Abstract
Background
Human papillomavirus (HPV)-related anal cancer lesions are often found adjacent to the squamocolumnar junction (SCJ). We have assessed the histopathology and associated HPV genotypes in anal SCJ lesions in surgically excised anal warts in HIV-negative and –positive patients.
Methods
Histopathology identified 47 squamous intraepithelial lesions (SILs) adjacent to the SCJ amongst a total of 145 cases of clinically diagnosed anal condylomata. The anal SCJ lesions were further analyzed with p16, CK7 and p63 immunohistochemistry and HPV genotyping.
Results
Sixteen (16/47) of the excised anal wart lesions contained HSIL; Three were HSIL and exclusively associated with oncogenic HPVs. A further thirteen (13/47) were mixed lesions. Of these eight were HSILs with LSIL and six were HSILs with papillary immature metaplasia (PIM); Ten of the mixed lesions were associated with one or more oncogenic HPVs, while three cases were exclusively associated with HPV6.
Conclusions
Clinically diagnosed anal warts cannot be assumed to be limited to low-grade lesions as anal warts of the SCJ often show heterogeneous lesions, with coexistence of LSIL, PIM, and HSIL. Lesions showing PIM, however, may mimic HSIL, because they are hypercellular, but lack the nuclear atypia and conspicuous mitotic activity of HSIL; and are p16 negative.
Keywords: Anal squamocolumnar junction, Low-grade squamous intraepithelial lesion (LSIL), High-grade squamous intraepithelial lesion (HSIL), Papillary immature metaplasia (PIM), HPV, HIV
Highlights
-
•
Anal warts of the SCJ are often heterogeneous; with LSIL, including PIM and HSIL.
-
•
Anal HSILs only with HPV6 may indicate that LR-HPVs have some role in oncogenesis.
-
•
Recognition of PIM is important given its potential for being confused with HSIL.
-
•
Clinical diagnosis of anal warts cannot be assumed to be limited to LSIL.
-
•
Microscopic examination of anal warts is encouraged with appropriate immunostains.
1. Introduction
Anal cancer is a relatively rare disease in the general population, with an incidence of 1–3% of all tumors of the digestive system [1], [2]. Anal canal squamous intraepithelial lesions (SILs) caused by certain human papillomaviruses (HPVs) play a fundamental role in anal carcinogenesis [3], [4], [5], [6]. Moreover, anal carcinoma and related precursor lesions have been increasing in incidence in the last five decades by 2% per year, for both men and women [2], [3]. In agreement with the proposed infectious etiology [7], anal cancer is especially common among men who have sex with men (MSM), as well as in patients infected by human immunodeficiency virus (HIV). HIV-positive MSM show the highest risk of anal cancer [3], [7], [8]. Women with cervical and vulvar cancer, and patients receiving immunosuppressive treatment have also been demonstrated to have an increased risk of anal cancer compared with the general population [3].
A two-faceted classification system for HPV-driven anogenital lesions has been previously established: i) low-grade squamous intraepithelial lesions (LSILs) such as condylomata and not considered cancer precursor lesions; and ii) high-grade squamous intraepithelial lesions (HSILs) and considered cancer precursor lesions [9], [10]. Interestingly, however, it has been shown that anogenital LSILs may carry foci of HSIL [11], [12], [13]. The juxtaposition of multiple histopathological patterns is interpreted as the confluence of adjacent independent lesions associated with independent HPV infections [13]. Such a view, follows the logic of the possible clonal origin of warts introduced by Murray and colleagues [14] and recently revisited as “one-virus, one-lesion” [15]. This pattern of mixed histopathology in the same lesion, often concurrent with the detection of multiple HPVs, is especially common in immunocompromised patients [13], [16]. Such complex lesions among anal LSILs, containing a HSIL component, pose a complex diagnostic problem [16], [17].
The microanatomy of anal mucosa includes transitional epithelia of the squamocolumnar junction (SCJ), but in contrast to the uterus cervix, with a unique population of stratified columnar cells covering the single layered basal cells [18]. These two different cell types in the anal transitional zone are not homogeneously susceptible to HPV-driven carcinogenesis [18]. Indeed, previous findings suggest that an abrasion of the columnar cells within the anal transition zone is required to expose the basal cells for persistent HPV-infection and further carcinogenic transformation [19].
The aim of this study was to histologically classify anal warts of the SCJ among HIV-negative and –positive patients in accordance with the LAST classification [9], [10] and standard terminology [20] and to associate the repertoire of HPV genotypes present within each SIL type classification.
2. Materials and methods
2.1. Sample biopsies
This study was approved (09/24/2010) by the Human Research Ethics Committee of Royal Perth Hospital (EC 2010/093) and the IDIBELL - Bellvitge Biomedical Research Institute/ Catalan Institute of Oncology as part of a retrospective review of HPV infections in anogenital lesions. This study was done in accordance with the Declaration of Helsinki, and written informed consent was obtained from each patient prior to surgical treatment. A total of 145 formalin-fixed paraffin embedded (FFPE) surgical anal canal lesion cases were analyzed from non-HPV-vaccinated patients with known HIV status, attending the Sexual Health Clinic at Royal Perth Hospital, Perth, Australia, between 1995 and 2011 (Fig. 1). Biopsy cases were collected from patients clinically identified with anal warts and who thus required surgical excisional treatment to remove the warts. All patients consented to surgery. Each FFPE sample was processed for histology and for viral DNA analysis, using a sandwich sectioning technique as previously reported [21].
Fig. 1.
Schematic diagram of the 47 FFPE anal squamous intraepithelial lesion (SIL) cases of the squamocolumnar junction (SCJ).
2.2. Histopathology diagnosis and case selection
One-hundred and forty five biopsies of the anal canal were reviewed by two pathologists (OC and VMM) using hematoxylin-eosin stained sections. Forty-seven cases showing diagnostic features of SIL, and involving the anal SCJ were selected for further histopathological classification. The histological features of the anal SCJ were identified using hematoxylin-eosin stained sections. To estimate the cellular origin of the HPV-related anal SILs we used CK7 immunostaining to identify CK7-positive SILs presumed to have arisen from SCJ columnar cells or related progeny [18], [22]. However, a note of caution is needed as CK7 is not entirely a specific marker to evaluated the explicit SCJ origin of the HPV-related anal or cervical lesions [18], [22]. Alternatively, cytokeratin 17 (CK17) and p63 have been suggested to be useful for anal lesion immunohistopathology but these biomarkers are mainly useful in cases of invasive anal squamous cell carcinomas and with limited use in cases of anal SILs [23], [24].
2.3. Histological classification in accordance with LAST
Criteria for LSIL [10], [25]: LSILs were lesions with proliferative squamous cells having abnormal nuclear features, including increased nuclear size, irregular nuclear membranes, and increased nuclear to cytoplasmic ratio. Lesions with little cytoplasmic maturation in the lower third of the epithelium, and maturation observed in the middle third and upper third of the epithelium. The presence of cytopathic effect of HPV (i.e. koilocytosis) could also be observed, including multinucleation, nuclear enlargement, and pleomorphism, accompanied by perinuclear halos.
In addition, cases classified as LSIL, but showing areas of Papillary Immature Metaplasia (PIM or "immature condyloma"), a distinct pathological subtype of LSIL, were identified [26], [27], [28], [29], [30]. PIM is not specifically addressed in the LAST classification, however, its recognition is important because it is a distinct subset of exophytic LSIL, typically p16-negative, but with a potential to mimic HSIL, which is recognized by the LAST [10], [28]. PIM lesions showed papillae with thin fibrovascular cores, lined by immature squamous cells, with only low levels of mitosis in basal cells, and typically showing CK7 positive columnar cells on the surface [26], [27], [28], [29], [30]. To further assess the mixed population of columnar and basal squamous cells often found in PIM lesions we performed p63 immunostaining, in addition with CK7, for selected cases.
Criteria for HSIL [10], [25]: Lesions with proliferative squamous cells with abnormal nuclear features, including increased nuclear size, irregular nuclear membranes, and increased nuclear to cytoplasmic ratios accompanied by mitotic figures. Lesions with little or no cytoplasmic maturation in the middle third and superficial third of the epithelium. In HSILs mitotic figures were not confined to the lower third of the epithelium, but were also found in the middle and/or superficial third of the epithelium. The presence of HSIL was further assessed with p16-positive IHC [31].
2.4. Immunohistochemistry
Immunohistochemical staining for CK7 and p16 biomarkers were performed on 3 µm sections of FFPE specimens using primary rabbit or mouse anti-human monoclonal antibody C clone SP52 for CK7 (Ventana Inc, US) and clone E6H4 for p16 (Ventana Inc, US) at a dilution of 0.5 mg/mL or 1.0 mg/mL, respectively. Additional immunohistochemical staining for p63 biomarker was perfomed using mouse anti-human monoclonal antibody clone 4A4 (Ventana Inc, US) at a dilution of 0.140 ng/mL. The staining was performed on a Ventana Bechmark LT automated immunostainer (Ventana Medical Systems Inc., Tucson, AZ) in accordance with standard protocols. Overexpression for p16 was considered positive when immunostaining was basal and ascending with strong nuclear and cytoplasmic positivity (Fig. 2B). When the staining was absent, patchy or discontinuous it was considered p16 negative (Fig. 2B). Expression of CK7 in multiple contiguous cells was classified as positive while diffuse or patchy staining was considered negative. Only nuclear staining for p63 was considered positive (Fig. 2C).
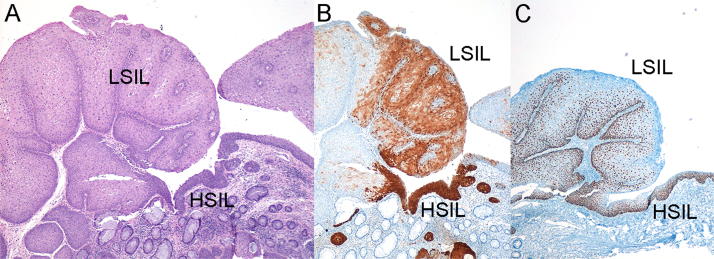
Fig. 2.
SIL of the anal SCJ showing coexistence of LSIL and HSIL in a HIV-positive patient: A) Hematoxylin-eosin staining. LSIL is exophytic, while HSIL is limited to a flat area; B) Strong and diffuse local p16 immunostaining in the HSIL component but only patchy staining in the LSIL component of the lesion; C) Nuclear p63 immunostaining. HPV genotyping was positive for HPV6. Original magnification x4.
2.5. HPV Genotyping
Total DNA was obtained from the FFPE samples as described [32] using proteinase K incubation and inactivation. Sample DNA quality and the presence of HPV DNA in the biopsies was further determined using two different detection methods, SPF10-DEiA-LiPA25 and HSL-PCR/Luminex systems as well as broad-papillomavirus PCR sequencing as previously reported [22]. In this study we referred to HPVs as oncogenic high-risk HPVs as classified by the International Agency for Research on Cancer into carcinogenic or probably or possibly carcinogenic agents, while HPVs commonly observed in anogenital infections but not yet considered carcinogenic were referred to as non-oncogenic low-risk HPVs [33].
2.6. Data analysis
Data analysis using STATA (StataCorp, US) was performed on the total of 47 anal SIL cases of the anal SCJ shown in Fig. 1. For association analysis between histopathological categories and HPV genotypes, a Fisher´s exact test with a significance level at 0.05 for p-value was used. Prevalence ratios of the histopathological categories and HPV genotypes between HIV-positive and –negative patients were estimated using the Poisson regression model with robust variance.
3. Results
To characterize the histopathology and associated HPV infections in anal SCJ lesions clinically diagnosed as genital warts we investigated 145 anal SIL cases previously reported [21] and shown in Fig. 1. From the total of 47 clinically diagnosed anal warts, involving the anal canal SCJ, 16 were diagnosed as HSIL and 31 as LSIL (Table 1).
Table 1.
Histopathology of the 47 anal squamous intraepithelial lesions (SILs) of the squamocolumnar junction.
| LAST | TYPE | N | % | p16+ |
HPV genotypes |
|||
|---|---|---|---|---|---|---|---|---|
| n | high-risk HPV | n | low-risk HPV | |||||
| HSIL 34% (16/47) | HSIL | 3 | 6.4 | 2/3 | 3/3 | HPV16 (x2), HPV52-91 | – | – |
| HSIL+LSIL | 8 | 17.0 | 8/8 | 76.9% (10/13) | HPV18-58, HPV18-34-39–56, HPV31-45, HPV51, HPV56, HPV6-51 | 23.1% (3/13) | HPV6 (x2) | |
| HSIL+LSIL+PIM | 5 | 10.6 | 5/5 | HPV16, HPV6-16, HPV6-18, HPV11-52 | HPV6 | |||
| LSIL 66% (31/47) | LSIL+PIM | 12 | 25.5 | 0/12 | 16.7% (2/12) | HPV6-18-40–43, HPV52 | 83.3% (10/12) | HPV6 (x3), HPV6-43, HPV6-11, HPV11 (x5) |
| LSIL | 19 | 40.4 | 0/7* | – | – | 19/19 | HPV6 (x9), HPV11 (x5), HPV6-11, HPV6-44, HPV6-7, HPV6-74, HPV43 | |
LAST, Lower Anogenital Squamous Terminology classification; HSIL, high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion. TYPE, lesion type classified at microscopic level as HSIL or LSIL; HSIL+LSIL, LSIL with foci of HSIL; HSIL+LSIL+PIM, mixed HSIL and LSIL including focal papillary immature metaplasia (PIM); LSIL+PIM, LSIL including focal PIM.
N, Number of cases; %, percentage of the lesion types; p16+, Number of positive p16 immunostaining cases in each category; HPV, Human Papillomavirus. In case of several samples within the same LAST category showed exactly the same HPV types the number of cases is indicated in the parenthesis (e.g. HPV6 (2x)). * Only seven cases were further tested with p16 immunostaining.
high-risk HPV, at least one oncogenic HPV type (i.e. HPV16, 18, 31, 34, 39, 45, 51, 52, 53, 56, 58) observed in the biopsy.
low-risk HPV, only non-oncogenic HPV types (i.e. HPV6, 11, 7, 40, 43, 44, 74, 91) observed in the biopsy.
Thirteen HSIL lesions combined features of LSIL (Fig. 1, Fig. 2, see also Supplementary Figs. 1 and 2), and five of these showed also PIM (Fig. 3, see also Supplementary Figs. 3 and 4). PIM lesions adopted a characteristic papillary growth pattern, while HSILs were flat (Fig. 3, see also Supplementary Figs. 3–5). At least one high-risk HPV type was identified in 13 HSILs, but three cases of mixed HSIL and LSIL were exclusively associated with HPV6 (Table 1, Fig. 2, see also Supplementary Fig. 3). Immunostaining of p16 was positive in the corresponding areas of HSIL in 15 cases (Figs. 2B and 3B, see also Supplementary Figs. 1–5), and negative in one case associated with HPV52 and HPV91 (Fig. 4, see also Table 1). All 31 cases with LSIL, including 12 with PIM (Fig. 5, see also Supplementary Figs. 6–10), were p16 negative. Of 31 biopsies classified as LSIL or LSIL with PIM, 29 were associated with non-oncogenic HPVs, and two cases with at least one oncogenic HPV (Table 1). CK7 showed positive immunostaining of columnar cells in all anal SCJ lesions likely arisen from SCJ columnar cells or related progeny (Figs. 4D and 5D, see also Supplementary Figs. 1,3,6–10). In addition, p63 biomarker showed wide nuclear immunostaining of anal SCJ SILs (Figs. 2C, 3C and 4E, see also Supplementary Figs. 2,4,5,8–10). Due to the demonstrated changes in host immune response and the higher risk of HPV related disease in HIV-infected patients [6] we further analyzed the data, stratifying by HIV-status. A significant difference (P=0.011) between HIV-negative and –positive patients was observed for the prevalence values of different SIL categories, associated either with at least one oncogenic or only with non-oncogenic HPVs (Table 2).
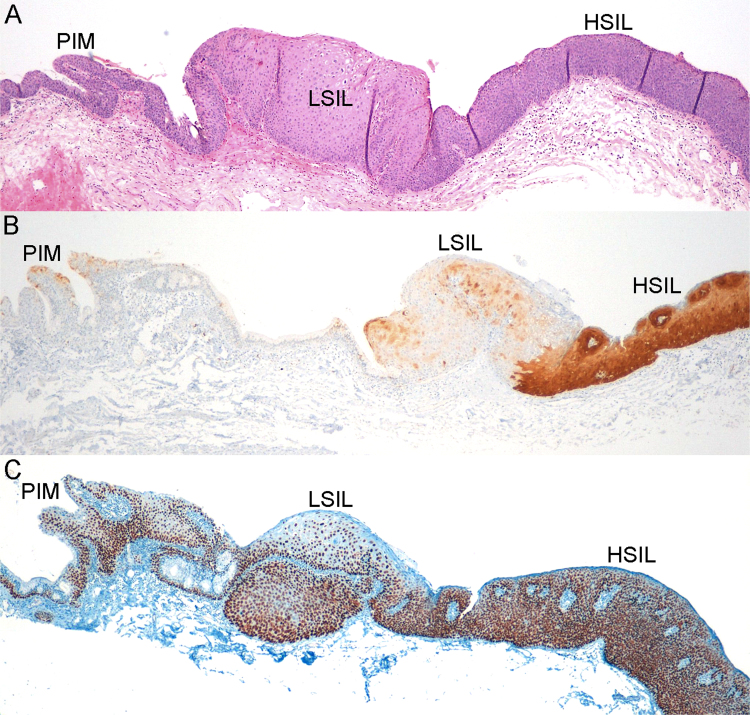
Fig. 3.
SIL of the anal SCJ showing coexistence of PIM, LSIL and HSIL in a HIV-positive patient: A) Hematoxylin-eosin staining; B) Strong and diffuse local p16 immunostaining limited to HSIL component of the lesion; C) Strong nuclear p63 immunostaining. HPV genotyping was positive for HPV6 and HPV16. Original magnification x4.
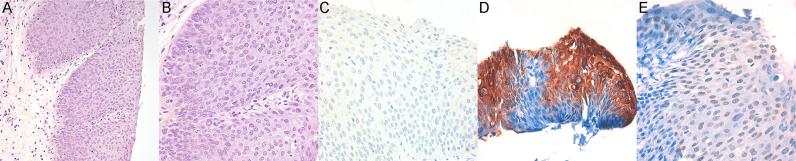
Fig. 4.
HSIL of the anal SCJ showing increased cellularity, and loss of surface maturation: A) Hematoxylin-eosin staining with 10 x original magnification; B) Higher magnification showing increased nuclear-cytoplasmic ratio and abnormal mitosis. Hematoxylin-eosin staining with 40 x original magnification; C) Negative p16 immunostaining, 20 x original magnification; D) Positive CK7 immunostaining, 40 x original magnification; E) Nuclear p63 immunostaining, 40 x original magnification. HPV genotyping was positive for HPV52 and HPV91.
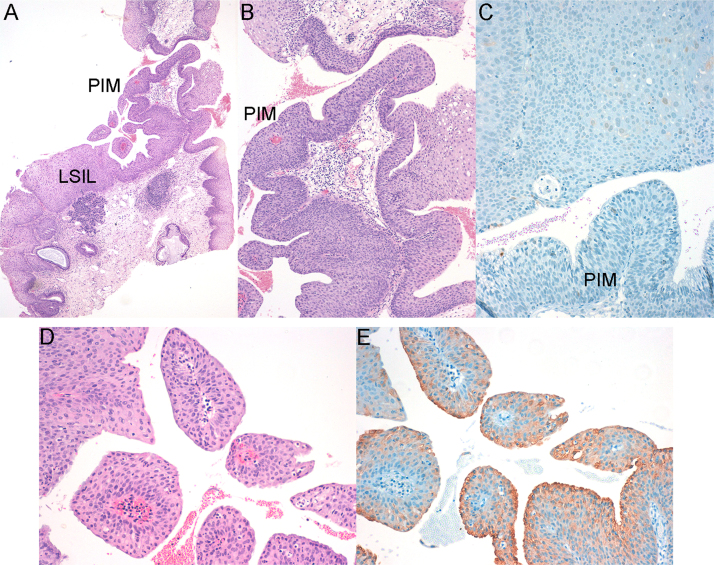
Fig. 5.
SIL of the anal SCJ showing LSIL, and contiguous area of PIM: A) SIL of the anal SCJ showing LSIL, and contiguous area of PIM. Original magnification x4; B) The area of PIM is densely cellular compared with LSIL. Original magnification x10; C) Negative p16 immunostaining, 20 x original magnification; D) PIM showing papillae with thin fibrovascular cores lined by stratified epithelium with columnar cells on the surface. Significant nuclear atypia or mitotic activity was not observed. Original magnification x20; E) Columnar cells on the surface of papillae were CK7 positive. Original magnification x20. HPV genotyping was positive for HPV6.
Table 2.
Prevalence contribution of anal squamous intraepithelial lesions (SILs) of the squamocolumnar junction and associated oncogenic/non-oncogenic HPV infections in HIV-negative and –positive patients.
| LSIL or HSIL | SILs and associated HPVs | N HIV- (%) | N HIV+(%) | PR | 95% CI |
|---|---|---|---|---|---|
| LSIL | LSIL and LR HPVs | 12 (46.2) | 7 (33.3) | Ref. | – |
| LSIL+PIM and LR HPVs | 9 (34.6) | 1 (4.8) | 0.3 | 0.04–1.95 | |
| LSIL+PIM and HR HPV | – | 2 (9.5) | 2.7 | 1.50–4.92 | |
| HSIL+LSIL | HSIL+LSIL and LR HPVs | 1 (3.9)* | 2 (9.5) | 1.8 | 0.66–4.94 |
| HSIL+LSIL and HR HPV | 1 (3.9) | 5 (23.8) | 2.3 | 1.1–4.5 | |
| HSIL+LSIL+PIM and HR HPV | 2 (7.7) | 2 (9.5) | 1.4 | 0.43–4.31 | |
| HSIL | HSIL and HR HPV | 1 (3.9) | 2 (9.5) | 1.8 | 0.66–4.94 |
| Fisher exact test p-value | 0.011 | ||||
| Wald test p-value | <0.002 | ||||
Prevalence ratio (PR) and 95% confidence interval (CI) for robust Poisson multivariate regression model. One multinomial variable with seven categories were used to evaluate the associations between lesion type, oncogenic HPVs and HIV-status of the patient.LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; LSIL+PIM, LSIL with focal PIM; HSIL+LSIL+PIM, mixed HSIL and LSIL with a focal PIM. LR HPVs, only non-oncogenic low-risk HPVs; HR HPVs, at least one oncogenic high-risk HPV observed in the case. HIV-, HIV-negative; HIV+, HIV-positive. LSILs with only low-risk HPVs was used as reference group (Ref) in the multinomial variable. Oncogenic high-risk HPV types observed in this study are presented in Table 1. *This sample also presented focal PIM.
Anal SCJ LSILs with PIM and linked with non-oncogenic HPV infection were more prevalent, although not significantly, in HIV-negative (34.6%) compared with HIV-positive (4.8%) patients. In contrast, anal SCJ LSILs with PIM and oncogenic HPV infection were only seen in HIV-positive patients. Mixed anal SCJ HSIL with LSIL cases associated with at least one oncogenic HPV were seen 2.3 times more frequently in HIV –positives compared with HIV-negative cases [95%CI: 1.10 – 4.50]. No trend with HIV co-infection was observed for the prevalence of HSIL with LSIL mixed lesions associated exclusively with non-oncogenic HPVs.
4. Discussion
The causal role of oncogenic HPVs in anal carcinogenesis, [6] particularly within SCJ, [34] is regarded analogous to the development of cervical cancer. [35] Persistent infection of oncogenic HPV in basal cells of the SCJ induces the local development of SIL and typically further carcinogenesis [36]. However, a recent study reveals significant anatomical differences between cervical and anorectal SCJ associated with HPV-related cancer risk [18]. In contrast to the single-layered columnar cells covering the basal cells of the cervical SCJ, the anal SCJ is lined by a multilayer of columnar cells. This difference between the SCJs of anus and cervix have been proposed as potentially explaining the 17-fold higher incidence of HPV-induced cervical cancers compared with anal cancers worldwide [18]. However, regarding the HPV-driven anal lesions, it is important to recall the correlation between the type of SIL and the HPV type detected [9], [10]. Clinically diagnosed condylomata, the most common subtype of LSILs, are mostly induced by non-oncogenic HPV types (mainly HPV6 or HPV11), and these lesions are not considered significant precursors of anal cancer not withstanding rare reports [10]. Conversely, HSILs (as well as many LSILs) are induced by oncogenic HPV types. These lesions are considered precursors of anal squamous cell carcinomas [6], [9]. Earlier studies, however, have shown that anal condyloma, considered a subset of LSIL, [3] may also harbour foci of HSIL or even invasive carcinoma [16]. Moreover, low-grade anal lesions can be caused by high-risk HPVs and vice versa [21], [37].
In this study, we found that 34.0% (16/47) of the clinically diagnosed anal warts of the SCJ were HSILs by histopathology, and 76.9% (13/16) of these HSILs were combined with LSIL. This is in line with previous studies of cervical and vulvar intraepithelial lesions showing that an extensive exophytic growth of low-grade lesions may obscure an adjacent/mixed high-grade lesion [11], [12], [13]. Moreover, we found that five of these cases containing both LSIL and HSIL contained also focal PIM. The association is of interest for two reasons. First, PIM has been previously reported to be a unique subset of exophytic LSIL, also been termed "immature condyloma", but with a potential to mimic HSIL [26], [27], [28], [29], [30]. In addition, PIM has been reported in association with HSIL. Second, PIM in the cervix is assumed to arise via infection of immature metaplastic epithelium with low-risk HPVs [27], [30]. Similar lesions can also be found near the urethra, manifesting with a transitional phenotype that may prompt the diagnosis of "transitional papilloma". Their frequent finding in the anus is of interest in as much as their presence there could signify infection of the immature squamous cells near the anal SCJ.
Taking into account the unique nature of the anal transformation zone and the fact that many of these patients are immunosuppressed, it is not unexpected that biopsies of anal warts of the SCJ are frequently heterogeneous, combining features of LSIL, including PIM, and HSIL in 27.6% (13/47) of the cases in this study. This heterogeneity was confirmed through p16 immunohistochemistry (IHC), showing overexpression limited to HSIL, and areas of LSIL and PIM being p16-negative (see Fig. 3). However, a note of caution is needed in the use of adjunct p16 IHC as according to LAST [11] 10–20% of less developed HSILs can be p16 negative. Indeed, in our study we observed one HSIL with p16 negative IHC (Fig. 4), but as recommended by LAST [10], histopathology diagnosis was not downgraded. In addition, lesions with PIM, often hypercellular and showing a mixed population of columnar and basal squamous cells, previously described in cervix and anus, [27], [28], [29], [30], [38] are of special interest in the study of anal warts due to their potential mimicry of HSIL. This in turn might further lead to failure in diagnosing clinically more important high-grade lesions, or overclassifying HSIL by failure to recognize PIM.
In this study, we identified a good concordance between the histological diagnosis of anal HSIL and the detection of oncogenic HPV types. All HSIL biopsies and 76.9% (10/13) of the mixed HSIL with LSIL cases were associated with oncogenic HPV infection. However, in three cases the diagnosis of focal HSIL, also with p16 positivity, was exclusively associated with HPV6. This is an interesting observation as some studies suggest that p16 expression with HPV6 may not always occur [39]. In accord, it has been demonstrated that non-oncogenic HPV6 or HPV11 infections can also be exclusively associated with anogenital HSIL and squamous carcinoma [40], [41], [42]. These results may indicate a role of HPV6 associated with malignant transformations of the anal SCJ. Finally, our cases showing LSIL and PIM (N=12), without HSIL, were associated with non-oncogenic HPV6 or HPV11, although, oncogenic HPV types were detected in two cases. These evidence, however, needs to be treated with caution as no further analysis were performed to identify the transcriptionally active HPV type within a particular anal lesion component.
Although the use of p16 immunostaining to predict HSIL is limited in the cervix by the multiplicity of high risk HPV types found with low-grade morphologies, p16 staining was particularly helpful in sorting out lesions combining both low and high risk HPVs in the anal SCJ, especially in the case of PIM (Fig. 3, see also Supplementary Figs. 3 and 4). Additional biomarkers CK7 and p63 were also helpful in identifying the mixed population of columnar and basal squamous cells respectively often found in PIM lesions (see Supplementary Fig. 10). However, these biomarkers were only of additional use for the histopathological distinction between HSIL and PIM.
Simultaneous infection with several oncogenic and/or non-oncogenic HPVs is commonly observed in lower genital tract SILs, especially among HIV-positive patients [6], [13], [16]. In agreement, we observed that more than half of the anal SCJ mixed LSIL with HSIL histology lesions showed the presence of at least two different HPV infections, mainly in HIV-infected patients. These result may further suggest that focal HSIL mixed with LSIL and/or PIM might be independent lesions caused by independent HPV infections, in accordance with the clonal etiology of HPV-induced lesions [14], [15]. Nevertheless, we are aware that our study is limited regarding the distinction between transcriptionally active HPVs and HPVs only present at the DNA level in cases associated with multiple HPVs infections. Moreover, with the current data, we can not specify for certainty if a particular HPV DNA is present only in a specific lesion component (i.e. HSIL/LSIL/PIM) in multiple HPV-infected mixed-lesion cases. Future work using a larger data set and also with RNA-level viral detection combined with laser capture microdissection would allow to detect the precise presence of transcriptionally active HPVs in different anal lesions of the SCJ, and thus, more accurately evaluate their connection with anal cancer development.
In summary excised anal lesions of the SCJ often were heterogeneous; with coexistence of LSIL, including PIM, and HSIL. The recognition of PIM, considered an immature variant of exophytic condyloma, was particularly important given its frequent association with low risk HPVs on one hand and its potential for being confused with or coexisting with HSILs. Our results also showed that mixed anal HSIL with LSIL lesions of the SCJ were at least twice more common in HIV-infected than in HIV-negative patients. The presence of low-risk HPV6 alone in cases of HSIL is of uncertain significance. However, rare anogenital HSILs and squamous carcinomas have been reported with HPV6 infection alone and such uncommon associations may bear further study (Huang E, Howitt B, Crum, CP, personal communication). Taken together that the SCJ is particularly susceptible to HPV infections in the cervix [36] and to a some extent in the anus [19] further indicates that this transitional epithelia retains a unique role in the development of SIL and cancer risk in connection to anal HPV infections. In biopsies of SIL in or around the anal SCJ, histology, p16 IHC, and HPV genotyping are helpful in assigning a precise classification and grade, particularly given the frequency of multiple infections and phenotypes seen in immunosuppressed individuals. These results indicate that a clinical diagnosis of anal warts in patients requiring surgical excision treatment cannot be assumed to be limited to low-grade lesions, and microscopic examination of anal wart tissue is encouraged, especially among anal cancer high-risk groups, with appropriate immunostains when the histologic picture is ambiguous.
Author contribution
Conceived the project: OC and VNP. Performed clinical diagnosis, tissue sampling and collected patient information: JM. Performed histopathology analysis and interpretation: OC VMM. Generated HPV data and performed the analysis: VNP. Contributed to the study design and data interpretation: JM VMM BQ IGB SS XFB. Supervised the project and wrote the manuscript: VNP. All authors assisted in the writing of the manuscript and had final approval of the submitted and published versions.
Funding
This research was financially supported by The European Society of Clinical Microbiology and Infectious Diseases (ESCMID Research Grant 2015 VNP), the late Spanish Ministry for Science and Innovation (Grant CGL2010-16713), Spanish Ministry for Economy and Competitivity (PFIS Grant FI12/0142; FIS Grant PI11/02096), and the Red Temática de Investigación Cooperativa en Cáncer (RTICC Grant RD06/0020/0095). None of these agencies had any role in the interpretation of the results, nor in the preparation of this manuscript.
Acknowledgements
The authors want to thank the patients willing to participate in the study, as well as Cecily Metcalf and Dugald McCallum for reporting on the original specimens in Australia. Laura Pallares, Richard Darwin, Gemma Florensa, Jo-Ellen Klaustermeyer, Marleny Vergara, Yolanda Florencia and Vanessa Camón are warmly thanked for technical support as well as Laia Alemany is greatly thanked for helpful comments of the manuscript. Silvia de Sanjose has received occasional travel grants from Merck and Qiagen. F. Xavier Bosch is on the Advisory Board (Merck and Co., Inc.); Speakers Bureau (GlaxoSmithKline); and has received Institutional Research Grants (Merck and Co., Inc., Sanofi Pasteur MSD, GlaxoSmithKline) not related to the study presented. Finally, the authors are indebted to Christopher Crum for his helpful review of the manuscript.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.pvr.2016.12.001.
Appendix A. Supplementary material
Supplementary Figure 1. Squamous intraepithelial lesion of the anal SCJ showing coexistence of LSIL and HSIL and associated with HPV6 and HPV18 in a HIV-negative patient : A) Hematoxylin-eosin staining, 10x original magnification; B) Strong local p16 immunostaining, 10x original magnification; C) Strong local CK7 immunostaining, 10x original magnification.
.
Supplementary Figure 2. SIL of the anal SCJ showing coexistence of LSIL and HSIL and associated with HPV6 and HPV16 in a HIV-positive patient: A) Hematoxylin-eosin staining, 10x original magnification; B) Strong and diffuse local p16 immunostaining limited to HSIL component, 10x original magnification; C) Local nuclear p63 immunostaining, x20 original magnification.
.
Supplementary Figure 3. SIL of the anal SCJ showing coexistence of LSIL, including PIM and HSIL and associated exclusively with HPV6 in a HIV-negative patient: A) Hematoxylin-eosin staining, 4x original magnification; B) Strong and diffuse local p16 immunostaining limited to HSIL component, 4x original magnification; C) Strong positive CK7 immunostaining, x4 original magnification; C) Higher magnification of positive CK7 immunostaining, x10 original magnification.
.
Supplementary Figure 4. SIL of the anal SCJ showing coexistence of PIM and HSIL and associated with HPV16 in a HIV-positive patient: A) Hematoxylin-eosin staining, 4x original magnification; B) Strong and diffuse local p16 immunostaining limited to HSIL component, 4x original magnification; C) Nuclear p63 immunostaining in the HSIL component of the lesion, x40 original magnification.
.
Supplementary Figure 5. High-grade squamous intraepithelial lesion (HSIL) of the anal squamocolumnar junction (SCJ) associated with HPV16 in a HIV-negative patient: A) Hematoxylin-eosin staining, 20x original magnification; B) Strong p16 immunostaining, 20x original magnification; C) Strong nuclear p63 immunostaining, x40 original magnification.
.
Supplementary Figure 6. LSIL of the anal SCJ showing PIM and associated with HPV6 and HPV43 in a HIV-negative patient: A) Hematoxylin-eosin staining, 4x original magnification; B) Negative p16 immunostaining, 4x original magnification; C) Positive CK7 immunostaining, x4 original magnification.
.
Supplementary Figure 7. LSIL of the anal SCJ showing PIM and associated with HPV11 in a HIV-negative patient: A) Hematoxylin-eosin staining, 10x original magnification; B) Negative p16 immunostaining, 10x original magnification; C) Strong positive CK7 immunostaining, x10 original magnification.
.
Supplementary Figure 8. LSIL of the anal SCJ showing PIM and associated with HPV11 in a HIV-negative patient: A) Hematoxylin-eosin staining, 4x original magnification; B) negative p16 immunostaining, 4x original magnification; C) Local positive CK7 immunostaining, x4 original magnification; D) Nuclear p63 immunostaining, x10 original magnification.
.
Supplementary Figure 9. LSIL of the anal SCJ showing PIM and associated with HPV6 in a HIV-negative patient: A) Hematoxylin-eosin staining, 10x original magnification; B) negative p16 immunostaining, 10x original magnification; C) Positive CK7 immunostaining, x10 original magnification; D) Nuclear p63 immunostaining, x10 original magnification.
.
Supplementary Figure 10. LSIL of the anal SCJ showing PIM and associated with HPV6 and HPV11 in a HIV-positive patient: A) Hematoxylin-eosin staining, 10x original magnification; B) Discontinuous negative p16 immunostaining, 10x original magnification; C) Positive CK7 immunostaining, x10 original magnification; D) Local nuclear p63 immunostaining, x10 original magnification.
.
References
- 1.J.K. Greenson, ed., Diagnostic Pathology Gastrointestinal, First Ed., Amirsys, Salt Lake City, Utah, 2010.
- 2.Johnson L.G., Madeleine M.M., Newcomer L.M., Schwartz S.M., Daling J.R. Anal cancer incidence and survival: the surveillance, epidemiology, and end results experience, 1973–2000. Cancer. 2004;101:281–288. doi: 10.1002/cncr.20364. [DOI] [PubMed] [Google Scholar]
- 3.Palefsky J.M., Giuliano A.R., Goldstone S., Moreira E.D., Aranda C., Jessen H., Hillman R., Ferris D., Coutlee F., Stoler M.H., Marshall J.B., Radley D., Vuocolo S., Haupt R.M., Guris D., Garner E.I.O. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N. Engl. J. Med. 2011;365:1576–1585. doi: 10.1056/NEJMoa1010971. [DOI] [PubMed] [Google Scholar]
- 4.Fenger C., Bichel P. Flow cytometric DNA analysis of anal canal epithelium and ano-rectal tumours. Acta Microbiol. Scand. A. 1981;89:351–355. doi: 10.1111/j.1699-0463.1981.tb00232.x. 〈http://www.ncbi.nlm.nih.gov/pubmed/7315332〉 (accessed 09.09.14) [DOI] [PubMed] [Google Scholar]
- 5.Abramowitz L., Benabderrahmane D., Ravaud P., Walker F., Rioux C., Jestin C., Bouvet E., Soulé J.-C., Leport C., Duval X. Anal squamous intraepithelial lesions and condyloma in HIV-infected heterosexual men, homosexual men and women: prevalence and associated factors. AIDS. 2007;21:1457–1465. doi: 10.1097/QAD.0b013e3281c61201. [DOI] [PubMed] [Google Scholar]
- 6.Berry J.M., Jay N., Cranston R.D., Darragh T.M., Holly E.A., Welton M.L., Palefsky J.M. Progression of anal high-grade squamous intraepithelial lesions to invasive anal cancer among HIV-infected men who have sex with men. Int. J. Cancer. 2014;134:1147–1155. doi: 10.1002/ijc.28431. [DOI] [PubMed] [Google Scholar]
- 7.Fenger C., Nielsen V.T. Intraepithelial neoplasia in the anal canal. The appearance and relation to genital neoplasia. Acta Pathol. Microbiol. Immunol. Scand. A. 1986;94:343–349. doi: 10.1111/j.1699-0463.1986.tb03003.x. [DOI] [PubMed] [Google Scholar]
- 8.Bosman F., Carneiro F., Hruban R., Theise N. Fourth ed. IARC Press; WHO: 2010. WHO Classification of Tumours of the Digestive System. [Google Scholar]
- 9.Northfelt D.W., Swift P.S., Palefsky J.M. Anal neoplasia. pathogenesis, diagnosis, and management. Hematol. Oncol. Clin. North Am. 1996;10:1177–1187. doi: 10.1016/s0889-8588(05)70392-0. 〈http://www.ncbi.nlm.nih.gov/pubmed/8880204〉 (accessed 14.10.14) [DOI] [PubMed] [Google Scholar]
- 10.Darragh T.M., Colgan T.J., Thomas Cox J., Heller D.S., Henry M.R., Luff R.D., McCalmont T., Nayar R., Palefsky J.M., Stoler M.H., Wilkinson E.J., Zaino R.J., Wilbur D.C. The lower anogenital squamous terminology Standardization project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and cervical pathology. Int. J. Gynecol. Pathol. 2013;32:76–115. doi: 10.1097/PGP.0b013e31826916c7. [DOI] [PubMed] [Google Scholar]
- 11.Park J., Sun D., Genest D.R., Trivijitsilp P., Suh I., Crum C.P. Coexistence of low and high grade squamous intraepithelial lesions of the cervix: morphologic progression or multiple papillomaviruses? Gynecol. Oncol. 1998;70:386–391. doi: 10.1006/gyno.1998.5100. [DOI] [PubMed] [Google Scholar]
- 12.Agorastos T., Miliaras D., Lambropoulos A.F., Chrisafi S., Kotsis A., Manthos A., Bontis J. Detection and typing of human papillomavirus DNA in uterine cervices with coexistent grade I and grade III intraepithelial neoplasia: biologic progression or independent lesions? Eur. J. Obstet. Gynecol. Reprod. Biol. 2005;121:99–103. doi: 10.1016/j.ejogrb.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 13.Maniar K.P., Ronnett B.M., Vang R., Yemelyanova A. Coexisting high-grade vulvar intraepithelial neoplasia (VIN) and condyloma acuminatum: independent lesions due to different HPV types occurring in immunocompromised patients. Am. J. Surg. Pathol. 2013;37:53–60. doi: 10.1097/PAS.0b013e318263cda6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray R.F., Hobbs J., Payne B. Possible clonal origin of common warts (Verruca vulgaris) Nature. 1971;232:51–52. doi: 10.1038/232051a0. [DOI] [PubMed] [Google Scholar]
- 15.Quint W., Jenkins D., Molijn A., Struijk L., van de Sandt M., Doorbar J., Mols J., Van Hoof C., Hardt K., Struyf F., Colau B. One virus, one lesion--individual components of CIN lesions contain a specific HPV type. J. Pathol. 2012;227:62–71. doi: 10.1002/path.3970. [DOI] [PubMed] [Google Scholar]
- 16.Longacre T.A., Kong C.S., Welton M.L. Diagnostic problems in anal pathology. Adv. Anat. Pathol. 2008;15:263–278. doi: 10.1097/PAP.0b013e318183234b. [DOI] [PubMed] [Google Scholar]
- 17.Palefsky J.M., Darragh T.M. Classification of anal squamous intraepithelial lesions. Pathol. Case Rev. 2013;18:200–208. [Google Scholar]
- 18.Yang E.J., Quick M.C., Hanamornroongruang S., Lai K., Doyle L.A., McKeon F.D., Xian W., Crum C.P., Herfs M. Microanatomy of the cervical and anorectal squamocolumnar junctions: a proposed model for anatomical differences in HPV-related cancer risk. Mod. Pathol. 2015;28:994–1000. doi: 10.1038/modpathol.2015.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts J.N., Buck C.B., Thompson C.D., Kines R., Bernardo M., Choyke P.L., Lowy D.R., Schiller J.T. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat. Med. 2007;13:857–861. doi: 10.1038/nm1598. [DOI] [PubMed] [Google Scholar]
- 20.Andrews J.C., Bogliatto F., Lawson H.W., Bornstein J. Speaking the Same Language: Using Standardized Terminology. J. Low. Genit. Trac. Dis. 2016;20:8–10. doi: 10.1097/LGT.0000000000000157. [DOI] [PubMed] [Google Scholar]
- 21.Pimenoff V.N., Félez-Sánchez M., Tous S., Clavero O., Godínez J.M., Klaustermeier J., Saunier M., Molijn A., Alemany L., Quint W., Bosch F.X., de Sanjosé S., McCloskey J., Bravo I.G. Disagreement in high-grade/low-grade intraepithelial neoplasia and high-risk/low-risk HPV infection: clinical implications for anal cancer precursor lesions in HIV-positive and HIV-negative MSM. Clin. Microbiol. Infect. 2015;21:e11–e19. doi: 10.1016/j.cmi.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Huang E.C., Tomic M.M., Hanamornroongruang S., Meserve E.E., Herfs M., Crum C.P. P16ink4 and cytokeratin 7 immunostaining in predicting HSIL outcome for low-grade squamous intraepithelial lesions: a case series, literature review and commentary. Mod. Pathol. 2016 doi: 10.1038/modpathol.2016.141. [DOI] [PubMed] [Google Scholar]
- 23.Owens S.R., Greenson J.K. Immunohistochemical staining for p63 is useful in the diagnosis of anal squamous cell carcinomas. Am. J. Surg. Pathol. 2007;31:285–290. doi: 10.1097/01.pas.0000213362.10756.d3. [DOI] [PubMed] [Google Scholar]
- 24.Nazarian R.M., Primiani A., Doyle L.A., Linskey K.R., Duncan L.M., Odze R.D., Zukerberg L.R. Cytokeratin 17: an adjunctive marker of invasion in squamous neoplastic lesions of the anus. Am. J. Surg. Pathol. 2014;38:78–85. doi: 10.1097/PAS.0000000000000111. [DOI] [PubMed] [Google Scholar]
- 25.Kurman R.J. Tumors of the cervix, vagina and vulva. AFIP Atlas of Tumor Pathology. 1st ed. The American Registry of Pathology in collaboration with the Armed Forces Institute of Pathology; Washinton, DC: 2010. [Google Scholar]
- 26.Kang G.H., Min K., Shim Y.H., Kim K.R. Papillary immature metaplasia of the uterine cervix: a report of 5 cases with an emphasis on the differential diagnosis from reactive squamous metaplasia, high-grade squamous intraepithelial lesion and papillary squamous cell carcinoma. J. Korean Med. Sci. 2001;16:762–768. doi: 10.3346/jkms.2001.16.6.762. 〈http://www.ncbi.nlm.nih.gov/pubmed/11748359〉 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang C.-W., Lin M.-C., Hsiao C.-H., Lin Y.-T., Kuo K.-T. Papillary squamous intraepithelial lesions of the uterine cervix: human papillomavirus-dependent changes in cell cycle expression and cytologic features. Hum. Pathol. 2010;41:326–335. doi: 10.1016/j.humpath.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 28.Roberts J.M., Cornall A.M., Ekman D., Law C., Poynten I.M., Jin F., Hillman R.J., Templeton D.J., Tabrizi S.N., Garland S.M., Thurloe J.K., Grulich A.E., Farnsworth A. Papillary immature metaplasia of the anal canal: a low-grade lesion that can mimic a high-grade lesion. Am. J. Surg. Pathol. 2015 doi: 10.1097/PAS.0000000000000549. [DOI] [PubMed] [Google Scholar]
- 29.Darragh T., Jay N., Berry M., Costa M.D., Ralston M., Holly E., Palefsky J. Papillary lesions of the anal canal. J. Low. Genit. Trac. Dis. 1999;3:36. doi: 10.1097/00128360-199901000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Ward B.E., Saleh A.M., Williams J.V., Zitz J.C., Crum C.P. Papillary immature metaplasia of the cervix: a distinct subset of exophytic cervical condyloma associated with HPV-6/11 nucleic acids. Mod. Pathol. 1992;5:391–395. 〈http://www.ncbi.nlm.nih.gov/pubmed/1323109〉 [PubMed] [Google Scholar]
- 31.Bala R., Pinsky B.A., Beck A.H., Kong C.S., Welton M.L., Longacre T.A. p16 is superior to ProEx C in identifying high-grade squamous intraepithelial lesions (HSIL) of the anal canal. Am. J. Surg. Pathol. 2013;37:659–668. doi: 10.1097/PAS.0b013e31828706c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Godínez J.M., Nicolás-Párraga S., Pimenoff V.N., Mengual-Chuliá B., Muñoz N., Bosch F.X., Sánchez G.I., McCloskey J., Bravo I.G. Phylogenetically related, clinically different: human papillomaviruses 6 and 11 variants distribution in genital warts and in laryngeal papillomatosis. Clin. Microbiol. Infect. 2014;20:406–413. doi: 10.1111/1469-0691.12420. [DOI] [PubMed] [Google Scholar]
- 33.IARC, Human papillomaviruses, IARC Monogr. Eval. Carcinog. Risks Hum. 90 (2007) pp. 1–636. [PMC free article] [PubMed]
- 34.Fritsch H., Zehm S., Illig R., Moser P., Aigner F. New insights into the development and differentiation of the human anorectal epithelia. Are there clinical consequences? Int. J. Colorectal Dis. 2010;25:1231–1242. doi: 10.1007/s00384-010-0986-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bosch F.X., Lorincz A., Muñoz N., Meijer C.J.L.M., Shah K.V. The causal relation between human papillomavirus and cervical cancer. J. Clin. Pathol. 2002;55:244–265. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herfs M., Yamamoto Y., Laury A., Wang X., Nucci M.R., McLaughlin-Drubin M.E., Münger K., Feldman S., McKeon F.D., Xian W., Crum C.P. A discrete population of squamocolumnar junction cells implicated in the pathogenesis of cervical cancer. Proc. Natl. Acad. Sci. USA. 2012;109:10516–10521. doi: 10.1073/pnas.1202684109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siegenbeek van Heukelom M.L., Richel O., de Vries H.J.C., van de Sandt M.M., Beck S., van den Munckhof H.A.M., Pirog E.C., de Koning M.N.C., Prins J.M., Quint K.D. Low- and high-risk human papillomavirus genotype infections in intra-anal warts in HIV-positive men-who-have-sex-with-men. Br. J. Dermatol. 2016 doi: 10.1111/bjd.14567. [DOI] [PubMed] [Google Scholar]
- 38.Trivijitsilp P., Mosher R., Sheets E.E., Sun D., Crum C.P. Papillary immature metaplasia (immature condyloma) of the cervix: a clinicopathologic analysis and comparison with papillary squamous carcinoma. Hum. Pathol. 1998;29:641–648. doi: 10.1016/s0046-8177(98)80016-6. [DOI] [PubMed] [Google Scholar]
- 39.Sano T., Oyama T., Kashiwabara K., Fukuda T., Nakajima T. Expression status of p16 protein is associated with human papillomavirus oncogenic potential in cervical and genital lesions. Am. J. Pathol. 1998;153:1741–1748. doi: 10.1016/S0002-9440(10)65689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cornall A.M., Roberts J.M., Garland S.M., Hillman R.J., Grulich A.E., Tabrizi S.N. Anal and perianal squamous carcinomas and high-grade intraepithelial lesions exclusively associated with “low-risk” HPV genotypes 6 and 11. Int. J. Cancer. 2013;133:2253–2258. doi: 10.1002/ijc.28228. [DOI] [PubMed] [Google Scholar]
- 41.Guimerà N., Lloveras B., Lindeman J., Alemany L., van de Sandt M., Alejo M., Hernandez-Suarez G., Bravo I.G., Molijn A., Jenkins D., Cubilla A., Muñoz N., de Sanjose S., Bosch F.X., Quint W. The occasional role of low-risk human papillomaviruses 6, 11, 42, 44, and 70 in anogenital carcinoma defined by laser capture microdissection/PCR methodology: results from a global study. Am. J. Surg. Pathol. 2013;37:1299–1310. doi: 10.1097/PAS.0b013e31828b6be4. [DOI] [PubMed] [Google Scholar]
- 42.Guimerà N., Lloveras B., Alemany L., Iljazovic E., Shin H.-R., Jung-Il S., de Sanjose S., Jenkins D., Bosch F.X., Quint W. Laser capture microdissection shows HPV11 as both a causal and a coincidental infection in cervical cancer specimens with multiple HPV types. Histopathology. 2013;63:287–292. doi: 10.1111/his.12137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Squamous intraepithelial lesion of the anal SCJ showing coexistence of LSIL and HSIL and associated with HPV6 and HPV18 in a HIV-negative patient : A) Hematoxylin-eosin staining, 10x original magnification; B) Strong local p16 immunostaining, 10x original magnification; C) Strong local CK7 immunostaining, 10x original magnification.
Supplementary Figure 2. SIL of the anal SCJ showing coexistence of LSIL and HSIL and associated with HPV6 and HPV16 in a HIV-positive patient: A) Hematoxylin-eosin staining, 10x original magnification; B) Strong and diffuse local p16 immunostaining limited to HSIL component, 10x original magnification; C) Local nuclear p63 immunostaining, x20 original magnification.
Supplementary Figure 3. SIL of the anal SCJ showing coexistence of LSIL, including PIM and HSIL and associated exclusively with HPV6 in a HIV-negative patient: A) Hematoxylin-eosin staining, 4x original magnification; B) Strong and diffuse local p16 immunostaining limited to HSIL component, 4x original magnification; C) Strong positive CK7 immunostaining, x4 original magnification; C) Higher magnification of positive CK7 immunostaining, x10 original magnification.
Supplementary Figure 4. SIL of the anal SCJ showing coexistence of PIM and HSIL and associated with HPV16 in a HIV-positive patient: A) Hematoxylin-eosin staining, 4x original magnification; B) Strong and diffuse local p16 immunostaining limited to HSIL component, 4x original magnification; C) Nuclear p63 immunostaining in the HSIL component of the lesion, x40 original magnification.
Supplementary Figure 5. High-grade squamous intraepithelial lesion (HSIL) of the anal squamocolumnar junction (SCJ) associated with HPV16 in a HIV-negative patient: A) Hematoxylin-eosin staining, 20x original magnification; B) Strong p16 immunostaining, 20x original magnification; C) Strong nuclear p63 immunostaining, x40 original magnification.
Supplementary Figure 6. LSIL of the anal SCJ showing PIM and associated with HPV6 and HPV43 in a HIV-negative patient: A) Hematoxylin-eosin staining, 4x original magnification; B) Negative p16 immunostaining, 4x original magnification; C) Positive CK7 immunostaining, x4 original magnification.
Supplementary Figure 7. LSIL of the anal SCJ showing PIM and associated with HPV11 in a HIV-negative patient: A) Hematoxylin-eosin staining, 10x original magnification; B) Negative p16 immunostaining, 10x original magnification; C) Strong positive CK7 immunostaining, x10 original magnification.
Supplementary Figure 8. LSIL of the anal SCJ showing PIM and associated with HPV11 in a HIV-negative patient: A) Hematoxylin-eosin staining, 4x original magnification; B) negative p16 immunostaining, 4x original magnification; C) Local positive CK7 immunostaining, x4 original magnification; D) Nuclear p63 immunostaining, x10 original magnification.
Supplementary Figure 9. LSIL of the anal SCJ showing PIM and associated with HPV6 in a HIV-negative patient: A) Hematoxylin-eosin staining, 10x original magnification; B) negative p16 immunostaining, 10x original magnification; C) Positive CK7 immunostaining, x10 original magnification; D) Nuclear p63 immunostaining, x10 original magnification.
Supplementary Figure 10. LSIL of the anal SCJ showing PIM and associated with HPV6 and HPV11 in a HIV-positive patient: A) Hematoxylin-eosin staining, 10x original magnification; B) Discontinuous negative p16 immunostaining, 10x original magnification; C) Positive CK7 immunostaining, x10 original magnification; D) Local nuclear p63 immunostaining, x10 original magnification.