Abstract
Spinal cord stimulation (SCS) is a promising treatment for disorders of consciousness (DOC), but the underlying mechanism and most effective procedures remain uncertain. To optimize the protocol, previous studies evaluated the frequency-specific effects of SCS on neurophysiological activities. However, whether and how the inter-stimulus interval (ISI) parameter affects the SCS neuromodulation in DOC remains unknown. We enrolled nine DOC patients who had implanted SCS devices and conducted three different durations of ISIs. Using functional near-infrared spectroscopy (fNIRS), we monitored the blood volume fluctuations in the prefrontal and occipital cortices during the SCS. The results showed that short stimuli (30 s) induced significant cerebral blood volume changes, especially in the prefrontal cortex, an important area in the consciousness system. By comparing the mean value of the responses from the first and the last block in each session, a shorter ISI was found to improve the blood volume in the prefrontal cortex. This phenomenon was more significant for the subgroup of patients with a favorable prognosis. These preliminary results imply that the ISI may be an important factor for SCS. The research paradigm proposed here also provides insights for further quantitative evaluations of the therapeutic effects of neuromodulation.
Abbreviations: ARAS, ascending reticular activating system; CBF, cerebral blood flow; DBS, deep brain stimulation; DOC, disorders of consciousness; EEG, electroencephalography; fMRI, functional magnetic resonance imaging; fNIRS, functional near-infrared spectroscopy; FWHM, full-width-at-half-maximum; GOS, Glasgow Outcome Scale; HbO, oxygenated hemoglobin; HbR, deoxygenated hemoglobin; HbT, total hemoglobin; ISI, inter-stimulus interval; JFKCRS-R, JFK Coma Recovery Scale; LTP, long-term potentiation; MBLL, modified Beer-Lambert law; MCS, minimally conscious state; MSN, medium spiny neuron; rCBV, regional cerebral blood volume; SCS, spinal cord stimulation; TMS, transcranial magnetic stimulation; VS, vegetative state
Keywords: Disorders of consciousness, Spinal cord stimulation, Functional near-infrared spectroscopy, Inter-stimulus interval, Prefrontal cortex
Highlights
-
•
Spinal cord stimulation rapidly evokes activity in consciousness-related brain areas.
-
•
Inter-stimulus interval of neuromodulation is important for treating disorders of consciousness.
-
•
Shorter inter-stimulus interval can better improve the blood volume in frontal area.
-
•
Near-infrared spectroscopy is feasible for evaluating neuromodulation effects.
1. Introduction
With great advancements in the emergency care of brain injury, the number of patients with disorders of consciousness (DOC), including the minimally conscious state (MCS) and the vegetative state (VS), has significantly increased (Georgiopoulos et al., 2010, Laureys et al., 2004). However, there is no effective evidence-based procedure for awakening or clinically assessing patients with DOC (Della Pepa et al., 2013, Georgiopoulos et al., 2010).
Alongside a variety of pharmacologic and non-pharmacologic interventions, neuromodulation techniques, such as deep brain stimulation (DBS) (Schiff et al., 2007) and spinal cord stimulation (SCS) (Kanno et al., 2009, Yamamoto et al., 2013, Yamamoto et al., 2012), have been used in the treatment of DOC patients. These techniques intervene directly in the central nervous system to improve the arousal and awareness system (e.g. the mesocircuit) (Della Pepa et al., 2013, Giacino et al., 2014, Guerra et al., 2014). Specifically, SCS utilizes a surgically implanted electrode in the epidural space (at C2-C4) to stimulate the ascending reticular activating system (ARAS) and regulate the awareness circuit (Della Pepa et al., 2013). Although experiments applying SCS to DOC have been few, the prior literature has shown some encouraging effects. The first study investigating the effects of SCS on VS patients reported that 33.3% (2 out of 6) patients displayed clinical improvement (recovered from the vegetative syndrome) (Funahashi et al., 1989). After a few decades, the improvement rate from VS patients after using SCS has been greatly improved to 54% (109 out of 201) (Kanno et al., 2009). As reported by Yamamoto, 70% (7 out of 10) of the patients had recovered from MCS following SCS therapy: they were able to carry out complete functional interactive communication consistently and reliably, and/or demonstrate the functional use of two different objects (Yamamoto et al., 2012). Compared to DBS, SCS is more cost-effective and less invasive with lower risk. SCS is also more feasible and has fewer limitations involving surgical instruments or the patients' condition.
SCS has been found to enhance global cerebral blood flow (CBF) and cerebral glucose metabolism by regulating the sympathetic system (Della Pepa et al., 2013, Visocchi et al., 2011). For instance, CBF can increase by an average of about 22% after SCS (Liu et al., 2008, Yamamoto et al., 2012). In addition, SCS has been found to promote the release of neurotransmitters and neuromodulators, such as dopamine and norepinephrine (but not epinephrine) (Georgiopoulos et al., 2010, Kaplitt, 2013, Liu et al., 2008, Visocchi et al., 2011), and to improve the nerve conduction and electrical activity of the cerebral cortex (Della Pepa et al., 2013, Kaplitt, 2013, Visocchi et al., 2011). However, these pioneering studies primarily focused on global-level effects; the link between consciousness and higher brain activity has not been completely determined.
Designing the parameters, especially the amplitude, frequency, pulse width, continuous or intermittent stimulation pattern, and the inter-stimulus interval (ISI), is a vital step for optimizing neuromodulation therapy using stimulation. Low frequencies (10–40 Hz) are known to induce excitation of neuronal populations, whereas high frequencies (> 60 Hz) inhibit them (Guerra et al., 2014, Yampolsky et al., 2012). However, except of frequency, the other parameters have seldom been investigated. As a matter of fact, designating the parameters in clinical is still primarily based on clinical observations, behavioral evaluations, and chief complaint. But the last one cannot be obtained for patients with DOC because of the lack of consciousness. Actually, which are the best parameters for the patient is still a question. Therefore, it is essential for developing a reliable quantitative evaluation system to assess the effects of neuromodulation and to provide guidance for designing the best parameters, especially for therapy for patients with DOC, who cannot give any subjective feedback. A quantitative evaluation is also essential for developing a closed-loop neuromodulation system. Finding the optimal ISI length (the length of the resting state period after each stimulation period) is, of all the parameters, the one that is most needed to prevent fatigue and damage to the neurons due to excessive continuous stimulation. In fact, the length of the interval between consecutive stimuli has been found to greatly affect the reactivity and persistence of neurons (Huettel et al., 2004, Zhang et al., 2008), but an investigation into different ISIs of the SCS has not yet been done in patients with DOC. Do different ISIs produce different effects? What ISI design is the most appropriate for SCS? To address these issues, it is necessary to investigate the influence of different ISIs of SCS quantitatively.
Functional magnetic resonance imaging (fMRI) and electroencephalography (EEG) are traditional techniques for measuring brain responses (Bruno et al., 2011, Jox et al., 2012). However, neither of these techniques is suitable for assessing real-time brain responses during SCS processing. However, functional near infrared spectroscopy (fNIRS) (Jobsis, 1977), a non-invasive optical neuroimaging technique, is more tolerant of movement artifacts and metal implants than fMRI, is superior to EEG in localizing and segmenting brain regions, and is not subject to interference from electrical stimulation. In addition, fNIRS is cost-effective and portable and can be used in a clinical environment, making it uniquely suitable for longitudinal monitoring and repeated experiments during SCS. Moreover, because fNIRS provides information about multiple specific physiological variables (e.g., the deoxygenated (HbR), oxygenated (HbO), and total (HbT) hemoglobin) (Zeff et al., 2007), it can provide a relatively comprehensive understanding of the hemodynamic changes during SCS. As mentioned above, fNIRS has potential to play a vital role in assessing the real-time modulation of SCS.
In this study, we measured hemodynamic activity using a multiple-channels fNIRS technique in patients with DOC during block-designed SCS with various ISIs. Our research goals were two-fold: to provide insight into the fundamental mechanism of SCS for patients with DOC and to quantitatively assess the neuromodulation effects of different ISIs of SCS to provide some guidance for designing the optimal parameters for SCS.
2. Materials and methods
2.1. Participants
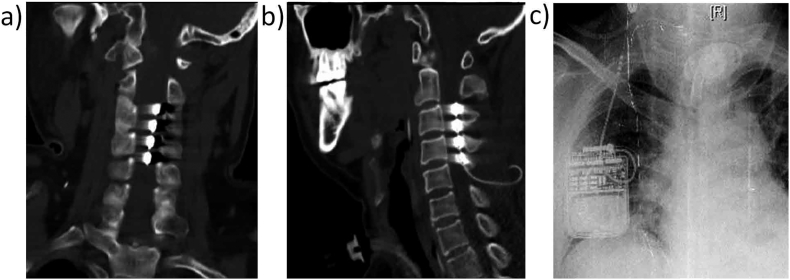
Nine patients (5 males and 4 females, ages 17–64 years) with DOC were recruited from the Department of Neurosurgery, PLA Army General Hospital for this study. The clinical data for all the patients are provided in Table 1. Each patient had had an implanted SCS device (3587A, Medtronic Inc., Minneapolis, Minnesota, USA, as shown in Fig. 1) for about 1 month but had not yet received any SCS treatment. Their consciousness state was assessed twice, before and 1 month after SCS surgery, using the JFK Coma Recovery Scale (JFK CRS-R) (Giacino, 2004). In addition, their prognosis was assessed using the Glasgow Outcome Scale (GOS) at 6 months after SCS surgery. GOS provides a measurement of outcome ranging from 1 to 5 (1, dead; 2, vegetative state/severe disability; 3, able to follow commands/unable to live independently/moderate disability; 4, able to live independently/unable to return to work or school; 5, good recovery/able to return to work or school) (Jennett and Bond, 1975). In this study, any GOS score less than or equal to 2 was defined as “unfavorable prognosis”, whereas a score from 3 to 5 was defined as “favorable prognosis” (PVS TM-STFo, 1994). No other treatments, including drugs that could modify cortical excitability, were administered. Written informed consent for each subject in the study was obtained from the patient's caregivers. The present study was approved by the ethics committee of the PLA Army General Hospital (No2015-026).
Table 1.
Clinical data of patients with disorders of consciousness treated by cervical SCS.
| No. | Diagnosis | Gender | Age (years) | Duration of DOC (months) | Postoperative time of SCS (days) | Etiology | Prognosis | GOS | CRS-R (T0) | CRS-R (T1) |
|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | VS | F | 17 | 11 | 28 | Head trauma | Favorable | 3 | 7(1-1-2-1-0-2) | 10(2-2-3-1-0-2) |
| Patient 2 | VS | M | 17 | 4 | 29 | Head trauma | Unfavorable | 2 | 6(1-0-2-1-0-2) | 6(1-0-2-1-0-2) |
| Patient 3 | VS | F | 64 | 24 | 30 | Cerebral hemorrhage | Unfavorable | 2 | 7(1-1-2-1-0-2) | 7(1-1-2-1-0-2) |
| Patient 4 | VS | M | 54 | 11 | 31 | Cerebral hemorrhage | Favorable | 3 | 8(1-2-2-1-0-2) | 11(2-3-3-1-0-2) |
| Patient 5 | VS | M | 53 | 12 | 30 | Cerebral trauma | Unfavorable | 2 | 6(1-0-2-1-0-2) | 7(1-1-2-1-0-2) |
| Patient 6 | VS | M | 42 | 6 | 29 | Stroke | Unfavorable | 2 | 7(1-1-2-1-0-2) | 7(1-1-2-1-0-2) |
| Patient 7 | MCS | F | 41 | 3 | 29 | Hypoxic ischemic encephalopathy | Favorable | 3 | 8(1-2-2-1-0-2) | 10(2-2-3-1-0-2) |
| Patient 8 | VS | M | 18 | 8 | 30 | Hypoxic ischemic encephalopathy | Unfavorable | 2 | 6(1-0-2-1-0-2) | 7(1-1-2-1-0-2) |
| Patient 9 | MCS | F | 29 | 28 | 31 | Hypoxic ischemic encephalopathy | Favorable | 3 | 9(2-2-2-1-0-2) | 11(2-3-3-1-0-2) |
CRS-R: Coma Recovery Scale- Revised; GOS: Glasgow Outcome Scale; MCS: minimally conscious state; SCS: spinal cord stimulation; T0: time before SCS surgery; T1: 1 months after SCS surgery; VS: vegetative state. The CRS-R includes six subscales addressing auditory, visual, motor, oromotor, communication, and arousal functions, which are summed to yield a total score ranging from 0 to 23. Patient 5 were excluded from post-processing analysis in this study because his data were too noisy maybe resulted from large and excessively head movements during experiments.
Fig. 1.
Location of implanted SCS devices in patient 6. a), b) the SCS electrode was implanted into the epidural space at the C2–C4 level. c) An impulse generator was placed subcutaneously on the anterior chest.
2.2. Study design
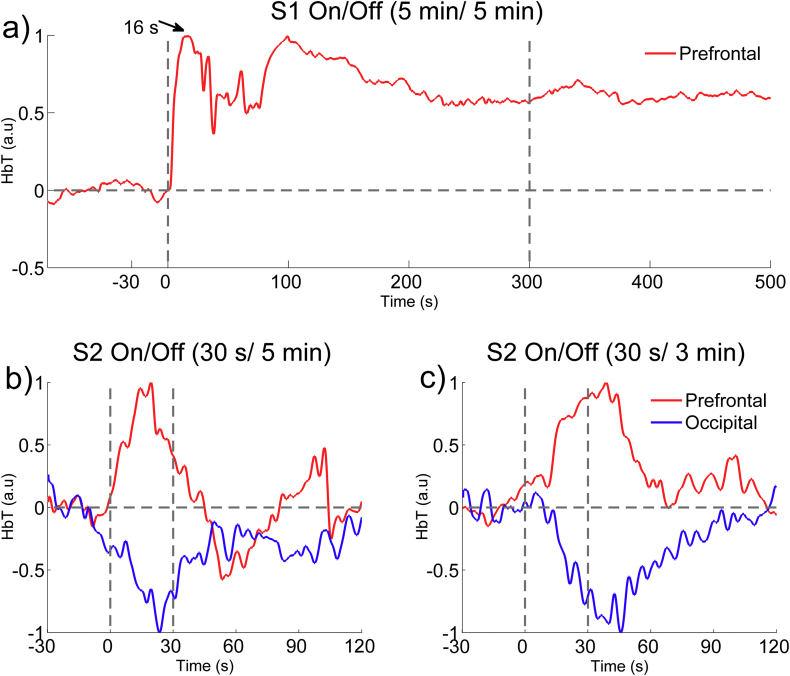
In this study, all the patients received 70 Hz (Bai et al., 2017, Kanno et al., 2009) SCS with a pulse width of 210 μs. The intensity was between 1.0 V and 5.0 V, depending on the patient's tolerance. According to our preliminary results in two pre-experiments with different time lengths for SCS “On” and “Off” periods (see Appendix A), we found the hemodynamic activity changed noticeably within a short period (30 s) during SCS and returned to the baseline in 60 s–90 s. Therefore, to control the overall length of the experiment and to ensure the comfort of the patients, the duration of the SCS was set at 30 s, which was enough to evoke a reliable hemodynamic response. The ISI was set at three levels, each of which was longer than 90 s: 2 min (short), 3 min (medium) and 5 min (long), as shown in Fig. 2 (a). To reduce the noise/artifacts, we repeated the stimulation four times. The SCS was turned off during the ISI “Off” period. The three different ISIs were presented in a pseudo-random order. Between each pair of sessions, the patients were given a 30 min rest to avoid superimposed effects. All the patients underwent the experimental protocol on up to three different days. To eliminate sequence effects, each of the three sessions used a different sequence of ISIs. All the experiments were carried out in a quiet, dimly illuminated, acoustically shielded room.
Fig. 2.
The stimulation paradigm for the experiments and an illustration of the fNIRS probes configuration under SCS. (a) The stimulation paradigm for the experiments. The paradigm consisted of three sessions. Each session consisted of an initial baseline period (1 min) followed by four blocks; each block consisted of an “On” period (30 s) and an “Off” period, which varied in lengths of 2, 3, and 5 min. The sessions were conducted in a randomized order, and the patient was given a 30 min rest after each session. (b) A photograph of our self-designed fNIRS system (NEG8). (c) An illustration of the arrangement of the probes over the prefrontal and occipital areas. (d) An experimental site photograph. Written consent for this photograph was obtained from this patient's caregivers.
2.3. fNIRS recording
The hemodynamic responses were measured using a custom-designed portable fNIRS system with eight channels (Si et al., 2015). As shown in Fig. 2 (c), two probe pads were arranged over the prefrontal and occipital areas. Each probe pad consisted of two sources and two detectors, yielding 4 optical channels. The distance between the source and the detector pairs was 3 cm, and the probe pad covered an area of approximately 3 × 3 cm2 for each brain area. fNIRS signals were recorded using a sampling rate of 100 Hz throughout each day's session of SCS experiments.
2.4. Data analysis
During data preprocessing, one subject (patient 5) were excluded from any further analysis because his data were too noisy maybe resulted from large and excessively head movements. The following data analysis processing was conducted on the MATLAB 2013a platform (The Mathworks Inc., Natick, Massachusetts, USA). First, the original raw optical data was converted to the relative concentration changes of HbO, HbR, and HbT hemoglobin using the modified Beer-Lambert law (MBLL) (Kocsis et al., 2006). We focused on the HbT concentration, because the changes in HbT concentration have been shown to be proportional to changes in regional cerebral blood volume (rCBV) (Ferrari et al., 1992, Ferrari et al., 1987), which is very closely related to CBF, which can be noticeably increased by SCS.
The HbT data were low-pass filtered at 0.3 Hz to remove high-frequency noise; then the data were segmented into blocks from 30 s before the SCS onset to 90 s after the SCS was turned off. The blocks with apparent artifacts (such as step noise resulting from head motion) were rejected based on a visual examination of the data. For the remaining 8 patients, the average trial rejection rate was 17.7%. After a baseline correction (the baseline was calculated based on the recordings during the 30 s period before the SCS onset), the block-averaged HbT concentrations were estimated for each individual and each length of ISI. Following this, the block-averaged responses were averaged across the channels located over the prefrontal and the occipital areas, separately. Finally, a group-averaged hemodynamic response was calculated.
To quantify the characteristics of the block-averaged responses, we estimated their peak amplitude, peak time, onset time, and full-width-at-half-maximum (FWHM) parameters.(Casanova et al., 2008, Thompson et al., 2014) Peak time was defined as the time at which the hemodynamic response reached its maximum amplitude. Onset time was defined as the time at which the response first reached 10% of its peak. FWHM was defined as the width of the curve at 50% of the peak response, indicating the shape of the hemodynamic response (wider or sharper) (Thompson et al., 2014).
3. Results
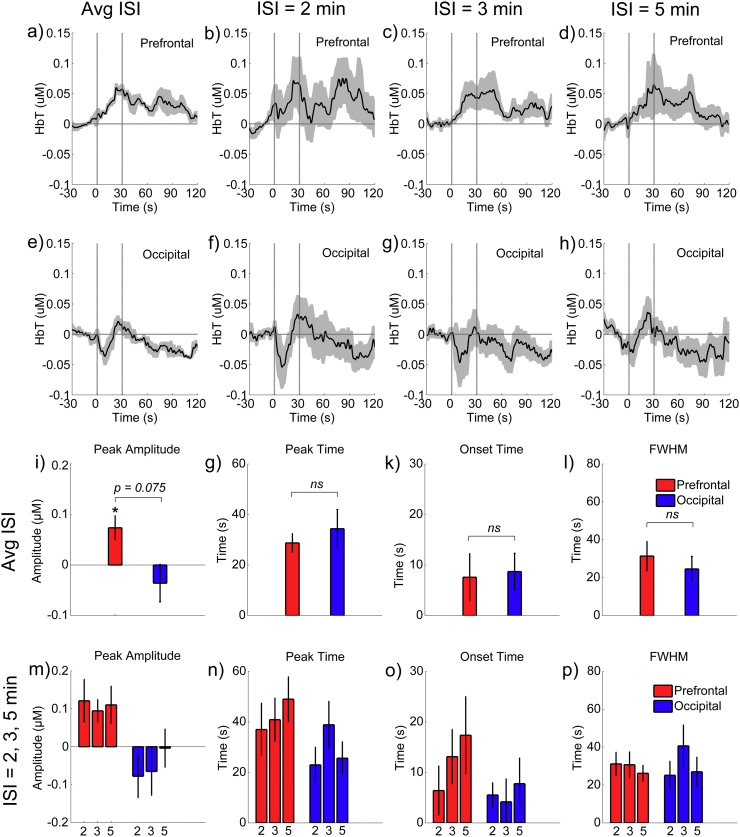
The group-averaged time courses of the hemodynamic responses for SCS in the prefrontal and occipital areas are illustrated in Fig. 3(a and e). Compared to the relatively stable activity during the baseline period (− 30–0 s), the HbT concentrations in both the frontal and occipital areas were noticeably changed after the SCS onset. Quantitatively, for the prefrontal areas, the increase in the HbT variability was significant both during the SCS (p = 0.050) and within 30 s after the SCS was turned off (p = 0.048). The peak amplitudes during the SCS were also significantly positive (p = 0.017) at the group level, as shown in Fig. 3(i). For the occipital area, the increase in HbT variability trended toward significance during the 30 s after the SCS was turned off (p = 0.099). After the SCS had been off for > 30 s, the variability returned to the baseline level (p > 0.1 for both areas). These findings clearly support the existence of a stimulation effect on the rCBV of the brain (at least in the prefrontal and the occipital areas).
Fig. 3.
The mean block-averaged responses to the SCS at the group level in the prefrontal and occipital areas. a) and e) The mean time courses among all the sessions and all the subjects. b, c, d) and f, g, h) the group-averaged time courses over the prefrontal and occipital areas for the sessions when the ISI was 2 min, 3 min, and 5 min, respectively. The shadow areas indicate the standard errors of the mean values across all the subjects and the space between the two vertical gray lines represents the period when the SCS was being administered. i, j, k, l) Comparison between the prefrontal and occipital areas for the mean parameters (peak amplitude, peak time, onset time, and FWHM, respectively) of the responses across all the sessions and the subjects. m, n, o, p) Comparison between the three sessions with different ISI values for the mean parameters (peak amplitude, peak time, onset time, and FWHM, respectively) of the responses across all the subjects. Error bars indicate the standard errors of the means.
The SCS-evoked responses differed between the prefrontal and the occipital areas. As shown in Fig. 3(a), the HbT concentrations in the prefrontal cortex consistently increased during the stimulation and gradually returned to the baseline after the SCS was turned off. These dynamic patterns were highly consistent with the typical hemodynamic responses of the prefrontal cortex to an external task stimulus in normal subjects.(Deepeshwar et al., 2014, Sato et al., 2013) However, such meaningful phenomena did not exist in the occipital cortex. The differences between the peak amplitudes also trended to significance (p = 0.075, as shown in Fig. 3(i). Their differences between the peak times, onset times, and FWHMs were insignificant, as shown in Fig. 3(g-l).
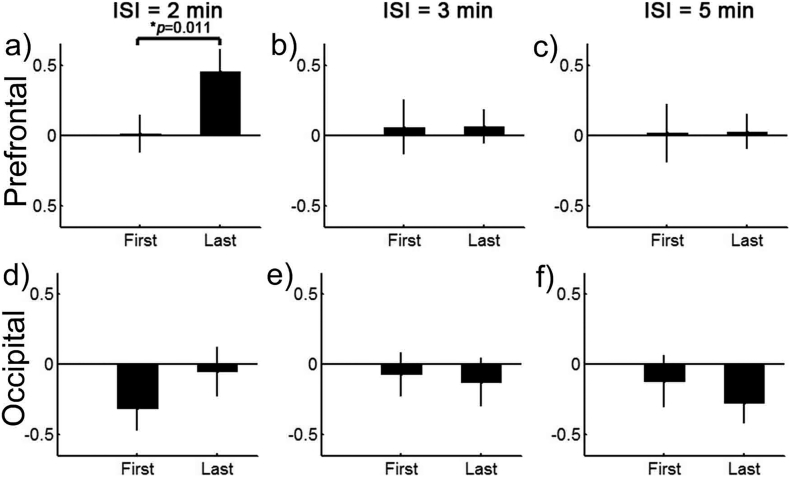
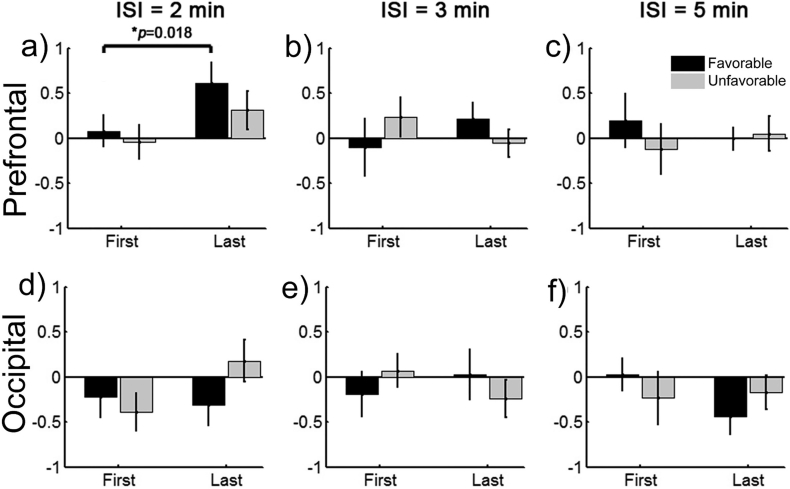
As shown in Fig. 3, the mean SCS-evoked hemodynamic responses were approximately coincident between the three sessions with different ISIs. There were no significant differences between them in peak amplitudes, peak time, onset time, or FWHM. However, the dynamic changes in the mean SCS-evoked amplitudes as the session progressed from the first to the last simulation blocks differed noticeably depending on the length of the ISI (Fig. 4). For clarity, we compared the stimuli-evoked amplitudes of the HbT between the first and the last (the fourth) blocks for each session and each subject. To reduce the effect of the variability between different sessions (maybe related to inherent individual variances and differences in subject's physiological condition between different time periods), we normalized the mean amplitudes of the HbT by dividing by the maximum value of the absolute mean amplitudes among these four blocks. There were a total of 20 sessions for each ISI condition, since the eight patients were involved in 1–3 repetitions of the experiments. As is shown in Fig. 4, for the prefrontal cortex, the mean amplitudes of the HbT concentration among the 20 sessions with an ISI of 2 min improved significantly from the first block to the last block (t = 2.9623, p = 0.011, paired t-test). However, as the blocks of stimulation for the ISI increased to 3 min and 5 min, the results showed a relatively stable distribution of low amplitudes. Although there were some slight changes in the occipital cortex, these differences were not statistically significant (p = 0.361 for 2 min, p = 0.563 for 3 min, and p = 0.371 for 5 min, paired t-test).
Fig. 4.
The dynamic changes in the mean SCS-evoked amplitudes as the stimulation blocks increased in the prefrontal (top row) and the occipital (bottom row) areas when the ISI was 2 min (left column), 3 min (middle column), and 5 min (right column). The bars represent the normalized mean amplitudes of the HbT from 10 s after the SCS onset to 10 s after the SCS off. Error bars indicate the standard errors of the means across all the sessions.
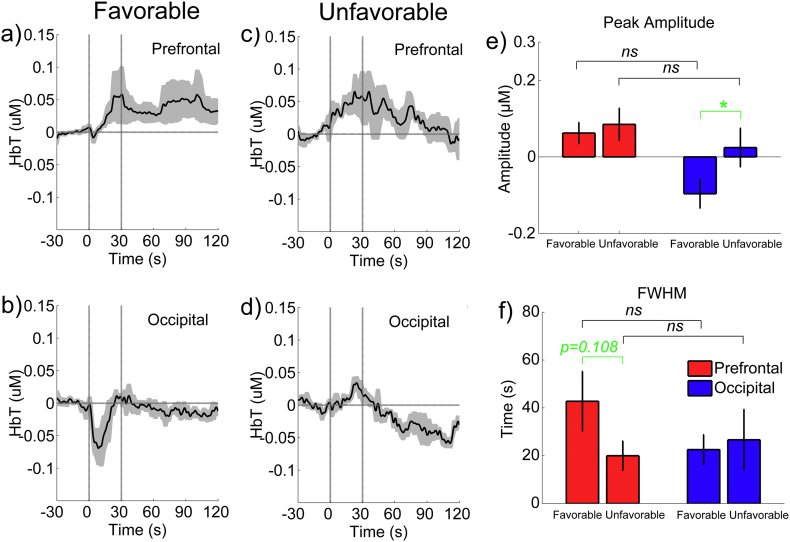
To assess the relationship between the stimulation effects on the rCBV and the prognosis of the individuals, we split the eight patients into two subgroups: one with a favorable prognosis and the other with an unfavorable prognosis (see Table 1). Fig. 5 demonstrates the mean time courses of the SCS-evoked responses for each subgroup. As shown in Figs. 5(a) and (c), the HbT concentration in the prefrontal areas increased consistently for both subgroups after the SCS onset. However, after the SCS was turned off, the HbT reduced rapidly for the subgroup with an unfavorable prognosis, while it appeared to be maintained for a longer period in the subgroup with a favorable prognosis. However the difference in the FWHM was statistically insignificant between the two subgroups (p = 0.108, paired t-test, as shown in Fig. 5 (f)). As shown in Figs. 5(b and d), the HbT concentration in the occipital area decreased rapidly and dramatically (within 10 s) after the SCS onset for the subgroup with a favorable prognosis. However, for the subgroup with an unfavorable prognosis, the HbT concentration in the occipital area did not change significantly during the period of the SCS but decreased dramatically after the SCS was turned off. The difference in the peak amplitude of the HbT between the two subgroups was statistically significant (p = 0.023, paired t-test, as shown in Fig. 5(e)).
Fig. 5.
The group-averaged hemodynamic responses to the SCS between the two subgroups in the prefrontal and occipital areas. “Favorable” and “Unfavorable” indicate the subgroups that had relatively favorable and unfavorable prognoses, respectively.
For each subgroup, we also evaluated the specific influence of the length of the ISI on the dynamic changes in the mean stimuli-evoked amplitudes within the stimulation blocks. As shown in Fig. 6, for the subgroup with a favorable prognosis, the mean amplitudes of the HbT in the prefrontal area improved significantly from the first block to the last block (p = 0.018, Wilcoxon sign rank test) when the ISI was 2 min. However, under the same conditions, although there was a slight improvement in the amplitudes in the prefrontal area for the subgroup with an unfavorable prognosis, it was statistically insignificant (p = 0.237, Wilcoxon sign rank test). The slight changes during the sessions were insignificant for both subgroups when the ISI was either 3 min or 5 min.
Fig. 6.
Dynamic changes in the mean SCS-evoked amplitudes across the stimulation blocks for the two subgroups. The bars represent the normalized mean amplitudes of the HbT from 10 s after the SCS onset to 10 s after the SCS was turned off. Error bars indicate the standard errors of the means of all the sessions.
4. Discussion
This work is the first study to our knowledge that used the fNIRS technique to investigate the ability of SCS to evoke brain activity in consciousness-related brain areas in patients with DOC. This study also quantitatively evaluated the influence of the ISI parameter for the effects of the SCS.
4.1. Effects of the varied ISIs on the hemodynamic responses
Previous research has reached no consistent conclusions about clinical applications of SCS. For instance, Yamamoto et al., 2012 applied SCS for 5 min every 30 min during the daytime, whereas some studies used a cyclic mode with 15 min on and 15 min off (Yamamoto et al., 2012). In fact, designing the parameters for SCS is still totally based on subjective clinical observations. Therefore, developing a reliable quantitative evaluation system to guide the design of the SCS parameters is urgent, especially for patients with DOC, who cannot give any subjective feedback to the neurologists. In this paper, we, for the first time to our knowledge, adopted fNIRS, an emerging non-invasive neuroimaging technique, to investigate the effects of varying the inter-stimulus intervals.
Our results indicated that, although the blood volumes during the mean time courses of the three different ISI sessions were very similar, the dynamic changes in the increases in the mean stimuli-evoked amplitude differed noticeably in the prefrontal area. The prefrontal cortex is an important component underlying awareness in the mesocircuit model (Giacino et al., 2014, Schiff, 2010). With a short ISI of 2 min, the mean amplitudes of the HbT concentration in the prefrontal cortex increased significantly from the first stimulation block to the last. Specifically, this phenomenon was more obvious for the subgroup of patients with a favorable prognosis. For the relatively longer ISIs (3 min, 5 min), the mean amplitudes of the HbT concentration was relatively stable at a low level, without any apparent improvement from the first block to the last. These results may imply that the long-term potentiation (LTP) effects evoked by the short-term SCS stimulations (only 30 s) in our experiments can last at least 2 min but may be depleted after 3 or 5 min. The LTP effect in the central nervous system has been found in animal models and in observations of humans undergoing surgery. A series of brief high-frequency external stimuli can induce a persistent enhancement of the synaptic transmission between two neurons, making the postsynaptic cell's response stronger to subsequent stimuli for a prolonged period of time (Bliss and Collingridge, 1993, Bliss and Lømo, 1973). LTP has been found from the spinal cord to the cerebral cortex and in a number of brain regions. It is considered to be one of the primary underlying mechanisms of learning and memory (Cooke and Bliss, 2006) and has been used to explore the mechanism behind brain disorders, such as Alzheimer's disease, and several neuromodulation techniques (Cooke and Bliss, 2006), such as transcranial magnetic stimulation (TMS) (Stefan et al., 2000) and DBS (van Hartevelt et al., 2014). In our work, LTP effects, specifically, the more blocks the higher the amplitude of the response in the prefrontal area, were only found with a relatively short ISI (2 min) (at least for the first four blocks). Longer ISIs, with a washout interval of 5 min, may be long enough to allow the LTP induced by the previous stimulation block to disappear completely. Therefore, we did not find any accumulation effect under these conditions. Collectively, these findings suggested the importance of the length of interval between the stimulation blocks for SCS, and, specifically, a shorter interval may be better. Excessive frequent stimulations of SCS risk fatiguing the patient, but the therapeutic effects are also limited if the interval is too long. Finding the trade-off between them is absolutely vital and necessary.
4.2. Functional system-specific responses induced by SCS
In our study, the blood volume fluctuations in the prefrontal cortex were significantly improved by intermittent SCS. After the SCS was turned off, the blood volume fluctuations in the prefrontal cortex gradually returned to baseline after remaining at a higher level for a short period. This pattern was consistent across the different sessions and closely resembled the typical hemodynamic response pattern of the prefrontal cortex to an external task stimulus (such as during attention (Deepeshwar et al., 2014) and working memory (Sato et al., 2013)) in normal individuals. Furthermore, compared with the subgroup of patients with an unfavorable prognosis, the subgroup with a favorable prognosis seemingly maintained a high level of blood flow in the prefrontal area for a longer period after the SCS and had greater improvement in blood flow with the stimulation. These findings imply that intermittent SCS can evoke hemodynamic activations in the prefrontal area and that the stimulus-evoked effect may have some functional significance and could relate to consciousness recovery.
In fact, studies have shown that the prefrontal area is an important part of the consciousness system. One piece of evidence comes from converging neuroimaging and neurophysiological data in normal individual studies. By measuring minimal experimental contrasts between nonconscious and conscious processing in normal individuals, “ignition” of a large-scale prefronto-parietal network has been found to be a significant sign of conscious activity, and a number of studies have even pointed to a causal involvement of the prefrontal cortex in conscious perception (Dehaene and Changeux, 2011, Dehaene et al., 2001, Haynes et al., 2005, Naghavi and Nyberg, 2005). Another piece of evidence comes from studies on the dissolution of consciousness resulting from severe acquired brain injury, which has provided a natural model from which key insights about consciousness can be found. For example, using 18F-fluorodeoxyglucose PET, which can be used to measure the brain's metabolic activity, researchers found that patients with DOC showed hypometabolism in the medial prefrontal cortex, frontoparietal associative areas, and thalamus (Laureys et al., 1999a, Laureys and Schiff, 2012, Nakayama et al., 2006). In addition, MRI spectroscopy has been used to measure several metabolic cortical neuronal dysfunctions in the left and right frontal cortex in DOC patients (Ricci et al., 1997). Laureys (2005)) reported that DOC may not be exclusively related to hypometabolism in the frontoparietal network but may also be related to decreased functional connectivity within this network and with the thalamus. Our group's previous study, which used EEG to investigate the effects of SCS, found significantly altered relative power and synchronization in the delta and gamma bands in the frontal area but no significant difference in other brain areas when 70 Hz SCS was conducted (Bai et al., 2017). Moreover, recovery of awareness of patients with DOC has been reported to be accompanied by a functional restoration of the frontoparietal network (Laureys et al., 1999b). To explain the mechanisms of DOC, a “mesocircuit” model with a frontal/prefrontal cortico-striatopallidal-thalamocortical loop system has been proposed (Giacino et al., 2014, Schiff, 2010). In this model, the frontal cortex is strongly innervated by the central thalamus through the thalamocortical projections and also has some efferent excitatory output back into the thalamus and striatum. In all severe brain injuries, widespread disconnection or neuronal death in the frontal/prefrontal cortico-striatopallidal-thalamocortical loop results in sufficient loss of afferent input to the medium spiny neurons (MSNs) of the striatum to prevent these neurons from reaching their firing threshold, which causes reduced regulation of the anterior forebrain function (Giacino et al., 2014, Schiff, 2010).
The noticeable hemodynamic activations in the prefrontal area found in our study may hint at a mechanism for SCS in the treatment of DOC: the electrical stimulus of the SCS emitted in the high-level cervical spinal cord may have a restorative effect on the anterior forebrain mesocircuit, at least in the prefrontal cortex. The electrical stimulus maybe transmitted through the connections from the spinal cord to the pedunculopontine nucleus, then to the central thalamus, striatum, and finally to the cortex. According to the mesocircuit model, such restorative activity of the prefrontal cortex, and possibly in other parts of the transmission pathways, could increase synaptic background activity in the forebrain and potentially arouse the firing of MSNs and other forebrain areas. Eventually, the SCS treatment may enhance cerebral blood flow, cause widespread cerebral glucose metabolism, and release organized behaviors.
For patients who have a favorable prognosis, the hemodynamic responses over the prefrontal area were maintained for a longer time. Moreover, a favorable prognosis is accompanied by a functional restoration of the frontoparietal network and some of its cortico-thalamo-cortical connections. Recent neuroimaging studies have illuminated the relationships between prognosis and global brain function, regional brain function, changes in functional connectivity, and cortical activation of primary versus associative areas in response to external stimulation, highlighting issues about the possible perception of pain (Bonsignore et al., 2014, Laureys et al., 2000, Laureys and Schiff, 2012). These findings may help to explain why the FWHM over the prefrontal area was larger for the favorable prognosis group.
The hemodynamic responses in the occipital cortex fluctuated noticeably after the onset of the SCS, especially in the favorable prognosis patients. It is likely that the SCS also influences the occipital cortex, but the time span of the hemodynamic response in the occipital area may be shorter than the response in the prefrontal area. Moreover, the improvement in the occipital area from the first block to the last block was not significant. This coincides with the mesocircuit hypothesis in which the status of the occipital cortex is not as important as the prefrontal area (Giacino et al., 2014, Laureys, 2005).
According to our results, compared with the increased HbT concentration in the prefrontal area, the changes in the HbT concentration in the occipital cortex dipped noticeably after the onset of the SCS (that is, it first decreased abruptly and greatly and then increased slowly to a low level that was lower than the initial baseline level). This may possibly have resulted from the global balance of cerebral blood volume within the whole brain. However, it may also have been related to systemic physiological changes (such as cardiac pulsation, respiratory signals, and blood pressure changes), which cannot be avoided in fNIRS recordings. SCS may be able to induce global physiological changes by stimulating the sympathetic nervous system (Della Pepa et al., 2013, Georgiopoulos et al., 2010, Visocchi et al., 2011). To identify the cause of this issue, future work should synchronously record physiological fluctuations and fNIRS data to determine whether SCS can induce global physiological changes by stimulating the sympathetic nervous system (Della Pepa et al., 2013, Georgiopoulos et al., 2010, Visocchi et al., 2011).
4.3. Limitations of current study
One of the limitations of this study is that, although the data was processed by a basic filtering algorithm, some noise, such as the physiological noise from the shallow layer of the scalp and the motion noise caused by the relative sliding between the scalp and the probes, could not be completely removed. In further studies, advanced data processing methods and improved experimental configurations should be used to record the hemodynamic responses. A second limitation is that the SCS treatment for each session was conducted only 4 times (blocks); this is a relatively small number to fully investigate the repeatable effects of SCS on the hemodynamic responses of patients with DOC. In further studies, an advanced experimental paradigm with more blocks should be used to provide more information about the effects of SCS. The third limitation is that the optical channels used in this paper were limited. Thus the brain areas measured were just the prefrontal and occipital areas; in further studies, more brain areas should be measured using a more advanced fNIRS system.
Although some limitations could be identified, this fNIRS study should be a meaningful step in the exploration of the effects of SCS for patients with DOC. Our current findings are just the tip of the iceberg. To make the effects of SCS clearer and more clinically applicable, we do not plan to stop at this stage but will adopt advanced technologies to measure the functional signals and to better understand the mechanism of the altered dynamics of circuits in patients with DOC. Moreover, advanced experimental paradigms and data processing methods will be utilized in further studies to investigate the effects of different parameters of SCS on patients with DOC and to provide some insights for clinicians. Last, but not least, more clinical data is needed to tailor the optimal stimulation parameters (frequency, amplitude, pulse width, washout period, etc.) for an individual patient with DOC to give him/her the best chance for recovery. Although these limitations reveal areas that can be improved in future studies, this research is the first study to our knowledge that used the fNIRS technique to investigate the ability of SCS to evoke brain activity in consciousness-related brain areas in patients with DOC. This study also quantitatively evaluated the influence of the ISI parameter on the effects of the SCS and may provide some insights for clinicians using neuromodulation.
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Acknowledgments
Acknowledgments
We appreciate the assistance that Rhoda and Edmund Perozzi, PhDs, provided in proofreading and critiquing this work. This work was partially supported by the National Key Research and Development Program of China (Grant No. 2017YFB1002502), National Natural Science Foundation of China (Grant No. 31571003, No. 81501550, and No. 81600919), and the National Natural Science Foundation of Beijing (Grant No. 7164302).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2017.09.017.
Contributor Information
Jianghong He, Email: he_jianghong@sina.cn.
Tianzi Jiang, Email: jiangtz@nlpr.ia.ac.cn.
Appendix A. Pre-experiments
The following are the supplementary data related to this article.
Supplementary material
Supplementary Fig. S1.
The time courses of the HbT responses to SCS in two pre-experiments. (a) Pre-experiment 1: the On/Off periods of SCS were set at 5 min/5 min. (b and c) Pre-experiment 2: the On/ Off periods of SCS were set at 30 s/5 min, and 30 s/3 min. The red and blue curves represent the normalized changes in the HbT concentration over the prefrontal and the occipital areas, respectively. The stimulus duration is indicated by the space between the two gray vertical lines.
References
- Bai Y., Xia X., Li X., Wang Y., Yang Y., Liu Y. Spinal cord stimulation modulates frontal delta and gamma in patients of minimally consciousness state. Neuroscience. 2017;346:247–254. doi: 10.1016/j.neuroscience.2017.01.036. [DOI] [PubMed] [Google Scholar]
- Bliss T.V., Collingridge G.L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bliss T.V.P., Lømo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsignore L.T., Macri S., Orsi P., Chiarotti F., Alleva E. Coma and vegetative states: state of the art and proposal of a novel approach combining existing coma scales. Ann. Ist. Super. Sanita. 2014;50:241–248. doi: 10.4415/ANN_14_03_07. [DOI] [PubMed] [Google Scholar]
- Bruno M.A., Fernandez-Espejo D., Lehembre R., Tshibanda L., Vanhaudenhuyse A., Gosseries O. Multimodal neuroimaging in patients with disorders of consciousness showing “functional hemispherectomy”. Prog. Brain Res. 2011;193:323–333. doi: 10.1016/B978-0-444-53839-0.00021-1. [DOI] [PubMed] [Google Scholar]
- Casanova R., Ryali S., Serences J., Yang L., Kraft R., Laurienti P.J. The impact of temporal regularization on estimates of the BOLD hemodynamic response function: a comparative analysis. NeuroImage. 2008;40:1606–1618. doi: 10.1016/j.neuroimage.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke S.F., Bliss T.V. Plasticity in the human central nervous system. Brain. 2006;129:1659–1673. doi: 10.1093/brain/awl082. [DOI] [PubMed] [Google Scholar]
- Deepeshwar S., Vinchurkar S.A., Visweswaraiah N.K., Nagendra H.R. Hemodynamic responses on prefrontal cortex related to meditation and attentional task. Front. Syst. Neurosci. 2014;8:252. doi: 10.3389/fnsys.2014.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S., Changeux J.P. Experimental and theoretical approaches to conscious processing. Neuron. 2011;70:200–227. doi: 10.1016/j.neuron.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Dehaene S., Naccache L., Cohen L., Bihan D.L., Mangin J.F., Poline J.B. Cerebral mechanisms of word masking and unconscious repetition priming. Nat. Neurosci. 2001;4:752–758. doi: 10.1038/89551. [DOI] [PubMed] [Google Scholar]
- Della Pepa G.M., Fukaya C., La Rocca G., Zhong J., Visocchi M. Neuromodulation of vegetative state through spinal cord stimulation: where are we now and where are we going? Stereotact. Funct. Neurosurg. 2013;91:275–287. doi: 10.1159/000348271. [DOI] [PubMed] [Google Scholar]
- Ferrari M., Zanette E., Sideri G., Giannini I., Fieschi C., Carpi A. Effects of carotid compression, as assessed by near infrared spectroscopy, upon cerebral blood volume and haemoglobin oxygen saturation. J. R. Soc. Med. 1987;80:83–87. doi: 10.1177/014107688708000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari M., Wilson D.A., Hanley D.F., Traystman R.J. Effects of graded hypotension on cerebral blood flow, blood volume, and mean transit time in dogs. Am. J. Phys. 1992;262:H1908–1914. doi: 10.1152/ajpheart.1992.262.6.H1908. [DOI] [PubMed] [Google Scholar]
- Funahashi K., Komai N., Ogura M., Kuwata T., Nakai M., Tsuji N. Effects and indications of spinal cord stimulation on the vegetative syndrome. No Shinkei Geka. 1989;17:917–923. [PubMed] [Google Scholar]
- Georgiopoulos M., Katsakiori P., Kefalopoulou Z., Ellul J., Chroni E., Constantoyannis C. Vegetative state and minimally conscious state: a review of the therapeutic interventions. Stereotact. Funct. Neurosurg. 2010;88:199–207. doi: 10.1159/000314354. [DOI] [PubMed] [Google Scholar]
- Giacino J.T. The vegetative and minimally conscious states: consensus-based criteria for establishing diagnosis and prognosis. NeuroRehabilitation. 2004;19:293–298. [PubMed] [Google Scholar]
- Giacino J.T., Fins J.J., Laureys S., Schiff N.D. Disorders of consciousness after acquired brain injury: the state of the science. Nat. Rev. Neurol. 2014;10:99–114. doi: 10.1038/nrneurol.2013.279. [DOI] [PubMed] [Google Scholar]
- Guerra A., Costantini E.M., Maatta S., Ponzo D., Ferreri F. Disorders of consciousness and electrophysiological treatment strategies: a review of the literature and new perspectives. Curr. Pharm. Des. 2014;20:4248–4267. [PubMed] [Google Scholar]
- van Hartevelt T.J., Cabral J., Deco G., Møller A., Green A.L., Aziz T.Z. Neural plasticity in human brain connectivity: the effects of long term deep brain stimulation of the subthalamic nucleus in Parkinson's disease. PLoS One. 2014;9 doi: 10.1371/journal.pone.0086496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes J.D., Driver J., Rees G. Visibility reflects dynamic changes of effective connectivity between V1 and fusiform cortex. Neuron. 2005;46:811–821. doi: 10.1016/j.neuron.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Huettel S.A., Obembe O.O., Song A.W., Woldorff M.G. The BOLD fMRI refractory effect is specific to stimulus attributes: evidence from a visual motion paradigm. NeuroImage. 2004;23:402–408. doi: 10.1016/j.neuroimage.2004.04.031. [DOI] [PubMed] [Google Scholar]
- Jennett B., Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- Jobsis F.F. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science. 1977;198:1264–1267. doi: 10.1126/science.929199. [DOI] [PubMed] [Google Scholar]
- Jox R.J., Bernat J.L., Laureys S., Racine E. Disorders of consciousness: responding to requests for novel diagnostic and therapeutic interventions. Lancet Neurol. 2012;11:732–738. doi: 10.1016/S1474-4422(12)70154-0. [DOI] [PubMed] [Google Scholar]
- Kanno T., Morita I., Yamaguchi S., Yokoyama T., Kamei Y., Anil S.M. Dorsal column stimulation in persistent vegetative state. Neuromodulation. 2009;12:33–38. doi: 10.1111/j.1525-1403.2009.00185.x. [DOI] [PubMed] [Google Scholar]
- Kaplitt M.G. Spinal cord stimulation for vegetative state: is this ready for prime time? Stereotact. Funct. Neurosurg. 2013;91:288–289. doi: 10.1159/000348272. [DOI] [PubMed] [Google Scholar]
- Kocsis L., Herman P., Eke A. The modified Beer-Lambert law revisited. Phys. Med. Biol. 2006;51:N91–98. doi: 10.1088/0031-9155/51/5/N02. [DOI] [PubMed] [Google Scholar]
- Laureys S. The neural correlate of (un)awareness: lessons from the vegetative state. Trends Cogn. Sci. 2005;9:556–559. doi: 10.1016/j.tics.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Laureys S., Schiff N.D. Coma and consciousness: paradigms (re)framed by neuroimaging. NeuroImage. 2012;61:478–491. doi: 10.1016/j.neuroimage.2011.12.041. [DOI] [PubMed] [Google Scholar]
- Laureys S., Goldman S., Phillips C., Van Bogaert P., Aerts J., Luxen A. Impaired effective cortical connectivity in vegetative state: preliminary investigation using PET. NeuroImage. 1999;9:377–382. doi: 10.1006/nimg.1998.0414. [DOI] [PubMed] [Google Scholar]
- Laureys S., Lemaire C., Maquet P., Phillips C., Franck G. Cerebral metabolism during vegetative state and after recovery to consciousness. J. Neurol. Neurosurg. Psychiatry. 1999;67:121. doi: 10.1136/jnnp.67.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laureys S., Faymonville M.E., Luxen A., Lamy M., Franck G., Maquet P. Restoration of thalamocortical connectivity after recovery from persistent vegetative state. Lancet. 2000;355:1790–1791. doi: 10.1016/s0140-6736(00)02271-6. [DOI] [PubMed] [Google Scholar]
- Laureys S., Owen A.M., Schiff N.D. Brain function in coma, vegetative state, and related disorders. Lancet Neurol. 2004;3:537–546. doi: 10.1016/S1474-4422(04)00852-X. [DOI] [PubMed] [Google Scholar]
- Liu J.T., Tan W.C., Liao W.J. Effects of electrical cervical spinal cord stimulation on cerebral blood perfusion, cerebrospinal fluid catecholamine levels, and oxidative stress in comatose patients. Acta Neurochir. Suppl. 2008;101:71–76. doi: 10.1007/978-3-211-78205-7_12. [DOI] [PubMed] [Google Scholar]
- Naghavi H.R., Nyberg L. Common fronto-parietal activity in attention, memory, and consciousness: shared demands on integration? Conscious. Cogn. 2005;14:390–425. doi: 10.1016/j.concog.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Nakayama N., Okumura A., Shinoda J., Nakashima T., Iwama T. Relationship between regional cerebral metabolism and consciousness disturbance in traumatic diffuse brain injury without large focal lesions: an FDG-PET study with statistical parametric mapping analysis. J. Neurol. Neurosurg. Psychiatry. 2006;77:856–862. doi: 10.1136/jnnp.2005.080523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PVS TM-STFo Medical aspects of the persistent vegetative state. N. Engl. J. Med. 1994;330:1499–1508. doi: 10.1056/NEJM199405263302107. [DOI] [PubMed] [Google Scholar]
- Ricci R., Barbarella G., Musi P., Boldrini P., Trevisan C., Basaglia N. Localised proton MR spectroscopy of brain metabolism changes in vegetative patients. Neuroradiology. 1997;39:313–319. doi: 10.1007/s002340050415. [DOI] [PubMed] [Google Scholar]
- Sato H., Yahata N., Funane T., Takizawa R., Katura T., Atsumori H. A NIRS–fMRI investigation of prefrontal cortex activity during a working memory task. NeuroImage. 2013;83:158–173. doi: 10.1016/j.neuroimage.2013.06.043. [DOI] [PubMed] [Google Scholar]
- Schiff N.D. Recovery of consciousness after brain injury: a mesocircuit hypothesis. Trends Neurosci. 2010;33:1–9. doi: 10.1016/j.tins.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff N.D., Giacino J.T., Kalmar K., Victor J.D., Baker K., Gerber M. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature. 2007;448:600–603. doi: 10.1038/nature06041. [DOI] [PubMed] [Google Scholar]
- Si J., Zhao R., Zhang Y., Zuo N., Zhang X., Jiang T. Proc. of SPIE. Vol 9305. 2015. A portable fNIRS system with eight channels. 93051B-93051B-93054. [Google Scholar]
- Stefan K., Kunesch E., Cohen L.G., Benecke R., Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123(Pt 3):572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- Thompson S.K., Engel S.A., Olman C.A. Larger neural responses produce BOLD signals that begin earlier in time. Front. Neurosci. 2014;8:159. doi: 10.3389/fnins.2014.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visocchi M., Della Pepa G.M., Esposito G., Tufo T., Zhang W., Li S. Spinal cord stimulation and cerebral hemodynamics: updated mechanism and therapeutic implications. Stereotact. Funct. Neurosurg. 2011;89:263–274. doi: 10.1159/000329357. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Katayama Y., Obuchi T., Kobayashi K., Oshima H., Fukaya C. Spinal cord stimulation for treatment of patients in the minimally conscious state. Neurol. Med. Chir. (Tokyo) 2012;52:475–481. doi: 10.2176/nmc.52.475. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Katayama Y., Obuchi T., Kobayashi K., Oshima H., Fukaya C. Deep brain stimulation and spinal cord stimulation for vegetative state and minimally conscious state. World Neurosurg. 2013;80 doi: 10.1016/j.wneu.2012.04.010. S30.e31-39. [DOI] [PubMed] [Google Scholar]
- Yampolsky C., Hem S., Bendersky D. Dorsal column stimulator applications. Surg. Neurol. Int. 2012;3:S275–S289. doi: 10.4103/2152-7806.103019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeff B.W., White B.R., Dehghani H., Schlaggar B.L., Culver J.P. Retinotopic mapping of adult human visual cortex with high-density diffuse optical tomography. Proc. Natl. Acad. Sci. U. S. A. 2007;104:12169–12174. doi: 10.1073/pnas.0611266104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N., Liu Z., He B., Chen W. Noninvasive study of neurovascular coupling during graded neuronal suppression. J. Cereb. Blood Flow Metab. 2008;28:280–290. doi: 10.1038/sj.jcbfm.9600531. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material