SEID, GBS and POTS in girls aged 11–15 years were obtained from the National Hospital Discharge Register during 2002–2012. We considered the following ICD-10 codes: G93.3 for CFS; G61.0 for GBS and G90.9, G90.8, G93.3, I49.8 for POTS. We calculated incidence rates per 100,000 person-years with 95% confidence intervals (CI).
Results
In total, 9 CFS/SEID, 19 GBS and 72 POTS cases were identified. The overall incidence rate was 0.53/100,000 (95% CI; 0.27–1.01) for CFS/SEID, 1.11 (95% CI; 0.71–1.74) for GBS and 4.21 (95%CI; 3.34–5.30) for POTS. Significant relative increase in annual incidence rate with a peak in 2012 was observed in CFS/SEID (33% (95% CI; 3.0–70.3: p=0.029) and POTS (16.5% (95% CI; 7.8–25.9: p<0.05), but not in GBS (5.4% (95% CI; −8.4–21.3: p=0.460).
Conclusions
Our findings provide baseline estimates of CFS/SEID, GBS and POTS incidences in Finland. However, rates based on register data should be interpreted with caution, especially for non-specific diagnostic entities for which internationally and even nationally agreed criteria are still being discussed. To assess the associations with HPV vaccine, methods using register linkage for cohort and self-controlled case series should be explored in addition to factors contributing to patients seeking care, treating physicians setting the diagnoses, and their preference of using of codes for these clinical entities.
Abbreviations: WHO, World Health Organization; HPV, human papillomavirus; AEFI, Adverse Events Following Immunization; GBS, Guillain Barré syndrome; CFS/SEID, Chronic fatigue syndrome/Systemic exertion intolerance disease; POTS, Postural orthostatic tachycardia syndrome; CRPS, Complex regional pain syndrome; ICD-10, International Classification of Diseases, 10th Revision; NIP, National Immunization Programme; HILMO, National Hospital Discharge Register; THL, National Institute for Health and Welfare; GACVS, WHO Global Advisory Committee on Vaccine Safety; EMA, European Medicines Agency; PRAC, Pharmacovigilance Risk Assessment Committee
Keywords: Papillomavirus vaccines, Vaccination adverse effects, Incidence rates, Guillain Barré syndrome, Chronic fatigue syndrome/Systemic exertion intolerance disease, Postural orthostatic tachycardia syndrome, Finland
Highlights
-
•
CFS/SEID, GBS and POTS can be found in all distinct age- and sex-related diagnostic entities.
-
•
Significant increase in CFS/SEID and POTS but not in GBS rates in years before HPV immunization.
-
•
Rates based on register data should be interpreted with caution, especially for non-specific diagnostic entities.
-
•
Factors contributing to clinicians setting the diagnoses and using different ICD-10 codes should be explored.
1. Introduction
The World Health Organization (WHO) recommends the human papillomavirus (HPV) vaccine as part of a coordinated strategy to prevent cervical cancer and other HPV-related diseases [1]. In Finland, the quadrivalent HPV (Gardasil) and bivalent HPV (Cervarix) vaccines were centrally authorized on the market based on their immunogenicity and acceptable safety profile [2], [3]. Since November 2013, Cervarix vaccine is included in the Finnish National Immunization Programme (NIP). It is routinely offered in three doses schedule free of charge via school-based programs for girls 11–12 years of age, with catch-up during the first two years of the programme among girls 13–15 years of age. According to the national vaccination register data until 13 June 2016, 339,803 HPV vaccines were administered within the NIP, with a mean of 66% coverage for the first dose with considerable regional variation [4]. Before this large scale use in NIP, HPV-vaccines were available for immunization on girls/ parents’ own initiative at own cost since 2006, but only approximately 30,000 doses were given prior to November 2013 and approximately 40,000 doses as part of a series of clinical trials [5].
HPV vaccination recommendations in the product label are based on analyses of clinical trials [6], [7], [8], [9] and the post-marketing periodic safety update reports (PSURs), which are required from marketing authorization holders. In the European Union, holders need to submit these to a central repository for PSURs [10]. To date the available data demonstrated acceptable safety of HPV vaccines [11], [12]. Nevertheless, concerns continue to surface around the world about possible adverse events following immunization (AEFI), including complex regional pain syndrome (CRPS), Guillain Barré syndrome (GBS), chronic fatigue syndrome/systemic exertion intolerance disease (CFS/SEID) and postural orthostatic tachycardia syndrome (POTS).
To detect vaccine safety alerts and signals, the National Institute for Health and Welfare in Finland (THL) maintains a register of AEFIs [13]. Until 31 May 2016 a total of 223 notifications have been reported to the register related to HPV vaccines, including 1 GBS and 1 POTS [14]. To further evaluate the associations and potential causality of these safety signals to vaccinations, THL uses different epidemiological methods, including population-based register data linkage studies.
In order to understand the association between a vaccine and the safety signal, assessment of the baseline rates of the suspected signal diseases in the target population for a specific product is of key importance. Comparisons of rates before and after vaccine introduction as well as a comparison among those vaccinated and non-vaccinated are needed for any hypotheses testing of possible AEFI [15]. The objective of this study was to establish the baseline age- and sex-specific incidence rates of GBS, CFS/SEID and POTS before HPV NIP was initiated in 2013 in Finland for further assessment of HPV vaccine safety among girls aged 11–15 years.
2. Methods
The study population was the complete population (average population of 5,300,000) with permanent residence in Finland with a focus on the target group, i.e. females of age 11–15 years between 2002 and 2012 registered in the Finnish Population Registry. We obtained the information on the CFS/SEID, GBS and POTS diagnoses from the National Hospital Discharge Register (HILMO), which since 1996 contains comprehensive individual level healthcare records on inpatients and since 1998 also outpatient visits provided by all hospitals in Finland. For each hospitalization and hospital outpatient visit, reporting of data to HILMO is mandatory. The basic variables in the register include personal identity number, area of residence, admission and discharge dates, outpatient visit dates and diagnoses of patients.
Since 1996, the discharge diagnoses are reported as International Classification of Diseases version 10 (ICD-10) codes. In the analysis we used the first registered ICD-10 code episode for each patient and we considered the following codes: G93.3 for CFS/SEID cases; G61.0 for GBS cases and G90.9, G90.8, G93.3, I49.8 for POTS cases. We calculated sex- and age-specific incidence rates per 100,000 person-years with 95% confidence intervals (CI) by dividing the number of CFS/SEID, GBS and POTS cases in each sex/age category by the total number of person years at risk in the same category. We estimated a relative increase in incidence rate per year by Poisson regression. For the data analysis we used the Stata software package, Version 14 (USA: StataCorp LP).
3. Results
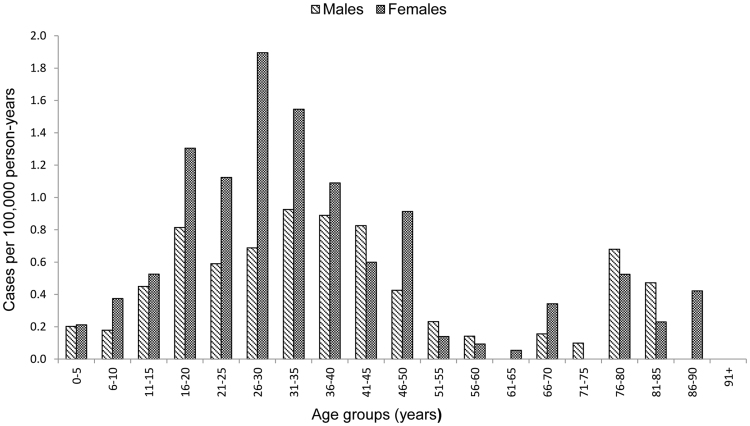
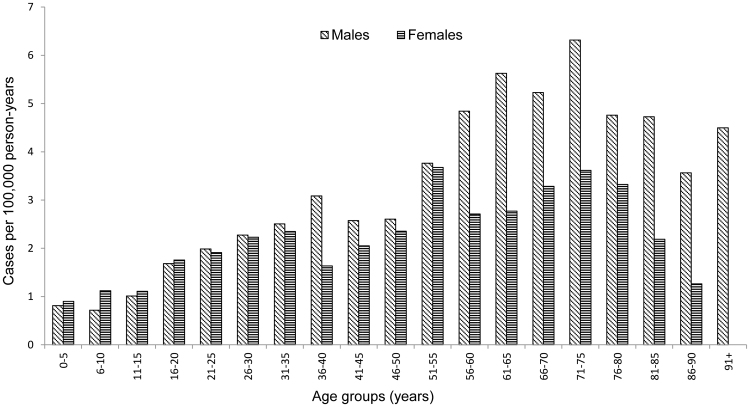
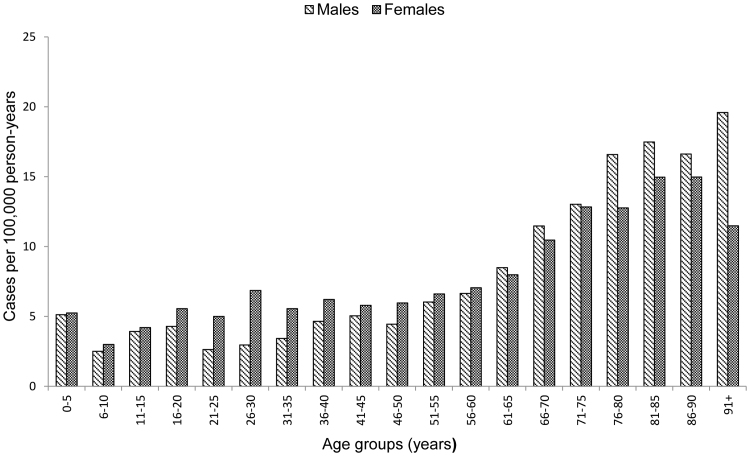
A total of 58,335 738 person-years for females and males in all age-groups in overall population of Finland, 3490 972 person-years for females and males of age 11–15 years and 1710 376 person-years for females of age 11–15 years were included in the analysis in the study period years 2002–2012. A total of 327 CFS/SEID, 1511 GBS and 3788 POTS cases were identified overall. The average annual incidence rate was 0.56/100,000 (95% CI; 0.50–0.62) for CFS/SEID, 2.59 (95% CI; 2.46–2.72) for GBS and 6.49 (95%CI; 6.29–6.70) for POTS. The average annual CFS/SEID incidence rate was higher among females than males with a peak observed among young adults in age group of 26–30 years (Fig. 1). The GBS rate increased with age and was higher among males with increasing variation between females and males from younger to the older age groups (Fig. 2). The POTS rate was highest in older age groups with higher rates among females in younger groups and higher rates among males in older groups above 61 years of age (Fig. 3).
Fig. 1.
Average annual incidence rate of chronic fatigue syndrome/systemic exertion intolerance disease (CFS/SEID) by age groups and sex during 2002–2012 in Finland.
Fig. 2.
Average annual incidence rate of Guillain Barré syndrome (GBS) by age groups and sex during 2002–2012 in Finland.
Fig. 3.
Average annual incidence rate of postural orthostatic tachycardia syndrome (POTS) by age groups and sex during 2002–2012 in Finland.
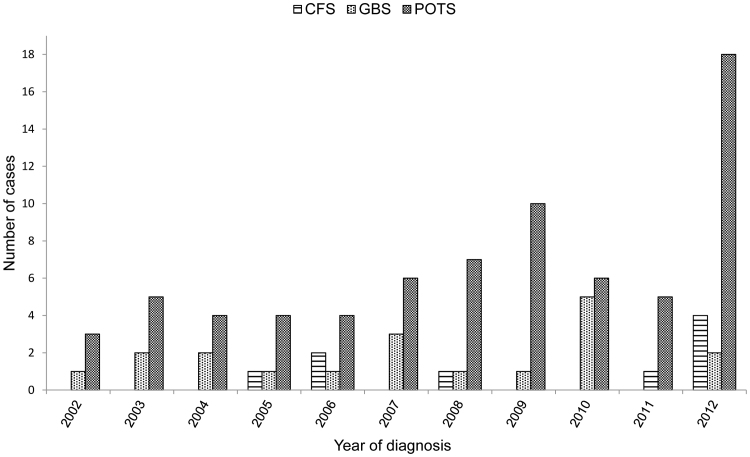
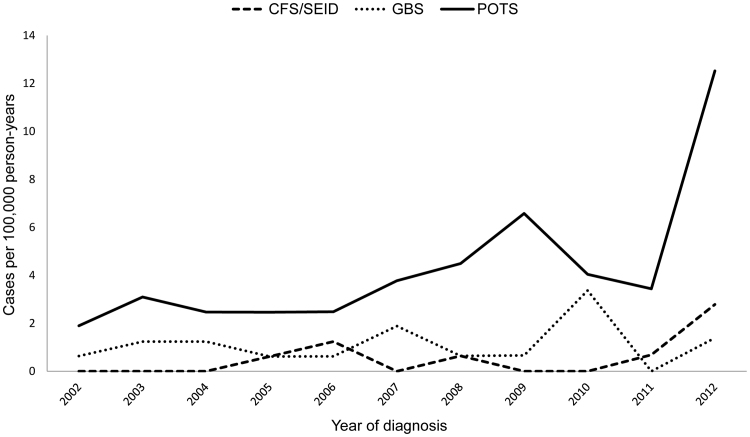
Fig. 4 shows the annual number of CFS/SEID, GBS and POTS cases among girls of age 11–15 years. In total, 9 CFS/SEID, 19 GBS and 72 POTS cases were identified during 2002–2012. Significant increase of POTS cases (n=18) and CFS/SEID (n=4) was observed in 2012.
Fig. 4.
Chronic fatigue syndrome/systemic exertion intolerance disease (CFS/SEID), Guillain Barré syndrome (GBS) and postural orthostatic tachycardia syndrome (POTS) in girls of age 11–15 years during 2002–2012 in Finland.
Fig. 5 shows the annual incidence rates of CFS/SEID, GBS and POTS among girls of age 11–15 years. During 2002–2012, a significant relative increase in annual incidence rate with a peak in 2012 was observed in CFS/SEID (33% (95% CI; 3.0–70.3: p=0.029) and POTS (16.5% (95% CI; 7.8–25.9: p<0.05), but not seen in GBS (5.4% (95% CI; −8.4–21.3: p=0.460). The annual incidence rate during years 2002–2011 compared to rate in 2012 varied for CFS/SEID from 0.32 (95% CI: 0.13–0.77) to 2.78 (95% CI: 1.04–7.41) respectively; for POTS from 3.45 (95% CI: 2.64–4.50) to 12.52 (95% CI: 7.89–19.87) respectively; with minor variation for GBS: 1.15 (95% CI: 0.72–1.82) during 2002–2011 vs. 1.39 (95% CI: 0.35–5.56) in 2012. The annual incidence rate during 2002–2012 was 0.53 (95%CI: 0.27–1.01) for CFS/SEID, 1.11 (95%CI: 0.71–1.74) for GBS, and 4.21 (95%CI; 3.34–5.30) for POTS.
Fig. 5.
Annual incidence rate of chronic fatigue syndrome/systemic exertion intolerance disease (CFS/SEID), Guillain Barré syndrome (GBS) and postural orthostatic tachycardia syndrome (POTS) in girls of age 11–15 years during 2002–2012 in Finland.
4. Discussion
This study assessed the register-based baseline rates of GBS, CFS/SEID and POTS over a period from 2002 to 2012 among the permanent population in Finland, including the adolescent girls of age 11–15 years. We identified the GBS, CFS/SEID and POTS in all distinct age- and sex-related diagnostic entities and a significant increase in CFS/SEID and POTS, but not in GBS among girls of age 11–15 years before HPV vaccines became available in the NIP in late 2013.
The GBS results from our study show that the overall GBS rates of 2.59 in Finland exceeds the normally reported rates of 0.89–1.89 in the Western countries [16], but not 0.16–3.0 per 100,000 person-years world estimates [17]. However, the reported GBS incidences worldwide vary considerably between the countries [16], [17]. Our findings on baseline GBS data were consistent with those demonstrated in published studies, i.e. an increase in incidence with advancing age, a higher risk of GBS among males [16] and secular trends indicating no significant increase or decrease [18]. A constant trend might be due to the fact that GBS is a well-known diagnostic entity. The GBS has been associated with several bacterial and viral infections, most recently e.g. Zika virus, where the cause-effect relation has been established [19], but though often associated, has not been established for vaccinations, e.g. influenza vaccine [20], [21], [22]. Very few pharmacoepidemiologic studies have evaluated the GBS association with the HPV vaccination and show contradictory results. A large Scandinavian study conducted in Denmark and Sweden, comparing almost 300,000 cases and 700,000 controls, found no increased risk for GBS following HPV vaccination [12]. A recent large observational study conducted in France, which compared unvaccinated to vaccinated 13–16 years old girls in a 2.2 million cohort, was the first study to have found association between the HPV vaccine and the GBS [23].
Our findings on the CFS/SEID slightly differed from a Norwegian study [24], which is to date the largest register-based study on CFS/SEID and the only study describing the distribution by both, sex and age. Contrary to our study, they found a fairly stable overall incidence rates over the years. There have been several attempts in the past to update case definition and diagnostic criteria for CFS/SEID [25]. It may be that the increased awareness among clinicians about CFS/SEID has led to increasing ascertainment and coding of these syndromes in Finland. While we observed an increasing trend in CFS/SEID during the years of this study (2002–2012), other diagnostic entities with overlapping symptoms, such as unspecified rheumatism, burn-out, and neurasthenia, had a decreasing trend during the same time period (data not shown). The increase in CFS/SEID and decrease of other codes describing similar symptoms during the same time period might be coincidental, but it is also possible that the CFS/SEID coding became increasingly used among physicians replacing the other previously used codes. The Norwegian study also found a much higher overall rate compared to our study (25.8 vs. 0.56 respectively), although their ICD-10 code used for the CFS/SEID case-search was the same as in our study (G93.3) and only first registered diagnosis was used in the analysis [24]. However, they also included the diagnoses assigned in the outpatient clinics, which might be a major reason for the differences with our rates, as HILMO does not capture information on the outpatient clinic visits done outside hospital. A higher incidence in women than in men is consistent in both studies, although the Norwegians found a peak of all cases in the age group 10–19 years and 30–39 years vs. the peak in age group 26–30 years and not observed among the 11–15 years old girls in our study. Their estimated incidence rate among women of age 10–14 years was approximately 60 compared to 0.53 among 10–15 years old girls in our study [24]. CFS/SEID has been associated with preceding infections [26] and vaccines, such as the pandemic influenza [26] and the HPV vaccination, but the association between the CFS/SEID with HPV vaccines has not been confirmed [27].
As in CFS/SEID, we observed an increase in POTS before the HPV vaccination was implemented in the NIP. POTS have been alleged as an adverse effect of HPV vaccine in Japan [28], [29], US, [30] Denmark [31] and other countries [32]. In July 2015, Denmark requested the European Medicines Agency's (EMA) Pharmacovigilance Risk Assessment Committee's (PRAC) for a safety review of HPV vaccines in terms of a causal link between POTS and HPV vaccinations [32]. After a careful review in November 2015, the EMA concluded that the benefits of HPV vaccinations outweigh the potential risks, and stated that POTS and complex regional pain syndrome (CRPS) in vaccinated girls is no higher than would be expected in girls in the general population [11]. Although the WHO Global Advisory Committee on Vaccine Safety (GACVS) in December 2015 also concluded that the benefit / risk of HPV vaccines is acceptable and that there is no reason to alter the recommendations for the use [33], the EMA´s conclusion was scientifically critiqued [34], [35]. It seems challenging to assess the association for both CFS/SEID and POTS given that clinical diagnostic criteria are not yet well-established, diagnosing is difficult and symptoms overlap between POTS and CFS/SEID [33]. Several factors, such as public confidence in vaccine safety, care seeking behavior, response of the media, anti-vaccine groups, governments and the medical profession could have influence on the increasing number of reported AEFIs and decreasing number of the vaccinated. Safety concerns have been identified as one of the important factors for low HPV vaccine uptake [36] and public controversy has led to decrease of HPV vaccination coverage for countries such as France [23], Denmark, [35] and Japan [28]. In France, the public controversy around the risks considerably decreased the full HPV vaccination coverage among 15–17 years’ girls from 29.9% in 2007 to 17% in 2011 (23). Controversy over suspected AEFIs of the HPV vaccines may have consequences even today. France is the country least confident in vaccines safety, with 41% of surveyed (more than three times the global average of 12%) disagreeing that vaccines are safe. (36) Japan is not far behind, with 25% of surveyed thinking that vaccines are not safe. (36) Even in the absence of any evidence of association between HPV vaccination and POTS and CRPS that emerged after HPV vaccination in Japan, the anti-vaccine groups, medical community, and social media aggravated public skepticism in vaccination and fears of AEFIs. Consequently, the Health Ministry suspended HPV vaccination in the NIP [28]. It is a question if a public with a low trust in vaccine safety reports more AEFIs, as well as how much influence the media has on heightened surveillance and awareness of AEFIs among the clinicians. In Finland, although the vaccination is part of the NIP, it remains voluntary and many parents are carefully considering whether their daughters should get the HPV shot after the narcolepsy scandal around the swine flu vaccine Pandemrix between 2009 and 10 [5], [37]. Skepticism caused by the Pandemrix might have influenced reporting of AEFIs. The results of this study can be subject to multiple interpretations. The observed increase of POTS before the HPV vaccination was introduced in NIP might be true, but it could also be due to multiple underlying contributing factors, such as increased awareness, and changing trends in the preferred use of ICD-10 codes among the clinicians. This is the first study in Finland on the baselines of GBS, CFS/SEID and POTS that can strengthen HPV vaccine safety assessment, and can help in providing prompt answers to concerns raised by public. This study gives register-based baseline rates for further assessment of HPV safety signals because simply comparing number of AEFIs in pre- and post-vaccination period can be misinterpreted if not taking above mentioned various factors in mind.
The limitation of this hospital register-based study is that the number CFS/SEID and POTS cases are likely to be under-reported as these two syndromes are often not diagnosed or misdiagnosed. The diagnoses in our study were also not validated by the review of the patient records, but will be reviewed in the framework of a future HPV vaccine safety study. Our study includes all information on inpatients and outpatient visits in hospitals, thus one of the limitations is that HILMO does not include the outpatient primary care visits, nor the visits in private health care. Since 2011, outpatient primary care visits are recorded in another National Register of Primary Health Care Visits (AvoHILMO), using both, the ICD-10 and the International Classification of Primary Care (ICPC-2) codes indicating a reason for the visit. We might have missed the less severe GBS, CFS/SEID and POTS from the primary care, but due to severity of these entities, which can mostly occur in the inpatient setting [18], and due to very few private health-care providers in Finland, we believe that we captured majority of severe cases from HILMO. To better understand the severity of these diagnoses, we could however further distinct between inpatient and outpatient visits in hospitals, as outlined in a Danish study [18].
5. Conclusion
Registry-based data can provide an important adjunct to clinical trial safety data collection, especially for rare diagnostic entities. These baseline rates must be considered with caution, however, when comparing pre-with post-vaccination data for assessing vaccine safety; especially for non-specific CFS/SEID and POTS diagnostic entities for which internationally and even nationally agreed criteria are still being formed. We hypothesize that clinicians’ increased awareness about POTS and CFS/SEID has led to increasing ascertainment and coding of these syndromes. To assess the associations with HPV vaccines, further analysis by register based linkage studies is needed, i.e. linking the national vaccination register and HILMO, as well as the collaboration with the neurologists and pediatric infectious disease specialists to further understand the access to care and diagnostics of these clinical entities as well as their preferred coding.
Authors’ contribution
JS participated in data analysis, data interpretation and drafted the manuscript. JO participated in data analysis. ER participated in data extraction from the national database. OL participated in reviewing the manuscript. HN participated in study conceptualization, data interpretation and reviewing the manuscript. All authors critically reviewed the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
None of the authors have any competing interests.
Acknowledgement
The authors would like to thank Dr. Alicia Barrasa Blanco, EPIET Scientific Coordinator, for reviewing the manuscript and Ms Rebecca Baiada for proofreading the manuscript.
Contributor Information
J. Skufca, Email: josie.skufca@gmail.com.
J. Ollgren, Email: jukka.ollgren@thl.fi.
E. Ruokokoski, Email: esa.ruokokoski@thl.fi.
O. Lyytikäinen, Email: outi.lyytikainen@thl.fi.
H. Nohynek, Email: hanna.nohynek@thl.fi.
References
- 1.World Health Organization Human papillomavirus vaccines: WHO position paper. Wkly. Epidemiol. Rec. 2014;43(89):465–492. [PubMed] [Google Scholar]
- 2.Paavonen J., Jenkins D., Bosch X., Naud P., Salmeron J., Wheeler C. Efficacy of a prophylactic adjuvanted L1 VLP vaccine against infection with HPV16 and 18 in young women: an interim analysis of a phase III double-blind, randomized controlled trial. Lancet. 2007;369:2161–2170. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 3.Villa L., Perez G., Kjaer S., Lehtinen M., Paavonen J., Muñoz N. Efficacy of a quadrivalent HPV types 8/11/16/18) L1 virus-like particle vaccine in the prevention of cervical intraepithelial neoplasia grades 2/3 and adenocarcinoma in situ: a randomized controlled trial. N. Engl. J. Med. 2007;356:1915–1927. [Google Scholar]
- 4.National Institute for Health and Welfare (THL), Department of Vaccination and Immune Protection. HPV vaccination coverage in Finland; 2016 . (Accessed 11 April 2016).
- 5.Finnish Medicines Agency (Fimea). Question E 9/2015 from the Nordic Council regarding adverse reactions to HPV-vaccine. Contribution from Finland to the question from the Nordic Council; 3 Nov 2015.
- 6.D. Descamps, K. Hardt, B. Spiessens, et al. Safety of human papillomavirus (HPV)-16/18 AS04 adjuvanted vaccine for cervical cancer prevention: integrated summary of 11 clinical trials. 26th Annual Meeting of the European Society for Pediatric Infectious Diseases (ESPID), Austria: Graz; 13–16 May, 2008.
- 7.Block S.L., Brown D.R., Chatterjee A. Clinical trial and post-licensure safety profile of a prophylactic human papillomavirus (types 6, 11, 16, and 18) l1 virus-like particle vaccine. Pediatr. Infect. Dis. J. 2010;29:95–101. doi: 10.1097/INF.0b013e3181b77906. [DOI] [PubMed] [Google Scholar]
- 8.Angelo M.G., Zima J., Tavares Da Silva F., Baril L., Arellano F. Post-licensure safety surveillance for human papillomavirus-16/18-AS04-adjuvanted vaccine: more than 4 years of experience. Pharmacoepidemiol. Drug Saf. 2014;23:456–465. doi: 10.1002/pds.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macartney K.K., Chiu C., Georgousakis M., Brotherton J.M. Safety of human papillomavirus vaccines: a review. Drug Saf. 2013;36(6):393–412. doi: 10.1007/s40264-013-0039-5. [DOI] [PubMed] [Google Scholar]
- 10.European Medicines Agency (EMA), Periodic safety update reports, 〈http://www.ema.europa.eu/ema/index.jsp?Curl=pages/regulation/document_listing/document_listing_000361.jsp〉 ; 2016. (Accessed 21 June 2016).
- 11.European Medicines Agency (EMA), Assessment report. Human papillomavirus (HPV) vaccines, 〈http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/HPV_vaccines_20/Opinion_provided_by_Committee_for_Medicinal_Products_for_Human_Use/WC500197129.pdf〉; Nov 11 2015. (Accessed 11 May 2016).
- 12.Arnheim-Dahlström L., Pasternak B., Svanström H., Sparén P., Hviid A. Autoimmune, neurological, and venous thromboembolic adverse events after immunisation of adolescent girls with quadrivalent human papillomavirus vaccine in Denmark and Sweden: cohort study. Br. Med. J. 2013;347:f5906. doi: 10.1136/bmj.f5906. (PMID: 24108159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ministry of Social Affairs and Health, Finland. Communicable Diseases Act. No. 583/1986, 〈http://www.finlex.fi/fi/laki/kaannokset/1986/en19860583.pdf〉; 25 Jul 1986 . (Accessed 25 May 2016).
- 14.National Institute for Health and Welfare (THL), Department of Vaccination and Immune Protection. Register of adverse effects of vaccines. April 2016.
- 15.Global Advisory Committee on Vaccine Safety (GACVS), Weekly epidemiological record. No. 7, 2014, 89, 53–60, 〈http://www.who.int/vaccine_safety/committee/reports/wer8907.pdf?ua=1〉; 11–12 December 2013. (Accessed 11 April 2016).
- 16.Sejvar J.J., Baughman A.L., Wise M., Morgan O.W. Population incidence of Guillain-Barré syndrome: a systematic review and meta-analysis. Neuroepidemiology. 2011;36:123–133. doi: 10.1159/000324710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGrogan A., Madle G.C., Seaman H.E., de Vries C.S. The epidemiology of Guillain-Barré syndrome worldwide. A systematic literature review. Neuroepidemiology. 2009;32:150–163. doi: 10.1159/000184748. [DOI] [PubMed] [Google Scholar]
- 18.Callréus T., Svanström H., Nielsen N.M., Poulsen S., Valentiner-Branth P., Hviid A. Human papillomavirus immunisation of adolescent girls and anticipated reporting of immune-mediated adverse events. Vaccine. 2009;27(22):2954–2958. doi: 10.1016/j.vaccine.2009.02.106. [DOI] [PubMed] [Google Scholar]
- 19.Cao-Lormeau V.-M., Blake A., Mons S. Guillain-Barré syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387:1531–1539. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alcalde-Cabero E., Almazán-Isla J., García López F.J. Guillain-Barré syndrome following the 2009 pandemic monovalent and seasonal trivalent influenza vaccination campaigns in Spain from 2009 to 2011: outcomes from active surveillance by a neurologist network, and records from a country-wide hospital discharge database. BMC Neurol. 2016;16:75. doi: 10.1186/s12883-016-0598-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghaderi S., Gunnes N., Bakken I., Magnus P., Trogstad L., Håberg S.E. Risk of Guillain-Barré syndrome after exposure to pandemic influenza A(H1N1)pdm09 vaccination or infection: a Norwegian population-based cohort study. Eur. J. Epidemiol. 2016;31(1):67–72. doi: 10.1007/s10654-015-0047-0. [DOI] [PubMed] [Google Scholar]
- 22.Committee to Review Adverse Effects of Vaccines . Institute of Medicine. In: Stratton K., Ford A., Rusch E., editors. Adverse Effects of Vaccines: Evidence and Causality. National Academies Press (US); Washington (DC): 2011. [PubMed] [Google Scholar]
- 23.French medicines agency (ANSM), Vaccins anti-HPV et risque de maladies auto-immunes: étude pharmaco-épidémiologique - Rapport final - Septembre 2015, 〈file:///C:/Users/jkue/AppData/Local/Temp/Ansm_Gardasil-Hpv2_Rapport_Septembre-2015.pdf〉; 14 Sept 2015.
- 24.Bakken I.J., Tveito K., Gunnes N., Ghaderi S., Stoltenberg C., Trogstad L. Two age peaks in the incidence of chronic fatigue syndrome/myalgic encephalomyelitis: a population-based registry study from Norway 2008–2012. BMC Med. 2014;12:167. doi: 10.1186/s12916-014-0167-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome; Board on the Health of Select Populations; Institute of Medicine. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness. Washington (DC): National Academies Press (US); 〈http://www.ncbi.nlm.nih.gov/books/NBK284897/〉; 10 Feb 2015.
- 26.Magnus P., Gunnes N., Tveito K., Bakken I.J., Ghaderi S., Stoltenberg C. Chronic fatigue syndrome/myalgic encephalomyelitis (CFS/SEID/ME) is associated with pandemic influenza infection, but not with an adjuvanted pandemic influenza vaccine. Vaccine. 2015;33(46):6173–6177. doi: 10.1016/j.vaccine.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 27.Donegan K., Beau-Lejdstrom R., King B. Bivalent human papillomavirus vaccine and the risk of fatigue syndromes in girls in the UK. Vaccine. 2013;31:4961–4967. doi: 10.1016/j.vaccine.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 28.R. Wilson, P. Paterson, J. Chiu, W. Schulz, H. Larson, HPV Vaccination in Japan. The continuing debate and Global Impacts. A Report of the CSIS Global Health Policy Center 2015, 〈http://csis.org/files/publication/150422_Wilson_HPVVaccination2_Web.pdf〉; April 2015. (Accessed 16 May 2016).
- 29.Kinoshita T., Abe R.T., Hineno A., Tsunekawa K., Nakane S., Ikeda S. Peripheral sympathetic nerve dysfunction in adolescent Japanese girls following immunization with the human papillomavirus vaccine. Intern. Med. 2014;53(19):2185–2200. doi: 10.2169/internalmedicine.53.3133. [DOI] [PubMed] [Google Scholar]
- 30.Blitshteyn S. Postural tachycardia syndrome following human papillomavirus vaccination. Eur. J. Neurol. 2014;21(1):135–139. doi: 10.1111/ene.12272. [DOI] [PubMed] [Google Scholar]
- 31.Brinth L.S., Pors K., Theibel A.C., Mehlsen J. Orthostatic intolerance and postural tachycardia syndrome as suspected adverse effects of vaccination against human papilloma virus. Vaccine. 2015;33(22):2602–2605. doi: 10.1016/j.vaccine.2015.03.098. [DOI] [PubMed] [Google Scholar]
- 32.European Medicines Agency, EMA to further clarify safety profile of human papillomavirus (HPV) vaccines. 〈http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/HPV_vaccines_20/Procedure_started/WC500189476.pdf〉; 13 July 2015. (Accessed 16 April 2016).
- 33.Global Advisory Committee on Vaccine Safety (GACVS). Statement on Safety of HPV vaccines, 〈http://www.who.int/vaccine_safety/committee/GACVS_HPV_statement_17Dec2015.pdf;17〉 December 2015 . (Accessed 21 May 2016).
- 34.Nordic Cochrane Centre. Complaint to the European Medicines Agency (EMA) over maladministration at the EMA, 〈http://nordic.cochrane.org/sites/nordic.cochrane.org/files/uploads/ResearchHighlights/Complaint-to-EMA-over-EMA.pdf〉; 26 May 2016. (Accessed 16 June 2016).
- 35.Statens Serum Institute. Annual reports on the Danish childhood vaccination programme, 〈http://www.ssi.dk/English/News/EPI-NEWS/2016/No%2017%20-%202016.aspx〉; 27 April 2016. (Accessed 12 May 2016).
- 36.Larson H.J. The state of vaccine confidence 2016: global insights through a 67-country survey. EBioMedicine. 2016;12:295–301. doi: 10.1016/j.ebiom.2016.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nohynek H., Jokinen J., Partinen M., Vaarala O., Kirjavainen T., Sundman J. ASO3 adjuvanted AH1N1 vaccine associated with an abrupt increase in the incidence of childhood narcolepsy in Finland. PLoS One. 2012;7(3):e33536. doi: 10.1371/journal.pone.0033536. [DOI] [PMC free article] [PubMed] [Google Scholar]