Abstract
MAGNEZIX® CS (Syntellix AG, Hanover, Germany) is a bioabsorbable compression screw made of a magnesium alloy (MgYREZr). Currently there are only two clinical studies reporting on a limited number of elective patients who received this screw in a hallux valgus operation. We applied MAGNEZIX® CS for fixation of distal fibular fracture in a trauma patient who had sustained a bimalleolar fracture type AO 44-B2.3. Clinical course was uneventful, fracture healing occurred within three months. Follow-up X-rays showed a radiolucent area around the implant for some months, yet this radiolucent area had disappeared in the 17-months follow-up X-ray.
Keywords: Magnesium, Bioabsorbable, Compression screw, Osteosynthesis, Ankle fracture
Introduction
A stable screw fixation needs a strong implant. Current implants made of steel or titanium are considered appropriate to reduce motion within the fracture gap to an acceptable range. However implant removal following fracture healing requires a secondary procedure accompanied especially with increased infection risk. Biodegradable materials are an option to overcome this issue. Such implants commonly consist of polymers, which might lack mechanical strength. Polymer degradation works by hydrolysis resulting in acidity, which again might induce phenomena like foreign body reactions [1], [2].
Biodegradable metal having a magnesium basis might be an innovative alternative. Several such alloys have recently been studied in animal experiments [3], [4], [5], [6], [7], [8], [9], [10]. In 2013, the compression screw MAGNEZIX® CS (Syntellix AG, Hanover, Germany) was the first magnesium implant to be approved for application in humans. The implant chemically consists of the alloy MgYREZr. In this case report the screw with a 3.2 mm diameter was used, which is available in lengths between 10 mm and 40 mm (in 2 mm increments) (Fig. 1). Currently there are only three clinical studies on this innovation, reporting about MAGNEZIX® CS fixations of Chevron osteotomies [2], [11], [12]. Comparing the results to a control group fixed with a conventional screw, no disadvantages were identified [2]. Up to now, there is no clinical report available on trauma applications for magnesium implants.
Fig. 1.

The MAGNEZIX® CS resembles a cannulated conventional compression screw. It is made of a completely bioabsorbable magnesium alloy (MgYREZr).
Case history
We report the case of a 43-year-old female horse rider who suffered a bimalleolar ankle fracture during a fall (AO-ASIF 44-A2.3). There was little displacement of the medial malleolus, however the CT scan revealed a notable impression zone and a loose intraarticular fragment. The lateral malleolar fracture consisted of a bony avulsion of the anterior talofibular and the calcaneofibular ligament (Fig. 2). As operative fixation of the medial malleolus seemed appropriate we decided on an additional stabilization of the lateral malleolus, aiming for rapid postoperative mobilization without cast.
Fig. 2.
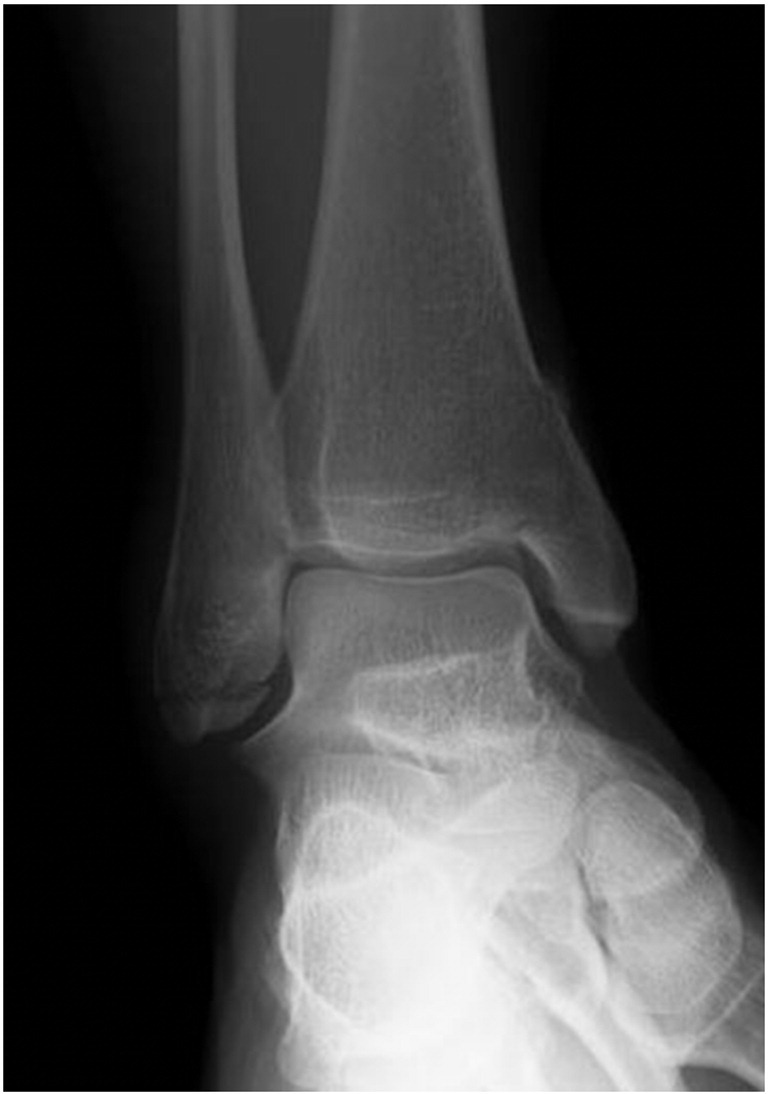
Ankle fracture type AO-ASIF 44-A2.3 in a 43-year-old healthy female.
The procedure was performed under spinal anaesthesia four days after trauma. We performed open reduction and internal fixation (two conventional cortical screws) for the medial malleolus. An osteochondral fragment was removed. The fracture of the lateral malleolus was addressed by a small incision, allowing for reduction and temporary fixation using a 1.5 mm Kirschner wire. Definitive fixation was achieved by a 24 mm long MAGNEZIX® CS 3.2 inserted over a guide wire (Ø 1.2 mm). Operative technique is completely equivalent to that in other cannulated compression screws (drilling Ø 2.5 mm, countersink Ø 3.5 mm, Ø 3.2 mm-screw insertion; all steps cannulated over guide wire).
Postoperative wound healing was uneventful. For mobilization range of motion was unrestricted, and the patient was advised for partial weight-bearing (15 kg). No cast, brace, or other immobilization aid was applied.
Six weeks postoperatively, a notable radiolucent zone appeared around the proximal part of the MAGNEZIX® screw. The fracture had not healed at this time point (Fig. 3), however full weight-bearing was allowed. Three months after trauma, fracture consolidation was confirmed by plain radiography with the radiolucent zone unchanged (Fig. 4). Metal removal at the medial malleolus was performed eight months postoperatively. At that time the radiolucent area had begun to decrease in size, still being detectable around the proximal third of the MAGNEZIX® screw only (Fig. 5).
Fig. 3.
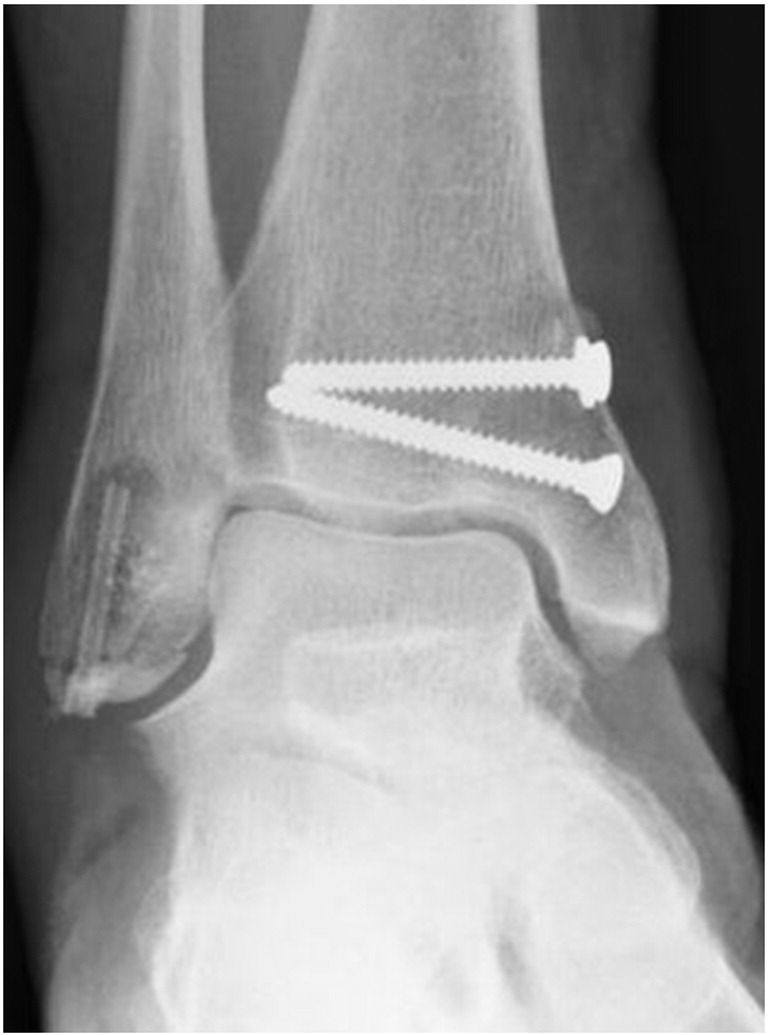
Six weeks postoperatively a radiolucent area occurred around the proximal part of the MAGNEZIX® screw. Fracture consolidation seemed incomplete.
Fig. 4.
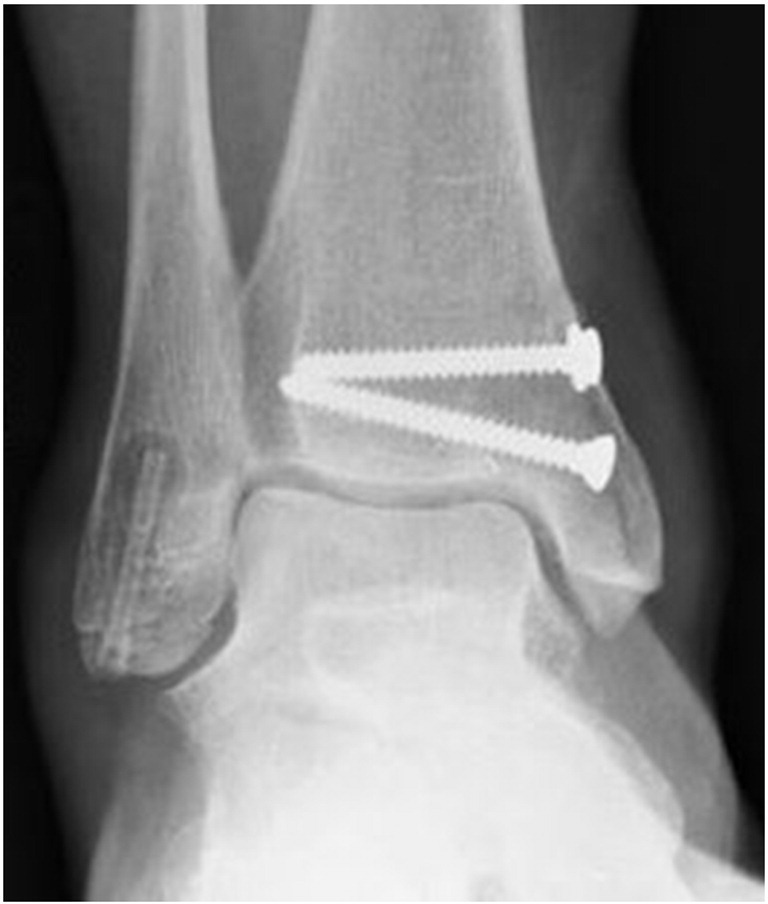
Three months postoperatively the fracture had healed. The radiolucency appears unchanged.
Fig. 5.
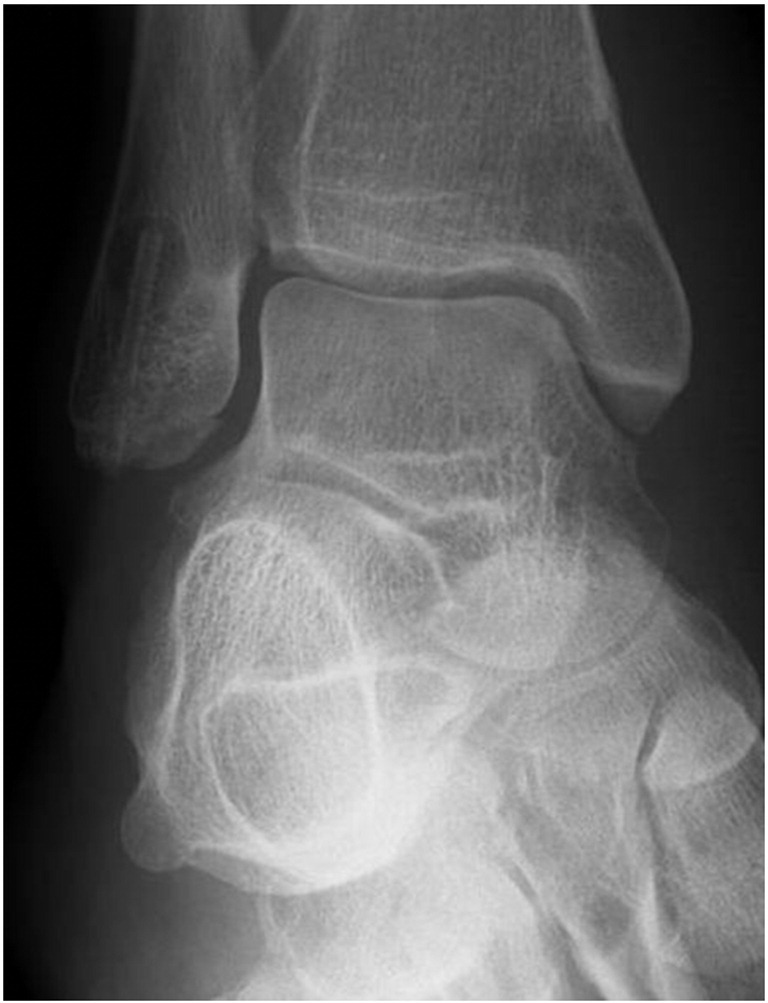
Metal removal was performed eight months postoperatively only at the medial malleolus. The radiolucency at the lateral side was still detectable at this time, however clearly decreasing in size.
At long-term follow-up 17 months later the patient displayed an excellent clinical result with unrestricted range of motion without pain, swelling, or other functional deficits. The radiolucency had nearly completely vanished at that time (Fig. 6).
Fig. 6.
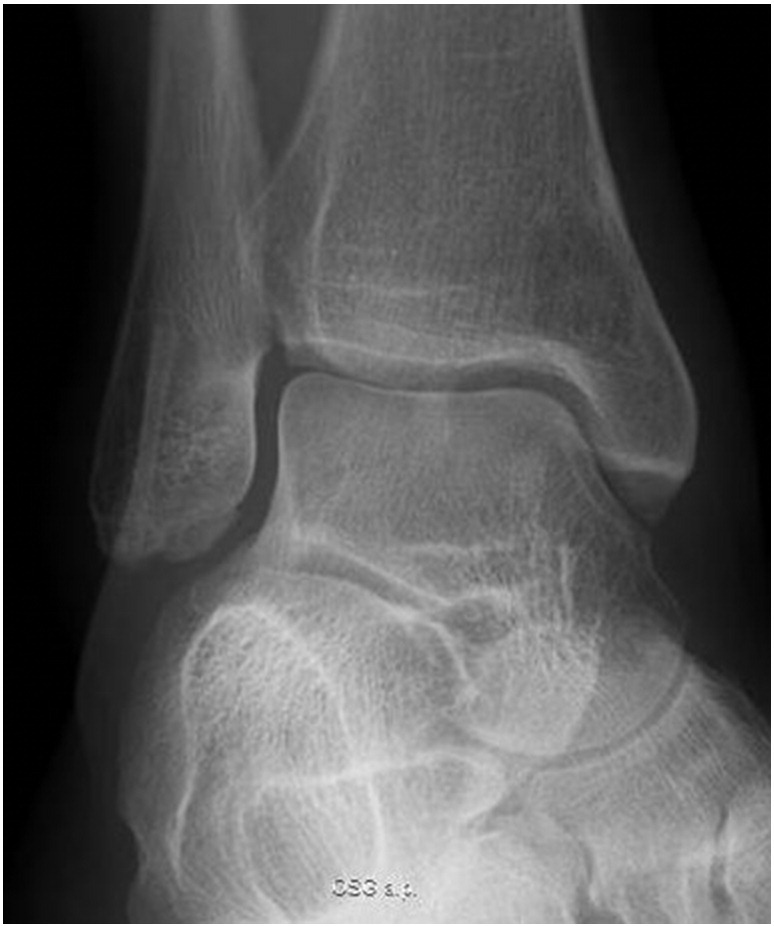
17 months postoperatively the radiolucent area was hardly detectable. Clinical outcome was excellent (ankle score of Olerud/Molander: 95).
Discussion
Metal removal may be challenging especially for small, percutaneously inserted implants. Considerable field damage may occur, thus the decision for implant removal needs to be made cautiously. Usage of biodegradable and nevertheless sufficiently strong metal screws would represent a remarkable advantage regarding this issue. Theoretical applications include all kinds of screw fixation in small bones as well as fixation of small fragments including osteochondral flakes. The MAGNEZIX® screw is approved for these indications in over 20 countries. The manufacturer explicitly recommends this implant for intra- and extraarticular fractures, non-unions, bone fusions, bunionectomies and osteotomies.
Up to now there are three publications on the clinical application of the MAGNEZIX® CS, examining fixation of Chevron-type osteotomies of the first metatarsal bone [2], [11], [12]. Our report is now the first one to deal with its application in the trauma field. After fixation of a distal fibular fracture with a MAGNEZIX® compression screw we observed uneventful healing both clinically and radiologically. A remarkable radiolucency around the implant appeared for several months but did not interfere with bone consolidation and almost disappeared in the late process of healing.
Experimental studies on the MgYREZr alloy proved biocompatibility and osteoconductivity. There seems to be no potential for allergic effects [1]. Several studies on magnesium implants even revealed an osteogenic potential [1], [2], [3].
Although we observed an uneventful consolidation of a malleolar fracture, the radiolucency around the MAGNEZIX® screw seems not to be consistent with the proposed osteoconductivity. A study of Windhagen et al. did not describe such radiolucencies around the Chevron osteotomies [2]. Degradation of MgYREZr is known to produce hydrogen, which can form cavities within the tissue [13]. However, animal experiments with 1-year follow-up indicated no associated bone loss [10]. Ultimately the radiolucency decreased eight months postoperatively in our case. It did not interfere with the fracture area.
Operative technique and handling of the MAGNEZIX® compression screw was completely equivalent to conventional metal screws made of titanium. Although magnesium alloys are generally less stiff than titanium [5], [10], applied torques and intraoperative stability appeared comparable to conventional implants. Degradation studies showed an implant mass reduction of less than 10% during the first six weeks, with the pull-out forces even increasing after four weeks [4], [7]. In animal experiments complete degradation of MgYREZr implants takes about one year [10].
Conclusion
The MAGNEZIX® CS 3.2 (Syntellix AG, Hanover, GERMANY) is a fully degradable implant made of the magnesium alloy MgYREZr. Reports on clinical applications are limited to Chevron osteotomies. For the first time ever we report on a trauma application, using the implant for lateral malleolar fracture fixation in an ankle fracture. The uneventful healing was accompanied by a radiolucency, which formed within six weeks postoperatively and had disappeared after 17 months. This phenomenon has not been reported yet. Further clinical reports are needed, as our finding is not quite consistent with former studies indicating osteoconductive properties of magnesium implants in humans.
Conflict of interest statement
None.
References
- 1.Luthringer B.J., Feyerabend F., Willumeit-Römer R. Magnesium-based implants: a mini-review. Magnes. Res. 2014;27:142–154. doi: 10.1684/mrh.2015.0375. [DOI] [PubMed] [Google Scholar]
- 2.Windhagen H., Radtke K., Weizbauer A., Diekmann J., Noll Y., Kreimeyer U., Schavan R., Stukenborg-Colsman C., Waizy H. Biodegradable magnesium-based screw clinically equivalent to titanium screw in hallux valgus surgery: short term results of the first prospective, randomized, controlled clinical pilot study. Biomed. Eng. Online. 2013;12:62. doi: 10.1186/1475-925X-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bondarenko A., Angrisani N., Meyer-Lindenberg A., Seitz J.M., Waizy H., Reifenrath J. Magnesium-based bone implants: immunohistochemical analysis of peri-implant osteogenesis by evaluation of osteopontin and osteocalcin expression. J. Biomed. Mater. Res. A. 2014;102:1449–1457. doi: 10.1002/jbm.a.34828. [DOI] [PubMed] [Google Scholar]
- 4.Erdmann N., Angrisani N., Reifenrath J., Lucas A., Thorey F., Bormann D., Meyer-Lindenberg A. Biomechanical testing and degradation analysis of MgCa0.8 alloy screws: a comparative in vivo study in rabbits. Acta Biomater. 2011;7:1421–1428. doi: 10.1016/j.actbio.2010.10.031. [DOI] [PubMed] [Google Scholar]
- 5.Erdmann N., Bondarenko A., Hewicker-Trautwein M., Angrisani N., Reifenrath J., Lucas A., Meyer-Lindenberg A. Evaluation of the soft tissue biocompatibility of MgCa0.8 and surgical steel 316L in vivo: a comparative study in rabbits. Biomed Eng. Online. 2010;9:63. doi: 10.1186/1475-925X-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reifenrath J., Angrisani N., Erdmann N., Lucas A., Waizy H., Seitz J.M., Bondarenko A., Meyer-Lindenberg A. Degrading magnesium screws ZEK100: biomechanical testing, degradation analysis and soft-tissue biocompatibility in a rabbit model. Biomed. Mater. 2013;8:045012. doi: 10.1088/1748-6041/8/4/045012. [DOI] [PubMed] [Google Scholar]
- 7.Weizbauer A., Modrejewski C., Behrens S., Klein H., Helmecke P., Seitz J.M., Windhagen H., Möhwald K., Reifenrath J., Waizy H. Comparative in vitro study and biomechanical testing of two different magnesium alloys. J. Biomater. Appl. 2014;28:1264–1273. doi: 10.1177/0885328213506758. [DOI] [PubMed] [Google Scholar]
- 8.Weizbauer A., Seitz J.M., Werle P., Hegermann J., Willbold E., Eifler R., Windhagen H., Reifenrath J., Waizy H. Novel magnesium alloy Mg-2La caused no cytotoxic effects on cells in physiological conditions. Mater. Sci. Eng., C. 2014;41:267–273. doi: 10.1016/j.msec.2014.04.063. [DOI] [PubMed] [Google Scholar]
- 9.Besdo S., Krüger A.K., Rössig C., Helmecke P., Waizy H., Wriggers P., Angrisani N., Reifenrath J. Finite element study of degradable intramedullary nails out of magnesium alloy for ovine tibiae. Biomed. Tech. 2013;58(Suppl. 1) doi: 10.1515/bmt-2013-4073. [DOI] [PubMed] [Google Scholar]
- 10.Waizy H., Diekmann J., Weizbauer A., Reifenrath J., Bartsch I., Neubert V., Schavan R., Windhagen H. In vivo study of a biodegradable orthopedic screw (MgYREZr-alloy) in a rabbit model for up to 12 months. J. Biomater. Appl. 2014;28:667–675. doi: 10.1177/0885328212472215. [DOI] [PubMed] [Google Scholar]
- 11.Plaass C., Modrejewski C., Ettinger S., Noll Y., Claassen L., Daniilidis K., Belenko L., Windhagen H., Stukenborg-Colsman C. Short-term results after distal metatarsal osteotomies for hallux valgus, using a biodegradable magnesium-implant. Fuß & Sprunggelenk. 2015;13:156–161. [Google Scholar]
- 12.Plaass C., Ettinger S., Sonnow L., Koenneker S., Noll Y., Weizbauer A., Reifenrath J., Claassen L., Daniilidis K., Stukenborg-Colsman C., Windhagen H. Early results using a biodegradable magnesium screw for modified chevron osteotomies. J. Orthop. Res. 2016 doi: 10.1002/jor.23241. http://dx.doi.org/10.1002/jor.23241 published online. [DOI] [PubMed] [Google Scholar]
- 13.Staiger M.P., Pietak A.M., Huadmai J., Dias G. Magnesium and its alloys as orthopedic biomaterials: a review. Biomaterials. 2006;27(9):1728–1734. doi: 10.1016/j.biomaterials.2005.10.003. [DOI] [PubMed] [Google Scholar]


