Abstract
Objective
HPV causes ~90% of anal cancer and HPV16 is the type most commonly associated with anal cancer. Gay and bisexual men (GBM) are at greatly increased risk. We investigated patterns of vaccine-preventable anal HPV in older GBM.
Methods
The Study of the Prevention of Anal Cancer (SPANC) is an ongoing, prospective cohort study of HIV-positive and HIV-negative Australian GBM. Participants completed questionnaires and underwent an anal swab for HPV genotyping using Roche Linear Array. We analysed baseline data from SPANC by HPV type, mean number of types, stratified by age and HIV status.
Results
Anal HPV results from 606 (98.2%) of 617 participants (median age 49 years, 35.7% HIV-positive) showed 525 (86.7%) had ≥1 HPV type and 178 (29.4%) had HPV16. Over one third of participants (214, 35.3%) had no nonavalent vaccine-preventable types detected. Two (0.3%) participants had all quadrivalent types and none had all nonavalent vaccine types. HIV-positive participants (p<0.001) and younger participants (p=0.059) were more likely to have more vaccine-preventable HPV types detected.
Conclusion
Anal HPV was highly prevalent in this largely community-based GBM cohort. Vaccine-preventable HPV16 was detected in approximately one third of participants. These findings suggest that the potential efficacy of HPV vaccination of older GBM should be explored.
Keywords: Human papillomavirus, HPV, Anal, Vaccine, Prevalence, Gay and bisexual men, MSM, HIV
1. Introduction
Squamous cell carcinoma of the anal canal (“anal cancer”) is rare, but its incidence has been increasing worldwide for decades [1], [2], [3], [4]. A key feature of anal cancer epidemiology is that its occurrence is heavily concentrated in certain population subgroups. Anal cancer is caused by anal exposure to human papillomavirus (HPV) and for this reason gay and bisexual men (GBM) are at particularly high risk, experiencing rates of anal cancer that are at least 20-fold higher than the general population [5]. The overall rates of anal cancer in people living with HIV are 30-fold higher than in the general population [6], but HIV positive GBM are the most affected population due to their combination of impaired immune function and increased anal HPV exposure, with up to a 100-fold higher incidence of anal cancer reported [7].
Anal cancer is believed to be preceded by persistent infection with high-risk types of HPV (HrHPV), with HPV16 comprising a large majority (75–80%) [8]. The strong association between anal HPV infection and cancer indicates that anal cancer is potentially preventable by HPV vaccination [9], with either the quadrivalent HPV vaccine (4vHPV) or the nonavalent HPV vaccine (9vHPV). There is evidence of HPV vaccine efficacy in prevention of cervical infection and disease in sexually active women aged up to 45 years [10], [11], [12] and in anal infection and disease in GBM aged up to 26 years [13]. Despite HPV-related anal cancer being one of the most pressing health issues in GBM, very few data exist to inform the potential efficacy of HPV vaccination in those aged older than 26 (“older GBM”). There is a concern that many older GBM may be currently HPV infected, and there is evidence that the vaccine does have an impact on current infections [14]. We explored patterns of vaccine-preventable anal HPV in a community-based cohort of older HIV-positive and HIV-negative Australian GBM.
2. Methods
2.1. Study design
The Study of Prevention of Anal Cancer (SPANC) is a prospective cohort study of the epidemiology of anal HPV infection and related cytological and histological anal abnormalities in GBM aged 35 years and older in Sydney, Australia. The methods of the study have been described in detail elsewhere [15]. Briefly, men aged ≥35 years who reported having sex with another man in their lifetime were eligible. Participants were recruited mainly from community-based settings in Sydney, including gay community social events and organizations, as well as referrals from other participants. At the baseline visit, participants completed a detailed risk factor questionnaire and had an anal swab for cytology and HPV DNA testing.
Signed, informed consent was provided by all participants. Ethics approval was granted by the Human Research Ethics Committees of St. Vincent's Hospital, Sydney, the Royal Prince Alfred Hospital, Sydney and the University of New South Wales.
2.2. Anal HPV genotyping
The PreservCyt anal swab specimens were tested for HPV DNA using the Roche Linear Array (LA) HPV genotyping test (Roche Molecular Systems, Alameda, CA, United States). DNA was extracted with the automated MagNA Pure isolation and purification system (Roche) by a modified protocol using 1 ml aliquots of PreservCyt specimens as described previously [16]. The LA HPV genotyping test involves PCR amplification of a 450 bp region of the HPV L1 gene and allows for the identification of 37 HPV types (6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51, 52, 53, 54, 55, 56, 58, 59, 61, 62, 64, 66, 67, 68, 69, 70, 71, 72, 73, 81, 82, 83, 84, IS39 (HPV 82v subtype), and CP6108 (HPV 89) as well as amplification of a 265 bp region of the human ß-globin gene, serving as an internal control. As an in-house modification, samples that produced a negative internal control were retested with half the volume of eluted DNA, in order to reduce inhibition due to bacterial DNA in those samples. In addition, due to possible cross-reactivity of the HPV-52 probe with types 33, 35, and 58 amplicons, samples positive for one or more of HPV-33, 35, and 58 probes were further tested using a real-time PCR assay with an HPV52 specific hydrolysis probe to confirm the presence or absence of HPV52 in addition to those types [17].
2.3. Statistical analyses
The exact binomial method was used to calculate 95% confidence intervals (CIs) for anal HPV prevalence values for each HPV type and vaccine-targeted HPV types, (4vHPV: HPV6, HPV11, HPV16 and HPV18 and 9vHPV: HPV6, HPV11, HPV16, HPV18, HPV31, HPV33, HPV45, HPV52 and HPV58). Generalised linear regression was used for univariate analyses to identify the association of number of vaccine-preventable anal HPV types with age and/or HIV status. A t-test was used to test for trend for means. A rank sum test was then performed as a sensitivity analysis, to test the robustness of the results. Among HIV-positive men, nadir and current CD4+ T-cell counts were also assessed. STATA Version 14 (StataCorp, College Station, Texas USA) was used for the analysis.
3. Results
A total of 617 men were enrolled in SPANC, with a median age of 49 years (range 35–79 years). Nearly all men (588, 95.3%) identified as gay or homosexual. Greater than one-third (220, 35.7%) were HIV-positive (Table 1). The great majority of these men (206, 93.6%) were currently receiving antiretroviral therapy, reported an undetectable viral load (197, 89.5%) and had a CD4 T-cell count of more than 350 cells/μL (194, 88.0%). Of the 582 men (94.3%) who completed a baseline question on HPV vaccination status, very few (15, 2.6%) reported receiving any prior vaccination.
Table 1.
SPANC participant baseline characteristics by HIV status (n=617).
|
Overall n=617 |
HIV negative n=397 |
HIV positive n=220 |
||
|---|---|---|---|---|
| Age (year groups) | ||||
| 35–44 | 198 | 134 | 64 | |
| 45–54 | 236 | 141 | 95 | |
| 55–64 | 124 | 76 | 48 | |
| ≥65 | 59 | 46 | 13 | |
| Cigarette smoking status | ||||
| Never | 334 | 232 | 102 | |
| Past smoker | 196 | 121 | 75 | |
| Current smoker | 87 | 44 | 43 | |
| Sexual identity | ||||
| Heterosexual or straight | 4 | 3 | 1 | |
| Bisexual | 17 | 11 | 6 | |
| Gay | 390 | 256 | 134 | |
| Queer | 8 | 4 | 4 | |
| Homosexual | 190 | 120 | 70 | |
| Other | 8 | 3 | 5 | |
| Lifetime number of male sexual partners | ||||
| <10 | 11 | 10 | 1 | |
| 11–50 | 102 | 81 | 21 | |
| 51–200 | 173 | 116 | 57 | |
| 201–500 | 122 | 76 | 46 | |
| >500 | 196 | 105 | 91 | |
| Lifetime number of R-CLAIapartners | ||||
| 0–1 | 84 | 74 | 10 | |
| 2–5 | 165 | 146 | 19 | |
| 6–10 | 125 | 84 | 41 | |
| >10 | 243 | 93 | 150 | |
| Number of male sexual partners in past 6 months | ||||
| None | 49 | 15 | 34 | |
| 1 | 123 | 90 | 33 | |
| 2–5 | 164 | 114 | 50 | |
| 6–10 | 106 | 65 | 41 | |
| >10 | 175 | 113 | 62 | |
| Number of R-CLAIapartners in past 6 months | ||||
| None | 289 | 194 | 95 | |
| 1 | 184 | 142 | 42 | |
| ≥2 | 144 | 61 | 83 | |
R-CLAI – participant reports receptive anal intercourse without a condom.
3.1. Prevalent anal HPV detection
Six-hundred and six (98.2%) participants had baseline anal HPV results available. Testing was inhibited on the remaining 11 (1.8%) participants and they were excluded from further analysis. Of the 606 men with an adequate samples, 525 (86.6%) had at least one anal HPV type detected. A single HPV type was detected in 101 participants (16.7%), while the rest had multiple HPV types detected. Nineteen participants (3.1%) had more than 10 HPV types detected. The prevalence of any anal HPV was 94.5% (207) in HIV-positive men and 82.2% (318) in HIV-negative men (p<0.001).
Only two participants (0.3%, both aged between 35 and 44 years) were positive for all 4vHPV types. No participant was positive for all 9vHPV types. Almost half the participants had no 4vHPV types detected (298, 49.2%) and this ranged from 42.1% (82) of men aged 35–44 years to 66.1% (39) of men aged 65 years or more. More than a third of participants had no 9vHPV vaccine types detected (214, 35.3%), ranging from 32.8% (64) of men aged 35–44 years to 50.8% (30) of men aged 65 years or more. Of the vaccine-preventable types, HPV16 was by far the most prevalent type, with a prevalence of 29.4% (178 participants), followed by HPV6 (18.0%, 109 participants) and HPV45 (13.9%, 84 participants) (Table 2).
Table 2.
Type-specific anal HPV prevalence, overall and by age (n=606).
| 9v vaccine HPV type | Overall prevalence |
Age (years) |
||||
|---|---|---|---|---|---|---|
| % (n) | 35–44 n=195 % (n) |
45–54 n=229 % (n) |
55–64 n=123 % (n) |
65+ n=59 % (n) |
P trend | |
| HPV16 | 29.4 (178) | 28.2 (55) | 30.6 (70) | 33.3 (41) | 20.3 (12) | 0.74 |
| HPV18 | 12.2 (74) | 18.5 (36) | 10.9 (25) | 8.1 (10) | 5.1 (3) | 0.001 |
| HPV31 | 5.9 (36) | 5.1 (10) | 7.0 (16) | 4.9 (6) | 6.8 (4) | 0.82 |
| HPV33 | 5.1 (31) | 5.1 (10) | 6.1 (14) | 4.9 (6) | 1.7 (1) | 0.40 |
| HPV45 | 13.9 (84) | 15.9 (31) | 14.0 (32) | 11.4 (14) | 11.9 (7) | 0.25 |
| HPV52 | 13.5 (82) | 15.4 (30) | 13.5 (31) | 12.2 (15) | 10.2 (6) | 0.25 |
| HPV58 | 11.1 (67) | 11.3 (22) | 10.5 (24) | 12.2 (15) | 10.2 (6) | 0.99 |
| HPV6 | 18.0 (109) | 22.1 (43) | 17.9 (41) | 13.8 (17) | 13.6 (8) | 0.04 |
| HPV11 | 9.6 (58) | 13.3 (26) | 8.7 (20) | 8.1 (10) | 3.4 (2) | 0.02 |
3.2. Predictors of prevalent anal vaccine-preventable HPV types
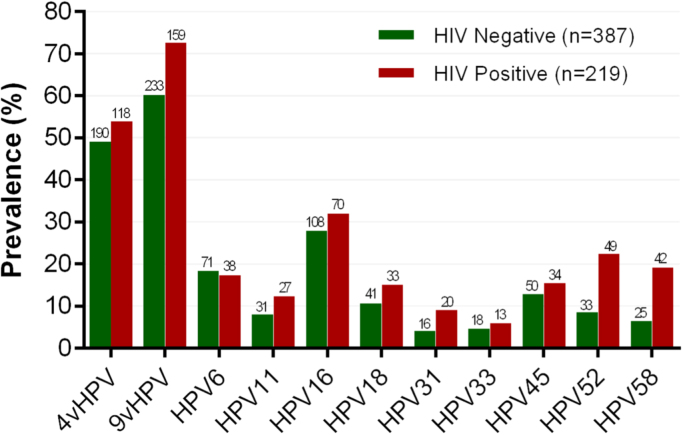
Of the 9vHPV types, HPV31 (p=0.012), HPV52 (p<0.001) and HPV58 (p<0.001) were significantly more prevalent in HIV-positive than HIV-negative participants. There was no difference in HPV16 prevalence by HIV status (p=0.29) (Fig. 1). Overall, prevalence was lower for all vaccine-preventable HPV types among men aged 65 and above. Prevalence of HPV18 (p=0.001), and the genital and anal wart-associated low risk HPV types, HPV6 (p=0.004) and HPV11 (p=0.002) declined across the four age groups (Table 2). Prevalence of the other types did not vary with age, however, the prevalence of HPV16 in men aged 65 years and older was significantly lower than in men younger than 65 years (p=0.042).
Fig. 1.
Prevalence1of vaccine-preventable anal HPV types by HIV status in gay and bisexual men. 1. 4vHPV=quadrivalent HPV types (HPV6, 11, 16 and 18), 9Vhpv=nonavalent HPV types (HPV6, 11, 16, 18, 31, 33, 45, 52 and 58). 2. HPV31 (p=0.012), HPV52 (p<0.001) and HPV58 (p<0.001) were significantly more prevalent among HIV positive participants. There was not a significant difference in prevalence for HPV16 (p=0.29), HPV18 (p=0.11), HPV33 (p=0.49), HPV45 (p=0.37), HPV6 (p=0.76) and HPV11 (p=0.083) by HIV status.
Among men with anal HPV, the mean number of 9vHPV types was significantly higher among HIV positive participants (1.69 in the HIV negative and 2.05 in the HIV positive, p<0.001) and higher among younger men (p trend=0.059) (Table 3). Younger men compared with older men and HIV-positive men compared with HIV-negative men, were more likely to have at least one vaccine preventable HPV type detected, (p=0.003 and p<0.001 respectively) (Table 2). Among HIV positive men, lower current CD4 T cell count was associated with detection of at least one vaccine preventable HPV type (p=0.008 for 4vHPV and p=0.003 for 9vHPV for CD4 count ≤350 cells/μL versus higher). Similarly, lower nadir CD4 T cell count was associated with detection of at least one vaccine preventable HPV type (p=0.013 for 4vHPV and p<0.001 for 9vHPV).
Table 3.
Number of 9v HPV vaccine types detected, overall and by HIV status and age (n=606).
| No of 9v vaccine HPV types | Prevalence |
Prevalence by HIV status |
Prevalence by age |
||||||
|---|---|---|---|---|---|---|---|---|---|
| % (n) | Negative n=386 % (n) |
Positive n=220 % (n) |
P value | 35–44 n=195 % (n) |
45–54 n=229 % (n) |
55–64 n=123 % (n) |
65+ n=59 % (n) |
P value (trend) |
|
| 0 | 35.3 (214) | 32.6 (126) | 27.7 (61) | <0.001 | 32.8 (64) | 34.1 (78) | 34.1 (42) | 50.8 (30) | 0.003 |
| 1 | 46.4 (182) | 32.4 (125) | 25.9 (57) | 28.7 (56) | 31.4 (72) | 32.5 (40) | 23.7 (14) | ||
| 2 | 32.9 (129) | 18.4 (71) | 26.4 (58) | 20.5 (40) | 21.0 (48) | 24.4 (30) | 18.6 (11) | ||
| 3 | 14.0 (55) | 7.0 (27) | 12.7 (28) | 10.8 (21) | 9.2 (21) | 8.1 (10) | 5.1 (3) | ||
| 4 | 4.3 (17) | 1.6 (6) | 5.0 (11) | 3.6 (7) | 3.5 (8) | 0.81 (1) | 1.7 (1) | ||
| 5 | 2.0 (8) | 0.78 (3) | 2.3 (5) | 3.1 (6) | 0.87 (2) | 0 | 0 | ||
| 6 | 0.26 (1) | 0.26 (1) | 0 | 0.51 (1) | 0 | 0 | 0 | ||
| 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Mean typesa | 1.69 | 2.05 | <0.001b | 2.00 | 1.81 | 1.65 | 1.69 | 0.059b | |
Among those men with at least one HPV type detected.
A t-test was used to obtain p values.
4. Discussion
The vast majority of this largely community-based GBM cohort had prevalent anal HPV (525 men, 86.6%). HPV16, the type which is most commonly associated with anal cancer and which is targeted by the 4vHPV and 9vHPV vaccines, was detected in nearly 30% (178 participants). More than one third had no 9vHPV types detected. Men younger than 45 years and HIV positive men were more likely to have at least one vaccine-preventable HPV type and to have higher mean numbers of 9vHPV types detected. Only two participants had all 4vHPV vaccine-related types detected and none had all 9vHPV vaccine-related types detected. A number of cross-sectional and prospective cohort studies around the world have measured anal HPV prevalence in GBM [18], [19], [20], [21], [22], [23], [24], [25], [26], [27]. Most report a similar prevalence of any anal HPV infection to that found in this cohort, although prevalence varies depending on the proportion of men who are HIV-positive. HIV-positive men generally have a higher prevalence of anal HPV [20], [26]. Similar rates of HPV16 detection have been reported in all studies discussed above, with prevalence between 27% and 35% [18], [19], [20], [21], [24]. However a cohort study of HIV-negative GBM in Brazil, Mexico and the USA found much lower prevalence of HPV16 of 6.3%, despite 63.5% of participants reporting a recent male anal sex partner [25]. These differences in anal HPV prevalence are likely to be at least partly explained by differences in HPV genotyping assays [18], [19], [20], [21], [22], [25], [27] or to demographic or behavioural differences in the study populations.
Although heterosexual men appear to benefit from substantial herd protection from HPV-related disease in settings with high rates of female vaccination, gay and bisexual men do not benefit from such herd protection [28]. In our study, only two participants were positive for all 4vHPV vaccine types and none was positive for all 9vHPV vaccine types. Less than one-third had HPV16, the type most likely to cause anal cancer. Almost half the participants had no 4vHPV vaccine types detected and more than one third of participants had no 9vHPV vaccine types detected. However, in serological studies of Australian GBM, older age was significantly associated with HPV16 seropositivity, indicating previous HPV16 infection [29]. Given the high exposure reported by SPANC participants, many anal HPV infections would have already cleared naturally, indicating that if they were reinfected they would be likely to clear the infection again. These findings, combined with the concentration of HPV-related cancers among GBM and the fact that very few data exist to inform the potential efficacy of HPV vaccination in older GBM, provide support for the need for randomized trials of HPV vaccination in older GBM.
A strength of the SPANC study is that recruitment was primarily through community sources; thus the study population is likely to be representative of the broader GBM community in Sydney. Both HIV-positive and HIV-negative men were enrolled, enabling us to determine difference in prevalence by HIV status. Apart from the clinic-based study by Schwartz et al., of 305 HIV-positive MSM in San Francisco [22], all other study populations discussed here had a median age of less than 44 years [19], [20], [21], [23], [24], [25], [26], [27]. Our older study population allows us to present data in decade strata in ages up to 65 years, and in a substantial number of even older men. This provides an important insight into anal HPV prevalence at the ages where the risk of anal cancer increases.
A potential limitation of the presented analysis is that HPV prevalence is measured at a single time point, and thus persistence is not assessed, meaning that many of these detections may represent short term acute infections rather than the chronic infection which is likely to precede cancer development. The prospective design of the SPANC study will allow us to address this in coming years, as testing at multiple endpoints will determine persistence and reinfection or reactivation. The Linear Array HPV genotyping may favour the detection of HPV16 when additional types are present, and in particular HPV31, HPV33, HPV51 and HPV59 detection may be impeded in the presence of high levels of HPV16 DNA [30].
In conclusion, anal HPV infection was highly prevalent in this older cohort of GBM. Two-thirds of men did not have HPV16 detected and none had all 9vHPV types detected. Thus, HPV vaccination in this highly sexually active population of older GBM should be investigated as an effective anal cancer prevention strategy.
Funding
This work was supported by the National Health and Medical Research Council Program Grant (Sexually transmitted infections: Causes, consequences and interventions Grant #568971); and a Cancer Council New South Wales Strategic Research Partnership Program Grant (Preventing morbidity and mortality from anal cancer Grant #13–11). Cytological testing materials were provided by Hologic (Australia) Pty Ltd. The Kirby Institute is affiliated with the Faculty of Medicine, University of New South Wales and funded by the Australian Government of Health and Ageing. The views expressed in this publication do not necessarily represent the position of the Australian Government.
Conflict of interest
Professor Andrew Grulich has received honoraria and research funding from CSL Biotherapies, honoraria and travel funding from MSD. Professor Christopher Fairley has received honoraria, travel funding and research funding from CSL and MSD, and owns shares in CSL Biotherapies. Professor Suzanne Garland have received advisory board fees and grant support from CSL and GlaxoSmithKline, and lecture fees from Merck, GSK and Sanofi Pasteur; in addition, has received funding through her institution to conduct HPV vaccine studies for MSD and GSK and is a member of the Merck Global Advisory Board as well as the Merck Scientific Advisory Committee for HPV. Associate Professor Richard J Hillman has received support from CSL Biotherapies and MSD. Professor Andrew Carr has received research funding from Bristol-Myers Squibb, Gilead Sciences, MSD, and ViiV Healthcare; consultancy fees from Gilead Sciences, Mayne Pharma, MSD, and ViiV Healthcare; lecture and travel sponsorships from Bristol-Myers Squibb, Gilead Sciences, MSD, and ViiV Healthcare; and has served on advisory boards for Gilead Sciences, MSD, and ViiV Healthcare. All other authors have no conflicts of interest to declare.
Acknowledgements
We would like to acknowledge and thank the participants of the SPANC study. The SPANC study team includes Brian Acraman, Marjorie Adams, Claire Biro, Andrew Carr, Susan Carroll, David Cooper, Alyssa Cornall, Leonie Crampton, Deborah Ekman, Christopher Fairley, Annabelle Farnsworth, Lance Feeney, Eddie Fraissard, Marko Garcia, Suzanne Garland, Andrew Grulich, Richard Hillman, Kirsten Howard, Fengyi Jin, Johann Kolstee, Carmella Law, Matthew Law, Dorothy Machalek, Kirsten McCaffery, Ross McDonald, Patrick McGrath, Robert Mellor, Susan Pendlebury, Kathy Petoumenos, Piero Pezzopane, Samuel Phillips, Mary Poynten, Garrett Prestage, Adele Richards, Jennifer Roberts, Daniel Seeds, Sepehr Tabrizi, David Templeton, Julia Thurloe, Winnie Tong and Rick Varma.
References
- 1.Robinson D., Coupland V., Møller H. An analysis of temporal and generational trends in the incidence of anal and other HPV-related cancers in Southeast England. Br. J. Cancer. 2009;100:527–531. doi: 10.1038/sj.bjc.6604871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forman D., de Martel C., Lacey C., I.S., Lortet-Tieulent J., Bruni L. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30(Suppl 5):SF12–SF23. doi: 10.1016/j.vaccine.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 3.Johnson L., Madeleine M., Newcomer L., Schwartz S., Daling J. Anal cancer incidence and survival: the surveillance, epidemiology, and end results experience, 1973–2000. Cancer. 2004;101:281–288. doi: 10.1002/cncr.20364. [DOI] [PubMed] [Google Scholar]
- 4.Jin F., Stein A., Conway E., Regan D., Law M., Brotherton J. Trends in anal cancer in Australia, 1982–2005. Vaccine. 2011;29:2322–2327. doi: 10.1016/j.vaccine.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 5.Daling J., Weiss N., Hislop T., Maden C., Coates R., Sherman K. Sexual practices, sexually transmitted diseases, and the incidence of anal cancer. N. Engl. J. Med. 1987;317:973–977. doi: 10.1056/NEJM198710153171601. [DOI] [PubMed] [Google Scholar]
- 6.Grulich A., van Leeuwen M., Falster M., Vajdic C. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 7.Machalek D., Poynten I., Jin F., Fairley C., Farnsworth A., Garland S. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol. 2012;13:487–500. doi: 10.1016/S1470-2045(12)70080-3. [DOI] [PubMed] [Google Scholar]
- 8.De Vuyst H., Clifford G., Nascimento M., Madeleine M., Franceschi S. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: a meta-analysis. Int. J. Cancer. 2009;124:1626–1636. doi: 10.1002/ijc.24116. [DOI] [PubMed] [Google Scholar]
- 9.Daling J., Madeleine M., Johnson L., Shera K., Wurscher M., Carter J. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer. 2004;101:270–280. doi: 10.1002/cncr.20365. [DOI] [PubMed] [Google Scholar]
- 10.Castellsagué X., Muñoz N., Pitisuttithum P., Ferris D., Monsonego J., Ault K. End-of-study safety, immunogenicity, and efficacy of quadrivalent HPV (types 6, 11, 16, 18) recombinant vaccine in adult women 24–45 years of age. Br. J. Cancer. 2011;105:28–37. doi: 10.1038/bjc.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skinner S., Szarewsk iA., Romanowski B., Garland S., Lazcano-Ponce E., Salmerón J. Efficacy, safety, and immunogenicity of the HPV 16/18 AS04-adjuvanted vaccine in women older than 25 years: 4-year interim follow-up of the phase 3, double-blind, randomised controlled VIVIANE study. Lancet. 2014;384:2213–2227. doi: 10.1016/S0140-6736(14)60920-X. [DOI] [PubMed] [Google Scholar]
- 12.Wheeler C., Skinner S., Del Rosario-Raymundo M., Garland S., Chatterjee A., Lazcano-Ponce E. Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 AS04-adjuvanted vaccine in women older than 25 years: 7-year follow-up of the phase 3, double-blind, randomised controlled VIVIANE study. Lancet Infect. Dis. 2016 doi: 10.1016/S1473-3099(16)30120-7. [DOI] [PubMed] [Google Scholar]
- 13.Giuliano A., Palefsky J., Goldstone S., Moreira E.J., Penny M., Aranda C. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N. Engl. J. Med. 2011;364:401–411. doi: 10.1056/NEJMoa0909537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hildesheim A., Herrero R., Wacholder S., Rodriguez A., Solomon D., Bratti M. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. J. Am. Med. Assoc. 2007;298:743–753. doi: 10.1001/jama.298.7.743. [DOI] [PubMed] [Google Scholar]
- 15.Machalek D., Grulich A., Hillman R., Jin F., Templeton D., Tabrizi S. The Study of the Prevention of Anal Cancer (SPANC): design and methods of a three-year prospective cohort study. BMC Public Health. 2013:13. doi: 10.1186/1471-2458-13-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tabrizi S., Stevens M., Chen S., Rudland E., Kornegay J., Garland S. Evaluation of a modified reverse line blot assay for detection and typing of human papillomavirus. Am. J. Clin. Pathol. 2005;123:896–899. doi: 10.1309/MPER-NJ0G-62RE-CHCQ. [DOI] [PubMed] [Google Scholar]
- 17.Stevens M., Garland S., Tabrizi S. Development and validation of a real-time PCR assay specifically detecting human papillomavirus 52 using the Roche Light Cycler 480 system. J. Virol. Methods. 2008;147:290–296. doi: 10.1016/j.jviromet.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 18.Videla S., Darwich L., Canadas M., Coll J., Pinol M., Garcia-Cuyas F. Natural history of Human Papillomavirus Infections involving anal, penile, and oral sites among HIV-positive men. Sex. Transm. Dis. 2013;40:3–10. doi: 10.1097/OLQ.0b013e31827e87bd. [DOI] [PubMed] [Google Scholar]
- 19.Sadlier C., Rowley D., Morley D., Surah S., O'Dea S., Delamere S. Prevalence of human papillomavirus in men who have sex with men in the era of an effective vaccine; a call to act. HIV Med. 2014;15:499–504. doi: 10.1111/hiv.12150. [DOI] [PubMed] [Google Scholar]
- 20.Schofield A., Sadler L., Nelson L., Gittins M., Desai M., Sargent A. A prospective study of anal cancer screening in HIV positive and negative men who have sex with men; results of analogy. AIDS. 2016 doi: 10.1097/QAD.0000000000001045. [DOI] [PubMed] [Google Scholar]
- 21.Nagata N., Watanabe K., Nishijima T., Tadokoro K., Watanabe K., Shimbo T. Prevalence of anal human Papillomavirus Infection and risk factors among HIV-positive patients in Tokyo, Japan. PLoS One. 2015;10:e0137434. doi: 10.1371/journal.pone.0137434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz L., Castle P., Follansbee S., Borgonovo S., Fetterman B., Tokugawa D. Risk factors for anal HPV infection and anal precancer in HIV-infected men who have sex with men. J. Infect. Dis. 2013;208:1768–1775. doi: 10.1093/infdis/jit374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donà M., Palamara G., Di Carlo A., Latini A., Vocaturo A., Benevolo M. Prevalence, genotype diversity and determinants of anal HPV infection in HIV-uninfected men having sex with men. J. Clin. Virol. 2012;54:185–189. doi: 10.1016/j.jcv.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 24.Torres M., González C., del Romero J., Viciana P., Ocampo A., Rodríguez-Fortúnez P. Anal human papillomavirus genotype distribution in HIV-infected men who have sex with men by geographical origin, age, and cytological status in a Spanish cohort. J. Clin. Microbiol. 2013;51:3512–3520. doi: 10.1128/JCM.01405-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nyitray A., Carvalho da Silva R., Baggio M., Lu B., Smith D., Abrahamsen M. Age-specific prevalence of and risk factors for anal human papillomavirus (HPV) among men who have sex with women and men who have sex with men: the HPV in men (HIM) study. J. Infect. Dis. 2011;203:49–57. doi: 10.1093/infdis/jiq021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu Y., Qian H., Sun J., Gao L., Yin L., Li X. Anal human papillomavirus infection among HIV-infected and uninfected men who have sex with men in Beijing, China. J. Acquir. Immune Defic. Syndr. 2013;64:103–114. doi: 10.1097/QAI.0b013e31829b6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Aar F., Mooij S., van der Sande M., Speksnijder A., Stolte I., Meijer C. Anal and penile high-risk human papillomavirus prevalence in HIV-negative and HIV-infected MSM. AIDS. 2013;27:2921–2931. doi: 10.1097/01.aids.0000432541.67409.3c. [DOI] [PubMed] [Google Scholar]
- 28.Donovan B., Franklin N., Guy R., Grulich A., Regan D., Ali H. Quadrivalent human papillomavirus vaccination and trends in genital warts in Australia: analysis of national sentinel surveillance data. Lancet Infect. Dis. 2011;11:39–44. doi: 10.1016/S1473-3099(10)70225-5. [DOI] [PubMed] [Google Scholar]
- 29.Poynten I., Jin F., Templeton D., Prestage G., Donovan B., Pawlita M. Prevalence, incidence, and risk factors for human papillomavirus 16 seropositivity in Australian homosexual men. Sex. Transm. Dis. 2012;39:726–732. doi: 10.1097/OLQ.0b013e31825d5cb8. [DOI] [PubMed] [Google Scholar]
- 30.Cornall A., Phillips S., Cummins E., Garland S., Tabrizi S. In vitro assessment of the effect of vaccine-targeted human papillomavirus (HPV) depletion on detection of non-vaccine HPV types: implications for post-vaccine surveillance studies. J. Virol. Methods. 2015;214:10–14. doi: 10.1016/j.jviromet.2014.12.007. [DOI] [PubMed] [Google Scholar]