Abstract
We evaluate the potential for using high-risk human papillomavirus (hr-HPV) testing-based screening for cervical intraepithelial neoplasia (CIN) in routine health services in Thailand; its accuracy in comparison to that of conventional cytology (CC); and the utility of HPV16/18 positive results and liquid-based cytology (LBC) triage for HPV-positive women in the detection of high-grade CIN. Women aged 30–60 years in Ubon Ratchathani province, Thailand were screened with CC and hr-HPV testing and those abnormal on either tests were referred for colposcopy and/or directed biopsies. The final diagnosis using COBAS was based on histology or colposcopy when histology was not available. Estimation of test accuracy parameters was done using latent class analysis using Bayesian models. Of the 5004 women were enrolled, 20 (0.4%) had abnormal CC and 174 (3.5%) women were HPV-positive. Among 185 women abnormal on CC or HPV-positive, 176 (95.1%) underwent colposcopy, of whom 101 (57.4%) had abnormal colposcopy findings. Ninety-seven women with abnormal and 69 with normal colposcopy had biopsies performed. All 21 women with histological CIN2 or worse had hr-HPV and none were abnormal on CC. The estimated sensitivity, specificity and positive predictive value were respectively 71.8%, 97.0% and 13.0% of HPV testing; 53%, 98.7% and 20.3% for triage of HPV-positive women with LBC; and 70.4%, 98.2% and 16.9% when test positivity was taken as HPV16/18 irrespective of LBC result or positive for hr-HPV non 16/18 types and LBC triage. Our study findings indicate poor performance of cytology screening and demonstrate the potential and utility of using HPV testing in public health services in Thailand as well as the utility of primary HPV testing and LBC triage in screening for cervical neoplasia.
Keywords: Cervix cancer, Early detection, HPV screening, Cytology screening, Accuracy
1. Introduction
Cervical cancer is the second most common cancer among Thai women, with an estimated 8200 new cases annually around 2012 which is expected to increase to 9200 cases around 2020 [1]. The age-standardized incidence rates across Thailand ranged from a high of 24.6 per 100,000 women in Lamphun to a low of 10.4 per 100,000 in Khon Kaen [2]. Wide spread implementation cytology screening has substantially reduced cervical cancer incidence and mortality in high-income countries in Europe, North America and Australia [3], [4], [5]. In Thailand, opportunistic cervical cytology screening has been on-going for several years since 1985. In 2002, the Ministry of Public Health (MoPH) and the National Health Security Office (NHSO) commenced providing countrywide cervical screening to all Thai women aged 35–60 years under universal health care coverage insurance scheme at 5-year intervals and integrated it within the routine health services of Thailand. Over the last two decades the incidence of cervical cancer has been slowly declining in Thailand [6], [7], [8], [9], [10], [11], [12].
It has been well documented in recent years that providing quality assured and effective cervical cytology screening is a challenging task and cytology screening programs have been less successful in reducing cervical cancer burden in low- and middle income countries (LMICs) [13]. The challenges in introducing high-quality, frequently repeated cytology screening and the well documented low sensitivity of cytology to detect cervical cancer and its precursors CIN 2 and CIN3 (cervical intraepithelial neoplasia grades 2 and 3) lesions in various settings have led to the evaluation of alternative screening approaches such as human papillomavirus (HPV) testing-based screening [13], [14], [15], [16], [17]. HPV testing alone or with cytology triage is currently increasingly being used as a primary screening approach for cervical neoplasia.
The causal role of persistent high-risk HPV (hr-HPV) infection in cervical carcinogenesis, the high accuracy of HPV testing in detecting cervical neoplasia, recent development of assays that permits the detection of hr-HPV DNA in cervical specimens, the high negative predictive value of negative HPV tests for CIN 2 or worse lesions and the potential value of HPV testing as an objective screening test in the post HPV vaccination era prompted us to evaluate its test performance, feasibility and acceptability in a cross-sectional study in Ubon Rachathani province. Moreover, the findings from such a study will be useful for and guide eventual national scale up of HPV testing as a primary screening test in future.
2. Material and methods
2.1. Study population
Eligible population were apparently healthy women aged 30–60 years who underwent Pap smear screening in 50 primary healthcare units in 7 districts in Ubon Ratchathani province in northern Thailand in the setting of the national cervical cytology screening program. The women were invited by the nurses in the primary care units to participate in the study when they presented for routine cytology screening. The study was explained in detail and all participants provided written informed consent before entering the study. The participants were recruited during the period July 2014 to January 2015. This study was approved by the ethics committee of the National Cancer Institute, Bangkok, Thailand.
2.2. Collection of sociodemographic details
After obtaining written informed consent, sociodemographic details including marital status, age at first child birth, number of children and screening history were obtained from the participants using a structured questionnaire in direct interviews administered by the nurses before obtaining a Pap smear.
2.3. Sample collection and HPV genotyping
After obtaining sociodemographic history, the examination procedure was described in detail to the women. A speculum examination was carried out under bright light from a halogen lamp and the cervix was visually inspected for any abnormalities such as signs of inflammation, ulceration and growth. After naked eye visual inspection, cervical cells were collected using an Ayre's spatula, and a conventional smear was prepared by spreading the cells in the spatula on a pre-labelled glass slide and fixed with spray fixative. Then the spatula was rinsed in a tube containing PreservCyt® solution (Hologic Inc., Marlborough MA, USA). A second cervical cell sample was collected using an endocervical brush, which was then rinsed and placed in the same vial containing the PreservCyt solution in order to collect more cervical cells.
2.4. Cytology evaluation
The cervical smear slides were transported to the provincial Government cytology laboratory in Ubon Ratchathani province where the smears were processed stained, read and reported. All the smears were initially read by a cytotechnician who categorised them as normal and abnormal smears. All abnormal smears and a 10% random sample of normal smears were then reviewed by a medically qualified cytopathologist and the results were reported using the Bethesda system. All women with cytological abnormalities at the atypical squamous cells of uncertain significance (ASCUS) threshold were referred for colposcopy by a gynaecology oncologist, and directed biopsies were obtained from colposcopically abnormal areas in women with colposcopic abnormalities. In those women with normal colposcopic findings cervical biopsies were randomly performed at 12 and 6 o clock positions.
2.5. HPV testing
The vials with cervical cell specimens in PreservCyt solution were stored at room temperature and were transported in room temperature to the National Cancer Institute (NCI), Bangkok for HPV testing using COBAS 4800 System (Roche Molecular Systems, Inc., Branchburg, NJ, USA). The COBAS 4800 HPV test simultaneously detects a total of 14 h-HPV types: HPV-16 individually, HPV-18 individually, and pooled high-risk (hr)-HPV genotypes other than HPV 16 and 18 (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68) (OHR), in addition to a separate high b-globin control. All HPV-positive samples were tested with reflex liquid based cytology (LBC) (Hologic Inc., Marlborough, MA, USA) and interpreted by a cytopathologist in the NCI, Bangkok. All HPV-positive women were referred for colposcopy by gynecologic oncologist and colposcopically directed biopsies were performed from the abnormal looking areas. In cases of normal colposcopic findings, cervical biopsies were randomly obtained.
2.6. Statistical analysis
Characteristics of study participants were presented as numbers and proportions. Five screening modalities were assessed: three involving primary screen testing: modality 1: conventional cytology; modality 2: hr-HPV testing where any type was taken as positive; and modality 3: hr-HPV testing where types 16/18 were taken as positive; and two HPV triaging options: modality 4 in which screen positivity was defined as HPV positive for any type and triage with liquid-based cytology was abnormal, and modality 5 where positivity was defined as HPV positive for 16/18 or if positive for other types other than 16/18 (OHR) and triage with positive LBC. The final diagnosis was based on histology report and colposcopy findings in case no or inadequate histology. We evaluated positivity rates of the screening and triaging modalities; participation in diagnosis (in terms of proportion of colposcopy done among screen-positives, and proportion of histology done among the women screen-positive and abnormal on colposcopy); proportion of abnormal colposcopy among the screen-positives; and performance characteristics of the each of the modalities described above (detection rates, approximate sensitivity, corrected specificity, and positive predictive value for detection of CIN 2 or worse lesions).
Since disease confirmatory investigations using colposcopy and/or biopsy were carried out only among women positive on any of the two screening tests (conventional cytology and hr-HPV testing), we could not calculate direct estimates of disease prevalence and performance characteristics without bias. To allow for estimation of these parameters for our screening program where there was no true disease verification for all participants, latent class analysis using Bayesian models was used. Two Bayesian models were constructed: the primary model involving assessment of Modalities 1, 2 and 4, and the secondary model involving modalities 1, 3 and 5. In these models the assumption of statistical independence conditional on the true disease status of the two HPV based modalities was relaxed. For each of the two models, each level of the four observed variables (based on the findings of the 3 screening modalities and colposcopy/biopsy) was constructed together with an unobserved or “latent” variable with two mutually exclusive categories, ‘diseased’ and ‘non-diseased’ that was used as a measure of true disease [18], [19], [20], [21], [22]. The corrected estimates of the disease prevalence, sensitivity and specificity of the screening test modalities were then estimated from this unobserved variable. Statistical analysis was carried out using STATA 13 software and Just Another Gibbs Sampler (JAGS) software.
3. Results
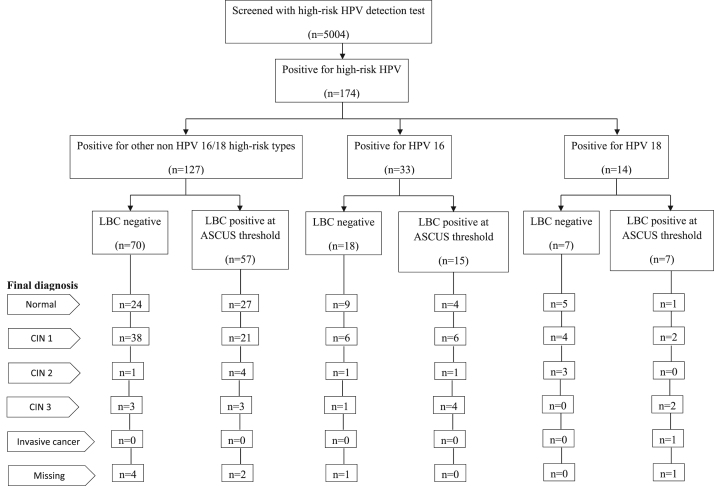
The characteristics of the 5004 women included in the study are given in Table 1. Vast majority of them had prior screening with Pap smear. A third of them had no knowledge about HPV. The flow chart of study outcomes in terms of HPV test results, reflex LBC results and number of women detected with CIN 2, CIN 3 and invasive cancer are given in Fig. 1. Women screened positive on Pap smear and HPV testing, colposcopy and histology findings stratified by 10-year age groups are given in Table 2. Only 20 (0.4%) of women were screened positive (ASCUS or worse) on Pap smear; 174 (3.5%) women were positive on HPV testing. Of the 185 women positive on Pap smear or HPV testing or both, 176 (95.1%) underwent colposcopy and 101 (57.4%) had abnormal colposcopy findings. Biopsies were directed in 97 of 101 women with abnormal colposcopy findings and in 69 of 75 women with normal colposcopy findings. Final diagnosis based on histology in 166 women who had biopsy and 10 women based on colposcopy findings alone are given in Table 3. Overall, 77 were diagnosed with CIN 1, 7 with CIN 2, and 13 with CIN 3 and 1 with invasive cancer.
Table 1.
Characteristics of women screened in the project in Ubon Ratchathani province.
| Characteristics | Number | Percentage |
|---|---|---|
| Women screened | 5004 | |
| Age | ||
| 30–39 | 1117 | 22.3 |
| 40–49 | 2340 | 46.8 |
| 50–59 | 1547 | 30.9 |
| District | ||
| Det Udom | 393 | 7.9 |
| Don Mot Daeng | 296 | 5.9 |
| Meuang | 1107 | 22.1 |
| Muang Sam Sip | 2289 | 45.7 |
| Samrong | 315 | 6.3 |
| Sawang Wirawong | 197 | 3.9 |
| Warin Chamrap | 407 | 8.1 |
| Marital status | ||
| Married | 4730 | 94.5 |
| Widowed | 135 | 2.7 |
| Separated | 99 | 2.0 |
| Unmarried | 40 | 0.8 |
| Age at first childa | ||
| <18 | 372 | 7.7 |
| 18–21 | 2187 | 45.5 |
| 22+ | 2244 | 46.7 |
| No. of childrena | ||
| 1 | 484 | 10.0 |
| 2–3 | 4017 | 83.3 |
| 4+ | 322 | 6.7 |
| Ever screened? | ||
| No | 236 | 4.7 |
| Yes | 4768 | 95.3 |
| No of years since previous screeninga | ||
| <5 | 3778 | 95.2 |
| 5+ | 189 | 4.8 |
| Modality used in previous screeninga | ||
| Pap smear | 4375 | 99.8 |
| VIA | 8 | 0.2 |
| HPV | 2 | 0.0 |
| Knowledge about HPV | ||
| No | 1804 | 36.1 |
| Yes | 3200 | 63.9 |
VIA: visual inspection with acetic acid; HPV: human papilloma virus.
Figures do not add up to total because of missing information.
Fig. 1.
Flowchart of number of women screened, HPV genotyping and liquid-based cytology results and final diagnosis among HPV positive women. Key: LBC: liquid-based cytology; ASCUS: atypical squamous cells of undetermined significance; HPV: human papilloma virus; CIN: cervical intraepithelial neoplasia.
Table 2.
Screening process.
| Age | Women | Women screen-positive | Colposcopy | Abnormal | Histology among abnormal | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| group | screened | Conv. | HPV | Positive | done | colposcopy | colposcopy in screen-positives | |||||||||||
| (years) | cytology | DNA | on | among | among | Performed | Normal | CIN | Invasive | |||||||||
| test | either | screen- | screen- | 1 | 2 | 3 | cancer | |||||||||||
| tests | positives | positives | ||||||||||||||||
| No. | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. | No. | No. | No. | No. | |||||||
| 30–39 | 1,117 | 8 | (0.7) | 54 | (4.8) | 57 | (5.1) | 52 | (91.2) | 31 | (59.6) | 31 | (100.0) | 2 | 22 | 3 | 4 | 0 |
| 40–49 | 2,340 | 6 | (0.3) | 68 | (2.9) | 74 | (3.2) | 71 | (95.9) | 40 | (56.3) | 38 | (95.0) | 1 | 28 | 3 | 5 | 1 |
| 50–59 | 1,547 | 6 | (0.4) | 52 | (3.4) | 54 | (3.5) | 53 | (98.1) | 30 | (56.6) | 28 | (93.3) | 0 | 24 | 1 | 3 | 0 |
| Total | 5,004 | 20 | (0.4) | 174 | (3.5) | 185 | (3.7) | 176 | (95.1) | 101 | (57.4) | 97 | (96.0) | 3 | 74 | 7 | 12 | 1 |
Conv.: Conventional; HPV: human papilloma virus; CIN: cervical intraepithelial neoplasia.
Table 3.
Final diagnosis among screen positive women and reference standard used.
| Final |
Reference standard used |
||
|---|---|---|---|
| diagnosis | Colposcopy | Histology | Total |
| Normal | 6 | 72 | 78 |
| CIN 1 | 3 | 74 | 77 |
| CIN 2 | 0 | 7 | 7 |
| CIN 3 | 1 | 12 | 13 |
| Invasive cancer | 0 | 1 | 1 |
| Total | 10 | 166 | 176 |
CIN: cervical intraepithelial neoplasia.
Number of women diagnosed with CIN and cancer among Pap smear positive women at ASCUS and low-grade squamous intraepithelial lesions (LSIL) threshold, hr-HPV positive women, and hr-HPV positive women triaged with LBC at ASCUS threshold are given in Table 4. Similar results for HPV 16 or 18 women and either HPV 16/18 positive women or women with hr-HPV types other than HPV 16/18 plus LBC positive women at ASCUS threshold (OHR) are given in Table 4. It is striking to note that none of the 21 CIN 2 or worse (CIN2+) cases was detected by Pap smear screening. On the other hand, all the 21 women with CIN 2+ lesions were detected by hr-HPV testing. Among HPV 16 and 18 positive women, 10 had CIN 2+ lesions and among HPV positive OHR women, 11 were detected with CIN 2+ lesions. Among HPV positive plus LBC positive women, 14 were detected with CIN 2+ lesions.
Table 4.
Numbers of women screened, screen-positives and cervical neoplasia detected screening test used.
| Screening test | Women | Women |
CIN detection (rate per 1000 women screened) |
||||
|---|---|---|---|---|---|---|---|
| screened | screen- | CIN 1 | CIN 2 | CIN 3 | CIN 2/3 | Invasive | |
| positive | cancer | ||||||
| (n=77) | (n=7) | (n=13) | (n=20) | (n=1) | |||
| Conventional cytology | |||||||
| ASCUS positivity threshold | 5004 | 20 | 8 | 0 | 0 | 0 | 0 |
| LSIL positivity threshold | 5004 | 6 | 4 | 0 | 0 | 0 | 0 |
| HPV test | |||||||
| Any positive | 5004 | 174 | 76 | 7 | 13 | 20 | 1 |
| Positive with type 16 | 5004 | 33 | 12 | 2 | 5 | 7 | 0 |
| Positive with type 18 | 5004 | 14 | 5 | 0 | 2 | 2 | 1 |
| Positive with types other than 16/18 | 5004 | 127 | 59 | 5 | 6 | 11 | 0 |
| HPVtest plus liquid based cytology triagea | 5004 | 79 | 29 | 5 | 9 | 14 | 1 |
| HPV 16/18b | 5004 | 47 | 17 | 2 | 7 | 9 | 1 |
| HPV 16/18 or other non 16/18 types (OHR) plus liquid based cytology triagec | 5004 | 104 | 38 | 6 | 10 | 16 | 1 |
ASCUS: atypical squamous cells of undetermined significance; LSIL: low-grade squamous intraepithelial lesion; HPV: human papilloma virus; CIN: cervical intraepithelial neoplasia; OHR: other high-risk.
Positive if both HPV test and liquid based cytology triage (at ASCUS threshold) are positive.
Positive if HPV test is positive for 16/18.
Positive if either HPV test is positive for 16/18 or HPV test is positive for other types other than 16/18 and liquid based cytology triage (at ASCUS threshold) is positive.
The performance characteristics of Pap smear, and different categories of hr-HPV testing women, with their 95% confidence intervals (95% CI) are given in Table 5. The sensitivity, specificity and positive predictive value (PPV) of HPV testing in this study were 71.8%, 97.0% and 13.0% respectively; these values for HPV positive women triaged by LBC were 53%, 98.7% and 20.3%; these values for HPV 16/18 positive or OHR women triaged with LBC were 70.4%, 98.2% and 16.9% respectively.
Table 5.
Approximate prevalence and screening test performance characteristics for detection CIN 2 or worse disease.
| Approximate screening test performance characteristics | Value (95% CI) | |||
|---|---|---|---|---|
| Prevalence of CIN 2 or worse disease (%) | 0.6 | (0.3 | – | 1.6) |
| Screen positivity (%) | ||||
| Conventional cytology (at ASCUS threshold) | 0.4 | (0.2 | – | 0.6) |
| HPV test | 3.4 | (2.9 | – | 3.9) |
| HPV test plus liquid based cytology triagea | 1.5 | (1.2 | – | 1.9) |
| HPV 16/18b | 1.0 | (0.7 | – | 1.3) |
| HPV 16/18 or other non 16/18 types (OHR) plus liquid based cytology triagec | 2.1 | (1.7 | – | 2.5) |
| Detection rate for CIN 2 or worse (per 1000 women screened) | ||||
| Conventional cytology (at ASCUS threshold) | 0.1 | (0.0 | – | 0.7) |
| HPV test | 4.4 | (2.8 | – | 6.5) |
| HPV test plus liquid based cytology triagea | 3.2 | (1.8 | – | 5.0) |
| HPV 16/18b | 2.2 | (1.1 | – | 3.8) |
| HPV 16/18 or other non 16/18 types (OHR) plus liquid based cytology triagec | 3.6 | (2.2 | – | 5.6) |
| Sensitivity for detection of CIN 2 or worse (%) | ||||
| Conventional cytology (at ASCUS threshold) | 2.2 | (0.1 | – | 12.6) |
| HPV test | 71.8 | (24.0 | – | 116.8) |
| HPV test plus liquid based cytology triagea | 53.0 | (17.7 | – | 87.3) |
| HPV 16/18b | 43.7 | (14.0 | – | 80.9) |
| HPV 16/18 or other non 16/18 types (OHR) plus liquid based cytology triagec | 70.4 | (23.2 | – | 120.1) |
| Specificity for detection of CIN 2 or worse (%) | ||||
| Conventional cytology (at ASCUS threshold) | 99.6 | (99.4 | – | 99.8) |
| HPV test | 97.0 | (96.3 | – | 97.7) |
| HPV test plus liquid based cytology triagea | 98.7 | (98.3 | – | 99.0) |
| HPV 16/18b | 99.2 | (98.9 | – | 99.5) |
| HPV (COBAS) 16/18 or other non 16/18 types (OHR) plus liquid based cytology triagec | 98.2 | (97.6 | – | 98.7) |
| Positive predictive value for detection of CIN 2 or worse (%) | ||||
| Conventional cytology (at ASCUS threshold) | 3.4 | (0.1 | – | 16.8) |
| HPV test | 13.0 | (8.4 | – | 18.6) |
| HPV test plus liquid based cytology triagea | 20.3 | (12.3 | – | 30.1) |
| HPV 16/18b | 22.5 | (12.3 | – | 35.7) |
| HPV 16/18 or other non 16/18 types (OHR) plus liquid based cytology triagec | 16.9 | (10.6 | – | 24.9) |
ASCUS: atypical squamous cells of undetermined significance; LSIL: low-grade squamous intraepithelial lesion; HPV: human papilloma virus; CIN: cervical intraepithelial neoplasia; OHR: other high-risk.
Positive if both HPV test and liquid based cytology triage (at ASCUS threshold) are positive.
Positive if HPV test is positive for 16/18.
Positive if either HPV test is positive for 16/18 or HPV test is positive for other types other than 16/18 and liquid based cytology triage (at ASCUS threshold) is positive.
4. Discussion
The objectives of this study were to address the potential and utility of using HPV testing based screening in routine health services in Thailand and to evaluate its accuracy in detecting high grade cervical neoplasia compared to Pap smear screening. The most striking finding in this cross-sectional study is the extremely poor performance of conventional cytology (Pap smear) in detecting high-grade cervical neoplasia. It may also raises the possibility of such poor performance of cytology in other settings in Thailand [23]. In fact, Pap smear failed to detect even a single case of 21 women with CIN 2+ lesions. The MoPH has provided opportunistic screening with Pap smears for more than 30 years. In 2002, the MoPH and the NHSO started providing countrywide screening of cervical cancer to all Thai women aged 35–60 years under universal health care coverage insurance scheme at 5-year intervals and integrated it within the routine health services of Thailand. Poor accuracy of Pap smear in our study setting in North Eastern Thailand exemplify the challenges in providing accurate Pap smear screening in Thailand and other LMICs as well as in populations with low frequency of hr-HPV positivity. The poor performance of Pap smear in this setting also calls for review of the current screening strategy based on cytology screening in Thailand. The observed slow decline in cervical cancer incidence rates in Thailand [6], [7], [8], [9], [10], [11], [12], [24] is more likely to be a result of declining parity and improving socio-economic conditions in Thailand over the last three decades rather than due to large scale Pap smear screening and further substantial decline in disease could be achieved if effective interventions such as HPV screening and HPV vaccination are introduced in Thailand.
Our study demonstrates the value of cervical cancer screening using hr-HPV testing as primary screening in general population in Ubon Ratchathani province in Northeastern Thailand. The prevalence of hr-HPV was 3.4%: 0.9% for HPV 16/18, 0.2% for HPV16 and 0.1% for HPV 18, indicating a low frequency of HPV positivity in Ubon Ratchathani as compared to other populations in Thailand. The prevalence of hr-HPV infection was 8.3% in Bangkok, 7.1% in Chiang Mai and 6.4% in Pathum Thani [25], [26]. The age-standardized incidence rate of cervical cancer in Chiang Mai is twice higher than that in Ubon Ratchathani (25.2/100,000 vs 13.4/100,000), reflecting the higher frequency of HPV infection in Chiang Mai [24].
Our results indicate that hr-HPV testing is much more sensitive than Pap smear screening. Higher sensitivity of HPV testing as compared to conventional cytology has been well established [14], [16], [27]. However, using HPV testing as a stand-alone primary screening test would require referring a large number of women for colposcopy, especially in populations with high HPV prevalence, overloading the public colposcopy services. Therefore, HPV-based primary screening requires the identification of an adequate triage method for hr-HPV positive women, so as to further stratify them by their risk of having high-grade cervical lesions.
Colposcopy is the preferred method of triaging screen positive women in Thailand and alternatives such as random biopsies following visual inspection with acetic acid (VIA) are not widely accepted in Thailand. To reduce colposcopy referral frequency, an hr-HPV screening strategy plus reflex LBC was explored. When LBC triaging at ASCUS-cut-off was considered, the number of women referred for colposcopy dropped from 166 to 79 and the detection rate of CIN2+ lesions declined from 4.4 to 3.2 per 1000 women screened. If one considers a scenario when all HPV16/18 positive cases plus other non 16/18 type with reflex LBC at ASCUS threshold, the number of women referred for colposcopy was 104 and the detection rate of CIN 2+ cases was 3.6/1000 women. In summary, HPV-based screening of Thai women aged 30–60 detected more high-grade CIN but decreased the screening specificity, and increased the demand for additional testing as compared triaging with LBC at ASCUS threshold. No participant refused to undergo HPV testing.
A major limitation of our study is the low frequency of high-grade cervical neoplasia on which the observations have been made. This is understandable given the fact that the study involved repeatedly screened population albeit with a poor performing cytology screen and the low frequency of HPV positivity compared to other populations in Thailand. A major strength is that the study has been mounted in real life settings in routine health services indicating the possibility to integrate HPV screening in routine health services in Thailand. Since it is a cross-sectional study with no follow-up component, we do not have information on retesting of those who were HPV positive but LBC negative at 1- year following primary screening. On the other hand, our study demonstrates the feasibility to integrate HPV testing in public health services in Thailand and its acceptability by the population as well as the utility of HPV testing and LBC triage in primary screening for cervical neoplasia. However, widespread public educational activities regarding the role of HPV infection in relation to development of cervical cancer should be implemented before scaling up a HPV testing based screening program since HPV positive women may get very anxious about HPV infection.
Acknowledgements
This project was partly supported by National Cancer Institute foundation. The authors gratefully acknowledge all women who participate this screening project. We would also like to thank all the staff of Ubon Ratchathani Provincial Public Health Office and Ubon Ratchathani Cancer Hospital.
References
- 1.J. Ferlay, I. Soerjomataram, M. Ervik, R.Dikshit, S. Eser, C. Mathers, et al., GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. 〈http://globocan.iarc.fr/〉, 2013 (accessed 22.05.16).
- 2.W. Imsamran, A. Chaiwerawattana, A. Wiangnon, K. Pongnikorn, K.Suwanrungraung, S.Sangrajrang, et al., Cancer in Thailand Volume VIII, 2010–2012, NCI, Bangkok, 2015.
- 3.IARC, IARC Handbook of Cancer Prevention Volume 10. Cervix CancerScreening, IARC, Lyon, 2005.
- 4.Arbyn M., Raifu A.O., Weiderpass E., Bray F., Anttila A. Trends of cervical cancer mortality in the member states of the European Union. Eur. J. Cancer. 2009;45:2640–2648. doi: 10.1016/j.ejca.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 5.Vaccarella S., Lortet-Tieulent J., Plummer M., Franceschi S., Bray F. Worldwide trends in cervical cancer incidence: impact of screening against changes in disease risk factors. Eur. J. Cancer. 2013;49:3262–3273. doi: 10.1016/j.ejca.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 6.IARC, Cancer Incidence in Five Continents Volumes I-X. 〈http://ci5.iarc.fr〉, 2016 (accessed 20.05.16).
- 7.V. Vatanasapt, N. Martin, H. Sripung, K. Chindavijak, S. Sontipong, S. Sriamporn, et al., Cancer in Thailand 1989–1991. IARC Technical Report No. 16, IARC, Lyon, 1993.
- 8.S. Deerasamee, N. Martin, S. Sontipong, S. Srimaporn, H. Sripung, P. Srivatabakul, et al., Cancer in Thailand Volume II, 1992–1994. IARC Technical Report No. 34, IARC, Lyon, 1999.
- 9.H. Sripung, S. Sontipong, N. Martin, S. Wiangnon, V. Vootiprux, A. Cheirsilpa, Cancer in Thailand Volume III, 1995–1997, NCI, Bangkok, 2003.
- 10.T. Khuhaprema, P. Srivatanakul, H. Sripung, S. Wiangnon, Y. Sumitsawan, P. Attasara, Cancer in Thailand Volume IV, 1998–2000, NCI, Bangkok, 2007.
- 11.T. Khuhaprema, P. Srivatanakul, P. Attasara, H. Sripung, S. Wiangnon, Y. Sumitsawan, Cancer in Thailand Volume V, 2001–2003, NCI, Bangkok, 2010.
- 12.T. Khuhaprema, P. Attasara, H. Sripung, S. Wiangnon, Y. Sumitasawan, S. Sangrajrang, Cancer in Thailand Volume VI, 2004–2006, NCI, Bangkok, 2012.
- 13.Sankaranarayanan R. Screening for cancer in low- and middle-income countries. Ann. Glob. Health. 2014;80:412–417. doi: 10.1016/j.aogh.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Cuzick J., Arbyn M., Sankaranarayanan R., Tsu V., Ronco G., Mayrand M.H. Overview of human papillomavirus-based and other novel options for cervical cancer screening in developed and developing countries. Vaccine. 2008;26(Suppl. 10):K29–K41. doi: 10.1016/j.vaccine.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 15.Sankaranarayanan R., Nene B.M., Shastri S.S., Jayant K., Muwonge R., Budukh A.M. HPV screening for cervical cancer in rural India. N. Engl. J. Med. 2009;360:1385–1394. doi: 10.1056/NEJMoa0808516. [DOI] [PubMed] [Google Scholar]
- 16.Arbyn M., Ronco G., Anttila A., Meijer C.J., Poljak M., Ogilvie G. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine. 2012;30(Suppl. 5):F88–F99. doi: 10.1016/j.vaccine.2012.06.095. [DOI] [PubMed] [Google Scholar]
- 17.Ronco G., Dillner J., Elfstrom K.M., Tunesi S., Snijders P.J., Arbyn M. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet. 2014;383:524–532. doi: 10.1016/S0140-6736(13)62218-7. [DOI] [PubMed] [Google Scholar]
- 18.Berry G., Smith C.L., Macaskill P., Irwig L. Analytic methods for comparing two dichotomous screening or diagnostic tests applied to two populations of differing disease prevalence when individuals negative on both tests are unverified. Stat. Med. 2002;21:853–862. doi: 10.1002/sim.1066. [DOI] [PubMed] [Google Scholar]
- 19.Chock C., Irwig L., Berry G., Glasziou P. Comparing dichotomous screening tests when individuals negative on both tests are not verified. J. Clin. Epidemiol. 1997;50:1211–1217. doi: 10.1016/s0895-4356(97)00122-4. [DOI] [PubMed] [Google Scholar]
- 20.Walter S.D. Estimation of test sensitivity and specificity when disease confirmation is limited to positive results. Epidemiology. 1999;10:67–72. [PubMed] [Google Scholar]
- 21.Qu Y., Tan M., Kutner M.H. Random effects models in latent class analysis for evaluating accuracy of diagnostic tests. Biometrics. 1996;52:797–810. [PubMed] [Google Scholar]
- 22.Menten J., Boelaert M., Lesaffre E. Bayesian latent class models with conditionally dependent diagnostic tests: a case study. Stat. Med. 2008;27:4469–4488. doi: 10.1002/sim.3317. [DOI] [PubMed] [Google Scholar]
- 23.Khuhaprema T., Attasara P., Srivatanakul P., Sangrajrang S., Muwonge R., Sauvaget C. Organization and evolution of organized cervical cytology screening in Thailand. Int. J. Gynaecol. Obstet. 2012;118:107–111. doi: 10.1016/j.ijgo.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 24.T. Khuhaprema, P. Attasara, H. Sripung, S. Wiangnon, S. Sangrajrang, Cancer in Thailand Volume VII, 2007–2009, NCI, Bangkok, 2013.
- 25.Swangvaree S.S., Kongkaew P., Rugsuj P., Saruk O. Prevalence of high-risk human papillomavirus infection and cytologic results in Thailand. Asian Pac. J. Cancer Prev. 2010;11:1465–1468. [PubMed] [Google Scholar]
- 26.Kantathavorn N., Mahidol C., Sritana N., Sricharunrat T., Phoolcharoen N., Auewarakul C. Genotypic distribution of human papillomavirus (HPV) and cervical cytology findings in 5906 Thai women undergoing cervical cancer screening programs. Infect. Agent. Cancer. 2015;10:7. doi: 10.1186/s13027-015-0001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rebolj M., Bonde J., Preisler S., Ejegod D., Rygaard C., Lynge E. Human papillomavirus assays and cytology in primary cervical screening of women aged 30 years and above. PLoS One. 2016;11:e0147326. doi: 10.1371/journal.pone.0147326. [DOI] [PMC free article] [PubMed] [Google Scholar]