Abstract
Objectives
Human papillomavirus (HPV) as a risk factor in oropharyngeal squamous cell carcinoma (OPSCC) is well established. However, accumulating data imply that the OPSCC concept is too unspecific with regard to HPV prevalence and clinical importance. To further study the role of HPV in OPSCC by sub-site, a systematic review and meta-analysis was performed.
Material and method
PubMed was searched and all studies reporting HPV data (p16/HPV DNA/RNA) in both “lymphoepithelial associated” (i.e. tonsillar and base of tongue cancer; TSCC and BOTSCC respectively) and “non-lymphoepithelial” (“other” OPSCC) OPSCC were included. Pooled odds ratios by HPV detection method were analysed using a random effects model.
Results
In total, 58 unique patient cohorts were identified. Total HPV prevalence in TSCC/BOTSCC was 56%, 95%CI: 55–57% (59%, 95%CI: 58–60% for TSCC only) as compared to 19%, 95%CI: 17–20%, in “other” OPSCC. Significant association of HPV to TSCC/BOTSCC vs. “other” OPSCC was observed no matter HPV detection method used, but statistical homogeneity was only observed when studies using algorithm based HPV detection were pooled.
Conclusion
HPV prevalence differs markedly between OPSCC sub-sites and while the role of HPV in TSCC/BOTSCC is strong, the role in “other” OPSCC is more uncertain and needs further evaluation.
Keywords: HPV, Tonsillar cancer, Base of tongue cancer, Oropharyngeal cancer, Meta-analysis, Prevalence
1. Introduction
Already in 1983 Syrjänen and colleagues published the first data suggesting that human papillomavirus (HPV) could be associated to a sub-group of head and neck squamous cell carcinoma (HNSCC) [1]. Since then, the field of HPV, especially HPV type 16, in HNSCC has emerged considerably. Subsequently, in 2009, due to a large body of evidence the International Agency of Research of Cancer (IARC) declared that “there is a strong epidemiological evidence for the casual role of HPV16 in the aetiology of cancer of the oropharynx and tonsil” [2]. Today, research on HPV and HNSCC in general has shifted and focuses on HPV in oropharyngeal squamous cell carcinoma (OPSCC). Moreover, recent accumulating data imply that HPV in the oropharynx context may still be too broad and un-specific and that it is biologically and clinically necessary to narrow down the concept of oropharynx to specific sub-sites, more specifically to tonsillar and base of tongue squamous cell carcinoma (TSCC and BOTSCC) [3], [4], [5], [6].
The oropharynx is namely a histological heterogeneous sub-site within the head and neck region that consists not only of the palatine tonsils and the base of tongue (including the lingual tonsils), but also the soft palate, the tonsillar pillars and the uvula. The histology of the palate, the pillars and the uvula is built up by a stratified squamous epithelium without a keratin layer, similar to what is observed in the oral cavity, whereas the histology of the tonsils and the tongue base is distinctly different. The tongue base and the tonsillar mucosa invaginates and forms “crypts” lined with reticulated epithelium, in which the basal lamina is discontinuous and the histological border between the epithelium and the underlying lymphoid stroma is indistinct (“lymphoepithelial tissue”) [7], [8]. These crypts are normally not observed at the other sites of the oropharynx (or in e.g. oral cavity). There is now evidence demonstrating that HPV positive carcinomas develop within the histological characteristic crypts in the oropharynx, while HPV negative carcinomas emerge mainly from the surface epithelium [7], [8]. Due to this morphological difference in tissue tropism and absence or presence of crypts, we speculate that HPV should be evaluated per sub-site in oropharynx. Here, a systematic review is presented of literature published 2013–2016 regarding HPV prevalence per cancer sub-site in the oropharynx, and we argue that sub-site within oropharynx matters.
2. Material and methods
2.1. Search strategy and data extraction
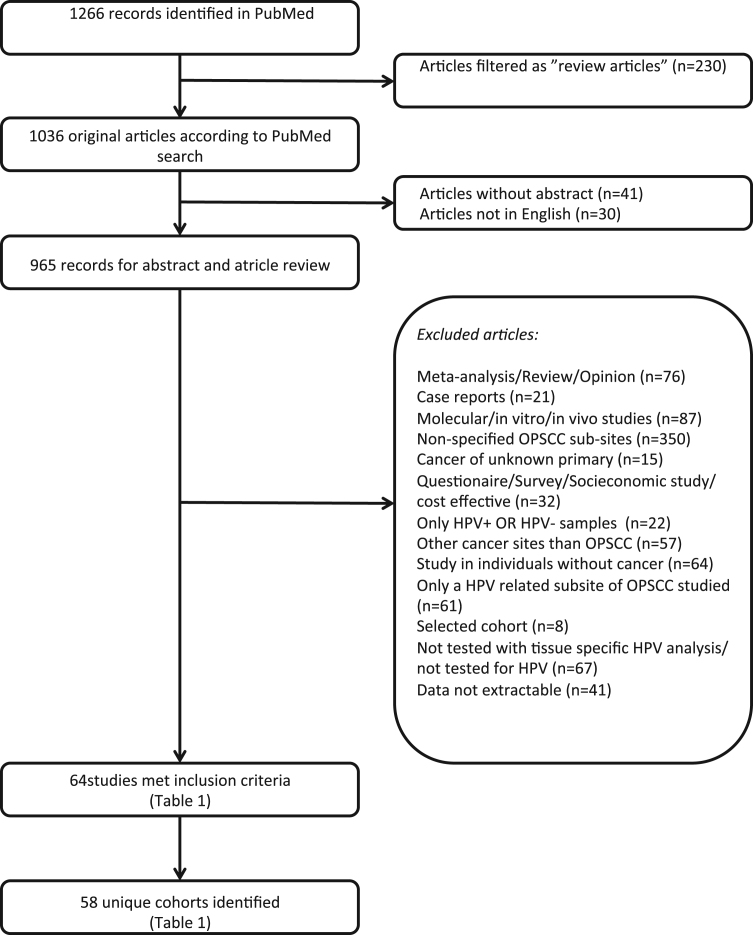
PubMed was searched for all studies published from 2013-01-01 to 2016-10-31 using the search terms (HPV OR Papillomaviridae[MeSH]) AND (oropharyngeal OR oropharynx OR tonsil OR tonsillar OR “base of tongue” OR “soft palate”) AND (cancer OR carcinoma) AND (2016[DP] OR 2015[DP] OR 2014[DP] OR 2013[DP]). The PRISMA statement was consulted to perform the search [9]. In total 1266 articles were identified and ultimately 64 met the inclusion criteria of which 58 unique cohorts were identified and for details see the flow chart in Fig. 1. More specifically, 965 articles remained initially for further analysis after filtering out 230 as review articles, 30 not written in English, and 41 without an abstract. Abstracts from these 965 articles were then reviewed by two researchers (AN and LH) and those reporting HPV data were then further reviewed by examining the “material and method” and the “result” section in the articles. Articles reporting HPV data by a molecular tissue specific method (PCR, ISH or p16 immunohistochemistry) in HPV related “lymphoepithelial” oropharyngeal sub-sites (i.e. tonsillar and base of tongue) and in HPV un-related “non-lymphoepithelial” oropharyngeal sub-sites (i.e. walls of oropharynx, uvula and soft palate) in an un-selected cohort (retrospective/prospective, randomized/non-randomized) were included (Fig. 1). For each study, only the cohort of OPSCC patients was considered and the numbers of patients with HPV positive and negative tumours per sub-site were calculated or extracted, together with the HPV detection method. A consensus was reached for each article. The main reason for exclusion was that the sub-sites of oropharynx were not specified (Fig. 1).
Fig. 1.
Flow diagram of study population identification and selection.
2.2. Statistical analysis
Differences in HPV positive and negative patient numbers were calculated by using Fisher's exact test (two-tailed) and Chi2-test (two-tailed) when appropriate. A p-value ≤0.05 was considered as significant. The metan command in Stata 11 (StataCorp, College Station, TX) was used to pool odds ratios (OR) with 95% confidence intervals (CI) across studies using the Der Simonian and Laird random-effects methods.
3. Results
3.1. Prevalence of HPV at different OPSCC sub-sites
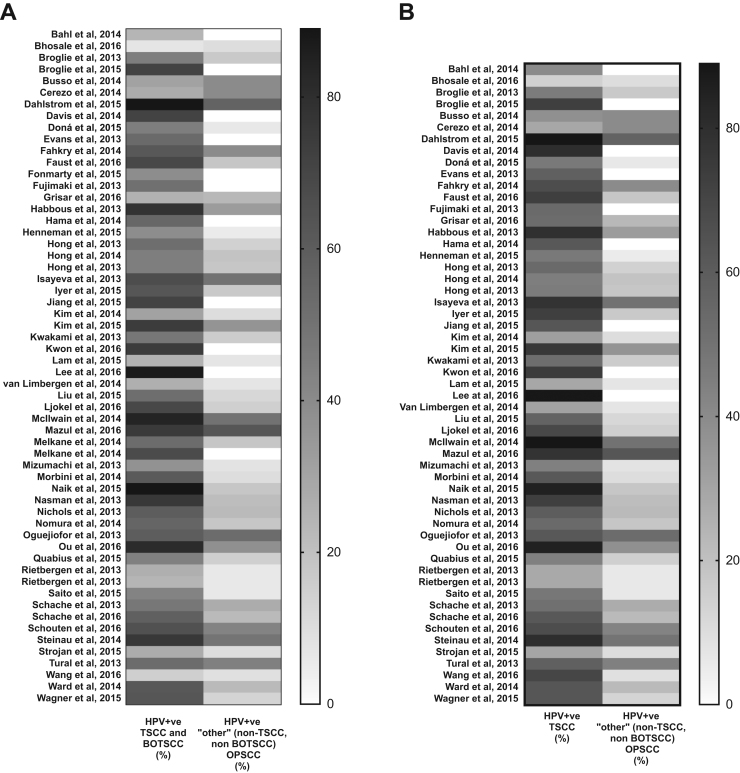
In total, 64 articles were included in the analysis, with a total of 11710 patients in these studies. The number of patients varied between 30 and 1474 (mean 202 patients per study) (Table 1) [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73]. The sub-sites tonsils and base of tongue dominated the oropharyngeal cancer sites (83%), whereas only a minority of the tumours were located in the soft palate and the oropharyngeal walls (17%). Total oropharyngeal HPV prevalence per study varied between 7% and 88% (Table 1). Notably, HPV was more commonly found in “lymphoepithelial” tissues (TSCC and BOTSCC) as compared to “non-lymphoepithelial” tissues (“other” OPSCC) of the oropharynx (Table 1, Fig. 2A). Total HPV prevalence in TSCC/BOTSCC was 56%, 95% CI: 55–57% (59%, 95% CI: 58–60% for TSCC only) as compared to 19%, 95% CI: 17–20%, HPV prevalence in “other” OPSCC (Table 1).
Table 1.
Studies and their patients included in the meta-analysis.
| Author, Year | Countrya | Oropharyngeal | HPV+ | HPV- | HPV | HPV | p-value* | p-value* |
|---|---|---|---|---|---|---|---|---|
| sub-site | tumours | tumours | prevalence | detection | (TSCC and BOTSCC vs.”other” OPSCC) | (TSCC only vs.”other” OPSCC) | ||
| Bahl et al., 2014 [10] | India | Base of tongue | 14 | 61 | 19% (18–20%) | PCR | NS | NS |
| Tonsil | 10 | 15 | 40% (36–44%) | |||||
| Soft palate | 0 | 5 | 0% (0–0%) | |||||
| Bhosale et al., 2016 [11] | India | Base of tongue | 0 | 23 | 0% (0–0%) | p16 IHC | NS | NS |
| Tonsil | 3 | 18 | 14% (11–18%) | |||||
| Soft palate | 0 | 5 | 0% (0–0%) | |||||
| Posterior wall | 1 | 4 | 20% (4–36%) | |||||
| Broglie et al., 2013 [13] | Switzerland | Base of tongue | 22 | 28 | 44% (42–46%) | p16 IHC | NS | NS |
| Tonsil | 31 | 37 | 46% (44–48%) | |||||
| Post wall/ soft palate | 1 | 5 | 17% (4–29%) | |||||
| Broglie et al., 2015 [12] | Switzerland | Base of tongue | 3 | 3 | 50% (34–66%) | p16 IHC | NS | NS |
| Tonsil | 36 | 13 | 73% (72–75%) | |||||
| Post wall | 0 | 2 | 0% (0–0%) | |||||
| Busso et al., 2014 [14] | Italy | Base of tongue | 1 | 10 | 9% (4–14%) | PCR | NS | NS |
| Tonsil | 13 | 21 | 38% (35–41%) | |||||
| Soft palate | 2 | 1 | 67% (36–97%) | |||||
| Posterior wall | 0 | 2 | 0% (0–0%) | |||||
| Cerezo et al., 2014 [16] | Spain | Base of tongue | 10 | 30 | 25% (23–27%) | p16 IHC | NS | NS |
| Tonsil | 11 | 27 | 29% (27–31%) | |||||
| Cerezo et al., 2014 [15] | Soft plate | 5 | 8 | 38% (31–46%) | ||||
| Pharyngeal wall | 1 | 1 | 50% (1–99%) | |||||
| Dahlstrom et al., 2015 [17] | United States of America | Base of tongue | 139 | 16 | 90% (89–90%) | p16 IHC and ISH with/without PCR | 0.04 | 0.04 |
| Tonsil | 172 | 22 | 89% (88–90%) | |||||
| Other | 4 | 3 | 57% (43–71%) | |||||
| Davis et al., 2014 [18] | United States of America | Base of tongue | 5 | 4 | 56% (45–66%) | p16 IHC | 0.003 | 0.002 |
| Tonsil | 12 | 3 | 80% (75–85%) | |||||
| Soft palate | 0 | 6 | 0% (0–0%) | |||||
| Doná et al., 2015 [19] | Italy | Base of tongue | 26 | 34 | 43% (42–45%) | PCR | 0.002 | 0.003 |
| Tonsil | 30 | 34 | 47% (45–48%) | |||||
| Other oropharynx | 1 | 15 | 6% (3–9%) | |||||
| Evans et al., 2013 [20] | United Kingdom | Base of tongue and vallecula | 15 | 20 | 43% (40–46%) | p16 IHC and PCR and/or ISH | 0.001 | 0.0003 |
| Tonsil | 54 | 39 | 58% (57–59%) | |||||
| Other oropharynx | 0 | 10 | 0% (0–0%) | |||||
| Fahkry et al., 2014 [21] | United States of America | Base of tongue | 52 | 36 | 59% (58–60%) | p16 IHC | 0.02 | 0.002 |
| Tonsil | 39 | 19 | 67% (66–69%) | |||||
| Soft palate | 0 | 3 | 0% (0–0%) | |||||
| Oropharynx NOS | 14 | 9 | 61% (57–65%) | |||||
| Faucial arch | 0 | 1 | 0% (0–0%) | |||||
| Pharyngeal oropharynx | 0 | 8 | 0% (0–0%) | |||||
| Faust et al., 2016 [22] | Sweden | Base of tongue | 15 | 12 | 56% (52–59%) | PCR | 0.001 | <0.001 |
| Tonsil | 75 | 28 | 73% (72–74%) | |||||
| Oropharynx NOS | 2 | 9 | 18% (11–25%) | |||||
| Fonmarty et al., 2015 [23] | Not specified | Anterior/lateral oropharynx (tonsil, base of tongue and glossotonsillar sulcus) | 20 | 31 | 39% (37–41%) | p16 IHC and PCR | <0.001 | – |
| Other oropharyngeal sites | 0 | 20 | 0% (0–0%) | |||||
| Fujimaki et al., 2013 [24] | Japan | Lateral | 27 | 23 | 54% (52–56%) | p16 IHC and ISH | NS | 0.05 |
| Anterior | 4 | 7 | 36% (28–45%) | |||||
| Posterior | 0 | 3 | 0% (0–0%) | |||||
| Superior | 0 | 2 | 0% (0–0%) | |||||
| Grisar et al., 2016 [26] | Belgium | Tongue base | 6 | 36 | 14% (13–16%) | p16 IHC | NS | 0.05 |
| Tonsil | 8 | 7 | 53% (47–60%) | |||||
| Soft palate | 1 | 4 | 20% (4–36%) | |||||
| Oropharynx NOS | 8 | 26 | 24% (21–26%) | |||||
| Habbous et al., 2013 [27] | Canada | Base of tongue | 159 | 50 | 76% (76–76%) | p16 IHC | <0.0001 | <0.0001 |
| Tonsil | 308 | 83 | 79% (79–79%) | |||||
| Other oropharynx | 27 | 56 | 33% (31–34%) | |||||
| Hama et al., 2014 [28] | Japan | Anterior | 6 | 20 | 23% (20–26%) | PCR | <0.0001 | <0.0001 |
| Lateral | 73 | 44 | 62% (62–63%) | |||||
| Upper | 0 | 10 | 0% (0–0%) | |||||
| Posterior | 0 | 4 | 0% (0–0%) | |||||
| Henneman et al., 2015 [29] | Netherlands | Base of tongue | 13 | 36 | 27% (25–28%) | PCR | 0.003 | 0.001 |
| Tonsil | 37 | 41 | 47% (46–49%) | |||||
| Other oropharynx | 1 | 18 | 5% (3–8%) | |||||
| Hong et al., 2013 [32] | Australia | Base of tongue | 11 | 18 | 38% (35–41%) | PCR and p16 IHC | 0.001 | <0.001 |
| Tonsil | 99 | 84 | 54% (54–55%) | |||||
| Other oropharynx | 3 | 18 | 14% (11–18%) | |||||
| Hong et al., 2014 [31] | Australia | Base of tongue | 29 | 30 | 49% (47–51%) | PCR and p16 IHC | <0.001 | <0.001 |
| Tonsil | 181 | 222 | 45% (45–45%) | |||||
| Other oropharynx | 10 | 43 | 19% (17–20%) | |||||
| Hong et al., 2013 [30] | Australia | Base of tongue | 15 | 31 | 33% (31–35%) | PCR and p16 IHC | <0.001 | 0.0001 |
| Tonsil | 253 | 298 | 46% (46–46%) | |||||
| Other oropharynx | 9 | 41 | 18% (16–20%) | |||||
| Isayeva et al., 2013 [33] | United States of America | Base of tongue | 20 | 16 | 56% (53–58%) | RT-PCR | NS | 0.03 |
| Tonsil | 31 | 9 | 78% (75–80%) | |||||
| Soft palate/uvula | 3 | 3 | 50% (34–66%) | |||||
| Oropharynx | 10 | 10 | 50% (45–55%) | |||||
| Iyer et al., 2015 [34] | United States of America | Base of tongue | 50 | 39 | 56% (55–57%) | p16 IHC | <0.0001 | <0.0001 |
| Tonsil | 48 | 18 | 73% (71–74%) | |||||
| Soft palate | 8 | 38 | 17% (16–19%) | |||||
| Jiang et al., 2015 [35] | United States of America | Base of tongue | 12 | 3 | 80% (75–85%) | ISH | <0.0001 | 0.0001 |
| Tonsil | 10 | 6 | 63% (57–68%) | |||||
| Soft palate | 0 | 10 | 0% (0–0%) | |||||
| Kim et al., 2014 [38] | Not specified | Base of tongue | 5 | 12 | 29% (24–35%) | PCR | NS | NS |
| Tonsil | 15 | 32 | 32% (30–34%) | |||||
| Soft palate | 1 | 9 | 10% (4–16%) | |||||
| Kim et al., 2015 [37] | South Korea | Base of tongue | 1 | 3 | 25% (4–46%) | p16 IHC | <0.001 | <0.001 |
| Tonsil | 79 | 25 | 76% (75–77%) | |||||
| Soft palate | 1 | 8 | 11% (4–18%) | |||||
| Oroharynx NOS | 8 | 8 | 50% (44–56%) | |||||
| Kwakami et al., 2013 [36] | Japan | Base of tongue | 4 | 9 | 31% (24–38%) | PCR | <0.001 | 0.001 |
| Tonsil | 31 | 29 | 52% (50–53%) | |||||
| Other oropharynx | 5 | 26 | 16% (14–18%) | |||||
| Kwon et al., 2016 [39] | New Zealand | Tonsil and tonguebase | 86 | 31 | 74% (73–74%) | p16 IHC | <0.0001 | – |
| Other oropharynx | 0 | 14 | 0% (0–0%) | |||||
| Lam et al., 2015 [40] | China | Base of tongue | 4 | 35 | 10% (9–12%) | PCR and E6*I mRNA | 0.01 | 0.003 |
| Tonsil | 36 | 88 | 29% (28–30%) | |||||
| Soft palate | 3 | 29 | 9% (8–11%) | |||||
| Other oropharyngeal walls | 0 | 12 | 0% (0–0%) | |||||
| Lee et al., 2016 [41] | South Korea | Base of tongue | 15 | 4 | 79% (75–83%) | p16 IHC | <0.0001 | <0.0001 |
| Tonsil | 89 | 12 | 88% (87–89%) | |||||
| Soft palate | 0 | 4 | 0% (0–0%) | |||||
| Posterior wall | 0 | 2 | 0% (0–0%) | |||||
| Van Limbergen et al., 2014 [70] | Belgium | Base of tongue | 16 | 67 | 19% (18–20%) | PCR and p16IHC | 0.002 | <0.001 |
| Tonsil | 33 | 72 | 31% (31–32%) | |||||
| Soft palate | 0 | 11 | 0% (0–0%) | |||||
| Pharyngeal wall | 1 | 30 | 3% (2–4%) | |||||
| Unclear | 3 | 16 | 16% (12–20%) | |||||
| Liu et al., 2015 [42] | Australia | Base of tongue | 7 | 13 | 35% (30–40%) | PCR and ISH | 0.002 | <0.001 |
| Tonsil | 39 | 29 | 57% (56–59%) | |||||
| Other oropharynx | 2 | 15 | 12% (8–15%) | |||||
| Ljokel et al., 2016 [43], [44] | Norway | Base of tongue | 28 | 13 | 68% (66–71%) | PCR | <0.0001** | <0.0001 |
| Tonsil | 86 | 39 | 69% (68–70%) | |||||
| Lybak et al., 2016 [45] | Tonsil pillar | 1 | 12 | 8% (4–12%) | ||||
| Overlapping tonsil | 1 | 0 | 100% (100–100%) | |||||
| Oropharynx (ICD-10 C10) | 3 | 22 | 12% (9–15%) | |||||
| Soft palate and overlapping lesion | 4 | 10 | 29% (22–35%) | |||||
| Uvula | 1 | 6 | 14% (4–24%) | |||||
| McIlwain et al., 2014 [47] | United States of America | Base of tongue | 26 | 8 | 76% (74–79%) | p16 IHC | 0.04 | 0.02 |
| Tonsil | 41 | 5 | 89% (88–90%) | |||||
| Soft palate | 4 | 2 | 67% (51–82%) | |||||
| Posterior wall | 0 | 2 | 0% (0–0%) | |||||
| Mazul et al., 2016 [46] | United States of America | Base of tongue | 50 | 23 | 68% (67–70%) | PCR | NS | NS |
| Tonsil | 115 | 33 | 78% (77–78%) | |||||
| Other oropharynx | 17 | 10 | 63% (59–66%) | |||||
| Melkane et al., 2014 [48] | France | Lymphoid location (tonsillar and base of tongue) | 65 | 57 | 53% (52–54%) | p16 IHC | 0.03 | – |
| Nonlymphoid location (posterior oropharyngeal wall and soft palate) | 2 | 9 | 18% (11–25%) | |||||
| Melkane et al., 2014 [49] | France | Lymphoid location | 28 | 13 | 68% (66–71%) | p16 IHC | <0.01 | – |
| Non-lymphoid location | 0 | 5 | 0% (0–0%) | |||||
| Mizumachi et al., 2013 [50] | Japan | Lateral wall | 18 | 23 | 44% (42–46%) | PCR | NS | 0.04 |
| Anterior wall | 4 | 14 | 22% (18–27%) | |||||
| Superior wall | 1 | 8 | 11% (4–18%) | |||||
| Posterior wall | 0 | 3 | 0% (0–0%) | |||||
| Morbini et al., 2014 [51] | Italy | Base of tongue | 6 | 4 | 60% (50–70%) | mRNA ISH | <0.01 | <0.01 |
| Tonsil | 13 | 8 | 62% (57–66%) | |||||
| Soft palate | 1 | 9 | 10% (4–16%) | |||||
| Naik et al., 2015 [52] | United States of America | Base of tongue | 70 | 6 | 92% (91–93%) | p16 IHC and/or ISH | NS | NS |
| Tonsil | 56 | 10 | 85% (84–86%) | |||||
| Other | 4 | 1 | 80% (64–96%) | |||||
| Nasman et al., 2013 [53] | Sweden | Base of tongue | 75 | 28 | 73% (72–74%) | PCR | <0.0001 | <0.0001 |
| Tonsil | 217 | 66 | 77% (76–77%) | |||||
| Other oropharynx | 4 | 27 | 13% (11–15%) | |||||
| Soft palate | 7 | 15 | 32% (28–36%) | |||||
| Nichols et al., 2013 [54] | United Kingdom | Base of tongue | 15 | 10 | 60% (56–64%) | PCR | <0.01 | 0.01 |
| Tonsil | 31 | 21 | 60% (58–61%) | |||||
| Other | 4 | 14 | 22% (18–27%) | |||||
| Nomura et al., 2014 [55] | Japan | Lateral wall | 29 | 25 | 54% (52–56%) | PCR and/or p16 IHC | 0.02 | 0.05 |
| Base of tongue | 8 | 4 | 67% (59–74%) | |||||
| Superior wall | 0 | 7 | 0% (0–0%) | |||||
| Posterior wall | 2 | 2 | 50% (26–74%) | |||||
| Oguejiofor et al., 2013 [56] | United Kingdom | Base of tonge | 32 | 27 | 54% (53–56%) | p16 IHC | NS | NS |
| Tonsil | 84 | 51 | 62% (62–63%) | |||||
| Other oropharynx | 9 | 8 | 53% (47–595) | |||||
| Ou et al., 2016 [57] | New Zealand | Base of tongue | 15 | 5 | 75% (71–79%) | p16 IHC and PCR | 0.02 | 0.02 |
| Tonsil | 23 | 4 | 85% (83–88%) | |||||
| Soft palate | 2 | 1 | 67% (36–97%) | |||||
| Oropharyngeal wall | 0 | 1 | 0% (0–0%) | |||||
| Oropharynx (unspecified) | 1 | 3 | 25% (4–46%) | |||||
| Quabius et al., 2015 [58], [59] | Germany | Tonsillar | 59 | 76 | 44% (43–44%) | PCR | 0.03 | 0.03 |
| Soft palate and posterior wall of oropharynx | 3 | 17 | 15% (12–18%) | |||||
| Rietbergen et al., 2013 [60] | Netherlands | Base of tongue | 51 | 161 | 24% (24–24%) | p16 IHC and PCR | <0.0001 | <0.0001 |
| Tonsil | 96 | 248 | 28% (28–28%) | |||||
| Soft palate | 9 | 115 | 7% (7–8%) | |||||
| Oropharynx NOS | 7 | 122 | 5% (5–6%) | |||||
| Rietbergen et al., 2013 [61] | Netherlands | Base of tongue | 13 | 54 | 19% (18–21%) | P16 IHC and PCR | <0.001 | <0.0001 |
| Tonsil | 23 | 60 | 28% (27–29%) | |||||
| Soft palate | 0 | 31 | 0% (0–0%) | |||||
| Oropharynx NOS | 5 | 54 | 8% (8–9%) | |||||
| Saito et al., 2015 [62] | Japan | Lateral wall | 45 | 48 | 48% (47–49%) | p16 IHC | 0.005 | 0.002 |
| Base of tongue | 12 | 29 | 29% (27–31%) | |||||
| Superior wall | 1 | 10 | 9% (4–14%) | |||||
| Posterior wall | 0 | 5 | 0% (0–0%) | |||||
| Schache et al., 2013 [64] | United Kingdom | Base of tongue | 5 | 8 | 38% (31–46%) | qRT-PCR | NS | NS |
| Tonsil | 22 | 21 | 51% (49–53%) | |||||
| Soft palate | 4 | 9 | 31% (24–38%) | |||||
| Oropharynx NOS | 2 | 7 | 22% (13–31%) | |||||
| Schache et al., 2016 [65] | United Kingdom | Base of tongue | 179 | 183 | 49% (49–50%) | p16 IHC and PCR or ISH | <0.0001 | <0.0001 |
| Tonsil | 528 | 326 | 62% (62–62%) | |||||
| Soft palate/uvula | 8 | 80 | 9% (8–10%) | |||||
| Oropharynx NOS | 49 | 121 | 29% (28–29%) | |||||
| Schouten et al., 2016 [66] | Not stated | Base of tongue | 12 | 7 | 63% (58–68%) | p16 IHC and PCR | NS | NS |
| Tonsil | 12 | 6 | 67% (62–72%) | |||||
| Oropharynx NOS | 3 | 4 | 43% (29–57%) | |||||
| Steinau et al., 2014b[67] | United States of America | Base of tongue | 149 | 64 | 70% (70–70%) | PCR | <0.0001 | <0.0001 |
| Saraiya et al., 2015b[63] | Tonsil | 201 | 49 | 80% (80–81%) | ||||
| Goodman et al., 2015b[25] | Other oropharynx | 46 | 48 | 49% (48–50%) | ||||
| Strojan et al., 2015 [68] | Slovenia | Base of tongue | 4 | 16 | 20% (16–24%) | E6/E7 mRNA ISH | NS | 0.05 |
| Tonsil | 12 | 28 | 30% (28–32%) | |||||
| Other oropharynx | 4 | 35 | 10% (9–12%) | |||||
| Tural et al., 2013 [69] | Turkey | Base of tongue | 12 | 15 | 44% (41–48%) | PCR | NS | NS |
| Tonsil | 26 | 19 | 58% (56–60%) | |||||
| Other | 4 | 5 | 44% (34–55%) | |||||
| Wang et al., 2016 [72] | China | Base of tongue | 6 | 68 | 8% (7–9%) | PCR | NS | <0.0001 |
| Tonsil | 7 | 3 | 70% (61–79%) | |||||
| Soft palate | 3 | 47 | 6% (5–7%) | |||||
| Oropharynx NOS | 6 | 48 | 11% (10–12%) | |||||
| Ward et al., 2014 [73] | United Kingdom | Base of tongue | 40 | 28 | 59% (57–60%) | p16 IHC and ISH | <0.0001 | <0.0001 |
| Tonsil | 99 | 57 | 63% (63–64%) | |||||
| Other oropharynx | 10 | 36 | 22% (20–23%) | |||||
| Wagner et al., 2015 [71] | Germany | Tonsil | 20 | 12 | 63% (60–65%) | P16 IHC and PCR and/or ISH | – | <0.0001 |
| Other than tonsil | 12 | 84 | 13% (12–13%) | |||||
p-value calculated by chi-2 test (tonsil and tongue base vs other oropharynx and soft palate; or tonsil vs other oropharynx and soft palate) after patient numbers had been extracted from article.
p-value calculated by chi-2 test (tonsil and tongue base, overlapping tonsil vs tonsil pillars other oropharynx and soft palate) after patient numbers been extracted from article.
Countries from which the patient material and data were collected.
Patients reported in Stainau et al. presented.
Fig. 2.
Heat map of HPV prevalence by oropharyngeal cancer sub-site. (A) Prevalence of HPV, defined by each included study, stratified by tonsillar (TSCC) and base of tongue (BOTSCC) squamous cell carcinomas vs. “other” (i.e. walls of oropharynx, uvulae and soft palate) oropharyngeal squamous cell carcinomas (OPSCC). (B) Prevalence of HPV, defined by each included study, stratified by TSCC only vs. “other” OPSCC.
Furthermore, since there is a risk of misclassification of large mobile tongue cancer into BOTSCC and vice versa, a sub-group analysis was performed comparing only TSCC and “other” OPSCC. The differences observed between “lymphoepithelial” and “non-lymphoepitelial” tissues were here even more pronounced (Table 1 and Fig. 2B).
In addition, a separate analysis including only studies reporting HPV prevalence data divided by tonsillar, base of tongue, soft palate/uvulae and oropharynx was performed. As depicted in Table 2, HPV prevalence was highest in TSCC, followed by BOTSCC, and lower at the other sites (Table 2).
Table 2.
HPV prevalence by oropharyngeal sub-site (data extracted only from studies reporting HPV data separated by tonsils, tongue base, soft palate/uvulae and oropharyngeal walls).
| Oropharyngeal sub-sitea | HPV+ | HPV- | HPV |
|---|---|---|---|
| tumours | tumours | prevalence (95% CI) | |
| Tonsilb | 1577 | 1238 | 56% (54–58%) |
| Base of tonguec | 590 | 881 | 40% (38–43%) |
| Soft palated | 59 | 429 | 12% (9–15%) |
| Posterior walle | 122 | 537 | 19% (16–22%) |
This table only presents data from studies that have divided by oropharyngeal sub-sites: base of tongue, tonsil, soft palate and posterior wall. Following studies where included: 11, 14–16, 21, 24, 26, 28, 33, 37, 40–41, 43–45, 47, 50, 53, 55, 57, 60–62, 64–65, 70, 72.
Includes tonsil, tonsil pillar, overlapping tonsil and lateral wall.
Includes base of tongue and anterior wall.
Includes soft palate, uvula, superior wall, upper, and soft palate with overlapping lesion.
Includes Posterior wall, Oropharyngeal NOS, pharyngeal wall and faucial arch.
3.2. HPV is significantly more prevalently found in TSCC and BOTSCC compared to other OPSCC sites
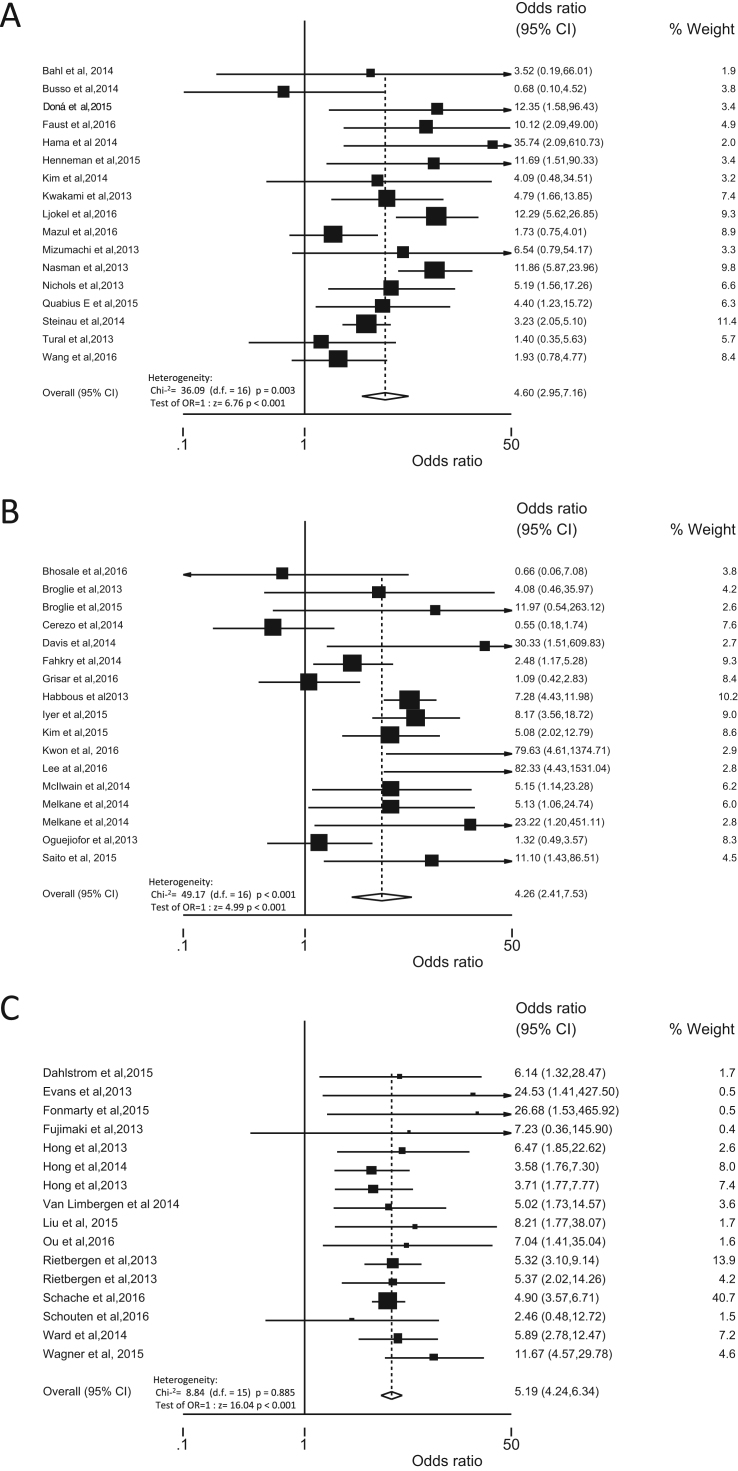
The odds ratio of having HPV in TSCC and BOTSCC as compared to “other” OPSCC was calculated and studies were grouped by HPV detection method, i.e. either HPV DNA PCR alone, or p16 IHC alone, or a HPV DNA based algorithm, i.e. combining HPV DNA and p16 overexpression. The odds having HPV in TSCC and BOTSCC as compared to “other” OPSCC was significantly higher, no matter which detection method that was used as depicted in Fig. 3 (PCR: OR 4.60 95% CI 2.95–7.16, p<0.001; p16 IHC: OR 4.26 95% CI 2.41–7.53, p<0.001; algorithm: OR 5.19 95% CI 4.24–6.34, p<0.001). Notably, no statistical heterogeneity (Chi2=8.84 (d.f.=15) p=0.885; Estimate of between-study variance Tau-squared=0.00) was observed when applying the algorithm using the presence of HPV in combination with p16 overexpression as defining positive HPV status (Fig. 3C). In contrast, when using either HPV DNA PCR positivity or p16 alone, gave significant statistical heterogenic results (PCR: Chi2=36.09 (d.f.=16) p=0.003; Estimate of between-study variance Tau-squared=0.39 and p16 IHC: Chi2=49.17 (d.f.=16) p<0.001; Estimate of between-study variance Tau-squared=0.76) (Fig. 3A and B).
Fig. 3.
Forrest plot with odds ratios (OR) of having HPV in tonsillar and base of tongue squamous cell carcinomas (TSCC and BOTSCC respectively) vs. “other” (i.e. walls of oropharynx, uvulae and soft palate) oropharyngeal squamous cell carcinoma (OPSCC) presented by molecular detection method. (A) OR (95% CI) of having HPV, defined by presence of HPV DNA by PCR, in TSCC/BOTSCC vs. “other” OPSCC. (B) OR (95% CI) of having HPV, defined by overexpression of p16 immunohistochemistry (IHC), in TSCC/BOTSCC vs. “other” OPSCC. (C) OR (95% CI) of having HPV, defined by an algorithm combining presence of HPV DNA and overexpression of p16 IHC, in TSCC/BOTSCC vs. “other” OPSCC.
4. Discussion
In this systematic review, HPV prevalence was significantly higher in “lymphoepithelial” sites of the oropharynx, i.e. tonsil and base of tongue, as compared to “non-lymphoepithelial” sites of the oropharynx, i.e. soft palate and oropharyngeal, irrespectively of HPV detection method.
Numerous previous studies have focused on differences in HPV prevalence between different head and neck cancer sites and different geographic areas [6], [74], but few have addressed the relevance of sub-sites within oropharynx. As there has been a focus on OPSCC in contrast to HNSCC in general, many studies have unfortunately not specified these oropharyngeal sub-sites and very few studies have verified the sub-sites by histopathology. Recently however, Garnaes et al. [4] subdivided TSCC into specified TSCC (“lymphoepithelial”) and non-specified TSCC (“non-lymphoepithelial”) by histomorphology. This study reported that HPV prevalence was higher and increased over time in specified TSCC, while the prevalence of HPV was lower and stable over time in non-specified TSCC. Notably, the authors also observed a significant discordant HPV DNA and p16 IHC positivity in non-specified TSCC as compared to specified TSCC. Likewise, Marklund et al. have also presented similar results with discordant p16 status and HPV DNA positivity by PCR in oropharyngeal sub-sites outside the tonsils and the tongue base [5]. Analogous data have also been conveyed in oral carcinomas [75]. Moreover, in a recent meta-analysis of HPV prevalence in different head and neck sites, 24.2% (18.7–30.2) of the oral carcinomas were reported to harbour HPV DNA [6]. Comparable prevalence data were here described for “other” OPSCC (19%, 95% CI: 17–20%), which – together with the overlapping histomorphology – may suggest that”other” OPSCC are more comparable with oral carcinomas than TSCC/BOTSCC. Hence, we argue that not only geographic region and detection method should be considered when reporting HPV prevalence, but also oropharyngeal sub-site.
Studies by others have shown that HPV status defined by only p16 IHC or PCR alone in OPSCC may be too unspecific, and that if the methods are combined in an algorithm there is a high concordance with presence of active HPV infection [61]. Although the odds ratios, reported in this study, of having HPV in TSCC and/or BOTSCC as compared to “other” OPSCC was higher independent of method used, there was a significant heterogeneity between studies using p16 or PCR alone. In contrast, statistical heterogeneity was not observed when uniting studies using an algorithm combining HPV DNA and p16 overexpression, which suggests that using only a PCR or p16 based HPV detection method is too unspecific and may detect false HPV positive samples in non-tonsillar non-base of tongue OPSCC.
Notably, HPV prevalence per oropharyngeal sub-site is not only of academic concern, it is in fact of clinical importance. In a recently published Danish study, patients with specified TSCC and BOTSCC had a better clinical outcome if their tumours were both HPV DNA and p16 positive as compared to being only p16 positive, while an analogous difference in clinical outcome was not observed in patients with non-specified TSCC [3]. Similar results were reported by Ljokjel et al.[44] In that study, patients with HPV positive TSCC and BOTSCC were reported to have a better clinical outcome, but no differences in clinical outcome were observed between patients with HPV positive and negative “other” OPSCC. Likewise, a study by Marklund et al.[5] showed that HPV infection was not correlated to patient outcome if the patients had a non-tonsillar, non-base of tongue OPSCC.
Currently, it is discussed whether oncological treatment can be tapered in patients with HPV positive OPSCC, and randomized controlled studies have shown a beneficial survival in patients with HPV positive OPSCC. However, since patients with TSCC and BOTSCC dominate the OPSCC patient group, there is a risk that patients with TSCC and BOTSCC in published survival studies supersede patients with “other OPSCC”. This could lead to that patients with “other OPSCC” could disfavour from the introduction of tapered treatment, as well as that de-escalated therapy could be offered to patients with HPV positive “other” OPSCC, where survival benefit is doubtful. Notably, according to the newest 8th AJCC staging system, all oropharyngeal malignancies should be staged depending on their p16 status [76]. In light of data presented and discussed here, this approach could potentially be problematic. Subsequently, sub-specific survival analysis studies in oropharynx are highly warranted.
There are recognisable limitations in this study. First of all, since OPSCC still is a relatively rare disease, there is a risk that same patients are included in different studies/cohorts. To reduce this risk, we have restricted our analysis to patient cohorts included in reports published during the three last years, still allowing for the inclusion of more than 11.000 patients. We also focused on the patient cohort description in the material and method sections, but there could still be a risk for non-described overlapping patients between studies. Secondly, there is also a possibility of misclassification of tumours within the oropharyngeal region. This is especially evident in the distinction between large mobile tongue carcinomas and BOTSCC, in which only the latter is HPV associated. Relatedly, sub-coding of TSCC is infrequently presented. As stated in the introduction section, the histology and, most likely, the HPV prevalence differs between specified TSCC (ICD-10 C09.0) and e.g. carcinomas of the tonsillar pillars (ICD-10 C09.1). Furthermore, few studies have sub-classified OPSCC by histo-morphology [4]. Nevertheless, misclassification of sub-sites would most likely only dilute the HPV prevalence numbers and thus reduce the HPV differences between TSCC/BOTSCC and “other” OPSCC. Lastly, it has been documented that HPV prevalence differs between geographic regions [6] and studies included in this report are obtained from different geographical regions with different risk factors. Nonetheless, since the difference in HPV prevalence between sub-sites is studied here, and not absolute numbers, the impact of patient nationality should be minor.
To conclude, combining HPV DNA and p16 overexpression is safer for defining HPV positivity compared to using HPV DNA or p16 alone, and with this algorithm HPV was significantly more prevalent in TSCC/BOTSCC as compared to “other OPSCC sites”. The clinical role of HPV in “other” OPSCC must be further evaluated before initiation of de-escalation trials in these patients.
Conflict of interest statement
None declared.
Acknowledgement
This work was supported by the Stockholm County Council (SLL), Karolinska Institutet (KI), Svenska läkarsällskapet (SLS), Stiftelsen Sigurd och Elsa Goljes Minne, Stiftelsen Tornspiran and Magnus Bergvalls stiftelse, the Swedish Cancer Foundation, the Stockholm Cancer Society and the Cancer and Allergy Foundation. Anders Näsman was supported by the Stockholm County Council (clinical postdoctorial appointment).
References
- 1.Syrjanen K., Syrjanen S., Lamberg M. Morphological and immunohistochemical evidence suggesting human papillomavirus (HPV) involvement in oral squamous cell carcinogenesis. Int. J. Oral. Surg. 1983;12:418–424. doi: 10.1016/s0300-9785(83)80033-7. [DOI] [PubMed] [Google Scholar]
- 2.IARC. A review of human carcinogens. monographs on the evaluation of carcinogenic risks to humans, 2009; 100B.
- 3.Garnaes E., Frederiksen K., Kiss K. Double positivity for HPV DNA/p16 in tonsillar and base of tongue cancer improves prognostication: insights from a large population-based study. Int. J. Cancer. 2016;139:2598–2605. doi: 10.1002/ijc.30389. [DOI] [PubMed] [Google Scholar]
- 4.Garnaes E., Kiss K., Andersen L. A high and increasing HPV prevalence in tonsillar cancers in Eastern Denmark, 2000–2010: the largest registry-based study to date. Int. J. Cancer. 2015;136:2196–2203. doi: 10.1002/ijc.29254. [DOI] [PubMed] [Google Scholar]
- 5.Marklund L., Nasman A., Ramqvist T. Prevalence of human papillomavirus and survival in oropharyngeal cancer other than tonsil or base of tongue cancer. Cancer Med. 2012;1:82–88. doi: 10.1002/cam4.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ndiaye C., Mena M., Alemany L. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta-analysis. Lancet Oncol. 2014;15:1319–1331. doi: 10.1016/S1470-2045(14)70471-1. [DOI] [PubMed] [Google Scholar]
- 7.Kim S.H., Koo B.S., Kang S. HPV integration begins in the tonsillar crypt and leads to the alteration of p16, EGFR and c-myc during tumor formation. Int. J. Cancer. 2007;120:1418–1425. doi: 10.1002/ijc.22464. [DOI] [PubMed] [Google Scholar]
- 8.Syrjanen S. HPV infections and tonsillar carcinoma. J. Clin. Pathol. 2004;57:449–455. doi: 10.1136/jcp.2003.008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moher D., Liberati A., Tetzlaff J. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bahl A., Kumar P., Dar L. Prevalence and trends of human papillomavirus in oropharyngeal cancer in a predominantly north Indian population. Head Neck. 2014;36:505–510. doi: 10.1002/hed.23317. [DOI] [PubMed] [Google Scholar]
- 11.Bhosale P.G., Pandey M., Desai R.S. Low prevalence of transcriptionally active human papilloma virus in Indian patients with HNSCC and leukoplakia. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2016;122:609–618. doi: 10.1016/j.oooo.2016.06.006. (e607) [DOI] [PubMed] [Google Scholar]
- 12.Broglie M.A., Soltermann A., Haile S.R. Human papilloma virus and survival of oropharyngeal cancer patients treated with surgery and adjuvant radiotherapy. Eur. Arch. Otorhinolaryngol. 2015;272:1755–1762. doi: 10.1007/s00405-014-3099-y. [DOI] [PubMed] [Google Scholar]
- 13.Broglie M.A., Soltermann A., Rohrbach D. Impact of p16, p53, smoking, and alcohol on survival in patients with oropharyngeal squamous cell carcinoma treated with primary intensity-modulated chemoradiation. Head Neck. 2013;35:1698–1706. doi: 10.1002/hed.23231. [DOI] [PubMed] [Google Scholar]
- 14.Bussu F., Sali M., Gallus R. Human papillomavirus (HPV) infection in squamous cell carcinomas arising from the oropharynx: detection of HPV DNA and p16 immunohistochemistry as diagnostic and prognostic indicators--a pilot study. Int. J. Radiat. Oncol. Biol. Phys. 2014;89:1115–1120. doi: 10.1016/j.ijrobp.2014.04.044. [DOI] [PubMed] [Google Scholar]
- 15.Cerezo L., de la Torre A., Hervas A. Oropharyngeal cancer related to Human Papilloma Virus: incidence and prognosis in Madrid, Spain. Clin. Transl. Oncol. 2014;16:301–306. doi: 10.1007/s12094-013-1074-5. [DOI] [PubMed] [Google Scholar]
- 16.Cerezo L., Lopez C., de la Torre A. Incidence of human papillomavirus-related oropharyngeal cancer and outcomes after chemoradiation in a population of heavy smokers. Head Neck. 2014;36:782–786. doi: 10.1002/hed.23366. [DOI] [PubMed] [Google Scholar]
- 17.Dahlstrom K.R., Bell D., Hanby D. Socioeconomic characteristics of patients with oropharyngeal carcinoma according to tumor HPV status, patient smoking status, and sexual behavior. Oral Oncol. 2015;51:832–838. doi: 10.1016/j.oraloncology.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis K.S., Vargo J.A., Ferris R.L. Stereotactic body radiotherapy for recurrent oropharyngeal cancer - influence of HPV status and smoking history. Oral Oncol. 2014;50:1104–1108. doi: 10.1016/j.oraloncology.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.M.G. Dona, G. Spriano, B. Pichi, et al. Human papillomavirus infection and p16 overexpression in oropharyngeal squamous cell carcinoma: a case series from 2010 to 2014. Future Microbiol; 10: 1283–1291, 2015. [DOI] [PubMed]
- 20.Evans M., Newcombe R., Fiander A. Human Papillomavirus-associated oropharyngeal cancer: an observational study of diagnosis, prevalence and prognosis in a UK population. BMC Cancer. 2013;13:220. doi: 10.1186/1471-2407-13-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fakhry C., Zhang Q., Nguyen-Tan P.F. Human papillomavirus and overall survival after progression of oropharyngeal squamous cell carcinoma. J. Clin. Oncol. 2014;32:3365–3373. doi: 10.1200/JCO.2014.55.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faust H., Eldenhed Alwan E., Roslin A. Prevalence of HPV types, viral load and physical status of HPV16 in head and neck squamous cell carcinoma from the South Swedish Health Care Region. J. Gen. Virol. 2016 doi: 10.1099/jgv.0.000611. [DOI] [PubMed] [Google Scholar]
- 23.Fonmarty D., Cherriere S., Fleury H. Study of the concordance between p16 immunohistochemistry and HPV-PCR genotyping for the viral diagnosis of oropharyngeal squamous cell carcinoma. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2015;132:135–139. doi: 10.1016/j.anorl.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Fujimaki M., Fukumura Y., Mitani K. Histological subtypes and characteristic structures of HPV-associated oropharyngeal carcinoma: study with Japanese cases. Diagn. Pathol. 2013;8:211. doi: 10.1186/1746-1596-8-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodman M.T., Saraiya M., Thompson T.D. Human papillomavirus genotype and oropharynx cancer survival in the United States of America. Eur. J. Cancer. 2015;51:2759–2767. doi: 10.1016/j.ejca.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grisar K., Dok R., Schoenaers J. Differences in human papillomavirus-positive and -negative head and neck cancers in Belgium: an 8-year retrospective, comparative study. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2016;121:456–460. doi: 10.1016/j.oooo.2015.10.035. [DOI] [PubMed] [Google Scholar]
- 27.Habbous S., Chu K.P., Qiu X. The changing incidence of human papillomavirus-associated oropharyngeal cancer using multiple imputation from 2000 to 2010 at a comprehensive cancer centre. Cancer Epidemiol. 2013;37:820–829. doi: 10.1016/j.canep.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 28.Hama T., Tokumaru Y., Fujii M. Prevalence of human papillomavirus in oropharyngeal cancer: a multicenter study in Japan. Oncology. 2014;87:173–182. doi: 10.1159/000360991. [DOI] [PubMed] [Google Scholar]
- 29.Henneman R., Van Monsjou H.S., Verhagen C.V. Incidence changes of human Papillomavirus in Oropharyngeal Squamous Cell Carcinoma and Effects on Survival in the Netherlands Cancer Institute, 1980–2009. Anticancer Res. 2015;35:4015–4022. [PubMed] [Google Scholar]
- 30.Hong A., Jones D., Chatfield M. HPV status of oropharyngeal cancer by combination HPV DNA/p16 testing: biological relevance of discordant results. Ann. Surg. Oncol. 2013;20(Suppl. 3):S450–S458. doi: 10.1245/s10434-012-2778-4. [DOI] [PubMed] [Google Scholar]
- 31.Hong A., Lee C.S., Jones D. Rising prevalence of human papillomavirus-related oropharyngeal cancer in Australia over the last 2 decades. Head Neck. 2016;38:743–750. doi: 10.1002/hed.23942. [DOI] [PubMed] [Google Scholar]
- 32.Hong A., Zhang M., Veillard A.S. The prognostic significance of hypoxia inducing factor 1-alpha in oropharyngeal cancer in relation to human papillomavirus status. Oral Oncol. 2013;49:354–359. doi: 10.1016/j.oraloncology.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Isayeva T., Xu J., Dai Q. African Americans with oropharyngeal carcinoma have significantly poorer outcomes despite similar rates of human papillomavirus-mediated carcinogenesis. Hum. Pathol. 2014;45:310–319. doi: 10.1016/j.humpath.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 34.Iyer N.G., Dogan S., Palmer F. Detailed analysis of clinicopathologic factors demonstrate distinct difference in outcome and prognostic factors between surgically treated HPV-Positive and negative oropharyngeal cancer. Ann. Surg. Oncol. 2015;22:4411–4421. doi: 10.1245/s10434-015-4525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang R., Ekshyyan O., Moore-Medlin T. Association between human papilloma virus/Epstein-Barr virus coinfection and oral carcinogenesis. J. Oral. Pathol. Med. 2015;44:28–36. doi: 10.1111/jop.12221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawakami H., Okamoto I., Terao K. Human papillomavirus DNA and p16 expression in Japanese patients with oropharyngeal squamous cell carcinoma. Cancer Med. 2013;2:933–941. doi: 10.1002/cam4.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim H.S., Lee J.Y., Lim S.H. Association between PD-L1 and HPV status and the prognostic value of PD-L1 in oropharyngeal squamous cell carcinoma. Cancer Res. Treat. 2016;48:527–536. doi: 10.4143/crt.2015.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim M.J., Ki M.S., Kim K. Different protein expression associated with chemotherapy response in oropharyngeal cancer according to HPV status. BMC Cancer. 2014;14:824. doi: 10.1186/1471-2407-14-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kwon H.J., Brasch H.D., Benison S. Changing prevalence and treatment outcomes of patients with p16 human papillomavirus related oropharyngeal squamous cell carcinoma in New Zealand. Br. J. Oral. Maxillofac. Surg. 2016;54:898–903. doi: 10.1016/j.bjoms.2016.05.033. [DOI] [PubMed] [Google Scholar]
- 40.Lam E.W., Chan J.Y., Chan A.B. Prevalence, clinicopathological characteristics, and outcome of Human Papillomavirus-associated oropharyngeal cancer in Southern Chinese Patients. Cancer Epidemiol. Biomark. Prev. 2016;25:165–173. doi: 10.1158/1055-9965.EPI-15-0869. [DOI] [PubMed] [Google Scholar]
- 41.Lee J., Chang J.S., Kwon H.J. Impact of p16 expression in oropharyngeal cancer in the postoperative setting: the necessity of re-evaluating traditional risk stratification. Jpn. J. Clin. Oncol. 2016;46:911–918. doi: 10.1093/jjco/hyw099. [DOI] [PubMed] [Google Scholar]
- 42.Liu J., Zhang M., Rose B. Ki67 expression has prognostic significance in relation to human Papillomavirus status in oropharyngeal Squamous cell carcinoma. Ann. Surg. Oncol. 2015;22:1893–1900. doi: 10.1245/s10434-014-4237-x. [DOI] [PubMed] [Google Scholar]
- 43.Ljokjel B., Haave H., Lybak S. The impact of HPV infection, smoking history, age and operability of the patient on disease-specific survival in a geographically defined cohort of patients with oropharyngeal squamous cell carcinoma. Acta Otolaryngol. 2014;134:964–973. doi: 10.3109/00016489.2014.927590. [DOI] [PubMed] [Google Scholar]
- 44.Ljokjel B., Lybak S., Haave H. The impact of HPV infection on survival in a geographically defined cohort of oropharynx squamous cell carcinoma (OPSCC) patients in whom surgical treatment has been one main treatment. Acta Otolaryngol. 2014;134:636–645. doi: 10.3109/00016489.2014.886336. [DOI] [PubMed] [Google Scholar]
- 45.Lybak S., Ljokjel B., Haave H. Primary surgery results in no survival benefit compared to primary radiation for oropharyngeal cancer patients stratified by high-risk human papilloma virus status. Eur. Arch. Otorhinolaryngol. 2016 doi: 10.1007/s00405-016-4203-2. [DOI] [PubMed] [Google Scholar]
- 46.Mazul A.L., Rodriguez-Ormaza N., Taylor J.M. Prognostic significance of non-HPV16 genotypes in oropharyngeal squamous cell carcinoma. Oral Oncol. 2016;61:98–103. doi: 10.1016/j.oraloncology.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McIlwain W.R., Sood A.J., Nguyen S.A., Day T.A. Initial symptoms in patients with HPV-positive and HPV-negative oropharyngeal cancer. JAMA Otolaryngol. Head Neck Surg. 2014;140:441–447. doi: 10.1001/jamaoto.2014.141. [DOI] [PubMed] [Google Scholar]
- 48.Melkane A.E., Auperin A., Saulnier P. Human papillomavirus prevalence and prognostic implication in oropharyngeal squamous cell carcinomas. Head Neck. 2014;36:257–265. doi: 10.1002/hed.23302. [DOI] [PubMed] [Google Scholar]
- 49.Melkane A.E., Mirghani H., Auperin A. HPV-related oropharyngeal squamous cell carcinomas: a comparison between three diagnostic approaches. Am. J. Otolaryngol. 2014;35:25–32. doi: 10.1016/j.amjoto.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 50.Mizumachi T., Kano S., Sakashita T. Improved survival of Japanese patients with human papillomavirus-positive oropharyngeal squamous cell carcinoma. Int. J. Clin. Oncol. 2013;18:824–828. doi: 10.1007/s10147-012-0469-6. [DOI] [PubMed] [Google Scholar]
- 51.Morbini P., Alberizzi P., Tinelli C. Identification of transcriptionally active HPV infection in formalin-fixed, paraffin-embedded biopsies of oropharyngeal carcinoma. Hum. Pathol. 2015;46:681–689. doi: 10.1016/j.humpath.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 52.Naik M., Ward M.C., Bledsoe T.J. It is not just IMRT: human papillomavirus related oropharynx squamous cell carcinoma is associated with better swallowing outcomes after definitive chemoradiotherapy. Oral Oncol. 2015;51:800–804. doi: 10.1016/j.oraloncology.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 53.Nasman A., Andersson E., Marklund L. HLA class I and II expression in oropharyngeal squamous cell carcinoma in relation to tumor HPV status and clinical outcome. PLoS One. 2013;8:e77025. doi: 10.1371/journal.pone.0077025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nichols A.C., Dhaliwal S.S., Palma D.A. Does HPV type affect outcome in oropharyngeal cancer? J. Otolaryngol. Head Neck Surg. 2013;42:9. doi: 10.1186/1916-0216-42-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nomura F., Sugimoto T., Kitagaki K. Clinical characteristics of Japanese oropharyngeal squamous cell carcinoma positive for human papillomavirus infection. Acta Otolaryngol. 2014;134:1265–1274. doi: 10.3109/00016489.2014.944272. [DOI] [PubMed] [Google Scholar]
- 56.Oguejiofor K.K., Hall J.S., Mani N. The prognostic significance of the biomarker p16 in oropharyngeal squamous cell carcinoma. Clin. Oncol. (R. Coll. Radiol.) 2013;25:630–638. doi: 10.1016/j.clon.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 57.Ou P., Gear K., Rahnama F. Human papillomavirus and oropharyngeal squamous cell carcinoma: a New Zealand cohort study. ANZ J. Surg. 2016 doi: 10.1111/ans.13759. [DOI] [PubMed] [Google Scholar]
- 58.Quabius E.S., Gorogh T., Fischer G.S. The antileukoprotease secretory leukocyte protease inhibitor (SLPI) and its role in the prevention of HPV-infections in head and neck squamous cell carcinoma. Cancer Lett. 2015;357:339–345. doi: 10.1016/j.canlet.2014.11.043. [DOI] [PubMed] [Google Scholar]
- 59.Quabius E.S., Haag J., Kuhnel A. Geographical and anatomical influences on human papillomavirus prevalence diversity in head and neck squamous cell carcinoma in Germany. Int. J. Oncol. 2015;46:414–422. doi: 10.3892/ijo.2014.2697. [DOI] [PubMed] [Google Scholar]
- 60.Rietbergen M.M., Brakenhoff R.H., Bloemena E. Human papillomavirus detection and comorbidity: critical issues in selection of patients with oropharyngeal cancer for treatment De-escalation trials. Ann. Oncol. 2013;24:2740–2745. doi: 10.1093/annonc/mdt319. [DOI] [PubMed] [Google Scholar]
- 61.Rietbergen M.M., Leemans C.R., Bloemena E. Increasing prevalence rates of HPV attributable oropharyngeal squamous cell carcinomas in the Netherlands as assessed by a validated test algorithm. Int. J. Cancer. 2013;132:1565–1571. doi: 10.1002/ijc.27821. [DOI] [PubMed] [Google Scholar]
- 62.Saito Y., Yoshida M., Omura G. Prognostic value of p16 expression irrespective of human papillomavirus status in patients with oropharyngeal carcinoma. Jpn. J. Clin. Oncol. 2015;45:828–836. doi: 10.1093/jjco/hyv085. [DOI] [PubMed] [Google Scholar]
- 63.Saraiya M., Unger E.R., Thompson T.D. US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines. J. Natl. Cancer Inst. 2015;107:djv086. doi: 10.1093/jnci/djv086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schache A.G., Liloglou T., Risk J.M. Validation of a novel diagnostic standard in HPV-positive oropharyngeal squamous cell carcinoma. Br. J. Cancer. 2013;108:1332–1339. doi: 10.1038/bjc.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schache A.G., Powell N.G., Cuschieri K.S. HPV-related oropharyngeal cancer in the United Kingdom: an evolution in understanding of disease etiology. Cancer Res. 2016 doi: 10.1158/0008-5472.CAN-16-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schouten C.S., Hakim S., Boellaard R. Interaction of quantitative (18)F-FDG-PET-CT imaging parameters and human papillomavirus status in oropharyngeal squamous cell carcinoma. Head Neck. 2016;38:529–535. doi: 10.1002/hed.23920. [DOI] [PubMed] [Google Scholar]
- 67.Steinau M., Saraiya M., Goodman M.T. Human papillomavirus prevalence in oropharyngeal cancer before vaccine introduction, United States. Emerg. Infect. Dis. 2014;20:822–828. doi: 10.3201/eid2005.131311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Strojan P., Zadnik V., Sifrer R. Incidence trends in head and neck squamous cell carcinoma in Slovenia, 1983–2009: role of human papillomavirus infection. Eur. Arch. Otorhinolaryngol. 2015;272:3805–3814. doi: 10.1007/s00405-014-3459-7. [DOI] [PubMed] [Google Scholar]
- 69.Tural D., Elicin O., Batur S. Human papillomavirus is independent prognostic factor on outcome of oropharyngeal squamous cell carcinoma. Tumour Biol. 2013;34:3363–3369. doi: 10.1007/s13277-013-0907-8. [DOI] [PubMed] [Google Scholar]
- 70.Van Limbergen E.J., Dok R., Laenen A. HPV-related oropharyngeal cancers in Flanders (Belgium): a multicenter study. B-ENT. 2014;10:7–14. [PubMed] [Google Scholar]
- 71.Wagner S., Wittekindt C., Reuschenbach M. CD56-positive lymphocyte infiltration in relation to human papillomavirus association and prognostic significance in oropharyngeal squamous cell carcinoma. Int. J. Cancer. 2016;138:2263–2273. doi: 10.1002/ijc.29962. [DOI] [PubMed] [Google Scholar]
- 72.Wang Z., Xia R.H., Ye D.X., Li J. Human Papillomavirus 16 infection and TP53 mutation: two distinct pathogeneses for oropharyngeal squamous cell carcinoma in an Eastern Chinese Population. PLoS One. 2016;11:e0164491. doi: 10.1371/journal.pone.0164491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ward M.J., Thirdborough S.M., Mellows T. Tumour-infiltrating lymphocytes predict for outcome in HPV-positive oropharyngeal cancer. Br. J. Cancer. 2014;110:489–500. doi: 10.1038/bjc.2013.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Castellsague X., Alemany L., Quer M. HPV involvement in Head and Neck cancers: comprehensive assessment of biomarkers in 3680 patients. J. Natl. Cancer Inst. 2016;108:djv403. doi: 10.1093/jnci/djv403. [DOI] [PubMed] [Google Scholar]
- 75.Zafereo M.E., Xu L., Dahlstrom K.R. Squamous cell carcinoma of the oral cavity often overexpresses p16 but is rarely driven by human papillomavirus. Oral Oncol. 2016;56:47–53. doi: 10.1016/j.oraloncology.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.O'Sullivan B., Huang S.H., Su J. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicentre cohort study. Lancet Oncol. 2016;17:440–451. doi: 10.1016/S1470-2045(15)00560-4. [DOI] [PubMed] [Google Scholar]