Abstract
High-risk human papillomaviruses (HR-HPV) infect basal keratinocytes, where in some individuals they evade host immune responses and persist. Persistent HR-HPV infection of the cervix causes precancerous neoplasia that can eventuate in cervical cancer. Dendritic cells (DCs) are efficient in priming/cross-priming antigen-specific T cells and generating antiviral and antitumor cytotoxic CD8+ T cells. However, HR-HPV have adopted various immunosuppressive strategies, with modulation of DC function crucial to escape from the host adaptive immune response. HPV E6 and E7 oncoproteins alter recruitment and localization of epidermal DCs, while soluble regulatory factors derived from HPV-induced hyperplastic epithelium change DC development and influence initiation of specific cellular immune responses. This review focuses on current evidence for HR-HPV manipulation of antigen presentation in dendritic cells and escape from host immunity.
1. Introduction
HPVs are epitheliotropic, double-stranded DNA viruses that infect basal keratinocytes (KCs) on surface epithelia of skin and mucosal membranes. Cervical and other anogenital cancers account for ~5% of the global cancer burden [1], [2] and are associated with infection of ‘high-risk’ HPVs; mainly HPV16 and HPV18. Together, HPV16 and 18 are responsible for ~70% of all cervical cancer cases worldwide, and approximately ~60% of oropharyngeal cancers are associated with HPV16 [2], [3], [4], [5], [6], [7]. More than 200 HPV genotypes have been identified. Mucosal HPVs are categorized based on their oncogenicity into high-risk (HR) and low-risk (LR) types [8], [9]. Persistence of HR-HPV infection is the key step in the transformation of normal epithelium to precancerous and cancerous lesions [10], [11]. The anogenital precancerous lesions, otherwise known as intraepithelial neoplasia, e.g. cervical intraepithelial neoplasia (CIN), can be subcategorized into low-grade (CIN1) and high-grade (CIN2/3) lesions. The development of HPV-related precancerous lesions, and cancer, is dependent on the expression of HR-HPV E6 and E7 oncoproteins; E6 and E7 oncoproteins disrupt the function of host cell cycle regulatory proteins in infected KCs and trigger cell transformation. These two oncoproteins enact cell cycle dysregulation via separate mechanisms. E6 binds to the host ubiquitin ligase E6-associated protein (E6AP/UBE3A) and promotes degradation of the p53 tumor suppressor gene product, a transcription factor promoting DNA repair, cell cycle arrest and apoptosis. In contrast, HPV E7 binds to retinoblastoma (pRb) and displaces the transcription control factor E2F, leading to constitutive expression of E2F-responsive genes, promoting cell cycle activation [12], [13], [14].
The immune system plays a key role during HPV-associated carcinogenesis. About 90% of immunocompetent HPV-infected individuals resolve a cervical infection spontaneously within three years and less than 1% develop invasive cervical cancer [15]. Cell-mediated immunity is considered to be crucial for clearance of HPV infections and HPV-related malignancy is more prevalent in immunocompromised individuals [16], [17]. The presence of a cytotoxic CD8+ T cell infiltrate in HPV-related tumors corresponds with improved patient survival [5], [18]. The entire HPV infection and life cycle of the virus is exclusively within epidermal KCs. KCs themselves are considered as a component of the innate immune system with immune sentinel functions [19], [20]. They express several toll-like receptors (TLRs) that recognize pathogen-associated molecular patterns (PAMPs) on pathogens, triggering production of type I interferon (IFN), defensins and proinflammatory cytokines such as interleukin 1 β (IL1-β) and tumor necrosis factor α (TNF-α) [21], [22].
2. Impact of HPV infection on KC susceptibility to immune responses
In vitro transfection of primary KC with episomal HPV, or HPV gene expression vectors, has demonstrated that HR-HPV gene products can prevent proinflammatory KC innate immune responses and susceptibility of KC to immune mediated elimination. Expression of the E6 and E7 genes of HR-HPV downregulates transcription and function of the viral DNA sensor, TLR9 [23], [24]. In addition, primary KCs transfected with HPV16 and HPV18 episomes show disrupted expression of inflammatory cytokine and chemokine genes [21]. HR-HPV E6/E7 oncoproteins inhibit NFκB activation and TLR-mediated proinflammatory cytokine and chemokine secretion, for proteins such as IFN-β, IL1-β, IL-8, CCL2, CCL5, and MIP3α, thus limiting innate immune cell trafficking and antigen (Ag)-specific effector cell activation. The HR-HPV oncoproteins inhibit NFκB signaling by blocking translocation of NFκB to the nucleus [25], [26], and suppressing NFκB nuclear transcriptional activities through enhancing interferon-related developmental regulator 1 (IFDR1) expression [27], and promote E6 dependent proteolytic destruction of IL-1β [28]. As a result, HPV-infected KCs fail to produce type-I IFN and the proinflammatory cytokines, TNF-α, IL-6, IL-8 and MIP3a [25], [26]. HR-HPV infection of primary human KCs also prevents IFN-γ-mediated cell-cycle arrest and blocks TNFα-mediated induction of necroptosis by downregulating interferon-induced transmembrane protein 1 (IFITM1) and receptor-interacting protein kinase 3 (RIPK3), respectively [29]. The HPV16 E5 early gene product has been shown, using immortalised HPV infected KCs, to downregulate expression of class I major histocompatibility complex (MHC-I) molecules [30], [31], reducing susceptibility of KC to CD8 T cell-mediated killing. HPV16 E7 protein expression in KCs also impairs IFN-γ-mediated enhancement of antigen (Ag) processing, presentation and cytotoxic T lymphocyte (CTL)-mediated lysis by impairing phosphorylation of STAT-1, resulting in suppression of IRF-1 mediated upregulation of TAP-1 [32], [33], [34].
3. Impact of HPV infection on antigen presentation to the adaptive immune system
To induce a cytotoxic T cell response against infected KCs, skin resident antigen presenting cells (APCs) must initiate and coordinate innate and adaptive immune responses. Skin resident professional APCs tissue-resident macrophages and dendritic cells (DCs). DCs are the only professional APCs that efficiently cross-present cell-associated Ags and transport processed Ag to the draining lymph nodes (LNs) to activate proliferation of naïve T cells [35], [36]. Tissue-resident-macrophages are major scavenger APCs responsible for phagocytosis of apoptotic cells and maintenance of local homeostasis. However they do not specifically migrate to the draining LNs and are less efficient in Ag presentation to T cells [36]. DCs are a highly heterogeneous group of APCs with functionally distinct subsets. Human skin DCs comprise epidermal Langerhans cells (LCs), dermal conventional DCs (cDCs) and monocyte-derived DCs, however, local inflammation is associated with a skin infiltrate of plasmacytoid DCs, the main source of type I IFN during viral infection [37], [38]. Murine models have been useful for understanding the biological functions, and dysfunctions, of antigen presenting cells in the skin under steady state and disease. It is important to note that there are fundamental differences in cellular machinery between mouse and human skin. Most studies on mouse models utilized haired skin where there is more densely packed hair follicles relative to human skin, or in the cervix, are absent. The epidermal layers of mouse and human skin are also different in thickness [39]. However, mouse skin and human skin contain similar immune cell types: Langerhans cells and tissue resident memory CD8+ T cells in the epidermis, and dermal DCs, macrophages, neutrophils, CD4+ T cells and ILCs in the dermal compartment [38]. Mouse skin also contains additional cell types such as dendritic epidermal T cells and gamma-delta T cells [40]. In healthy skin, LCs are the only resident DCs in the epidermis and account for 3–5% of epidermal nucleated cells [41], [42]. They originate from embryonic precursors before birth and, in contrast to other DC subsets, are radio-resistant. They continuously repopulate locally in the basement membrane under the epidermal layer without the need for continuous input from bone marrow precursors. Both human and murine LCs can be identified through the expression of CD11b, epithelial cell adhesion molecule (EpCAM) and langerin (CD207) [43], [44]. Dermal cDCs arise from hematopoietic precursors and mainly comprise murine CD11c+ CD11b+ and CD103+ DCs and their functionally equivalent human CD1c+ and CD141+ DCs, respectively. Mouse and human skin DCs, in both steady-state and inflammatory conditions, capture and transport cutaneous Ag to draining LNs of the T cell zone and initiate Ag-specific T cell responses [45], [46], [47]. The focus of the remainder of this review is on the influence of HPV infection on Ag presentation by professional APC in skin.
3.1. The effects of HPV on dendritic cell frequency and distribution
Regulating DC differentiation and function is an essential mechanism for tumor escape from immune surveillance [48]. Several studies have demonstrated that progression of HPV-induced carcinogenesis is associated with alteration of frequency and distribution of LCs, the epidermal DCs which normally reside where HPV infection occurs [49]. HR-HPV positive cancer cells, and E6- and E7-expressing cells can inhibit differentiation of monocytes into LCs in vitro [50]. In cervical precancerous lesions and invasive cancers, active HR-HPV infection and/or expression of E6/E7 oncoproteins were correlated with depletion of intraepithelial LCs [51], [52]. Similarly, the expression of HPV16 E7 protein alone in murine epidermis has been associated with significantly reduced numbers of epidermal LCs [53]. Besides HR-HPV-related lesions, in immunohistochemical studies of patient skin biopsies, the density of LCs was reduced in HPV8-positive Epidermodysplasia Verruciformis patient skin lesions [54] and in chronic HPV-associated skin warts APCs gradually disappeared from the epidermis with their retention below the basement membrane of the epidermal layer [55]. Significantly, accumulation of LCs in cervical biopsies is associated with clearance of cervical HPV infection [56]. In addition, although the number of intratumoral LC in head and neck squamous cell cancer (HNSCC) patients was higher relative to precancerous lesions, HPV-positive HNSCC patients contained a smaller number of intratumoral LCs as compared to HPV-negative HNSCC patients and increase in LC frequency has been correlated with recurrence-free survival [57]. Collectively, these data suggest that epidermal-resident LCs might be important for control of HPV-induced lesions and inhibition of LC infiltration to HPV-infected epidermis could be an immune escape mechanism of the virus.
One mechanism that could produce the altered number and distribution of LCs may be the observed downregulation in HR-HPV-infected KCs of the expression of the chemokines CCL20/MIP3α and E-cadherin [58], [59] in conjunction with inhibition of KC proinflammatory and chemotactic cytokines response [25], [26]. KCs play a significant role in the development and maintenance of epidermal LCs. KCs express CCL20 that results in migration of immature LCs to the epidermis, while E-cadherin expressed by KCs mediates adherence of LCs to KCs [60], [61], [62]. Under homeostatic conditions, IL-34 derived from KCs regulates the local replenishment of LCs [63], while KCs help maintain LCs in the epidermis via integrin-mediated activation of transforming growth factor β (TGF-β). TGF-β, an autocrine LC transcriptional factor required for its epidermal localization, is activated by integrin αvβ6 and αvβ8 derived from KCs [64]. Furthermore, in vitro experiments revealed that the KC-to-LC interaction mediated by E-cadherin is required for LC differentiation [65]. In HPV16-infected cervical epithelium, E6 and E7 expression and disease severity were correlated with downregulation of CCL20 expression [52] and HPV16 E6 and E7 silencing in vitro promotes CCL20 secretion and LC migration into HPV-infected epithelium [66]. Moreover, in HPV-positive HNSCC patients, immunohistochemical profiling showed that reduced CCL20 expression is correlated with a significant decrease in LC infiltration into tumors [57]. Most importantly, in vitro gene transfection studies in human primary KCs have demonstrated that CCL20 expression is inhibited by HPV viral proteins via downregulation of NF-κB signaling [58] and inhibition of binding of the cellular transcription factor CCAAT/enhancer binding protein (C/EBP) to a CCL20 promoter [67]. In this study, C/EBP from normal KCs colocalized with and enhanced the expression of CCL20. However, human KCs transfected with HPV8 E7 protein exhibited decreased CCL20 expression by preventing binding of C/EBP to the CCL20 promoter [67], suggesting a possible mechanism for HPV oncoprotein interference with KC-derived CCL20, which attracts LCs. Similarly, lower E-cadherin expression on HPV-infected KCs is correlated with cervical lesion severity [59], [68], [69] and reduced numbers of LCs in HPV-infected epidermis [60], [70]. In vitro, inhibition of HPV16 E6 and E7 oncoproteins restored E-cadherin expression and LC adhesion to KCs [69], [70]. Together, these data imply that recruitment and localization of DCs to HPV-infected epithelia may hamper accessibility of HPV proteins to APCs and this may in part contribute to failure to generate effective anti-HPV CTL responses.
3.2. The effects of HPV on dendritic cells activation
DC functional maturation is instrumental in generating Ag-specific immune responses. DCs continuously sample the peripheral tissues and recognize pathogens or danger-signals from dying or infected cells and undergo immunogenic maturation, characterized by transport of endosomal MHC-II to the cell membrane, up-regulation of CD80 (B7-1) and CD86 (B7-2) co-stimulatory molecules and secretion of instructive proinflammatory cytokines, including IL-1α/β, IL-6, IL-12 and TNF-α, and chemokines, including CCL2, CCL3, and CCR4 [71], [72]. Mature DCs present processed Ags in association with MHC molecules to naïve T cells, and instruct T cell proliferation by providing co-stimulatory signals and cytokines which aid the differentiation of T cells [73], [74]. However, several studies have demonstrated that HR-HPV infection leads to impaired DC maturation, thus limiting their capacity to stimulate a CTL response and this promotes lesion progression [51], [75]. T cells which encounter their cognate peptide on immature DCs that lack the co-stimulatory surface markers and/or proinflammatory cytokines are tolerized, a critical mechanism which the tumor uses to escape from host immune attacks [76].
In the context of chronic HPV infection, in addition to the low density of LCs, expression of MHC-ΙΙ and langerin on LCs in HR-HPV positive cervical low-grade CIN1 lesions was significantly reduced as compared to the normal cervical epithelium [51]. Further, in patients with HR-HPV-mediated CIN, downregulation of MHC and co-stimulatory molecules on cervical intraepithelial CD11c+ DC was significantly correlated with lesion severity [75]. An in vitro model using self-assembled HPV viral-like particles (VLPs) was designed to study skin-resident APC-mediated anti-HPV immune responses. In vitro exposure of immature LCs isolated from CIN2/3 patients [77] or healthy volunteers [78] to HPV16 L1L2 VLPs failed to up-regulate MHC and co-stimulatory molecules and did not promote secretion of proinflammatory cytokines and chemokines [77], [78]. The in vitro suppressive effect of VLPs is not limited to HPV16, but exposure to many HR-HPV and LR-HPV VLPs also inhibits activation of LCs [79]. In the absence of an inflammatory stimulus, CD8+ T cells co-cultured with HPV VLP exposed LCs did not proliferate or secrete the effector cytokine IFN-γ [77], [78]. On the other hand, while exposure to HPV16 L1 major capsid VLP alone triggers activation of human monocyte-derived LCs, HPV16 VLPs that contain the minor capsid protein L2, individually or with L1 were unable to induce LC activation [80]. However, human monocyte-derived DCs incubated with either HPV16 L1 or L1L2 VLPs upregulated activation molecules and were able to stimulate proliferation of T cells [80], [81]. The minor capsid protein L2-mediated interaction of HPV16 with annexin A2 heterotetramer (A2t) on LC is suggested as one possible mechanism for HPV-induced interference with LC maturation [82], leading to a failure to generate specific cellular immune responses during early HPV infection. This LC-specific inhibition by HPV L2 VLP may indicate one HPV immune escape mechanism. However, full maturation and Ag-specific CD8 T cell stimulatory capacity could be achieved when HPV VLP exposed LC were also stimulated with TLR agonists [77], [79] or cell-derived cytokine-based biologic (IRX-2) consisting of IL-1β, IL-2, IL-6, IL-8, TNF-α, GM-CSF, and IFN-γ [83], suggesting that administration of local inflammatory stimuli restore HPV capsid protein tolerized LC activation. Together, APC dysfunction during HR-HPV infection appears to be associated with accumulation of “immature” APCs, or depletion of mature DCs, in the local environment, which may consequently adversely impact on T cell priming in the draining LNs and mounting of an appropriate immune response against infected cells.
The effect of HPV-induced malignancy has been modelled in a murine skin grafting model where mice are engineered to express a HPV16 E7 transgene within basal KCs under the control of keratin 14 (K14) transcriptional promoter (K14E7). K14E7 skin displays epithelial hyperplasia that mimics HPV-related human CIN [84], [85], [86]. Similar to LCs isolated from human cervical epithelium, epidermal LCs from K14E7 transgenic mice exhibit low expression of MHC-ΙΙ and langerin/CD207, but the levels of CD40, CD80 and CD86 surface molecules were up-regulated as compared to non-transgenic mouse skin [87], [88]. Despite their partially activated state, in vivo Ag-processing and T cell priming capacity of K14E7 skin DCs was impaired [88]. Further, LCs in K14E7 epidermis showed a regulatory phenotype characterized by expression of indoleamine 2, 3-dioxygenase 1(IDO-1) and arginase 1 (Arg-1) [88], [89]. However, in murine epidermis transduced with HPV16 E7, depletion of LCs had no effect on suppression of CTL responses by HPV16 E7 [53]. In a murine contact hypersensitivity model, activation of skin effector T cells required antigen presentation by dermal DCs, but not LCs [90]. Therefore, whether the dysregulation of epidermal LCs is relevant to failure to initiate HPV-specific CD8+ T cell responses requires further investigation.
4. Chronic HPV infection, immunoregulatory cytokine milieu and DCs
4.1. Immunoregulatory soluble factors
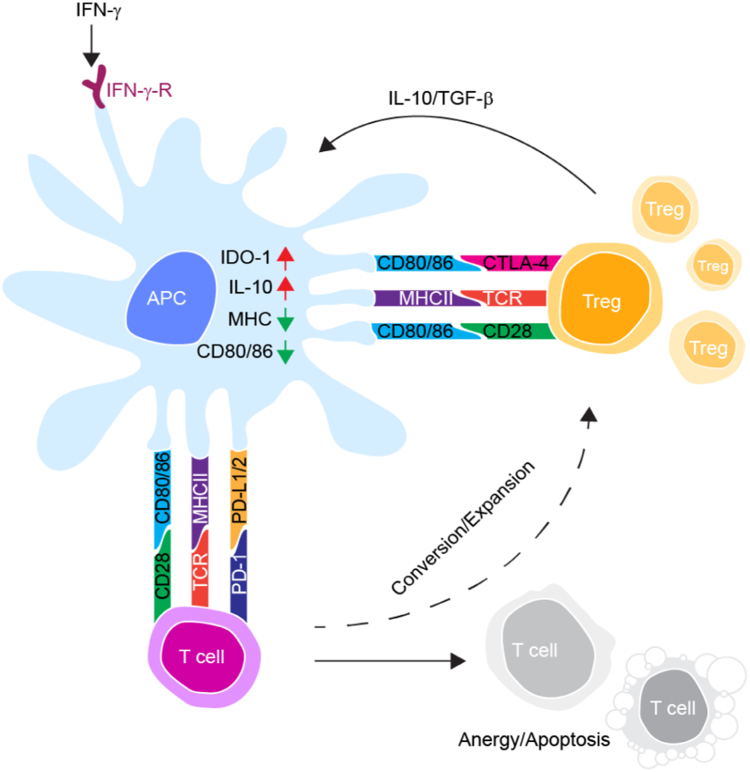
As indicated in Fig. 1 and Table 1, local immunosuppressive signaling established during precancerous epithelial neoplasia seems to play a significant role in HPV-related carcinogenesis, possibly through modulating APC activation and function. HPV-transformed cells produce immunoregulatory soluble factors and recruit suppressive cells, tumor-associated macrophages, myeloid-derived suppressor cells and regulatory T (Treg) cells, which instruct tolerogenic DC differentiation and promote lesion progression [91], [92]. HPV-transformed cell lines and tissues studied from patients with cervical cancer and high-grade CINs have demonstrated that the soluble mediators, IL-10, TGF-β and prostaglandins (PG), play a key role in the establishment of an immunosuppressive milieu [93], [94], [95], [96]. PGE2 produced by HPV-driven malignant epithelium inhibits DC maturation and Ag presentation [97]. In the HPV-associated neoplastic epithelium, accumulation of TGF-β and IL-10, chief regulator of DC development and function, was correlated with disease severity [92], [98]. IL-10 secreted from Treg cells downregulated maturation of DCs by up-regulating the E3 ubiquitin ligase MARCH-I expression, which increased ubiquitination and degradation of MHC and costimulatory molecule [99]. The possible DC suppressive effect of IL-10 and TGF-β in a chronic HPV-infected local microenvironment is evidenced by a correlation between elevated levels of TGF-β and IL-10 positive Treg cells, regulatory DCs and impaired CTL responses in cervical cancer epithelium compared to normal cervix [95]. This was also seen in mouse models where HPV Ag-experienced experimental mice produced IFN-γ, favouring the generation of IL-10+ CD4+ T cells and impaired CTL responses [100]. Additionally, in vitro examination of HPV-positive cervical cancer cell lines demonstrated that IL-10 and TGF-β derived from cancer cells suppress MHC-I expression in tumor cells [101], preventing CTL-mediated clearance of HPV-transformed KCs. Moreover, the expression of co-stimulatory and MHC-II in DCs co-cultured with genital squamous cell carcinoma (SCC) cell lines was significantly reduced via a receptor activator of nuclear factor kappa-B ligand (RANKL) cytokine-mediated effect although cells retained their ability to up-regulate lymphoid homing receptor CCR7, thereby inducing tolerogenic DC development [102]. The chemokine receptor CCR7 is expressed by mature DCs and plays a role in mediating DC trafficking in response to LN homing CCL19 and CCL21 chemokines [103]. Upregulated expression of CCR7 on DCs within HPV-infected squamous epithelium and with a low level of co-stimulatory markers may induce migration of immunologically inactive DCs to draining LNs [104]. In addition, HR-HPV-positive cervical cancer cell lines induced monocyte recruitment and differentiation to immature DCs in vitro. However, the migration of mature DCs was impaired by cervical cancer cell-derived IL-6, which led to suppression of CCR7 [105].
Fig. 1.
Schematic illustration of tumor -infiltrating T cell interactions with dendritic cells. Chronic IFN-γ secretion from tumor infiltrating cells and cell to cell interaction through CTLA-4 on Treg cells with CD80/CD86 on DCs induce IDO expression and activities. The increased activity of IDO inhibits effector function of T cells through depleting tryptophan and increased kynurenine catabolite, kynurenine. Increase production of the immune inhibitory molecules IL-10, TGF-β and CTLA-4 in Treg cells downregulate maturation of DCs. Ligation of TCR with cognate peptide-loaded MHC molecules on dendritic cells that lack the co-stimulatory surface markers and/or PD-1-PD-L1 interactions lead to T cell proliferation arrest or anergy. APC: antigen presenting cell; IDO-1: Indoleamine 2, 3-dioxygenase; IL-10: interlunkin-10; IFN-γ: Interferon γ; IFN-γ-R: IFN-γ receptor; CTLA-4: cytotoxic lymphocyte-associated antigen-4; TCR: T cell receptor; PD-1/PD-L1: programmed death receptor 1/programmed death ligand 1; TGF-β: Tumor growth factor β.
Table 1.
Summary of some of the common regulatory mediators in HR-HPV- induced malignancies.
| Immunoregulatory factors | Main source | Mechanisms of immune regulation | Possible effect | Reference |
|---|---|---|---|---|
| Arg-1 | DCs and other myeloid cells |
|
|
[88] |
| TGF-β & IL-10 | Tumor cells, TAM, Treg cells, DCs |
|
|
[91], [93], [94], [95], [96] |
| IFN-γ | Activated T cells |
|
|
[88], [89], [95] |
| PD-L1 | Tumor cells, APCs |
|
|
[75], [93], [130] |
| PD-1 | T cells |
|
|
[93], [132] |
| CTLA-4 | T cells |
|
|
[107], [108], [110] |
| IDO-1 | Tumor cells, DCs |
|
|
[88], [89], [122], [123], [124] |
Antigen-experienced Treg cells modulate the maturation of DCs via cytotoxic T lymphocyte-antigen-4 (CTLA-4)-mediated down-regulation of CD80 and CD86 expression. CTLA-4 binds to CD80 and CD86 with higher affinity than CD28, and Treg cell interaction with DCs via CTLA-4 downregulates CD80 and CD86 expression, thus limiting the ability of DCs to stimulate CD8+ T cells [106]. The expression of CTLA-4, the immune checkpoint receptor, on Treg cells provides an important strategy for different tumor cells to evade host immune surveillance. Accumulation of CTLA-4+ Treg cells in the HPV-positive HNSCC tumor microenvironment [107] and in the peripheral blood of advanced cervical cancer [108] patients, suggests a repressive role for CTLA-4 during HPV infection. The clinical benefits of blocking the CTLA-4 immune inhibitory pathway is also under clinical evaluation in cervical cancer [109] and HNSCC patients [110].
HPV16 E7-expressing mouse premalignant skin has also shown suppressed immune responses. Unlike skin expressing mOVA from an epidermal keratin 5 (K5) promoter- [111] or K14-driven human growth hormone (K14hGH) neo-self-antigens [112], E7-expressing murine skin is not rejected when engrafted onto immunocompetent syngeneic, non-transgenic recipient hosts [86], and an E7 immunization induced CD8+ T cell response, was not sufficient to enable graft rejection [113]. Administration of Listeria monocytogenes or bacterial endotoxin at the time of grafting of E7 transgenic skin promoted graft rejection [114]. This suggests that local application of proinflammatory stimuli may overcome a suppressive environment by sustaining stimulatory signals that help rescue immunogenic DC activation [115]. However, although CD4+CD25+ T cell depletion increased Ag-specific CD8 T cell responses in E7- expressing mice [116], these were insufficient to induce graft rejection [117]. Natural killer T (NKT) cells inhibit rejection of E7-grafts, and DCs isolated from NKT cell deficient mice recipient of E7 skin grafts have shown enhanced Ag-specific T cell stimulatory function in draining LNs [117]. Invariant NKT cell interaction with immature DCs can program maturation of tolerogenic DC [118] expressing immunoregulatory molecules including IDO. An increased NKT cell infiltration with increased levels of IFN-γ expression has also been observed in cervical tissues of patients with high-grade (CIN2/3) lesions [119], although the protective or suppressive nature of this response is unknown. Altogether, immune infiltrates into the tumor microenvironment may suppress anti-HPV responses by promoting regulatory DC development, thus inducing expansion of ineffective tumor infiltrating CD8+ T cells.
4.2. Indoleamine 2, 3-dioxygenase
Prolonged local IFN-γ-signaling contributes to E7-induced immunosuppression in K14E7 animals, as IFN-γ-deficient K14E7 skin grafts were spontaneously rejected by non-transgenic immunocompetent hosts [117]. IFN-γ exhibits anti-inflammatory and immunomodulatory functions and is the primary inducer of indoleamine 2, 3-dioxygenase (IDO) production from various cell types, including APCs and tumor cells [120]. IDO is an immunoregulatory enzyme that breaks down L-tryptophan, an important amino acid required for T cell effector function, to the immune inhibitory metabolite called kynurenine. Increased activity of IDO leads to local depletion of tryptophan and an increase in kynurenine, which subsequently inhibits the immune response by inducing apoptosis of effector T cells, converting naïve T cells into Treg cells or leading to cell cycle arrest [120], [121].
In a cervical cancer patient biopsy analysis, IDO1 gene expression and kynurenine to tryptophan ratio was significantly elevated as compared to healthy controls [122]. Increased expression and activities of serum IDO [123] and cervical tumor cell IDO expression [124] were negatively associated with survival of cervical cancer patients. Markedly higher infiltration of stromal immature IDO+ DCs were observed from cervical tissues of high-grade cervical neoplastic disease and cancer [95]. Although the number of IFN-γ+ cells were lower in patients with cervical cancer compared to CIN, IFN-γ+ cells were significantly higher in high-grade CIN compared to normal cervix biopsies [95]. Indeed, IFN-γ promotes antitumor immune responses and IFN-γ producing effector T cells are important positive predictors of improved HPV-related cancer patient survival [125], [126]. However, the number of T cell populations within HPV-positive high grade CINs may not be different [119]. Further, despite the effector role of IFN-γ in antitumor immune response, there is increasing evidence that chronic IFN-γ release could promote immune suppression by upregulation of immunomodulatory signals such as PD-L1 and IDO in HPV-positive precancerous lesions [127]. Adding to the complexity of the role of IFN-γ, IFN-γ can be produced by a variety of cells, which, in combination with different tissue microenvironments, can cause different outcomes to the immune response. One source of IFN-γ is from increased numbers of invariant NKT cells, as seen in HPV-positive high-grade CIN compared to HPV-positive low-grade CINs and HPV-negative cervical tissues [119]. Invariant NKT cells and IFN-γ-dependent upregulated expression and activity of IDO-1 were detected in the K14E7 murine skin DCs and blockade of IDO-1 activities with 1-methyltryptophan (1-MT) led to rejection of E7 transgenic skin grafts [88], [89]. Additionally, in an experimental murine model of subcutaneously inoculated cervical tumor cells, though in vitro tumor cell growth was not affected, inhibition of IDO activity promotes significant intratumoral accumulation of natural killer (NK) cells and regression of tumor growth [128]. Collectively these data strongly suggest that prolonged or chronic IFN-γ signaling might be responsible for IDO-1 expression by HPV-infected tissue DCs and dysfunction of T cell response can be attributed to IFN-γ-induced differentiation of regulatory DCs.
4.3. Programmed death ligand inhibitory signaling
DCs exposed to the HR-HPV-induced tumor microenvironment may attenuate effector T cell functions through programmed death receptor 1 (PD-1)/programmed death ligand 1(PD-1/PD-L1) immune inhibitory signaling [75]. Normally, the inhibitory signaling is important to terminate cell-mediated immune responses and is responsible for preventing adverse immune reactions, such as immune-mediated destruction of normal cells during infections. The interaction between PD-1 on T cells and its ligands PD-L1 (B7-H1) and PD-L2 (B7-DC) on APCs or tumor cells delivers a negative signal that results in apoptosis and anergy or exhaustion of T cells [106]. Aberrantly high PD-1/PD-L1 signaling is associated with immune escape in cancer and HIV infection [106], [129]. K14E7 transgenic mouse skin DCs do not demonstrate elevated expression of PD-L1 [88]. However, a correlation of increased PD-1/PD-L1 expression and tumor -infiltrating T cell immune dysfunction during HR-HPV-induced carcinogenesis of both cervical [75], [93], [130] and oropharyngeal [131], [132] cancer patients has been reported. Likewise, there is significantly elevated expression of PD-1 on T cells and PD-L1 on DCs in cervical tissues [75], [93] and draining LNs [133] from patients with high-grade CIN, as compared to low-grade CIN and CIN-negative patients. Furthermore, infiltration of functionally impaired PD-1+ CD8+ T cells is observed in HNSCC [132] and cervical cancer [93] patients. In HPV-infected tissue, PD-L1-expressing DCs have shown regulatory phenotypes, characterized by reduced co-stimulatory molecule expression and IL-12 cytokine production, but up-regulated secretion of IL-10 [75]. Interaction between DCs and T cells via ligation of PD-L1/PD-1 and immunoregulatory cytokine secretion may allow for immune escape during chronic HR-HPV infection. Immune checkpoint blockade therapy can promote tumor clearance by the host immune system. In vitro studies involving human cell lines expressing HPV16 E7 Ags have shown that blockade of PD-1/PD-L1 signaling significantly enhanced CTL responses [93], [130]. Pre-clinical studies have also demonstrated that the combination of HPV16 E7 DNA-based therapeutic vaccines with co-blockade of PD-1/PD-L1 signaling in E6- and E7-expressing transplantable TC-1 tumor bearing mice can significantly enhance the tumor -specific CTL response and improve tumor regression [134], [135], [136]. Currently, there are several clinical trials targeting blockade of PD-1/PD-L1 in HNSCCs and nivolumab (anti-PD1 antibody) has been approved by the FDA for the treatment of recurrent or metastatic HNSCC patients [110], [137]. However, although pembrolizumab [138] and nivolumab [139] anti-PD-1 monoclonal antibodies are under phase I/II trials for the treatment of cervical and other anogenital cancers, it remains to be determined if these strategies can offer a therapeutic advantage for clearance of HPV-related tumors in patients.
5. Conclusion
HPV utilizes multiple strategies to evade host immune responses. HR-HPV- infected KCs escape from CTL recognition through downregulation of MHC-I molecules and the associated Ag-processing machinery. Subverting the KC’s inflammatory response by HR-HPV E6 and E7 non-structural oncoproteins contributes to the ineffectiveness of the host immune response, thus promoting persistent HPV infection and KC transformation. HPV E6 and E7 affect epidermal recruitment and localization of LCs through disrupting KC-to-LC networks. The various cell types recruited to HR-HPV infected lesions appear to interact with resident (or recruited) APCs, setting up a feed-forward loop of immunoregulation to favour persistence of infected cells. In both pre-clinical animal models and cervical cancer patients, HPV-related epithelial dysplasia is associated with infiltration of immature DCs with reduced ability to present Ags and stimulate T cell proliferation. Most strikingly, within HR-HPV-infected or E6/E7-expressing hyperproliferative epithelia, DCs exhibit regulatory phenotypes associated with production of IL-10, IDO-1, and PD-L1. Persistent immunosuppressive signaling through chronic IFN-γ, IL-10, and TGF-β in the HR-HPV-infected hyperproliferative lesion is presumably responsible for recruiting and programming tolerogenic DCs. Multiple clinical trials targeting blockade of PD-1/PD-L1 and CTLA-4 immune checkpoints are being conducted and may provide crucial help in diminishing the burden of HPV- associated diseases. However, it is not yet clear whether HR-HPV-induced malignancies directly suppress the CTL response or DCs conditioned by the regulatory milieu actively divert T cell responses towards tolerance. Thus, further investigation of the interaction between APCs and tumor -derived soluble mediators within HPV-induced malignant epithelia may help with targeting DCs for therapeutic intervention.
References
- 1.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Walhart T. Human papillomavirus biology, pathogenesis, and potential for drug discovery: a literature review for HIV nurse clinical scientists. J. Assoc. Nurses AIDS Care. 2015;26:693–702. doi: 10.1016/j.jana.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Cancer Genome Atlas Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–582. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang K.W., Alaei-Mahabadi B., Samuelsson T., Lindh M., Larsson E. The landscape of viral expression and host gene fusion and adaptation in human cancer. Nat. Commun. 2013;4:2513. doi: 10.1038/ncomms3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masterson L., Lechner M., Loewenbein S., Mohammed H., Davies-Husband C., Fenton T. CD8+ T cell response to human papillomavirus 16 E7 is able to predict survival outcome in oropharyngeal cancer. Eur. J. Cancer. 2016;67:141–151. doi: 10.1016/j.ejca.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Song D., Li H., Li H., Dai J. Effect of human papillomavirus infection on the immune system and its role in the course of cervical cancer. Oncol. Lett. 2015;10:600–606. doi: 10.3892/ol.2015.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaravinos A. An updated overview of HPV-associated head and neck carcinomas. Oncotarget. 2014;5:3956–3969. doi: 10.18632/oncotarget.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egawa N., Egawa K., Griffin H., Doorbar J. Human papillomaviruses; epithelial tropisms, and the development of neoplasia. Viruses. 2015;7:3863–3890. doi: 10.3390/v7072802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Institute of Health. Papillomavirus Episteme. 〈https://pave.niaid.nih.gov/#search/search_database〉 (Accessed 6 August 2017), 2017.
- 10.Cho H.W., So K.A., Lee J.K., Hong J.H. Type-specific persistence or regression of human papillomavirus genotypes in women with cervical intraepithelial neoplasia 1: a prospective cohort study. Obstet. Gynecol. Sci. 2015;58:40–45. doi: 10.5468/ogs.2015.58.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woodman C.B., Collins S.I., Young L.S. The natural history of cervical HPV infection: unresolved issues. Nat. Rev. Cancer. 2007;7:11–22. doi: 10.1038/nrc2050. [DOI] [PubMed] [Google Scholar]
- 12.Martinez-Zapien D., Ruiz F.X., Poirson J., Mitschler A., Ramirez J., Forster A. Structure of the E6/E6AP/p53 complex required for HPV-mediated degradation of p53. Nature. 2016;529:541–545. doi: 10.1038/nature16481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balsitis S., Dick F., Lee D., Farrell L., Hyde R.K., Griep A.E. Examination of the pRb-dependent and pRb-independent functions of E7 in vivo. J. Virol. 2005;79:11392–11402. doi: 10.1128/JVI.79.17.11392-11402.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brenna S.M., Syrjanen K.J. Regulation of cell cycles is of key importance in human papillomavirus (HPV)-associated cervical carcinogenesis. Sao Paulo Med. J. 2003;121:128–132. doi: 10.1590/S1516-31802003000300009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sasagawa T., Takagi H., Makinoda S. Immune responses against human papillomavirus (HPV) infection and evasion of host defense in cervical cancer. J. Infect. Chemother. 2012;18:807–815. doi: 10.1007/s10156-012-0485-5. [DOI] [PubMed] [Google Scholar]
- 16.Varada S., Posnick M., Alessa D., Ramirez-Fort M.K. Management of cutaneous human papillomavirus infection in immunocompromised patients. Curr. Probl. Dermatol. 2014;45:197–215. doi: 10.1159/000357187. [DOI] [PubMed] [Google Scholar]
- 17.Choudhury S.A., Choudhury N.A., Humphrey A.D., Berthaud V., Ladson G., Tucker V.A. Higher prevalence of human papillomavirus-related cervical precancerous abnormalities in HIV-infected compared to HIV-uninfected women. J. Natl. Med. Assoc. 2016;108:19–23. doi: 10.1016/j.jnma.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Vos van Steenwijk P.J., Ramwadhdoebe T.H., Goedemans R., Doorduijn E.M., van Ham J.J., Gorter A. Tumor-infiltrating CD14-positive myeloid cells and CD8-positive T-cells prolong survival in patients with cervical carcinoma. Int. J. Cancer. 2013;133:2884–2894. doi: 10.1002/ijc.28309. [DOI] [PubMed] [Google Scholar]
- 19.Dai X., Tohyama M., Murakami M., Sayama K. Epidermal keratinocytes sense dsRNA via the NLRP3 inflammasome, mediating interleukin (IL)-1beta and IL-18 release. Exp. Dermatol. 2017 doi: 10.1111/exd.13334. [DOI] [PubMed] [Google Scholar]
- 20.Sugita K., Kabashima K., Atarashi K., Shimauchi T., Kobayashi M., Tokura Y. Innate immunity mediated by epidermal keratinocytes promotes acquired immunity involving Langerhans cells and T cells in the skin. Clin. Exp. Immunol. 2007;147:176–183. doi: 10.1111/j.1365-2249.2006.03258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karim R., Meyers C., Backendorf C., Ludigs K., Offringa R., van Ommen G.J. Human papillomavirus deregulates the response of a cellular network comprising of chemotactic and proinflammatory genes. PLoS One. 2011;6:e17848. doi: 10.1371/journal.pone.0017848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lebre M.C., van der Aar A.M., van Baarsen L., van Capel T.M., Schuitemaker J.H., Kapsenberg M.L. Human keratinocytes express functional Toll-like receptor 3, 4, 5, and 9. J. Investig. Dermatol. 2007;127:331–341. doi: 10.1038/sj.jid.5700530. [DOI] [PubMed] [Google Scholar]
- 23.Pacini L., Savini C., Ghittoni R., Saidj D., Lamartine J., Hasan U.A. Downregulation of toll-like receptor 9 expression by beta human papillomavirus 38 and implications for cell cycle control. J. Virol. 2015;89:11396–11405. doi: 10.1128/JVI.02151-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasan U.A., Zannetti C., Parroche P., Goutagny N., Malfroy M., Roblot G. The Human papillomavirus type 16 E7 oncoprotein induces a transcriptional repressor complex on the Toll-like receptor 9 promoter. J. Exp. Med. 2013;210:1369–1387. doi: 10.1084/jem.20122394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richards K.H., Wasson C.W., Watherston O., Doble R., Blair G.E., Wittmann M. The human papillomavirus (HPV) E7 protein antagonises an Imiquimod-induced inflammatory pathway in primary human keratinocytes. Sci. Rep. 2015;5:12922. doi: 10.1038/srep12922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karim R., Tummers B., Meyers C., Biryukov J.L., Alam S., Backendorf C. Human papillomavirus (HPV) upregulates the cellular deubiquitinase UCHL1 to suppress the keratinocyte's innate immune response. PLoS Pathog. 2013:9. doi: 10.1371/journal.ppat.1003384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tummers B., Goedemans R., Pelascini L.P.L., Jordanova E.S., van Esch E.M.G., Meyers C. The interferon-related developmental regulator 1 is used by human papillomavirus to suppress NFκB activation. Nat. Commun. 2015;6:6537. doi: 10.1038/ncomms7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niebler M., Qian X., Höfler D., Kogosov V., Kaewprag J., Kaufmann A.M. Post-translational control of IL-1β via the human papillomavirus type 16 E6 oncoprotein: a novel mechanism of innate immune escape mediated by the E3-ubiquitin ligase E6-AP and p53. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma W., Tummers B., van Esch E.M., Goedemans R., Melief C.J., Meyers C. Human papillomavirus downregulates the expression of IFITM1 and RIPK3 to escape from IFNgamma- and TNFalpha-mediated antiproliferative effects and necroptosis. Front. Immunol. 2016;7:496. doi: 10.3389/fimmu.2016.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campo M.S., Graham S.V., Cortese M.S., Ashrafi G.H., Araibi E.H., Dornan E.S. HPV-16 E5 down-regulates expression of surface HLA class I and reduces recognition by CD8 T cells. Virology. 2010;407:137–142. doi: 10.1016/j.virol.2010.07.044. [DOI] [PubMed] [Google Scholar]
- 31.Cortese M.S., Ashrafi G.H., Campo M.S. All 4 di-leucine motifs in the first hydrophobic domain of the E5 oncoprotein of human papillomavirus type 16 are essential for surface MHC class I downregulation activity and E5 endomembrane localization. Int. J. Cancer. 2010;126:1675–1682. doi: 10.1002/ijc.25004. [DOI] [PubMed] [Google Scholar]
- 32.Zhou F., Leggatt G.R., Frazer I.H. Human papillomavirus 16 E7 protein inhibits interferon-gamma-mediated enhancement of keratinocyte antigen processing and T-cell lysis. FEBS J. 2011;278:955–963. doi: 10.1111/j.1742-4658.2011.08011.x. [DOI] [PubMed] [Google Scholar]
- 33.Zhou F., Chen J., Zhao K.N. Human papillomavirus 16-encoded E7 protein inhibits IFN-gamma-mediated MHC class I antigen presentation and CTL-induced lysis by blocking IRF-1 expression in mouse keratinocytes. J. Gen. Virol. 2013;94:2504–2514. doi: 10.1099/vir.0.054486-0. [DOI] [PubMed] [Google Scholar]
- 34.Hong S., Mehta K.P., Laimins L.A. Suppression of STAT-1 expression by human papillomaviruses is necessary for differentiation-dependent genome amplification and plasmid maintenance. J. Virol. 2011;85:9486–9494. doi: 10.1128/JVI.05007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bedoui S., Whitney P.G., Waithman J., Eidsmo L., Wakim L., Caminschi I. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat. Immunol. 2009;10:488–495. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- 36.Tamoutounour S., Guilliams M., Montanana Sanchis F., Liu H., Terhorst D., Malosse C. Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity. 2013;39:925–938. doi: 10.1016/j.immuni.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Gregorio J., Meller S., Conrad C., Di Nardo A., Homey B., Lauerma A. Plasmacytoid dendritic cells sense skin injury and promote wound healing through type I interferons. J. Exp. Med. 2010;207:2921–2930. doi: 10.1084/jem.20101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heath W.R., Carbone F.R. The skin-resident and migratory immune system in steady state and memory: innate lymphocytes, dendritic cells and T cells. Nat. Immunol. 2013;14:978–985. doi: 10.1038/ni.2680. [DOI] [PubMed] [Google Scholar]
- 39.Summerfield A., Meurens F., Ricklin M.E. The immunology of the porcine skin and its value as a model for human skin. Mol. Immunol. 2015;66:14–21. doi: 10.1016/j.molimm.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 40.Sumaria N., Roediger B., Ng L.G., Qin J., Pinto R., Cavanagh L.L. Cutaneous immunosurveillance by self-renewing dermal gammadelta T cells. J. Exp. Med. 2011;208:505–518. doi: 10.1084/jem.20101824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merad M., Ginhoux F., Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat. Rev. Immunol. 2008;8:935–947. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- 42.Bigley V., McGovern N., Milne P., Dickinson R., Pagan S., Cookson S. Langerin-expressing dendritic cells in human tissues are related to CD1c+ dendritic cells and distinct from Langerhans cells and CD141high XCR1+ dendritic cells. J. Leukoc. Biol. 2015;97:627–634. doi: 10.1189/jlb.1HI0714-351R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Merad M., Manz M.G., Karsunky H., Wagers A., Peters W., Charo I. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat. Immunol. 2002;3:1135–1141. doi: 10.1038/ni852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kanitakis J., Petruzzo P., Dubernard J.M. Turnover of epidermal Langerhans' cells. N. Engl. J. Med. 2004;351:2661–2662. doi: 10.1056/NEJM200412163512523. [DOI] [PubMed] [Google Scholar]
- 45.Jongbloed S.L., Kassianos A.J., McDonald K.J., Clark G.J., Ju X., Angel C.E. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J. Exp. Med. 2010;207:1247–1260. doi: 10.1084/jem.20092140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wylie B., Seppanen E., Xiao K., Zemek R., Zanker D., Prato S. Cross-presentation of cutaneous melanoma antigen by migratory XCR1+CD103- and XCR1+CD103+ dendritic cells. Oncoimmunology. 2015;4:e1019198. doi: 10.1080/2162402X.2015.1019198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vander Lugt B., Khan A.A., Hackney J.A., Agrawal S., Lesch J., Zhou M. Transcriptional programming of dendritic cells for enhanced MHC class II antigen presentation. Nat. Immunol. 2014;15:161–167. doi: 10.1038/ni.2795. [DOI] [PubMed] [Google Scholar]
- 48.Tran Janco J.M., Lamichhane P., Karyampudi L., Knutson K.L. Tumor-infiltrating dendritic cells in cancer pathogenesis. J. Immunol. 2015;194:2985–2991. doi: 10.4049/jimmunol.1403134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leong C.M., Doorbar J., Nindl I., Yoon H.S., Hibma M.H. Loss of epidermal Langerhans cells occurs in human papillomavirus alpha, gamma, and mu but not beta genus infections. J. Investig. Dermatol. 2010;130:472–480. doi: 10.1038/jid.2009.266. [DOI] [PubMed] [Google Scholar]
- 50.Lijima N., Goodwin E.C., Dimaio D., Iwasaki A. High-risk human papillomavirus E6 inhibits monocyte differentiation to Langerhans cells. Virology. 2013;444:257–262. doi: 10.1016/j.virol.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jimenez-Flores R., Mendez-Cruz R., Ojeda-Ortiz J., Munoz-Molina R., Balderas-Carrillo O., de la Luz Diaz-Soberanes M. High-risk human papilloma virus infection decreases the frequency of dendritic Langerhans' cells in the human female genital tract. Immunology. 2006;117:220–228. doi: 10.1111/j.1365-2567.2005.02282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang B., Xue M. Correlation of E6 and E7 levels in high-risk HPV16 type cervical lesions with CCL20 and Langerhans cells. Genet. Mol. Res. 2015;14:10473–10481. doi: 10.4238/2015.September.8.8. [DOI] [PubMed] [Google Scholar]
- 53.Jemon K., Leong C.M., Ly K., Young S.L., McLellan A.D., Hibma M.H. Suppression of the CD8 T cell response by human papillomavirus type 16 E7 occurs in Langerhans cell-depleted mice. Sci. Rep. 2016;6:34789. doi: 10.1038/srep34789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sperling T., Oldak M., Walch-Ruckheim B., Wickenhauser C., Doorbar J., Pfister H. Human papillomavirus type 8 interferes with a novel C/EBPbeta-mediated mechanism of keratinocyte CCL20 chemokine expression and Langerhans cell migration. PLoS Pathog. 2012;8:e1002833. doi: 10.1371/journal.ppat.1002833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reva I.V., Reva G.V., Yamamoto T., Girya O.Y., Grakhova N.V., Maloman N.Y. Distribution of antigen-presenting cells CD68 in papillomavirus infection in the skin. Bull. Exp. Biol. Med. 2014;157:56–61. doi: 10.1007/s10517-014-2491-3. [DOI] [PubMed] [Google Scholar]
- 56.Shannon B., Yi T.J., Perusini S., Gajer P., Ma B., Humphrys M.S. Association of HPV infection and clearance with cervicovaginal immunology and the vaginal microbiota. Mucosal Immunol. 2017 doi: 10.1038/mi.2016.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kindt N., Descamps G., Seminerio I., Bellier J., Lechien J.R., Pottier C. Langerhans cell number is a strong and independent prognostic factor for head and neck squamous cell carcinomas. Oral Oncol. 2016;62:1–10. doi: 10.1016/j.oraloncology.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 58.Guess J.C., McCance D.J. Decreased migration of Langerhans precursor-like cells in response to human keratinocytes expressing human papillomavirus type 16 E6/E7 is related to reduced macrophage inflammatory protein-3alpha production. J. Virol. 2005;79:14852–14862. doi: 10.1128/JVI.79.23.14852-14862.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.D'Costa Z.J., Jolly C., Androphy E.J., Mercer A., Matthews C.M., Hibma M.H. Transcriptional repression of E-cadherin by human papillomavirus type 16 E6. PLoS One. 2012;7:e48954. doi: 10.1371/journal.pone.0048954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hubert P., Caberg J.H., Gilles C., Bousarghin L., Franzen-Detrooz E., Boniver J. E-cadherin-dependent adhesion of dendritic and Langerhans cells to keratinocytes is defective in cervical human papillomavirus-associated (pre)neoplastic lesions. J. Pathol. 2005;206:346–355. doi: 10.1002/path.1771. [DOI] [PubMed] [Google Scholar]
- 61.Le Borgne M., Etchart N., Goubier A., Lira S.A., Sirard J.C., van Rooijen N. Dendritic cells rapidly recruited into epithelial tissues via CCR6/CCL20 are responsible for CD8+ T cell crosspriming in vivo. Immunity. 2006;24:191–201. doi: 10.1016/j.immuni.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 62.Dieu-Nosjean M.C., Massacrier C., Homey B., Vanbervliet B., Pin J.J., Vicari A. Macrophage inflammatory protein 3α Is expressed at inflamed epithelial surfaces and is the most potent chemokine known in attracting langerhans cell precursors. J. Exp. Med. 2000;192:705–718. doi: 10.1084/jem.192.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Y., Bugatti M., Ulland T.K., Vermi W., Gilfillan S., Colonna M. Nonredundant roles of keratinocyte-derived IL-34 and neutrophil-derived CSF1 in Langerhans cell renewal in the steady state and during inflammation. Eur. J. Immunol. 2016;46:552–559. doi: 10.1002/eji.201545917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mohammed J., Beura L.K., Bobr A., Astry B., Chicoine B., Kashem S.W. Stromal cells control the epithelial residence of DCs and memory T cells by regulated activation of TGF-beta. Nat. Immunol. 2016;17:414–421. doi: 10.1038/ni.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mayumi N., Watanabe E., Norose Y., Watari E., Kawana S., Geijtenbeek T.B.H. E-cadherin interactions are required for Langerhans cell differentiation. Eur. J. Immunol. 2013;43:270–280. doi: 10.1002/eji.201242654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Caberg J.H., Hubert P., Herman L., Herfs M., Roncarati P., Boniver J. Increased migration of Langerhans cells in response to HPV16 E6 and E7 oncogene silencing: role of CCL20. Cancer Immunol. Immunother. 2009;58:39–47. doi: 10.1007/s00262-008-0522-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sperling T., Ołdak M., Walch-Rückheim B., Wickenhauser C., Doorbar J., Pfister H. Human papillomavirus type 8 interferes with a novel C/EBPβ-mediated mechanism of keratinocyte CCL20 chemokine expression and langerhans cell migration. PLoS Pathog. 2012:8. doi: 10.1371/journal.ppat.1002833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cavalcante J.R., Sampaio J.P.A., Maia Filho J.T.A., Vieira R.B., Eleutério Júnior J., Lima Júnior R.C.P. Progressive loss of E-cadherin immunoexpression during cervical carcinogenesis. Acta Cir. Bras. 2014;29:667–674. doi: 10.1590/s0102-8650201400160007. [DOI] [PubMed] [Google Scholar]
- 69.Hu D., Zhou J., Wang F., Shi H., Li Y., Li B. HPV-16 E6/E7 promotes cell migration and invasion in cervical cancer via regulating cadherin switch in vitro and in vivo. Arch. Gynecol. Obstet. 2015;292:1345–1354. doi: 10.1007/s00404-015-3787-x. [DOI] [PubMed] [Google Scholar]
- 70.D'Costa Z.J., Leong C.M., Shields J., Matthews C., Hibma M.H. Screening of drugs to counteract human papillomavirus 16 E6 repression of E-cadherin expression. Investig. New Drugs. 2012;30:2236–2251. doi: 10.1007/s10637-012-9803-0. [DOI] [PubMed] [Google Scholar]
- 71.Kupz A., Guarda G., Gebhardt T., Sander L.E., Short K.R., Diavatopoulos D.A. NLRC4 inflammasomes in dendritic cells regulate noncognate effector function by memory CD8+ T cells. Nat. Immunol. 2012;13:162–169. doi: 10.1038/ni.2195. [DOI] [PubMed] [Google Scholar]
- 72.Lee C.H., Hajishengallis G., Connell T.D. Dendritic Cell-Mediated Mechanisms Triggered by LT-IIa-B5, a Mucosal Adjuvant Derived from a Type II Heat-Labile Enterotoxin of Escherichia coli. J. Microbiol. Biotechnol. 2017;27:709–717. doi: 10.4014/jmb.1611.11072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Verma V., Kim Y., Lee M.C., Lee J.T., Cho S., Park I.K. Activated dendritic cells delivered in tissue compatible biomatrices induce in-situ anti-tumor CTL responses leading to tumor regression. Oncotarget. 2016;7:39894–39906. doi: 10.18632/oncotarget.9529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Coquerelle C., Moser M. DC subsets in positive and negative regulation of immunity. Immunol. Rev. 2010;234:317–334. doi: 10.1111/j.0105-2896.2009.00887.x. [DOI] [PubMed] [Google Scholar]
- 75.Yang W., Song Y., Lu Y.L., Sun J.Z., Wang H.W. Increased expression of programmed death (PD)-1 and its ligand PD-L1 correlates with impaired cell-mediated immunity in high-risk human papillomavirus-related cervical intraepithelial neoplasia. Immunology. 2013;139:513–522. doi: 10.1111/imm.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Almand B., Clark J.I., Nikitina E., van Beynen J., English N.R., Knight S.C. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J. Immunol. 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 77.Da Silva D.M., Woodham A.W., Skeate J.G., Rijkee L.K., Taylor J.R., Brand H.E. Langerhans cells from women with cervical precancerous lesions become functionally responsive against human papillomavirus after activation with stabilized Poly-I:C. Clin. Immunol. 2015;161:197–208. doi: 10.1016/j.clim.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Woodham A.W., Yan L., Skeate J.G., van der Veen D., Brand H.H., Wong M.K. T cell ignorance is bliss: t cells are not tolerized by Langerhans cells presenting human papillomavirus antigens in the absence of costimulation. Papillomavirus Res. 2016;2:21–30. doi: 10.1016/j.pvr.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Da Silva D.M., Movius C.A., Raff A.B., Brand H.E., Skeate J.G., Wong M.K. Suppression of Langerhans cell activation is conserved amongst human papillomavirus α and β genotypes, but not a μ genotype. Virology. 2014;0:279–286. doi: 10.1016/j.virol.2014.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fahey L.M., Raff A.B., Da Silva D.M., Kast W.M. A major role for the minor capsid protein of human papillomavirus type 16 in immune escape. J. Immunol. 2009;183:6151–6156. doi: 10.4049/jimmunol.0902145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Langers I., Renoux V., Reschner A., Touze A., Coursaget P., Boniver J. Natural killer and dendritic cells collaborate in the immune response induced by the vaccine against uterine cervical cancer. Eur. J. Immunol. 2014;44:3585–3595. doi: 10.1002/eji.201444594. [DOI] [PubMed] [Google Scholar]
- 82.Woodham A.W., Raff A.B., Raff L.M., Da Silva D.M., Yan L., Skeate J.G. Inhibition of Langerhans cell maturation by human papillomavirus type 16: a novel role for the annexin A2 heterotetramer in immune suppression. J. Immunol. 2014;192:4748–4757. doi: 10.4049/jimmunol.1303190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Da Silva D.M., Woodham A.W., Naylor P.H., Egan J.E., Berinstein N.L., Kast W.M. Immunostimulatory activity of the cytokine-based biologic, IRX-2, on human papillomavirus-exposed langerhans cells. J. Interferon Cytokine Res. 2016;36:291–301. doi: 10.1089/jir.2015.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Doan T., Chambers M., Street M., Fernando G.J., Herd K., Lambert P. Mice expressing the E7 oncogene of HPV16 in epithelium show central tolerance, and evidence of peripheral anergising tolerance, to E7-encoded cytotoxic T-lymphocyte epitopes. Virology. 1998;244:352–364. doi: 10.1006/viro.1998.9128. [DOI] [PubMed] [Google Scholar]
- 85.Herber R., Liem A., Pitot H., Lambert P.F. Squamous epithelial hyperplasia and carcinoma in mice transgenic for the human papillomavirus type 16 E7 oncogene. J. Virol. 1996;70:1873–1881. doi: 10.1128/jvi.70.3.1873-1881.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Choyce A., Yong M., Narayan S., Mattarollo S.R., Liem A., Lambert P.F. Expression of a single, viral oncoprotein in skin epithelium is sufficient to recruit lymphocytes. PLoS One. 2013;8:e57798. doi: 10.1371/journal.pone.0057798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Abd Warif N.M., Stoitzner P., Leggatt G.R., Mattarollo S.R., Frazer I.H., Hibma M.H. Langerhans cell homeostasis and activation is altered in hyperplastic human papillomavirus type 16 E7 expressing epidermis. PLoS One. 2015;10:e0127155. doi: 10.1371/journal.pone.0127155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chandra J., Miao Y., Romoff N., Frazer I.H. Epithelium expressing the E7 oncoprotein of HPV16 attracts immune-modulatory dendritic cells to the skin and suppresses their antigen-processing capacity. PLoS One. 2016;11:e0152886. doi: 10.1371/journal.pone.0152886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mittal D., Kassianos A.J., Tran L.S., Bergot A.S., Gosmann C., Hofmann J. Indoleamine 2,3-dioxygenase activity contributes to local immune suppression in the skin expressing human papillomavirus oncoprotein e7. J. Investig. Dermatol. 2013;133:2686–2694. doi: 10.1038/jid.2013.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Natsuaki Y., Egawa G., Nakamizo S., Ono S., Hanakawa S., Okada T. Perivascular leukocyte clusters are essential for efficient activation of effector T cells in the skin. Nat. Immunol. 2014;15:1064–1069. doi: 10.1038/ni.2992. [DOI] [PubMed] [Google Scholar]
- 91.Stone S.C., Rossetti R.A., Lima A.M., Lepique A.P. HPV associated tumor cells control tumor microenvironment and leukocytosis in experimental models. Immun. Inflamm. Dis. 2014;2:63–75. doi: 10.1002/iid3.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Prata T.T., Bonin C.M., Ferreira A.M., Padovani C.T., Fernandes C.E., Machado A.P. Local immunosuppression induced by high viral load of human papillomavirus: characterization of cellular phenotypes producing interleukin-10 in cervical neoplastic lesions. Immunology. 2015;146:113–121. doi: 10.1111/imm.12487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen Z., Pang N., Du R., Zhu Y., Fan L., Cai D. Elevated expression of programmed death-1 and programmed death ligand-1 negatively regulates immune response against cervical cancer cells. Mediat. Inflamm. 2016;2016:6891482. doi: 10.1155/2016/6891482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mangino G., Chiantore M.V., Iuliano M., Fiorucci G., Romeo G. Inflammatory microenvironment and human papillomavirus-induced carcinogenesis. Cytokine Growth Factor Rev. 2016 doi: 10.1016/j.cytogfr.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 95.Kobayashi A., Weinberg V., Darragh T., Smith-McCune K. Evolving immunosuppressive microenvironment during human cervical carcinogenesis. Mucosal Immunol. 2008;1:412–420. doi: 10.1038/mi.2008.33. [DOI] [PubMed] [Google Scholar]
- 96.Torres-Poveda K., Bahena-Roman M., Madrid-Gonzalez C., Burguete-Garcia A.I., Bermudez-Morales V.H., Peralta-Zaragoza O. Role of IL-10 and TGF-beta1 in local immunosuppression in HPV-associated cervical neoplasia. World J. Clin. Oncol. 2014;5:753–763. doi: 10.5306/wjco.v5.i4.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Herfs M., Herman L., Hubert P., Minner F., Arafa M., Roncarati P. High expression of PGE2 enzymatic pathways in cervical (pre)neoplastic lesions and functional consequences for antigen-presenting cells. Cancer Immunol. Immunother. 2009;58:603–614. doi: 10.1007/s00262-008-0584-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Arantes D.A.C., Costa N.L., Mendonça E.F., Silva T.A., Batista A.C. Overexpression of immunosuppressive cytokines is associated with poorer clinical stage of oral squamous cell carcinoma. Arch. Oral Biol. 2016;61:28–35. doi: 10.1016/j.archoralbio.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 99.Chattopadhyay G., Shevach E.M. Antigen-specific induced T regulatory cells impair dendritic cell function via an IL-10/MARCH1-dependent mechanism. J. Immunol. 2013;191:5875–5884. doi: 10.4049/jimmunol.1301693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu X.S., Leerberg J., MacDonald K., Leggatt G.R., Frazer I.H. IFN-gamma promotes generation of IL-10 secreting CD4+ T cells that suppress generation of CD8 responses in an antigen-experienced host. J. Immunol. 2009;183:51–58. doi: 10.4049/jimmunol.0802047. [DOI] [PubMed] [Google Scholar]
- 101.Garcia-Rocha R., Moreno-Lafont M., Mora-Garcia M.L., Weiss-Steider B., Montesinos J.J., Pina-Sanchez P. Mesenchymal stromal cells derived from cervical cancer tumors induce TGF-beta1 expression and IL-10 expression and secretion in the cervical cancer cells, resulting in protection from cytotoxic T cell activity. Cytokine. 2015;76:382–390. doi: 10.1016/j.cyto.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 102.Demoulin S.A., Somja J., Duray A., Guenin S., Roncarati P., Delvenne P.O. Cervical (pre)neoplastic microenvironment promotes the emergence of tolerogenic dendritic cells via RANKL secretion. Oncoimmunology. 2015;4:e1008334. doi: 10.1080/2162402X.2015.1008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vicari A.P., Vanbervliet B., Massacrier C., Chiodoni C., Vaure C., Ait-Yahia S. In vivo manipulation of dendritic cell migration and activation to elicit antitumour immunity. Novartis Found. Symp. 2004;256(241–254):254–269. doi: 10.1002/0470856734.ch18. (discussion) [DOI] [PubMed] [Google Scholar]
- 104.Xin H., Zhu J., Miao H., Gong Z., Jiang X., Feng X. Adenovirus-mediated CCR7 and BTLA overexpression enhances immune tolerance and migration in immature dendritic cells. Biomed. Res. Int. 2017;2017 doi: 10.1155/2017/3519745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pahne-Zeppenfeld J., Schroer N., Walch-Ruckheim B., Oldak M., Gorter A., Hegde S. Cervical cancer cell-derived interleukin-6 impairs CCR7-dependent migration of MMP-9-expressing dendritic cells. Int. J. Cancer. 2014;134:2061–2073. doi: 10.1002/ijc.28549. [DOI] [PubMed] [Google Scholar]
- 106.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Montler R., Bell R.B., Thalhofer C., Leidner R., Feng Z., Fox B.A. OX40, PD-1 and CTLA-4 are selectively expressed on tumor-infiltrating T cells in head and neck cancer. Clin. Transl. Immunol. 2016;5:e70. doi: 10.1038/cti.2016.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kosmaczewska A., Bocko D., Ciszak L., Wlodarska-Polinska I., Kornafel J., Szteblich A. Dysregulated expression of both the costimulatory CD28 and inhibitory CTLA-4 molecules in PB T cells of advanced cervical cancer patients suggests systemic immunosuppression related to disease progression. Pathol. Oncol. Res. 2012;18:479–489. doi: 10.1007/s12253-011-9471-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.National Cancer Institute, A Phase I Trial of Sequential Ipilimumab After Chemoradiation for the Primary Treatment of Patients With Locally Advanced Cervical Cancer Stages IB2/IIA With Positive Para-Aortic Lymph Nodes Only and Stage IIB/IIIB/IVA With Positive Lymph Nodes. 〈https://clinicaltrials.gov/ct2/show/NCT01711515〉 (Accessed 7 August 2017). ClinicalTrials.gov Identifier: NCT01711515.
- 110.Dogan V., Rieckmann T., Munscher A., Busch C.J. Current studies of immunotherapy in head and neck cancer. Clin. Otolaryngol. 2017 doi: 10.1111/coa.12895. [DOI] [PubMed] [Google Scholar]
- 111.Rahimpour A., Mattarollo S.R., Yong M., Leggatt G.R., Steptoe R.J., Frazer I.H. gammadelta T cells augment rejection of skin grafts by enhancing cross-priming of CD8 T cells to skin-derived antigen. J. Investig. Dermatol. 2012;132:1656–1664. doi: 10.1038/jid.2012.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Broom J.K., Lew A.M., Azukizawa H., Kenna T.J., Leggatt G.R., Frazer I.H. Antigen-specific CD4 cells assist CD8 T-effector cells in eliminating keratinocytes. J. Investig. Dermatol. 2010;130:1581–1589. doi: 10.1038/jid.2010.17. [DOI] [PubMed] [Google Scholar]
- 113.Matsumoto K., Leggatt G.R., Zhong J., Liu X., de Kluyver R.L., Peters T. Impaired antigen presentation and effectiveness of combined active/passive immunotherapy for epithelial tumors. J. Natl. Cancer Inst. 2004;96:1611–1619. doi: 10.1093/jnci/djh301. [DOI] [PubMed] [Google Scholar]
- 114.Frazer I.H., De Kluyver R., Leggatt G.R., Guo H.Y., Dunn L., White O. Tolerance or immunity to a tumor antigen expressed in somatic cells can be determined by systemic proinflammatory signals at the time of first antigen exposure. J. Immunol. 2001;167:6180–6187. doi: 10.4049/jimmunol.167.11.6180. [DOI] [PubMed] [Google Scholar]
- 115.Lee S.J., Song L., Yang M.C., Mao C.P., Yang B., Yang A. Local administration of granulocyte macrophage colony-stimulating factor induces local accumulation of dendritic cells and antigen-specific CD8+ T cells and enhances dendritic cell cross-presentation. Vaccine. 2015;33:1549–1555. doi: 10.1016/j.vaccine.2015.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Narayan S., Choyce A., Linedale R., Saunders N.A., Dahler A., Chan E. Epithelial expression of human papillomavirus type 16 E7 protein results in peripheral CD8 T-cell suppression mediated by CD4+CD25+ T cells. Eur. J. Immunol. 2009;39:481–490. doi: 10.1002/eji.200838527. [DOI] [PubMed] [Google Scholar]
- 117.Mattarollo S.R., Yong M., Gosmann C., Choyce A., Chan D., Leggatt G.R. NKT cells inhibit antigen-specific effector CD8 T cell induction to skin viral proteins. J. Immunol. 2011;187:1601–1608. doi: 10.4049/jimmunol.1100756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Caielli S., Conforti-Andreoni C., Di Pietro C., Usuelli V., Badami E., Malosio M.L. On/off TLR signaling decides proinflammatory or tolerogenic dendritic cell maturation upon CD1d-mediated interaction with invariant NKT cells. J. Immunol. 2010;185:7317–7329. doi: 10.4049/jimmunol.1000400. [DOI] [PubMed] [Google Scholar]
- 119.Hu T., Yang P., Zhu H., Chen X., Xie X., Yang M. Accumulation of invariant NKT cells with increased IFN-gamma production in persistent high-risk HPV-infected high-grade cervical intraepithelial neoplasia. Diagn. Pathol. 2015;10:20. doi: 10.1186/s13000-015-0254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mellor A.L., Munn D.H. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat. Rev. Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 121.Mulley W.R., Nikolic-Paterson D.J. Indoleamine 2,3-dioxygenase in transplantation. Nephrol. (Carlton) 2008;13:204–211. doi: 10.1111/j.1440-1797.2007.00921.x. [DOI] [PubMed] [Google Scholar]
- 122.Hascitha J., Priya R., Jayavelu S., Dhandapani H., Selvaluxmy G., Sunder Singh S. Analysis of Kynurenine/Tryptophan ratio and expression of IDO1 and 2 mRNA in tumour tissue of cervical cancer patients. Clin. Biochem. 2016;49:919–924. doi: 10.1016/j.clinbiochem.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 123.Ferns D.M., Kema I.P., Buist M.R., Nijman H.W., Kenter G.G., Jordanova E.S. Indoleamine-2,3-dioxygenase (IDO) metabolic activity is detrimental for cervical cancer patient survival. Oncoimmunology. 2015;4:e981457. doi: 10.4161/2162402X.2014.981457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Inaba T., Ino K., Kajiyama H., Shibata K., Yamamoto E., Kondo S. Indoleamine 2,3-dioxygenase expression predicts impaired survival of invasive cervical cancer patients treated with radical hysterectomy. Gynecol. Oncol. 2010;117:423–428. doi: 10.1016/j.ygyno.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 125.Seresini S., Origoni M., Lillo F., Caputo L., Paganoni A.M., Vantini S. IFN-gamma produced by human papilloma virus-18 E6-specific CD4+ T cells predicts the clinical outcome after surgery in patients with high-grade cervical lesions. J. Immunol. 2007;179:7176–7183. doi: 10.4049/jimmunol.179.10.7176. [DOI] [PubMed] [Google Scholar]
- 126.Stevanovic S., Draper L.M., Langhan M.M., Campbell T.E., Kwong M.L., Wunderlich J.R. Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells. J. Clin. Oncol. 2015;33:1543–1550. doi: 10.1200/JCO.2014.58.9093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bonin C.M., Padovani C.T.J., Ferreira A.M.T., Ãvila L.S., Machado A.P., Prata T.T.M. Predominant overexpression of CD25/FOXP3, IFN-³, and suppressive cytokines in high-grade lesion samples infected with human papillomavirus. J. Bras. Patol. Med. Lab. 2017;53:53–60. [Google Scholar]
- 128.Sato N., Saga Y., Mizukami H., Wang D., Takahashi S., Nonaka H. Downregulation of indoleamine-2,3-dioxygenase in cervical cancer cells suppresses tumor growth by promoting natural killer cell accumulation. Oncol. Rep. 2012;28:1574–1578. doi: 10.3892/or.2012.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Postow M.A., Callahan M.K., Wolchok J.D. Immune checkpoint blockade in cancer therapy. J. Clin. Oncol. 2015;33:1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu C., Lu J., Tian H., Du W., Zhao L., Feng J. Increased expression of PD-L1 by the human papillomavirus 16 E7 oncoprotein inhibits anticancer immunity. Mol. Med. Rep. 2016 doi: 10.3892/mmr.2016.6073. [DOI] [PubMed] [Google Scholar]
- 131.Leng C., Li Y., Qin J., Ma J., Liu X., Cui Y. Relationship between expression of PD-L1 and PD-L2 on esophageal squamous cell carcinoma and the antitumor effects of CD8(+) T cells. Oncol. Rep. 2016;35:699–708. doi: 10.3892/or.2015.4435. [DOI] [PubMed] [Google Scholar]
- 132.Lyford-Pike S., Peng S., Young G.D., Taube J.M., Westra W.H., Akpeng B. Evidence for a role of the PD-1: PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013;73:1733–1741. doi: 10.1158/0008-5472.CAN-12-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Heeren A.M., Koster B.D., Samuels S., Ferns D.M., Chondronasiou D., Kenter G.G. High and interrelated rates of PD-L1+CD14+ antigen-presenting cells and regulatory T cells mark the microenvironment of metastatic lymph nodes from patients with cervical cancer. Cancer Immunol. Res. 2015;3:48–58. doi: 10.1158/2326-6066.CIR-14-0149. [DOI] [PubMed] [Google Scholar]
- 134.Rice A.E., Latchman Y.E., Balint J.P., Lee J.H., Gabitzsch E.S., Jones F.R. An HPV-E6/E7 immunotherapy plus PD-1 checkpoint inhibition results in tumor regression and reduction in PD-L1 expression. Cancer Gene Ther. 2015;22:454–462. doi: 10.1038/cgt.2015.40. [DOI] [PubMed] [Google Scholar]
- 135.Liu Z., Zhou H., Wang W., Fu Y.X., Zhu M. A novel dendritic cell targeting HPV16 E7 synthetic vaccine in combination with PD-L1 blockade elicits therapeutic antitumor immunity in mice. Oncoimmunology. 2016;5:e1147641. doi: 10.1080/2162402X.2016.1147641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chandra J., Dutton J.L., Li B., Woo W.P., Xu Y., Tolley L.K. DNA vaccine encoding HPV16 oncogenes E6 and E7 induces potent cell-mediated and humoral immunity which protects in tumor challenge and drives E7-expressing skin graft rejection. J. Immunother. 2017;40:62–70. doi: 10.1097/CJI.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ferris R.L., Blumenschein G., Jr., Fayette J., Guigay J., Colevas A.D., Licitra L. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2016;375:1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Merck Sharp, Dohme Corp, A Clinical Trial of Pembrolizumab (MK-3475) Evaluating Predictive Biomarkers in Subjects with Advanced Solid Tumors (KEYNOTE 158). 〈https://clinicaltrials.gov/ct2/show/NCT02628067〉 (Accessed 7 August 2017). ClinicalTrials.gov Identifier: NCT02628067.
- 139.Bristol-Myers Squibb, Non-Comparative, Open-Label, Multiple Cohort, Phase 1/2 Study of Nivolumab Monotherapy and Nivolumab Combination Therapy in Subjects With Virus-Positive and Virus-Negative Solid Tumors. 〈https://clinicaltrials.gov/ct2/show/NCT02488759〉 (Accessed 7 August 2017). ClinicalTrials.gov Identifier: NCT02488759.