Abstract
Background
The immunogenicity profile of the 9-valent HPV (9vHPV) vaccine was evaluated across five phase III clinical studies conducted in girls and boys 9–15 years of age and young women 16–26 years of age. The effect of baseline characteristics of subjects on vaccine-induced HPV antibody responses was assessed.
Methods
Immunogenicity data from 11,304 subjects who received ≥1 dose of 9vHPV vaccine in five Phase III studies were analyzed. Vaccine was administered as a 3-dose regimen. HPV antibody titers were assessed 1 month after dose 3 using a competitive Luminex immunoassay and summarized as geometric mean titers (GMTs). Covariates examined were age, gender, race, region of residence, and HPV serostatus and PCR status at day 1.
Results
GMTs to all 9 vaccine HPV types decreased with age at vaccination initiation, and were otherwise generally similar among the demographic subgroups defined by gender, race and region of residence. For all subgroups defined by race or region of residence, GMTs were higher in girls and boys than in young women. Vaccination of subjects who were seropositive at day 1 to a vaccine HPV type resulted in higher GMTs to that type, compared with those in subjects who were seronegative for that type at day 1.
Conclusions
9vHPV vaccine immunogenicity was robust among subjects with differing baseline characteristics. It was generally comparable across subjects of different races and from different regions. Greater immunogenicity in girls and boys versus young women (the population used to establish 9vHPV vaccine efficacy in clinical studies) indicates that the anti-HPV responses generated by the vaccine in adolescents from all races or regions were sufficient to induce high-level protective efficacy. This immunogenicity profile supports a widespread 9vHPV vaccination program and early vaccination.
Abbreviations: HPV, human papillomavirus; VLP, virus-like particle; 9vHPV, 9-valent human papillomavirus; cLIA, competitive Luminex immunoassay; GMTs, geometric mean titers; CI, confidence interval; mMU/mL, milli-Merck units per milliliter; qHPV, quadrivalent human papillomavirus
Studies in the meta-analysis: V503-001, V503-002, V503-005, V503-007, V503-009/GDS01C
Clinical trials.gov identifier: NCT00543543, NCT00943722, NCT00988884, NCT01073293, NCT01304498
Keywords: Human papillomavirus, 9v HPV vaccine, Immunogenicity, Clinical trial
1. Introduction
Human papillomavirus (HPV) is the cause of nearly all cervical cancers and a substantial proportion of anal, vulvar, vaginal, penile and oropharyngeal cancers; thus, it is responsible of approximately 5% of the global cancer burden [1]. The identification of HPV as a primary cause of anogenital cancers created an opportunity for cancer prevention through vaccination. First generation HPV vaccines, including the quadrivalent HPV (types 6/11/16/18) (qHPV) vaccine and the bivalent HPV (types 16/18) vaccine were initially developed [2]. A 9-valent HPV (types 6/11/16/18/31/33/45/52/58) (9vHPV) vaccine (Gardasil 9, Merck & Co., Inc., Kenilworth, NJ) was subsequently developed to provide protection against the HPV types already covered by the qHPV vaccine and the next five most common oncogenic types associated with cervical cancer worldwide (types 31/33/45/52/58) [3]. The 9vHPV vaccine could potentially prevent approximately 90% of cervical cancers, HPV-related vulvar, vaginal and anal cancers and genital warts worldwide [4], [5], [6], [7], [8], [9]. The 9vHPV vaccine was licensed in 2014 in the US, in 2015 in Canada, the EU and Australia, and in 2015 and 2016 in other countries.
In a clinical trial conducted in women 16–26 years of age, the 9vHPV vaccine prevented infection and disease caused by HPV 31/33/45/52/58. It also induced anti-HPV 6/11/16/18 antibody responses that were non-inferior to responses induced by the qHPV vaccine; efficacy of the 9vHPV vaccine against infection and disease caused by HPV 6/11/16/18 was inferred based on these results [10], [11], [12]. In another clinical trial, the 9vHPV vaccine induced non-inferior antibody responses to HPV 6/11/16/18/31/33/45/52/58 in girls and boys 9–15 years of age vs. women 16–26 years of age; efficacy of the 9vHPV vaccine against infection and disease caused by the 9 vaccine HPV types in girls and boys 9–15 years of age was inferred based on these results [13].
HPV infection is a global health concern; prophylactic HPV vaccination is included in the national immunization programs of at least 80 countries [14], and used in diverse settings worldwide. It is anticipated that the 9vHPV vaccine will be widely licensed and recommended. Thus, it is useful to evaluate the impact of demographic parameters on the immunogenicity of the 9vHPV vaccine. Of relevant note, a similar study examining the impact of demographic parameters on the immunogenicity of the qHPV vaccine was published shortly after the initial licensure of the qHPV vaccine [15]. This report summarizes a combined analysis of five Phase III clinical trials conducted in girls and boys 9–15 years of age and women 16–26 years of age to examine antibody responses in subgroups for which individual studies may have had limited sample size. Thus, these analyses are novel and may be of interest to many as the 9vHPV vaccine becomes more widely available. Immunogenicity of the 9vHPV vaccine in young men 16–26 years of age was not included in these analyses; it will be the topic of another report so that the additional complexities specific to that population (i.e., lower HPV antibody responses in men having sex with men than in heterosexual men [16], [17]) can be fully explored.
2. Materials and methods
2.1. Enrollment and vaccination
An analysis of the combined immunogenicity database of Phase III studies submitted to regulatory agencies in support of the licensure of the 9vHPV vaccine was conducted. This analysis included 11,304 subjects who received 9vHPV vaccine in five Phase III studies (Table 1). These studies contained three separate populations: virginal girls 9–15 years of age, virginal boys 9–15 years of age, and young women 16–26 years of age, most of whom were sexually active. Eligible subjects received a 3-dose vaccination regimen given as intramuscular injections at day 1, month 2 and month 6. Each study was conducted in accordance with principles of Good Clinical Practice and was approved by the institutional review board at each participating institution and by regulatory agencies. Written informed consent was provided by all adult subjects and by a parent or legal guardian of subjects who were minors, assent was also obtained from minors in conformity with applicable national and local requirements. Baseline characteristics for the overall population of subjects who were randomized to receive the 9vHPV vaccine are presented in Table 2.
Table 1.
Phase III studies of the 9vHPV vaccine contributing to the combined immunogenicity analysis.
| Study | Key objectives | Experimental arm | Control arm | Included in analysesa |
|---|---|---|---|---|
| 001 | Immunogenicity, efficacy vs. qHPV | Women age 16–26 years randomized to 9vHPV vaccine (N=6799)b | Women age 16–26 years randomized to qHPV vaccine (N=6799)b | N=6792b, c |
| 002 | Adult-to-adolescent immunobridging | Girls and boys age 9–15 years (N=2604) enrolled to receive 9vHPV vaccine | Women age 16–26 years enrolled to receive 9vHPV vaccine (N=470) | N=3066 |
| 005 | Co-administration with Menactra/Adacel | Girls and boys age 11–15 years randomized to concomitant arm (N=621) | Girls and boys age 11–15 years randomized to non-concomitant arm (N=620) | N=618d |
| 007 | Co-administration with Repevax | Girls and boys age 11–15 years randomized to concomitant arm (N=526) | Girls and boys age 11–15 years randomized to non-concomitant arm (N=528) | N=528d |
| 009 | qHPV-to-9vHPV immunobridging | Girls age 9–15 years randomized to 9vHPV vaccine (N=300) | Girls age 9–15 years randomized to qHPV vaccine (N=300) | N=300c |
Study 001: NCT00543543 [10].
Study 002: NCT00943722 [13].
Study 005: NCT00988884 [22].
Study 007: NCT01073293 [23].
Study 009/GDS01C: NCT01304498 [12].
Subjects who received at least one vaccination with 9vHPV vaccine. A total of 11,304 subjects who received at least one 9vHPV vaccination are included in these analyses. Most subjects (97.7% [11,046 of 11,304]) received the three vaccinations.
Subjects who received the low-dose, mid-dose or high-dose formulation of 9vHPV vaccine during the dose selection portion of the study [10], [43] are not included; immunogenicity results in these subjects are reported in [44].
Subjects randomized to the 9vHPV vaccine who received ≥1 dose of vaccine.
Table 2.
Subject characteristics (all randomized subjects).
| Females 9–15 years of age |
Males 9–15 years of age |
Females 16–26 years of age |
||||
|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | |
| Subjects in population | 2809 | 1243 | 7269 | |||
| Age (years) | ||||||
| Subjects with data | 2809 | 1243 | 7269 | |||
| Mean | 12 | 12 | 22 | |||
| SD | 2 | 2 | 2 | |||
| Median | 12 | 12 | 22 | |||
| Range | 9 to 15 | 9 to 15 | 16 to 26 | |||
| Race | ||||||
| Asian | 470 | (16.7) | 224 | (18.0) | 1113 | (15.3) |
| Black | 182 | (6.5) | 60 | (4.8) | 281 | (3.9) |
| White | 1749 | (62.3) | 665 | (53.5) | 4004 | (55.1) |
| Othera | 408 | (14.5) | 294 | (23.7) | 1871 | (25.7) |
| Region | ||||||
| Africa | 95 | (3.4) | 30 | (2.4) | 40 | (0.6) |
| Asia-Pacific | 458 | (16.3) | 219 | (17.6) | 998 | (13.7) |
| Europe | 1102 | (39.2) | 373 | (30.0) | 2531 | (34.8) |
| Latin America | 545 | (19.4) | 286 | (23.0) | 2319 | (31.9) |
| North America | 609 | (21.7) | 335 | (27.0) | 1381 | (19.0) |
Study participants were from 26 countries (Austria, Belgium, Brazil, Canada, Chile, Colombia, Costa Rica, Denmark, Finland, Germany, Hong Kong, India, Italy, Japan, Korea, Mexico, New Zealand, Norway, Peru, Poland, South Africa, Spain, Sweden, Taiwan, Thailand, and the United States [including Puerto Rico]).
The category 'Other' for the race variable includes Multi-Racial, American Indian or Alaska Native, Native Hawaiian or Pacific Islander, Unknown and missing race information. Most subjects in that category are Multi-Racial.
2.2. Immunogenicity evaluation
Serum samples were obtained at day 1 and month 7 for anti-HPV antibody testing. The serum samples were assessed for antibodies to HPV VLP types 6/11/16/18/31/33/45/52/58 by a multiplexed competitive Luminex Immunoassay (cLIA; HPV-9 cLIA Version 2.0; performed by PPD Vaccines and Biologics Lab, Wayne, PA, USA), as described previously [18]. Antibody titers for each individual HPV type were determined through competition with type-specific monoclonal antibodies, so it is not possible to directly compare assay results across HPV types. In addition, cervical and external genital swabs collected at day 1 and month 7 in young women 16–26 years of age for testing by polymerase chain reaction (PCR) for type-specific detection of HPV DNA; PCR testing included the 9 vaccine types and 5 additional oncogenic HPV types (HPV 35/39/51/56/59)[19], [20]. HPV seropositivity at day 1 or PCR positivity at day 1 and month 7 was not a reason for exclusion from the study; however, the results were part of the criteria to define analysis populations.
2.3. Data analysis
The serum samples from day 1 and PCR samples from day 1 and month 7 were analyzed for each vaccine HPV type prior to enrollment to identify participants who were positive to one or more HPV types, and these participants were subsequently excluded from the per-protocol immunogenicity analysis for the corresponding HPV type(s). To be included in the HPV type specific per-protocol immunogenicity analysis populations, subjects had to meet the following requirements: (1) be seronegative at day 1 and (for 16- to 26-year-old women) PCR-negative from day 1 through month 7 only for the HPV type being analyzed (for HPV 6 and HPV 11 immunogenicity analyses, because of extensive cross-reactivity due to the high amino acid sequence identity [92%] between HPV 6 and HPV 11 L1 proteins [21], subjects had to be seronegative and, for women 16–26 years of age, PCR-negative for both HPV 6 and HPV 11); (2) receive all 3 doses of the correct clinical material within acceptable day ranges; (3) have a post-dose 3 serology result within acceptable day ranges and (4) have no protocol violation that could potentially interfere with the immunogenicity evaluation as judged by the study director. Seropositive was defined as anti-HPV serum cLIA levels ≥30, 16, 20, 24, 10, 8, 8, 8, 8 milli-Merck units per milliliter (mMU/mL) for HPV types 6, 11, 16, 18, 31, 33, 45, 52 and 58 respectively. Subjects who incidentally received a concomitant vaccination in studies 001, 002 and 009, were excluded from per-protocol immunogenicity analyses as concomitant vaccination was prohibited per protocol in these studies [10], [12], [13]. Thus, for consistency across studies, subjects in the concomitant arm of studies 005 and 007 (studies to assess co-administration of 9vHPV vaccine with diphtheria, tetanus, pertussis, poliomyelitis and meningococcal vaccines) were also excluded from this combined immunogenicity analysis. Immunogenicity results from the concomitant and non-concomitant arms of studies 005 and 007 have been reported [22], [23]. Subjects excluded from per-protocol analyses are summarized in Supplementary material Table 1. Although the per-protocol analyses excluded subjects who were HPV positive (seropositive and/or PCR positive) for vaccine HPV types at baseline, a separate analysis was performed including these subjects to elucidate the differences in immune response between baseline HPV positive and HPV negative subjects. To be included in this analysis, subjects had to meet the following requirements: (1) have a day 1 serology result and a day 1 PCR result; (2) receive all 3 doses of the correct clinical material; and (3) have a post-dose 3 serology result within acceptable day ranges. These analyses were done in subjects (women 16–26 years) enrolled in study 001.
Geometric mean titers (GMTs) with associated 95% confidence intervals were computed and compared across categories of baseline subject characteristics. Cohorts analyzed included subjects given the 9vHPV vaccine stratified into the following three age/sex groups: boys 9–15 years of age, girls 9–15 years of age, and young women 16–26 years of age. Baseline covariates analyzed included age, sex, race, region of residence, and baseline HPV seropositivity and PCR-positivity. All of these evaluations were exploratory in nature; therefore, no statistical tests of hypotheses were performed. Non overlapping 95% confidence intervals were used as indicators of differences of immune response.
It has been previously observed that HPV antibody response to HPV vaccination declines with increasing age [2], [13], [15]. Analyses were conducted to explore whether this relationship varies with race and geographic region. Linear regression model was fitted on the logarithm (base 10) of HPV antibody titer at Month 7 as a function of age at vaccination dose 1 to model the relationship of HPV antibody response at Month 7 with age. Graphical methods were used to display the regression line representing the estimated mean log10-HPV antibody response as a function of age together with the 95% confidence band around the regression line. The regression line and 95% confidence band were displayed by race and geographic region to graphically compare trends of the relationship of HPV antibody response by age across race and geographic region. For each HPV type, two analyses were conducted: an analysis of mean HPV antibody response by race irrespective of geographic region (this analysis is relevant given that race is a reasonable surrogate for geographic region); a second analysis of mean HPV antibody response by race and geographic region. No formal statistical testing was done on the trends of the relationship of mean HPV antibody response by age across race or race and geographic region.
In studies 001 and 009, subjects were randomized to receive 9vHPV vaccine or qHPV vaccine [10], [12]. This report considers only subjects who received the 9vHPV vaccine. Immunogenicity of qHPV vaccine by baseline covariates has already been reported [15].
3. Results
The population included in the present analysis was ethnically diverse and resided in both high-income and low- and middle-income countries. The baseline characteristics among the age/sex cohorts are shown in Table 2. Overall, 98.8% (11,304 of 11,321) of subjects randomized to 9vHPV vaccine received at least one dose of vaccine and 97.6% (11,046 of 11,321) of subjects received all three doses of vaccine. Principal reasons for exclusion from per-protocol immunogenicity analyses included seropositivity and/or PCR positivity to HPV vaccine types and missing serology samples (Supplementary material Table 1).
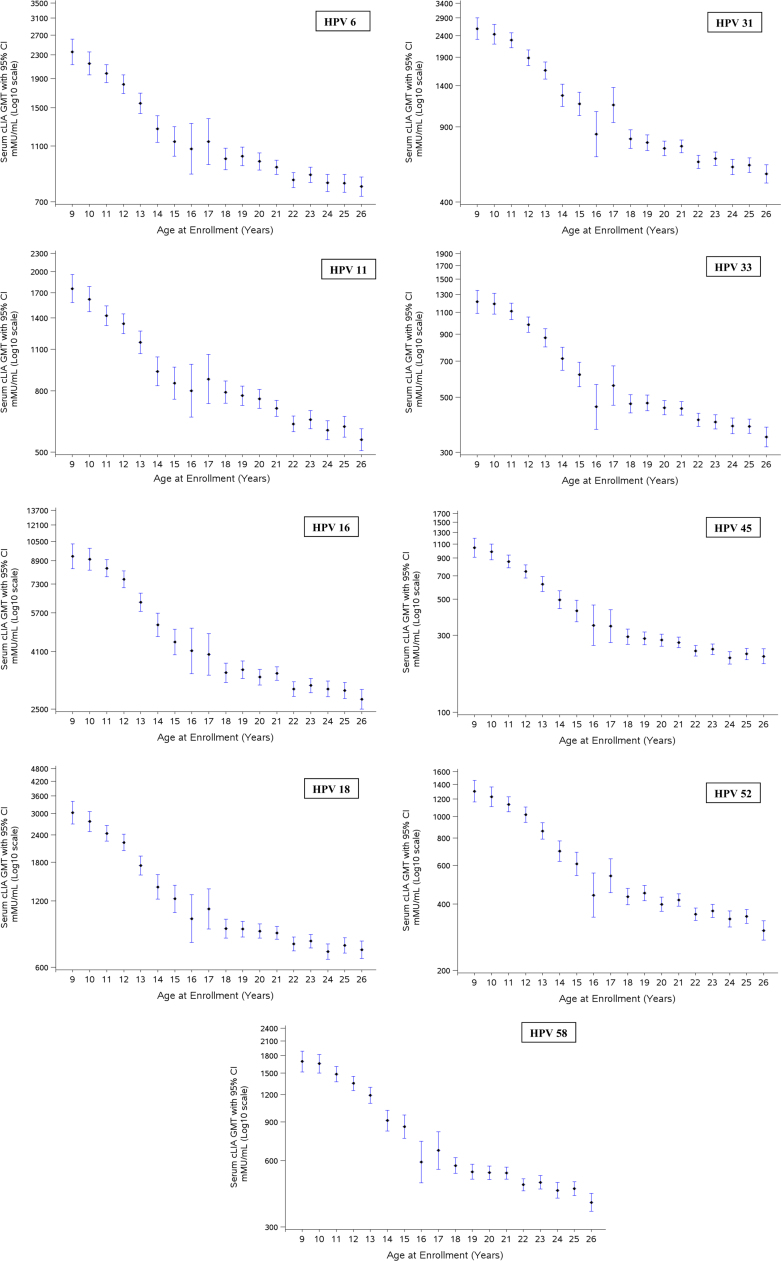
Table 3 summarizes the serum anti-HPV responses at month 7 in the three populations analyzed. For all subjects, seroconversion rates at month 7 ranged from 99.6% to 100%. As seen previously in study 002 [13], geometric mean titers (GMTs) at month 7 were markedly higher in girls and boys than in young women for all 9 vaccine HPV types; and among the adolescents, administration of the vaccine to boys generally resulted in marginally higher anti-HPV GMTs than girls of the same age. Fig. 1 summarizes the serum anti-HPV responses at month 7 in girls and women, stratified by age at enrollment. GMTs decreased with increasing age. Relatively large variations for ages 16 and 17 years were observed, likely representing random variations due to the limited numbers of subjects enrolled in this age range (~2% of total girls and women enrollment). Supplementary material Fig. 1 provides a similar analysis for boys. For each of the 9 HPV types, month 7 GMTs decreased as the age at first vaccination increased.
Table 3.
Per-protocol summary of month 7 anti-HPV geometric mean titers in subjects who received 3 doses of 9vHPV vaccine.
| Population | N | n | % Seropositive (95% CI) | GMT (95% CI) |
|---|---|---|---|---|
| Anti-HPV 6 | ||||
| 9- through 15-year-old girls | 2805 | 2349 | 99.7 (99.4, 99.9) | 1744.6 (1684.7, 1806.7) |
| 9- through 15-year-old boys | 1239 | 1055 | 99.9 (99.5, 100) | 2085.3 (1984.2, 2191.6) |
| 16- through 26-year-old women | 7260 | 4321 | 99.8 (99.6, 99.9) | 893.7 (873.5, 914.3) |
| Anti-HPV 11 | ||||
| 9- through 15-year-old girls | 2805 | 2350 | 99.9 (99.7, 100) | 1289.7 (1244.3, 1336.8) |
| 9- through 15-year-old boys | 1239 | 1055 | 100 (99.7, 100) | 1469.2 (1397.7, 1544.4) |
| 16- through 26-year-old women | 7260 | 4327 | 100 (99.9, 100) | 669.3 (653.6, 685.4) |
| Anti-HPV 16 | ||||
| 9- through 15-year-old girls | 2805 | 2405 | 99.9 (99.7, 100) | 7159.9 (6919.7, 7408.5) |
| 9- through 15-year-old boys | 1239 | 1076 | 100 (99.7, 100) | 8444.9 (8054.2, 8854.5) |
| 16- through 26-year-old women | 7260 | 4361 | 100 (99.9, 100) | 3159.0 (3088.6, 3231.1) |
| Anti-HPV 18 | ||||
| 9- through 15-year-old girls | 2805 | 2420 | 99.9 (99.6, 100) | 2085.5 (2002.2, 2172.3) |
| 9- through 15-year-old boys | 1239 | 1074 | 100 (99.7, 100) | 2620.4 (2474.3, 2775.2) |
| 16- through 26-year-old women | 7260 | 4884 | 99.8 (99.7, 99.9) | 809.9 (789.2, 831.1) |
| Anti-HPV 31 | ||||
| 9- through 15-year-old girls | 2805 | 2397 | 100 (99.8, 100) | 1883.3 (1811.3, 1958.1) |
| 9- through 15-year-old boys | 1239 | 1069 | 100 (99.7, 100) | 2173.5 (2057.0, 2296.6) |
| 16- through 26-year-old women | 7260 | 4806 | 99.8 (99.6, 99.9) | 664.8 (647.4, 682.6) |
| Anti-HPV 33 | ||||
| 9- through 15-year-old girls | 2805 | 2418 | 99.9 (99.7, 100) | 960.6 (927.5, 994.9) |
| 9- through 15-year-old boys | 1239 | 1076 | 100 (99.7, 100) | 1178.6 (1120.9, 1239.4) |
| 16- through 26-year-old women | 7260 | 5056 | 99.7 (99.5, 99.8) | 419.2 (409.6, 429.1) |
| Anti-HPV 45 | ||||
| 9- through 15-year-old girls | 2805 | 2430 | 99.8 (99.6, 100) | 728.7 (697.6, 761.2) |
| 9- through 15-year-old boys | 1239 | 1079 | 100 (99.7, 100) | 841.7 (790.0, 896.7) |
| 16- through 26-year-old women | 7260 | 5160 | 99.6 (99.4, 99.7) | 254.1 (247.0, 261.5) |
| Anti-HPV 52 | ||||
| 9- through 15-year-old girls | 2805 | 2426 | 99.9 (99.7, 100) | 978.2 (942.8, 1015.0) |
| 9- through 15-year-old boys | 1239 | 1077 | 100 (99.7, 100) | 1062.2 (1007.2, 1120.2) |
| 16- through 26-year-old women | 7260 | 4792 | 99.8 (99.6, 99.9) | 382.4 (373.0, 392.0) |
| Anti-HPV 58 | ||||
| 9- through 15-year-old girls | 2805 | 2397 | 99.9 (99.7, 100) | 1306.0 (1259.8, 1354.0) |
| 9- through 15-year-old boys | 1239 | 1072 | 100 (99.7, 100) | 1545.8 (1470.6, 1624.8) |
| 16- through 26-year-old women | 7260 | 4818 | 99.8 (99.6, 99.9) | 489.2 (477.5, 501.2) |
N=number of individuals randomized to the respective vaccination group who received at least 1 injection.
n=number of individuals contributing to the analysis.
mMU=milli-Merck units.
CI=confidence interval.
GMT=geometric mean titers (given in milli-Merck units per milliliter).
Fig. 1.
Plots of month 7 anti-HPV geometric mean titers (GMTs) responses in females to component human papillomavirus (HPV) vaccine types, by age at enrollment. GMTs with associated 95% confidence intervals are presented for the per-protocol immunogenicity population. cLIA, competitive Luminex-based immunoassay; mMU, milli-Merck units.
Small numeric differences in month 7 anti-HPV GMTs were observed among subpopulations of women 16–26 years of age defined by race. In particular, black women tended to have higher anti-HPV GMTs than Asian or white women or women of other races. However, no consistent pattern was demonstrated across all 9 vaccine types (Table 4). Subjects in Africa, Latin America and North America tended to have higher anti-HPV GMTs than subjects in Asia, and Europe (Table 5). Analyses of month 7 GMTs by race (Supplementary material Tables 2 and 3) and by region (Supplementary material Tables 4 and 5) in girls and boys 9–15 years of age provided similar results. Month 7 GMTs were markedly higher in girls and boys 9–15 years of age than in women 16–26 years of age for all subgroups defined by race or region for all 9 HPV types.
Table 4.
Per-protocol summary of anti-HPV geometric mean titers at month 7 by race in women 16–26 years of age who received 3 doses of 9vHPV vaccine.
|
Race |
||||||||
|---|---|---|---|---|---|---|---|---|
|
Asian |
Black |
White |
Other |
|||||
| Assay | n | GMT (95% CI) | n | GMT (95% CI) | n | GMT (95% CI) | n | GMT (95% CI) |
| HPV 6 | 763 | 837.4 (793.1, 884.0) |
123 | 935.3 (817.1, 1070.6) |
2415 | 895.4 (868.5, 923.1) |
1020 | 929.0 (886.5, 973.7) |
| HPV 11 | 764 | 594.5 (561.9, 629.0) |
122 | 670.1 (581.8, 771.7) |
2421 | 691.1 (669.5, 713.3) |
1020 | 677.7 (645.4, 711.6) |
| HPV 16 | 792 | 3071.2 (2913.4, 3237.7) |
152 | 3983.9 (3531.7, 4493.9) |
2361 | 3077.8 (2985.2, 3173.4) |
1056 | 3307.7 (3159.9, 3462.3) |
| HPV 18 | 831 | 850.9 (799.4, 905.7) |
170 | 995.0 (866.8, 1142.3) |
2669 | 755.6 (729.7, 782.3) |
1214 | 886.2 (841.6, 933.2) |
| HPV 31 | 858 | 710.7 (667.6, 756.6) |
169 | 786.4 (683.0, 905.5) |
2631 | 619.5 (597.8, 642.1) |
1148 | 725.1 (686.9, 765.4) |
| HPV 33 | 856 | 420.2 (397.1, 444.6) |
189 | 414.6 (367.6, 467.5) |
2733 | 417.1 (404.1, 430.5) |
1278 | 424.0 (404.8, 444.0) |
| HPV 45 | 885 | 281.4 (262.9, 301.3) |
190 | 345.0 (297.9, 399.6) |
2824 | 227.3 (218.8, 236.1) |
1261 | 290.1 (274.1, 307.2) |
| HPV 52 | 788 | 353.8 (332.7, 376.1) |
166 | 444.0 (388.4, 507.5) |
2690 | 388.2 (375.6, 401.4) |
1148 | 381.0 (362.1, 400.8) |
| HPV 58 | 814 | 520.3 (490.5, 551.9) |
161 | 493.1 (431.9, 562.9) |
2720 | 483.6 (468.2, 499.4) |
1123 | 480.8 (457.2, 505.5) |
GMT, geometric mean titer (given in milli-Merck units per milliliter). CI, confidence interval.
Table 5.
Per-protocol summary of month 7 anti-HPV geometric mean titers by region in women 16–26 years of age who received 3 doses of 9vHPV vaccine.
|
Region |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Africa |
Asia-Pacific |
Europe |
Latin America |
North America |
||||||
| Assay | n | GMT (95% CI) | n | GMT (95% CI) | n | GMT (95% CI) | n | GMT (95% CI) | n | GMT (95% CI) |
| HPV 6 | 16 | 1029.5 (708.5, 1496.1) |
691 | 817.9 (772.7, 865.8) |
1454 | 850.9 (818.2, 884.9) |
1292 | 946.6 (908.1, 986.8) |
868 | 953.2 (906.1, 1002.8) |
| HPV 11 | 15 | 764.4 (511.2, 1142.9) |
692 | 588.9 (555.0, 624.8) |
1458 | 660.0 (633.7, 687.5) |
1292 | 686.0 (656.9, 716.4) |
870 | 729.5 (692.0, 769.1) |
| HPV 16 | 20 | 5218.2 (3746.9, 7267.3) |
722 | 2943.1 (2785.2, 3109.8) |
1412 | 2929.3 (2816.1, 3047.1) |
1336 | 3355.0 (3221.8, 3493.8) |
871 | 3412.8 (3245.7, 3588.5) |
| HPV 18 | 21 | 1376.9 (930.3, 2037.7) |
758 | 827.4 (775.1, 883.2) |
1604 | 714.2 (682.9, 747.0) |
1537 | 887.6 (847.9, 929.2) |
964 | 838.4 (791.3, 888.3) |
| HPV 31 | 23 | 986.1 (673.5, 1443.9) |
785 | 673.6 (631.0, 719.0) |
1584 | 578.7 (552.7, 605.9) |
1454 | 725.5 (691.6, 761.2) |
960 | 717.3 (676.1, 760.9) |
| HPV 33 | 18 | 442.9 (300.3, 653.3) |
782 | 407.1 (383.8, 431.9) |
1635 | 396.9 (381.1, 413.4) |
1616 | 429.3 (412.0, 447.3) |
1005 | 450.9 (428.1, 475.0) |
| HPV 45 | 24 | 518.1 (343.1, 782.3) |
810 | 267.7 (249.4, 287.4) |
1714 | 207.8 (197.9, 218.2) |
1604 | 291.0 (276.7, 306.1) |
1008 | 272.0 (255.2, 289.8) |
| HPV 52 | 20 | 552.8 (376.5, 811.7) |
717 | 340.6 (319.4, 363.2) |
1620 | 365.1 (349.8, 381.0) |
1465 | 390.8 (373.7, 408.8) |
970 | 432.1 (409.0, 456.7) |
| HPV 58 | 15 | 706.9 (458.1, 1090.8) |
743 | 497.3 (467.5, 528.9) |
1647 | 468.2 (449.2, 487.9) |
1425 | 482.8 (461.7, 504.7) |
988 | 527.2 (499.8, 556.1) |
GMT, geometric mean titer (given in milli-Merck units per milliliter). CI, confidence interval.
In exploratory analyses, the inverse relationship between mean HPV antibody responses at month 7 and age was seen regardless of race and geographic region (Supplementary material Fig. 2). Even though small differences were observed among subgroups defined by race or race and region, no consistent pattern was demonstrated across all 9 vaccine types.
Table 6 displays the anti-HPV levels at month 7 in subject groups defined by day 1 HPV serostatus and PCR status. Inclusion of subjects regardless of baseline HPV status permitted a comparison of vaccine-induced immune responses with those generated in response to an HPV infection. Robust antibody responses were observed in all groups for all HPV types. GMTs appeared to be the highest in the group which was seropositive and PCR negative on day 1 (i.e., subjects who were seropositive at enrollment likely due to a prior exposure to HPV).
Table 6.
Month 7 anti-HPV geometric mean titers by day 1 serostatus and PCR status in women, 16–26 years of age who completed the 3-dose 9vHPV vaccine regimena.
|
Females 16–26 years of age (N=7260) |
|||||
|---|---|---|---|---|---|
| HPV type | Day 1 serostatus | Day 1 PCR status | n | GMT (mMU/mL) | (95% CI) |
| HPV 6 | Negative | Negative | 4720 | 901.4 | (881.8, 921.4) |
| Negative | Positive | 113 | 1025.2 | (881.8, 1192.0) | |
| Positive | Negative | 807 | 1876.0 | (1742.2, 2020.1) | |
| Positive | Positive | 109 | 1509.5 | (1287.3, 1770.1) | |
| HPV 11 | Negative | Negative | 4723 | 675.7 | (660.3, 691.4) |
| Negative | Positive | 13 | 618.4 | (331.1, 1155.3) | |
| Positive | Negative | 188 | 1065.9 | (935.0, 1215.0) | |
| Positive | Positive | 14 | 1110.9 | (652.9, 1890.2) | |
| HPV 16 | Negative | Negative | 4799 | 3177.3 | (3109.4, 3246.7) |
| Negative | Positive | 323 | 2941.4 | (2676.1, 3233.1) | |
| Positive | Negative | 492 | 5248.6 | (4848.6, 5681.5) | |
| Positive | Positive | 260 | 4374.0 | (3968.9, 4820.6) | |
| HPV 18 | Negative | Negative | 5334 | 815.9 | (796.0, 836.3) |
| Negative | Positive | 178 | 941.2 | (829.7, 1067.7) | |
| Positive | Negative | 266 | 1917.2 | (1714.3, 2144.0) | |
| Positive | Positive | 86 | 1472.5 | (1236.3, 1753.8) | |
| HPV 31 | Negative | Negative | 5254 | 668.2 | (651.5, 685.3) |
| Negative | Positive | 184 | 625.3 | (553.5, 706.4) | |
| Positive | Negative | 327 | 964.4 | (877.3, 1060.1) | |
| Positive | Positive | 112 | 798.5 | (690.3, 923.7) | |
| HPV 33 | Negative | Negative | 5503 | 424.1 | (414.8, 433.7) |
| Negative | Positive | 101 | 443.3 | (375.7, 523.1) | |
| Positive | Negative | 215 | 665.7 | (582.5, 760.7) | |
| Positive | Positive | 59 | 616.0 | (482.4, 786.7) | |
| HPV 45 | Negative | Negative | 5620 | 255.0 | (248.1, 262.0) |
| Negative | Positive | 130 | 256.9 | (216.8, 304.4) | |
| Positive | Negative | 82 | 354.0 | (279.1, 449.1) | |
| Positive | Positive | 29 | 285.8 | (195.7, 417.4) | |
| HPV 52 | Negative | Negative | 5259 | 385.1 | (376.1, 394.3) |
| Negative | Positive | 275 | 303.5 | (272.8, 337.5) | |
| Positive | Negative | 220 | 547.1 | (488.5, 612.7) | |
| Positive | Positive | 121 | 319.8 | (271.4, 376.7) | |
| HPV 58 | Negative | Negative | 5267 | 493.8 | (482.5, 505.4) |
| Negative | Positive | 158 | 423.3 | (376.1, 476.3) | |
| Positive | Negative | 373 | 618.9 | (559.6, 684.6) | |
| Positive | Positive | 82 | 669.8 | (548.4, 818.0) | |
N=number of subjects who received at least 1 injection of 9vHPV vaccine; n=number of subjects contributing to the analysis.
GMT, geometric mean titer (given in milli-Merck units per milliliter). CI, confidence interval.
This analysis population includes subjects who received all 3 doses of correct clinical material, had serology & PCR results at day 1 for the relevant HPV type and had a post-dose 3 or month 7 serology result within acceptable day ranges.
GMTs were analyzed over time in the per-protocol population and in subjects seropositive and PCR negative at day 1 in study 001. Table 7 enumerates anti-HPV GMTs at all time- points from month 3 (1 month post-dose 2) and month 7 (1 month post-dose 3) to month 42 in subjects that were seropositive and PCR negative at day 1. Notably, in this population, the anti-HPV GMTs generated by the 9vHPV vaccine were dramatically increased after 2 doses (month 3) or 3 doses (month 7), and were substantially higher (as evidenced by non-overlapping 95% CI) than GMTs observed in the per-protocol population for all time points from month 3 to month 42.
Table 7.
Month 7 anti-HPV geometric mean titers in women, 16–26 years of age who completed the 3-dose 9vHPV vaccine regimen in study 001.
|
Per protocol immunogenicity population |
Day 1 seropositive and PCR-negative |
|||
|---|---|---|---|---|
|
Assay time point |
n | GMT (95% CI) | n | GMT (95% CI) |
| HPV 6 | ||||
| Day 1 | 3993 | <16 (<16, <16) | 917 | 108.1 (102.2, 114.3) |
| Month 3 | 788 | 734.0 (692.8, 777.7) | 188 | 2384.0 (1982.9, 2866.1) |
| Month 7 | 3993 | 893.1 (871.7, 915.1) | 752 | 1874.8 (1737.6, 2022.8) |
| Month 12 | 800 | 330.6 (312.2, 350.1) | 213 | 951.9 (807.3, 1122.3) |
| Month 24 | 715 | 208.6 (195.5, 222.7) | 182 | 634.3 (529.6, 759.8) |
| Month 36 | 685 | 163.9 (153.0, 175.6) | 155 | 462.9 (382.5, 560.2) |
| Month 42 | 692 | 147.2 (137.3, 157.8) | 163 | 444.1 (369.2, 534.3) |
| HPV 11 | ||||
| Day 1 | 3995 | <6 (<6, <6) | 208 | 44.6 (39.2, 50.8) |
| Month 3 | 790 | 529.1 (499.7, 560.1) | 44 | 1666.4 (1229.9, 2257.9) |
| Month 7 | 3995 | 666.3 (649.6, 683.4) | 175 | 1050.2 (914.9, 1205.6) |
| Month 12 | 810 | 212.4 (200.1, 225.6) | 49 | 609.9 (465.7, 798.9) |
| Month 24 | 763 | 123.3 (115.8, 131.2) | 40 | 360.7 (264.9, 491.0) |
| Month 36 | 690 | 89.6 (83.3, 96.3) | 35 | 214.4 (149.4, 307.7) |
| Month 42 | 696 | 84.9 (79.0, 91.3) | 34 | 201.9, (140.6, 289.8) |
| HPV 16 | ||||
| Day 1 | 4032 | <12 (<12, <12) | 564 | 126.6 (115.1, 139.3) |
| Month 3 | 794 | 2435.8 (2303.5, 2575.6) | 104 | 7064.1 (5775.9, 8639.7) |
| Month 7 | 4032 | 3131.1 (3057.1, 3206.9) | 458 | 5149.6 (4752.9, 5579.5) |
| Month 12 | 819 | 1041.7 (979.9, 1107.4) | 134 | 3079.9 (2582.1, 3673.6) |
| Month 24 | 778 | 520.7 (484.7, 559.4) | 98 | 1824.4 (1436.6, 2316.9) |
| Month 36 | 695 | 386.5 (356.3, 419.4) | 82 | 1228.6 (939.5, 1606.6) |
| Month 42 | 709 | 346.8 (319.3, 376.7) | 90 | 1186.7 (934.8, 1506.4) |
| HPV 18 | ||||
| Day 1 | 4539 | <8 (<8, <8) | 296 | 83.0 (74.3, 92.8) |
| Month 3 | 908 | 470.8 (442.8, 500.7) | 58 | 1920.1 (1464.2, 2517.9) |
| Month 7 | 4539 | 804.6 (782.7, 827.1) | 241 | 1883.4 (1670.6, 2123.4) |
| Month 12 | 929 | 198.6 (184.9, 213.4) | 72 | 1012.7 (773.7, 1325.6) |
| Month 24 | 886 | 86.0 (79.0, 93.6) | 52 | 537.2 (390.1, 739.8) |
| Month 36 | 789 | 78.5 (71.9, 85.6) | 48 | 397.0 (285.8, 551.5) |
| Month 42 | 806 | 70.8 (64.8, 77.3) | 47 | 374.9 (268.6, 523.2) |
| HPV 31 | ||||
| Day 1 | 4466 | <4 (<4, <4) | 373 | 38.7 (35.0, 42.6) |
| Month 3 | 881 | 437.6 (406.7, 470.8) | 83 | 825.6 (609.4, 1118.4) |
| Month 7 | 4466 | 658.4 (636.7, 680.9) | 295 | 952.8 (831.5, 1091.8) |
| Month 12 | 909 | 196.5 (183.5, 210.4) | 90 | 452.2 (340.3, 600.8) |
| Month 24 | 863 | 101.9 (94.9, 109.5) | 71 | 242.0 (174.5, 335.6) |
| Month 36 | 772 | 72.7 (67.5, 78.4) | 62 | 182.1 (128.4, 258.4) |
| Month 42 | 783 | 70.4 (65.3, 75.9) | 68 | 163.7 (119.9, 223.4) |
| HPV 33 | ||||
| Day 1 | 4702 | <4 (<4, <4) | 248 | 24.9 (22.1, 28.0) |
| Month 3 | 937 | 287.8 (272.9, 303.5) | 50 | 647.0 (459.2, 911.4) |
| Month 7 | 4702 | 415.9 (405.6, 426.4) | 196 | 660.4 (550.1, 792.9) |
| Month 12 | 958 | 126.2 (119.9, 132.9) | 68 | 372.9 (276.8, 502.4) |
| Month 24 | 909 | 65.3 (61.7, 69.0) | 52 | 243.3 (169.1, 349.8) |
| Month 36 | 813 | 46.8 (44.0, 49.8) | 45 | 184.0 (124.1, 272.9) |
| Month 42 | 835 | 44.3 (41.6, 47.1) | 39 | 172.6 (111.8, 266.5) |
| HPV 45 | ||||
| Day 1 | 4792 | <3 (<3, <3) | 97 | 23.7 (20.2, 27.9) |
| Month 3 | 956 | 160.4 (151.7, 169.7) | 14 | 389.1 (197.5, 766.4) |
| Month 7 | 4792 | 252.8 (246.2, 259.6) | 76 | 346.8 (244.3, 492.4) |
| Month 12 | 976 | 69.2 (65.4, 73.3) | 18 | 203.5 (105.3, 393.5) |
| Month 24 | 928 | 33.0 (31.0, 35.0) | 13 | 115.5 (51.6, 258.2) |
| Month 36 | 835 | 22.9 (21.4, 24.4) | 11 | 60.8 (23.7, 155.8) |
| Month 42 | 846 | 21.1 (19.8, 22.5) | 13 | 87.7 (36.9, 208.3) |
| HPV 52 | ||||
| Day 1 | 4455 | <3 (<3, <3) | 244 | 22.3 (20.3, 24.6) |
| Month 3 | 895 | 241.3 (229.7, 253.4) | 41 | 877.2 (543.0, 1417.0) |
| Month 7 | 4455 | 379.7 (371.6, 388.0) | 199 | 534.7 (447.2, 639.3) |
| Month 12 | 916 | 118.9 (113.0, 125.0) | 53 | 450.3 (320.3, 633.0) |
| Month 24 | 867 | 57.9 (54.7, 61.2) | 40 | 265.6 (175.1, 402.8) |
| Month 36 | 777 | 47.9 (45.0, 50.9) | 34 | 177.7 (120.2, 262.8) |
| Month 42 | 791 | 43.2 (40.6, 46.0) | 33 | 178.0 (120.8, 262.3) |
| HPV 58 | ||||
| Day 1 | 4486 | <4 (<4, <4) | 419 | 21.7 (19.9, 23.8) |
| Month 3 | 884 | 281.1 (265.3, 297.7) | 85 | 546.2 (436.4, 729.4) |
| Month 7 | 4486 | 482.5 (469.9, 495.3) | 342 | 606.2 (539.3, 681.5) |
| Month 12 | 905 | 153.3 (145.5, 161.6) | 108 | 273.4 (219.0, 341.2) |
| Month 24 | 852 | 80.3 (75.7, 85.3) | 84 | 147.4 (114.8, 189.3) |
| Month 36 | 765 | 55.0 (51.4, 58.8) | 79 | 101.3 (78.0, 131.6) |
| Month 42 | 784 | 52.0 (48.7, 55.6) | 76 | 95.1 (73.6, 122.9) |
n=number of subjects contributing to the analysis.
GMT, geometric mean titer (given in milli-Merck units per milliliter).
CI, confidence interval.
4. Discussion
A combined analysis of the immunogenicity in five Phase III clinical studies showed that a 3-dose regimen of the 9vHPV vaccine was highly immunogenic in girls and boys 9–15 years of age and young women 16–26 years of age, with seroconversion rates at 1 month post-dose 3>99% in these three populations. GMTs at 1 month post-dose 3 in the combined database were higher in girls and boys than in young women, a finding consistent with results previously seen in study 002 [13]. Moreover, the 9vHPV vaccine induced robust HPV antibody responses to all 9 vaccine HPV types in all subgroups of subjects defined by age, race, and geographic region of residence. GMTs at 1 month post-dose 3 steadily decreased with increasing age at the start of vaccination. Small numeric differences in GMTs were observed among subpopulations defined by race and region of residence. In groups of subjects who were seropositive and PCR negative for a given HPV type at day 1, higher GMTs were seen for this HPV type compared with groups of subjects who were seronegative for this HPV type at day 1. HPV seropositivity at baseline likely reflects a humoral immune response following prior infection with HPV; PCR negativity likely indicates the absence of ongoing infection with HPV. The higher GMTs in this population are suggestive of an anamnestic response following 9vHPV vaccine administration [24]. Recently, Scherer et al. reported that a single dose of the qHPV vaccine improved the B cell memory of persons with pre-existing antibodies to HPV 16. In this study, a single dose of qHPV vaccine administered to HPV 16-seropositive women boosted antibody levels 24- to 930-fold at one month post-vaccination and elicited HPV 16-specific memory B cells that expressed type specific neutralizing antibodies [25]. These data indicate that vaccination augments natural HPV immunity by not only boosting antibody levels but also eliciting a quantitatively and qualitatively superior memory B cell response. Of note, the qHPV vaccine has been shown to protect seropositive women against subsequent disease due to the corresponding vaccine HPV type [26]. Taken together these results indicate that in subjects previously infected with certain HPV vaccine types and who have cleared infection, vaccination could potentially prevent reinfection and disease due to these types. It must be noted though, regardless of the HPV seropositivity status prior to vaccination, all subjects exhibited a robust boost in GMTs post-vaccination.
In a Phase III clinical study (study 001), the 9vHPV vaccine prevented infection and disease related to HPV 31/33/45/52/58 in young women 16–26 years of age from multiple races and regions [10]. In the same study, the efficacy findings established with qHPV vaccine for HPV 6/11/16/18 in earlier clinical studies [27], [28], [29] were extended to the 9vHPV vaccine based on the demonstration of non-inferior HPV 6/11/16/18 antibody responses. In additional analyses, both qHPV and 9vHPV vaccine were found to be highly efficacious against infection and disease in subgroups of young women 16–26 years of age differing by age, race, and region of residence [29], [30], [31], [32], [33], [34], [35] . Therefore, the small differences in 9vHPV vaccine immunogenicity by age, race or region of residence shown in this report in young women 16–26 years of age are unlikely to have a clinical significance.
As seen in this report, anti-HPV GMTs at month 7 are substantially higher in all subgroups of girls and boys 9–15 years of age defined by age, race, and region of residence compared with HPV antibody responses in young women 16–26 years of age in the combined database (Table 3) or previously reported in study 001 [10]. As previously reported, prophylactic administration of the 9vHPV vaccine to 16–26-year-olds was highly effective in preventing infection and disease due to vaccine HPV types [10]. Thus, the anti-HPV responses generated by the vaccine in adolescents were sufficient to induce high-level protective efficacy. Overall, 9vHPV vaccine efficacy can be inferred in all subgroups and the small differences in 9vHPV vaccine immunogenicity by age, race or region of residence shown in this report are unlikely to have a clinical significance in girls and boys 9–15 years of age.
There are several limitations to this combined analysis of immunogenicity. Even though the studies included in these analyses enrolled subjects from six continents, they enrolled only a limited number of subjects from Africa and South Asia. Therefore, it will be important to further evaluate the immunogenicity of the 9vHPV vaccine in these regions, especially given the prevalence of HIV infection or other co-infections and malnutrition in these regions which may impact immune response to the vaccine. Of note, studies of the qHPV vaccine in sub-Saharan Africa, India, and Vietnam, and a study in HIV-infected children demonstrated robust immunogenicity in these populations [36], [37], [38], [39]. Given that the 9vHPV vaccine and qHPV vaccine have comparable immunogenicity profiles [10], [11], [12], similar results are expected with the 9vHPV vaccine.
This combined analysis assessed the immunogenicity of a 3-dose regimen of 9vHPV vaccine. The use of an alternative 2-dose regimen for HPV vaccines has been recommended in 2014 by the World Health Organization in 9- to 14-year-olds [40]. The 2-dose schedule for the previously developed bivalent and quadrivalent HPV vaccines has been implemented in several countries [41]. A Phase III study to assess a 2-dose regimen of the 9vHPV vaccine in girls and boys 9–14 years of age has recently provided relevant immunogenicity data [42].
In summary, the 9vHPV vaccine induced robust HPV antibody responses to all 9 vaccine HPV types in subjects from all ages, races, and geographic regions represented in the aforementioned five Phase III studies of the vaccine. This comprehensive immunogenicity profile provides compelling evidence for administration of the 9vHPV vaccine at an early age (i.e., before exposure to HPV) and supports a widespread 9vHPV vaccination program regardless of race or region of residence.
5. Conclusion
In clinical trials, the 9vHPV vaccine was highly immunogenic in subjects aged 9–26 years. The 9vHPV vaccine immunogenicity was robust among subjects with differing baseline characteristics (age, gender, race, region of residence, and HPV serostatus and PCR status at day 1). Its immunogenicity profile was similar to that of the qHPV vaccine. The demonstrated efficacy, safety and immunogenicity profile of the 9vHPV vaccine supports widespread vaccination programs.
Funding source & sponsors' role
This study was funded by Merck & Co., Inc., Kenilworth, NJ, USA (sponsor). All co-authors approved the final version of the manuscript.
Financial disclosures
Other than employees of Merck & Co., Inc., Kenilworth, NJ, USA (as indicated on the title page), all authors have been investigators for the sponsor. Employees of Merck & Co., Inc., Kenilworth, NJ, USA may hold stock and/or stock options in the company.
Author contribution
Study concept and design: RM, EM, AL.
Acquisition of data: LKP, EDM, OEI, PP, PVD, EAJ, S-EO, DF, SB, ARG.
Analysis and interpretation of data: All authors.
Manuscript Preparation: All Authors.
Statistical analysis: OMB, DH, RM.
Conflicts of interest
LKP: was advisory board member for Sanofi Pasteur and investigator at the vaccine trials funded by MSD.
JAR: Nothing to disclose.
EDM: has received research grants from and is a member of a speaker’s bureau for Merck & Co., Inc, Kenilworth, NJ, USA.
O-EI: has received compensation from Merck to conduct vaccine clinical trials and scientific advisory board fees.
PP: No potential conflicts of interest to disclose.
PVD: Acts as chief and principal investigator for vaccine trials conducted on behalf of the University of Antwerp, for which the University obtains research grants from vaccine manufacturers; speakers fees for presentations on vaccines are paid directly to an educational fund held by the University of Antwerp. PVD receives no personal remuneration for this work.
EAJ : Reports having received grant support paid to his institution from Merck and GlaxoSmithKline and advisory board fees from Merck and Sanofi Pasteur MSD.
S-EO: has received grants from Merck according to contracts to perform studies with HPV-vaccines.
DF: has received grant support from Merck through his institution and personal fees for consultancy and advisory boards for Merck.
SB: has received research grants from and is a member of a speaker’s bureau for Merck & Co., Inc., Kenilworth, NJ, USA and has served as a paid expert witness and consultant for Merck.
ARG: has received fees for serving on an advisory board and grant support through her institution from Merck.
XB: has received institutional research and educational grants from Sanofi Pasteur MSD and GlaxoSmithKline and personal travel grant and speakers honorarium from Sanofi Pasteur MSD and GlaxoSmithKline.
SP: has received travel expenses from Sanofi Pasteur MSD.
JC: has received fees for serving on advisory boards from Merck, Abbott, Gen-Probe Hologic, and Becton Dickinson, lecture fees from GlaxoSmithKline, and grant support from Roche, Abbott, Gen- Probe Hologic, Becton Dickinson, and Qiagen.
SMG: has received Grants to her institution from Commonwealth Dept. of Health for HPV genoprevalance surveillance post-vaccination, Merck and GSK to perform phase 3 clinical vaccine trials: Merck to evaluate HPV in RRP post-vaccination program &, CSL for HPV in cervical cancer study, & VCA for a study on effectiveness of public health HPV vaccine study plus a study on associations of early onset cancers. Received speaking fees from MSD and SPMSD for work performed in her personal time. Merck paid for travel & accommodation to present at HPV Advisory board meetings.
WH: has received fees for serving on advisory board from Merck.
SKK: has received speaker’s and advisory board fees from Sanofi Pasteur MSD and Merck, advisory board fees from BD, and unrestricted research grants through her institution from Merck.
OMB, DH, EM, HQ, AL: are employees of Merck & Co., Inc., Kenilworth, NJ, USA.
RM, CR: were employees of Merck & Co., Inc., Kenilworth, NJ, USA at the time of the study.
Acknowledgements
The authors are indebted to all the participants and their caregivers involved with this study. The expert assistance of Amita Joshi, Ph.D. and Karyn Davis, BA of Merck & Co., Inc., Kenilworth, NJ, USA in preparation and editing of the manuscript is also much appreciated.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.pvr.2017.03.002.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Forman D., de Martel C., Lacey C.J., Soerjomataram I., Lortet-Tieulent J., Bruni L. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30:F12–23. doi: 10.1016/j.vaccine.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 2.Schiller J.T., Castellsague X., Garland S.M. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine. 2012;30(5) doi: 10.1016/j.vaccine.2012.04.108. SF123–S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Sanjose S., Quint W., Alemany L., Geraets D., Klaustermeier J., Lloveras B. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–1056. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 4.Serrano B., Alemany L., Tous S., Bruni L., Clifford G., Weiss T. Potential impact of a nine-valent vaccine in human papillomavirus related cervical disease. Infect. Agent Cancer. 2012;7:38. doi: 10.1186/1750-9378-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Sanjose S., Alemany L., Ordi J., Tous S., Alejo M., Bigby S. Worldwide human papillomavirus genotype attribution in over 2000 cases of intraepithelial and invasive lesions of the vulva. Eur. J. Cancer. 2013;49:3450–3461. doi: 10.1016/j.ejca.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 6.Alemany L., Saunier M., Alvarado-Cabrero I., Quirós B., Salmeron J., Shin H. Human papillomavirus DNA prevalence and type distribution in anal carcinomas worldwide. Int. J. Cancer. 2014;10:98–107. doi: 10.1002/ijc.28963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alemany L., Saunier M., Tinoco L., Quirós B., Alvarado-Cabrero B.I., Alejo M. Large contribution of human papillomavirus in vaginal neoplastic lesions: a worldwide study in 597 samples. Eur. J. Cancer. 2014;21:2846–2854. doi: 10.1016/j.ejca.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 8.Serrano B., deSanjose S., Tous S., QuirosB B., Muñoz N., Bosch X. Human papillomavirus genotype attribution for HPVs 6, 11, 16, 18, 31, 33, 45, 52 and 58 in female anogenital lesions. Eur. J. Cancer. 2015;51:1732–1741. doi: 10.1016/j.ejca.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Lacey C., Lowndes C., Shah K. Chapter 4: burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine. 2006;24:S35–S41. doi: 10.1016/j.vaccine.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 10.Joura E., Giuliano A., Iversen O.-E., Bouchard C., Mao C., Mehlsen J. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N. Engl. J. Med. 2015;372:711–723. doi: 10.1056/NEJMoa1405044. [DOI] [PubMed] [Google Scholar]
- 11.Van Damme P., Meijer C., Kienenger D., Schuyleman A., Thomas S., Luxembourg A. A phase III clinical study to compare the immunogenicity and safety of the 9-valent and quadrivalent HPV vaccines in men. Vaccine. 2016;34:4205–4212. doi: 10.1016/j.vaccine.2016.06.056. [DOI] [PubMed] [Google Scholar]
- 12.Vesikari T., Brodszki N., Van Damme P., Diez-Domingo J., Icardi G., Petersen L. A randomized, double-blind, phase III study of the immunogenicity and safety of a 9-valent human papillomavirus L1 virus-like particle vaccine (V503) versus Gardasil(R)in 9-15-year-old girls. Pediatr. Infect. Dis. J. 2015;34:992–998. doi: 10.1097/INF.0000000000000773. [DOI] [PubMed] [Google Scholar]
- 13.Van Damme P., Olsson S.-E., Block S., Castellsague X., Gray G., Herrera T. Immunogenicity and safety of a 9-valent HPV vaccine. Pediatrics. 2015;136:e28–e39. doi: 10.1542/peds.2014-3745. [DOI] [PubMed] [Google Scholar]
- 14.Wigle J., Fontenot H.B., Zimet G.D. Global delivery of human papillomavirus vaccines. Pediatr. Clin. N. Am. 2016;63:81–95. doi: 10.1016/j.pcl.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Giuliano A., Lazcano-Ponce E., Villa L. Impact of baseline covariates on the immunogenicity of a quadrivalent (types6, 11, 16, and 18) human papillomavirus virus-like-particle vaccine. J. Infect. Dis. 2007;196:1153–1162. doi: 10.1086/521679. [DOI] [PubMed] [Google Scholar]
- 16.Castellsague X., Giuliano A., Goldstone S., Guevara A., Mogensen O., Palefsky J. Immunogenicity and safety of the 9-valent HPV vaccine in men. Vaccine. 2015;33:6892–6901. doi: 10.1016/j.vaccine.2015.06.088. [DOI] [PubMed] [Google Scholar]
- 17.Hillman R., Giuliano A., Palefsky J., Goldstone S., Moreira E., Vardas E. Immunogenicity of the quadrivalent human papillomavirus (type 6/11/16/18) vaccine in males 16 to 26 years old. Clin. Vaccin. Immunol. 2012;19:261–267. doi: 10.1128/CVI.05208-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts C., Green T., Hess E., Matys K., Brown M., Haupt R. Development of a human papillomavirus competitive immunoassay for nine HPV types. Hum. Vaccin. Immunother. 2014;10:2168–2174. doi: 10.4161/hv.29205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts C., Swoyer R., Bryan J., Taddeol F. Comparison of real-time multiplex human papillomavirus (HPV) PCR assays with the linear array HPV genotyping PCR assay and influence of DNA extraction method on HPV detection. J. Clin. Microbiol. 2011;49:1899–1906. doi: 10.1128/JCM.00235-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Else E., Swoyer R., Zhang Y., Taddeo F., Bryan J., Lawson J. Comparison of real-time multiplex human papillomavirus PCR assay with INNO-LiPA HPV genotyping extra assay. J. Clin. Microbiol. 2011;49:1907–1912. doi: 10.1128/JCM.00236-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown M., Seitz H., Towne V., Müller M., Finnefrock A. Development of neutralizing monoclonal antibodies for oncogenic human papillomavirus types 31, 33, 45, 52, and 58. Clin. Vaccin. Immunol. 2014;21:587–593. doi: 10.1128/CVI.00773-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schilling A., Macias Parra M., Guiterrez M., Restrepo M.J., Ucros S., Herrera T. Coadministration of a 9-valent human papillomavirus vaccine with meningococcal and Tdap vaccines. Pediatrics. 2015;136:e563–e572. doi: 10.1542/peds.2014-4199. [DOI] [PubMed] [Google Scholar]
- 23.Kosalaraksa P., Mehlsen J., Vesikari T., Forstén A., Helm K., Van Damme P. An open-label, randomized study of a 9-valent human papillomavirus vaccine given concomitantly with diphtheria, tetanus, pertussis and poliomyelitis vaccines to healthy adolescents 11-15 years of age. Pediatr. Infect. Dis. J. 2015;34:627–634. doi: 10.1097/INF.0000000000000694. [DOI] [PubMed] [Google Scholar]
- 24.Olsson S.-E., Villa L., Costa R., Petta C., Andrade R., Malm C. Induction of immune memory following administration of a prophylactic quadrivalent human papillomavirus (HPV) types 6/11/16/18 L1 virus like particle (VLP) vaccine. Vaccine. 2007;25:4931–4939. doi: 10.1016/j.vaccine.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 25.Scherer E., Smith R., Gallego D., Carter J., Wipf G., Hoyos M. A single human papillomavirus vaccine dose improves B cell memory in previously infected subjects. EBioMedicine. EBioMedicine. 2016 doi: 10.1016/j.ebiom.2016.06.042. e pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olsson S.-E., Kjaer S., Sigurdsson K., Iversen O.-E., Hernandez-Avila M., Wheeler C. Evaluation of the quadrivalent HPV 6/11/16/18 vaccine efficacy against cervical and anogenital disease in subjects with serological evidence of prior vaccine HPV type infection. Hum. Vaccin. 2009;5:696–704. doi: 10.4161/hv.5.10.9515. [DOI] [PubMed] [Google Scholar]
- 27.Garland S., Hernandez-Avila M., Wheeler C., Perez G., Harper D., Leodolter S. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N. Engl. J. Med. 2007;356:1928–1943. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 28.FUTURE II Study Group Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N. Engl. J. Med. 2007;356:1915–1927. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 29.Kjaer S., Sigurdsson K., Iversen O.-E., Hernandez-Avila M., Wheeler C., Perez G. A pooled analysis of continued prophylactic efficacy of quadrivalent human papillomavirus (Types 6/11/16/18) vaccine against high-grade cervical and external genital lesions. Cancer Prev. Res. 2009;2:868–878. doi: 10.1158/1940-6207.CAPR-09-0031. [DOI] [PubMed] [Google Scholar]
- 30.Barr E., Gause C., Bautista O., Railkar R., Lupinacci L., Insinga R. Impact of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, 18) L1 virus-like particle vaccine in a sexually active population of North American women. Am. J. Obstet. Gynecol. 2008;198 doi: 10.1016/j.ajog.2007.09.001. (261.e.1-261.e.11) [DOI] [PubMed] [Google Scholar]
- 31.Perez G., Lazcano-Ponce E., Hernandez-Avila M., García P., Muñoz N., Villa L. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) L1 virus-like-particle vaccine in Latin American women. Int. J. Cancer. 2008;122:1311–1318. doi: 10.1002/ijc.23260. [DOI] [PubMed] [Google Scholar]
- 32.Tay E., Garland S., Tang G., Nolan T., Huang L., Orloski L. Clinical trial experience with prophylactic HPV 6/11/16/18 VLP vaccine in young women from the Asia-Pacific region. Int. J. Gynaecol. Obstet. 2008;102:275–283. doi: 10.1016/j.ijgo.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 33.Majewski S., Bosch F., Dillner J., Iversen O.-E., Kjaer S., Muñoz N. The impact of a quadrivalent human papillomavirus (types 6, 11, 16, 18) virus-like particle vaccine in European women aged 16 to 24. J. Eur. Acad. Dermatol. Venereol. 2009;23:1147–1155. doi: 10.1111/j.1468-3083.2009.03266.x. [DOI] [PubMed] [Google Scholar]
- 34.Clark L., Myers E., Huh W., Joura E., Paavonen J., Perez G. Clinical trial experience with prophylactic human papillomavirus 6/11/16/18 vaccine in young black women. J. Adolesc. Health. 2013;52:322–329. doi: 10.1016/j.jadohealth.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 35.E. Joura, A. Giuliano, O.-E. Iversen, O. Bautista, J. Chen, E. Moeller, et al., End-of-study efficacy and immunogenicity of a novel 9-valent HPV L1 virus-like particle vaccine in 16–26 year old women, European Research Organization on Genital Infection and Neoplasia February 4–7, OC 6–7, 2015, Available at: 〈http://www.eurogin.com/2015/images/pdf/eurogin_2015_abstracts_part_2.pdf〉 (Accessed 31 December 2015).
- 36.Neuzil K., Canh do G., Thiem V., Janmohamed A., Huong V., Tang Y. Immunogenicity and reactogenicity of alternative schedules of HPV vaccine in Vietnam: a cluster randomized noninferiority trial. JAMA. 2011;305:1424–1431. doi: 10.1001/jama.2011.407. [DOI] [PubMed] [Google Scholar]
- 37.Mugo N., Ansah N., Marino D., Saah A., Garner E. Evaluation of safety and immunogenicity of a quadrivalent human papillomavirus vaccine in healthy females between 9 and 26 years of age in Sub-Saharan Africa. Hum. Vaccin. Immunother. 2015;11:1323–1330. doi: 10.1080/21645515.2015.1008877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sankaranarayanan R., Prabhu P., Pawlita M., Gheit T., Bhatla N., Muwonge R. Immunogenicity and HPV infection after one, two, and three doses of quadrivalent HPV vaccine in girls in India: a multicentre prospective cohort study. Lancet Oncol. 2016;17:67–77. doi: 10.1016/S1470-2045(15)00414-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levin M., Moscicki A.-B., Song L.-Y., Fenton T., Meyer W., Read J. Safety and immunogenicity of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine in HIV-infected children 7 to 12 years old. Acquir. Immune Defic. Syndr. 2010;55:197–204. doi: 10.1097/QAI.0b013e3181de8d26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Human papillomavirus vaccines: WHO position paper, Weekly Epidemiol. Rec., 89, October 2014, pp. 465–491. [PubMed]
- 41.Donken R., Bogaards J., van der Klis F., Meijer C., de Melker H. An exploration of individual- and population-level impact of the 2-dose HPV vaccination schedule in pre-adolescent girls. Hum. Vaccin. Immunother. 2016;12:1381–1393. doi: 10.1080/21645515.2016.1160978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iversen O.-E., Miranda M.J., Ulied A. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA. 2016;316:2411–2421. doi: 10.1001/jama.2016.17615. [DOI] [PubMed] [Google Scholar]
- 43.Chen J., Gesser R., Luxembourg A. A seamless phase IIB/III adaptive outcome trial: design rationale and implementation challenges. Clin. Trials. 2015;12:84–90. doi: 10.1177/1740774514552110. [DOI] [PubMed] [Google Scholar]
- 44.Luxembourg A., Brown D., Bouchard C., Giuliano A., Iversen O.-E., Joura E. Phase II studies to select the formulation of a multivalent HPV L1 virus-like particle (VLP) vaccine. Hum. Vaccin. Immunother. 2015;11:1313–1322. doi: 10.1080/21645515.2015.1012010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material