Abstract
Background
Anal intraepithelial neoplasia is associated with high-risk human papillomavirus (hrHPV) as a precursor to anal cancer. However, factors other than hrHPV are likely to be involved and further study of cofactors is required because of the possibility of syndemic interactions.
Methods
Three hundred and fourteen patients underwent 457 operations. Histopathology and hrHPV testing using the Digene Hybrid Capture 2 (HC 2) method were performed. Demographic factors and sexually transmissible infections (STIs) were recorded.
Results
Results showed that hrHPV alone was associated with HSIL (OR = 4.65, p < 0.001). None of the other STIs were alone associated with HSIL but amplification of risk was found when hrHPV infection occurred with HIV (OR = 11.1); syphilis (OR = 5.58); HSV 2 (OR = 7.85); gonorrhoea (OR = 6.45) and some other infections.
Conclusions
These results suggest that hrHPV is a sufficient cause of anal HSIL. Seropositivity for HIV, HSV 2, T. pallidum, HBV and HCV and a history of gonorrhoea or chlamydia exert a powerful amplifying factor increasing the risk of HSIL above the risk with hrHPV alone. Other co-factors which are associated with an increased risk of HSIL are increased age, male gender, MSM behaviour and self-reported history of more than 50 sexual partners. This pattern of disease in patients with warts is characteristic of a syndemic with potential serious increased risk of anal carcinoma.
Keywords: HSIL, Anal warts, Sexually transmissible infections, High-risk HPV, HIV, Syndemic
Highlights
-
•
High-risk HPV is a necessary and sufficient cause of progression from LSIL to HSIL.
-
•
HIV, HSV 2, HBV and HCV are associated with an amplified risk of hrHPV induced HSIL.
-
•
Gonorrhoea, chlamydia, and syphilis are associated with increased odds HSIL.
-
•
HSIL shows syndemic interaction patterns with STIs and behavioural/social factors.
1. Introduction
Anal intraepithelial neoplasia is thought to be associated with high-risk human papillomavirus (hrHPV) as a precursor to anal cancer. Anal warts are associated with high rates of intraepithelial neoplasia especially in human immunodeficiency virus (HIV)-infected patients [1], [2]. Anal cancer is increasing in many parts of the world in both men and women, particularly in men with HIV infection. Screening of the anal canal to detect probable precursors is being promoted for men with HIV infection where the standardised incidence rate of anal cancer has increased 4 fold between 1992 and 2003 to 78.2/100,000 person years [3]. In addition the introduction of antiretroviral therapy (ART) has not been demonstrated to reduce the prevalence of anal squamous intraepithelial lesions [4] except in one study [5]. Anal warts are also recognised to be linked to the development of anal cancer [6]. Acquisition of genital warts has also been linked to progression to a higher level of squamous intraepithelial lesions (HSIL) in both HIV-positive and negative men [7]. Gonorrhoea was found to be associated with the development of anal cancer but to date no association with anal pre-cancer has been described [8]. Chlamydia has been previously associated with cervical intraepithelial neoplasia [9], [10]. Because of the high rates of intraepithelial neoplasia (IN) associated with anal warts [1] and the fact that hrHPV has not been found in all anal cancers [11], we explored other possible associations with HSIL.
The emerging concept of a disease syndemic [12] where the disease/outcome risk is seen as a combination of biological, behavioural and social factors appears to apply to anal cancer as the existing literature indicates.
There are many risk factors associated with the development of anal cancer. In addition to HPV infection [13], [14], [15], [16], other risk factors include receptive anal intercourse (before the age of 30) [6], [16], [17], lifetime number of sexual partners [6], [17], female gender [18], current cigarette smoking [15], [19], [20], genital warts [6], [20], immune-suppression post-organ transplantation and HIV infection [16], [21], [22], [23]. Persistence of cervical HrHPV in women with genital warts has been observed in a recent Danish population study [24]. Anal fistulae and epithelial trauma are also associated, possibly because of access for HPV to the basement membrane [25], [26], [27].
A history of genital warts on the anus has a strong association with the development of anal cancer (OR 15.1, 95%CI: 6.8–33.5) [6]. Anal warts preceded anal cancer by about ten years with a shorter time interval for homosexual men compared to heterosexual men and women [6]. Anal squamous cell carcinoma has been demonstrated to arise out of anal warts [28], [29], [30], [31], [32], [33]. In addition the presence of HSIL in association with anal warts has been under-recognised, with HSIL being present in 52% of HIV positive men, compared to 20% in HIV negative men, and 2.8% in HIV negative women with warts. Further evidence of an interaction between high and low-risk viruses comes from the EXPLORE study where the odds of developing high-grade squamous intraepithelial lesions in HIV-negative men was 23 times higher (95% CI 9.6–53) with combined high and low- risk HPV, compared to lower risks for high-risk HPV alone OR 6.4 (95% CI 2.7–15) and low-risk HPV alone OR 5.8 (95% CI 2.3–14) [34]. Similarly, Hessol et al. also found a 7.7 times increased risk (95% CI 5–38) of developing HSIL in women infected with both low and high-risk HPV. The increase in anal cancer in the last 30 years has been attributed to changed sexual patterns [35], [36] including increased anal sex [36], [37] and increased partner number for both MSM, women, and HIV infection [38].
MSM have a high prevalence of HPV infection in the anal canal (47.2–88.9%; pooled 63.9%) and HIV-positive men have even higher rates (74.6–97.7%, pooled 92.6 [39], [40]). Anal HPV infection is almost universal in HIV-positive MSM [4], [40], [41], [42]. Rates of anal HPV have been consistently demonstrated to be higher in HIV positive men compared to HIV-negative men [5], [39], [41], [43], [44], [45], [46], [47], and in studies that compare HIV-positive to negative men, HPV has been associated with HIV infection. Whether ART alters the risk of anal cancer remains to be determined with some studies describing progression [48], others regression [49], [50]. A more recent study found there may be some benefit with prolonged ART (OR = 0.32 95% CI: 0.16-0.63, p = 0.001) [51].
Patients with genital warts have a significantly increased risk of anogenital cancer and other cancers such as head and neck cancers [52]. This paper explores the role of infectious and demographic cofactors for HSIL as a syndemic in a case series of men and women with anogenital warts requiring surgical treatment and as such it is unique in that it is able to address behaviour and HrHPV confounding that exists in other studies.
2. Materials and methods
2.1. Study sample and design
In this case series, 460 operations were performed in 317 patients undergoing scissor excision of perianal and/or anal condylomata acuminata or mapping biopsies under general anaesthesia between December 1995 and November 2016. Patients with missing surgical material were excluded from the analysis (4). We examined the Royal Perth Hospital Anogenital Wart Database (established in December 1995) for variables that might be associated with HSIL. Demographic data were collected on each patient including age, sex, sexual preference, lifetime sexual partners (0–10, 11–50, > 50), cigarette smoking (current, ever, never), and a history of gonorrhoea and chlamydia, wherever possible confirmed by evidence from the patient clinical record at the time they were enrolled for surgery. Serological data were obtained for the following infections: HIV 1 and 2 antibodies, syphilis [Treponema pallidum haemaglutination assay (TPHA), Rapid Reagin Index (RPR)], past or present HBV infection (HBsAg, HBcAb), HSV 2 antibodies (EIA), and HCV antibodies.
2.2. HPV testing
HPV testing of the anal canal by Digene Hybrid Capture-2 (HC2) for high-risk strains was used as a standard part of the surgical procedure from June 2005 onwards. Just prior to surgery, a proctoscope was inserted and using direct visualisation a HC2 sampler for hrHPV was then rotated from just above the dentate line to the anal and perianal skin as the proctoscope was removed. The Digene brush was placed into the Digene Transport buffered container for HPV testing. Excised surgical material labelled by site (perianal, anal) was placed in formalin and then processed in paraffin. No material was discarded at the bedside.
2.3. Reporting of surgical material
Biopsy material was stained with haematoxylin and eosin then routinely reported with other surgical specimens. Lesions were classified using the same concepts and criteria described in the LAST criteria [53]. Accordingly, HSIL in this study comprises AIN 2 and AIN 3 together with anal cancer (Table 2).
Table 2.
Histopathology by HPV status.
| Histology grade | HPV -ve | HPV +ve | Odds ratio (CL95%) | p | ||
|---|---|---|---|---|---|---|
| n (%) | n (%) | % | ||||
| LSIL | Negative | 3 (2.61) | 4 (1.17) | 306 (67.0) | 6.55 (3.39–12.6) | < 0.00001 |
| Atypia, etc. | 79 (68.7) | 134 (39.2) | ||||
| AIN1 | 22 (19.1) | 64 (18.7) | ||||
| HSIL | AIN2 | 7 (6.09) | 68 (19.9) | 151 (33.0) | ||
| AIN3 | 4 (3.48) | 70 (20.5) | ||||
| Cancer | 0 | 2 (0.58) | ||||
| Total | 115 (100) | 342 (100) | 457 | |||
2.4. Statistical analysis
Samples with hrHPV results were examined for associations between hrHPV, other STIs and HSIL. Statistical analysis used percentages and appropriate means with confidence intervals for descriptive purposes. For variables that had a log-normal distribution the geometric mean was used because it is an unbiased estimate of the average under these circumstances. The chi-squared statistic and clustered linear logistic regression analysis were used to determine p-values with interaction terms to examine the independence of possible predictive variables. Because the data was clustered with samples nested within patients robust multi-level analyses were conducted. Multivariable logistic regression analysis was used to examine associations with HSIL and ordinal logistic regression analysis was used for histopathology grade, both with control for possible confounding factors. During the analysis it became clear that the STIs were not independent of each other and this multi-co-linearity was addressed by using boosted regression analysis [54] and structural equation models. The influence of missing data was assessed using the ‘missing-completely-at-random’ test of Little [55]. A p-value that was less than 0.05 was considered to be statistically significant. Statistical analysis was conducted using the Stata software package (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP). The research was approved by the Royal Perth Hospital Ethics Committee.
3. Results
The RPH Genital Wart Database contained records for 463 patients who were examined during 536 operations. High-risk HPV data was available for 314 patients from 460 operations and this sample was used for most of this analysis (Table 1). Sixty eight percent of these patients had a single operation, 20% had two operations and 12% had more than two operations over the period from December 1995 to November 2016.
Table 1.
Description of patients with HPV serology or history by gender and HPV status.
| Men | P | Women | P | |||
|---|---|---|---|---|---|---|
| n = 261 (%) | HPV +ve vs. HPV -ve | n = 53 (%) | Men vs. women | |||
| Digene HC2 (high-risk) | HPV +ve 195 (74.7) | HPV - ve 66 (25.3) | HPV +ve 21 (39.6) | HPV - ve 32 (60.4) | < 0.001 | |
| Median age (years) | 33.1 | 30.0 | 0.62 | 33.1 | 31.8 | 0.004 |
| Age range | 13.7–76.3 | 18.8–71.7 | 19.2–62.4 | 17.0–71.2 | ||
| Sexual preference | ||||||
| Heterosexual | 24 (47.1) | 27 (52.9) | < 0.001 | 20 (39.2) | 31 (60.8) | |
| MSM | 171 (81.4) | 39 (18.6) | ||||
| Lifetime Number of Sexual Partners | ||||||
| 1–10 | 38 (20.4) | 13 (20.4) | 0.85 | 11 (57.9) | 27 (90.0) | < 0.001 |
| 11–50 | 71 (38.2) | 24 (40.7) | 6 (31.6) | 2 (6.67) | ||
| > 50 | 77 (41.4) | 22 (37.3) | 2 (10.5) | 1 (3.33) | ||
| Smoking status | ||||||
| Never smoked | 28 (42.4) | 92 (47.2) | 0.80 | 9 (42.9) | 16 (50.0) | 0.67 |
| Current | 29 (43.9) | 78 (40.0) | 9 (42.9) | 15 (46.9) | ||
| Ex-smoker | 9 (13.6) | 25 (12.8) | 3 (14.3) | 1 (3.13) | ||
| History of STDs | ||||||
| Gonorrhoea | 9 (13.6) | 44 (22.6) | 0.12 | 0 | 0 | 0.001 |
| Chlamydia | 41 (21.0) | 11 (16.7) | 0.44 | 3 (15.0) | 3 (9.38) | 0.034 |
| Serology | ||||||
| HIV | 77 (39.5) | 5 (7.58) | < 0.001 | 0 | 0 | < 0.001 |
| Syphilis | 24 (12.3) | 3 (4.55) | 0.073 | 0 | 0 | 0.004 |
| HSV-2 | 47 (24.1) | 11 (16.7) | 0.21 | 6 (30.0) | 3 (9.38) | 0.15 |
| HBV | 18 (9.42) | 1 (1.54) | 0.036 | 0 | 0 | 0.005 |
| HCV | 9 (4.66) | 3 (4.76) | 0.97 | 1 (4.0) | 0 | 0.30 |
| Wart site for first operation | ||||||
| Perianal only | 10 (5.13) | 20 (30.3) | < 0.001 | 5 (23.8) | 15 (46.9) | < 0.001 |
| Anal only | 75 (38.5) | 12 (18.2) | 4 (19.1) | 2 (6.25) | ||
| Perianal & anal | 109 (55.9) | 34 (51.5) | 12 (57.1) | 14 (43.8) | ||
| Number of operations | ||||||
| 1 | 195 (62.9) | 66 (79.5) | 0.030 | 21 (63.6) | 32 (94.1) | 0.026 |
| 2 | 69 (22.3) | 11 (13.3) | 8 (24.2) | 2 (5.88) | ||
| 3 | 25 (8.06) | 3 (3.61) | 1 (1.49) | 0 | ||
| 4 or more | 21 (6.77) | 3 (3.61) | 3 (9.09) | 0 | ||
n signifies the number of patients.
3.1. General characteristics
A description of the patients with known hrHPV status sub-divided by gender and hrHPV status is shown in Table 1. Almost three quarters of men were hrHPV positive (74.7%) compared with one third of women (39.6%) (p < 0.001). A little over eighty percent of hrHPV positive men had a history of sex with other men (MSM) compared with 19% of hrHPV negative men (p < 0.001). The lifetime number of sexual partners was no different for hrHPV positive and hrHPV negative men (p = 0.85) but it was very different for men and women (p < 0.001) with many more men reporting more than fifty sexual partners. Smoking status showed little difference between men and women or between hrHPV groups. Men were significantly older than women (p = 0.004) but there was no significant difference in age between HIV negative men and women (p = 0.23).
None of the female patients was HIV positive. High-risk HPV status was significantly associated with HIV (p < 0.001). But their bivariate joint association was quite different for most other STIs: syphilis and HIV (p < 0.001) and hrHPV (p = 0.073); gonorrhoea and HIV (p = 0.001) and hrHPV (p = 0.119); chlamydia and HIV (p = 0.308) and hrHPV (p = 0.443); HBV and HIV (p < 0.001) and hrHPV (p = 0.036); HCV and HIV (p = 0.046) and hrHPV (p = 0.974). High-risk HPV infection was significantly less likely for perianal warts (p = 0.035).
3.2. Association of HSIL with HPV and HIV
Histopathology showed that 30% of samples were HSIL (Table 2). Two cases of anal cancer were detected.
There was a statistically significant association between histopathology grade and hrHPV status (Table 2) with an OR of 4.01 for increasing grade (95% CI: 2.60–6.19, p < 0.001) and OR of 6.55 for HSIL versus LSIL (95% CI: 3.39–12.6, p < 0.001).
3.3. Association of infectious agents
Most of the STIs showed associations with each other (Table 3) and a principle components analysis indicated that there were three separate components: (1) HPV/HIV/HSV 2/HBV; (2) HCV/syphilis; (3) gonorrhoea/chlamydia (Table 4). The test for missing at random indicated that the results were not influenced by missing values.
Table 3.
Tetrachoric correlation matrix for STIs.
| Statistic | HPV | HIV | HSV 2 | HBV | HCV | Syphilis | GC | CHL | |
|---|---|---|---|---|---|---|---|---|---|
| HPV | rho | 1 | |||||||
| n | 460 | ||||||||
| HIV | rho | 0.6102 | 1 | ||||||
| n | 455 | 640 | |||||||
| p | < 0.0001 | ||||||||
| HSV 2 | rho | 0.2014 | 0.4406 | 1 | |||||
| n | 459 | 633 | 639 | ||||||
| p | 0.0419 | < 0.0001 | |||||||
| HBV | rho | 0.5133 | 0.548 | 0.4619 | 1 | ||||
| n | 446 | 617 | 611 | 619 | |||||
| p | 0.0012 | < 0.0001 | < 0.0001 | ||||||
| HCV | rho | − 0.1371 | 0.3219 | 0.1635 | 0.6301 | 1 | |||
| n | 441 | 576 | 572 | 563 | 576 | ||||
| p | 0.3242 | 0.0031 | 0.1575 | < 0.0001 | |||||
| Syphilis | rho | 0.3128 | 0.441 | 0.4311 | 0.2382 | − 1 | 1 | ||
| n | 460 | 637 | 636 | 616 | 573 | 644 | |||
| p | 0.0339 | < 0.0001 | < 0.0001 | 0.0522 | 0.2460 | ||||
| GC | rho | 0.266 | 0.3835 | 0.1853 | 0.2667 | 0.2171 | 0.2404 | 1 | |
| n | 458 | 638 | 637 | 617 | 574 | 642 | 645 | ||
| p | 0.0123 | < 0.0001 | 0.0320 | 0.0140 | 0.0642 | 0.0276 | |||
| CHL | rho | 0.1297 | 0.1292 | 0.0807 | 0.0397 | − 0.085 | 0.4854 | 0.4902 | 1 |
| n | 458 | 638 | 637 | 617 | 574 | 642 | 645 | 645 | |
| p | 0.2392 | 0.1236 | 0.3802 | 0.6588 | 0.7843 | < 0.0001 | < 0.0001 |
rho: the value for the tetrachoric correlation coefficient.
n signifies the number of samples.
p: the p value for the test that rho = 0.
Table 4.
Tetrachoric principal components for STIs.
| Variable | Component A | Component B | Component C |
|---|---|---|---|
| HPV | 0.445 | ||
| HIV | 0.515 | ||
| HSV 2 | 0.437 | ||
| HBV | 0.518 | ||
| HCV | 0.702 | ||
| Syphilis | 0.630 | ||
| GC | 0.668 | ||
| CHL | 0.723 |
3.4. Association of HSIL with hrHPV, HIV and other infectious agents
An analysis of the joint effect of high-risk HPV (hrHPV) and HIV showed that HIV alone was not significantly associated with HSIL (OR = 1.58 (95% CI: 0.17–14.5), p = 0.68), whereas hrHPV infection was significantly associated with HSIL (OR = 4.65 (95% CI: 2.28–9.48), p < 0.001). A significant interaction was found when HIV infection occurred with hrHPV infection (OR = 11.1, 95% CI: 5.30–23.2, p < 0.001). These estimates were only slightly reduced when adjusted for lifetime number of sexual partners: HIV adjusted OR = 2.03 (95% CI: 0.20–20.4), p = 0.55; hrHPV adjusted OR = 4.36 (95% CI: 2.10–9.05), p < 0.001; hrHPV plus HIV; adjusted OR = 10.9 (95% CI: 5.03–23.7), p < 0.001. No other STIs were alone associated with HSIL but many showed some modification of the risk associated with hrHPV with or without HIV (Table 5). A history of syphilis in the presence of hrHPV and HIV was associated with an increased risk of HSIL (OR = 18.6 (95% CI: 5.67–61.0), p < 0.001), and also in the absence of HIV (OR = 5.58 (95% CI: 1.33–23.4), p = 0.019). A similar risk of HSIL in HIV negative patients was seen for HSV 2 (OR = 7.85 (95%CI: 2.84–21.7)), p < 0.001; gonorrhoea (OR = 6.45 (95% CI: 2.32–17.9)); a history of chlamydia (OR = 4.80 (95% CI: 1.78–13.03), p = 0.002).
Table 5.
Association between HSIL and hrHPV, HIV and other STIs.
| Other infection | Other infection only | HR-HPV only | HIV only | HR-HPV + HIV | HR-HPV + other | HIV + other | HR-HPV + HIV + other | |
|---|---|---|---|---|---|---|---|---|
| HSV 2 | OR | NA | 4.99 | NA | 14.3 | 7.85 | NA | 9.89 |
| 95% CI | 2.27–11.0 | 6.20–33.1 | 2.84–21.7 | 3.85–25.4 | ||||
| p | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||||
| n = 447 | n | 11 | 178 | 3 | 88 | 32 | 4 | 42 |
| Syphilis | OR | NA | 4.51 | NA | 9.83 | 5.58 | NA | 18.6 |
| 95% CI | 2.03–10.1 | 4.20–23.0 | 1.33–23.4 | 5.67–61.0 | ||||
| p | < 0.001 | < 0.001 | 0.019 | < 0.001 | ||||
| n = 358 | n | 3 | 203 | 7 | 109 | 8 | 0 | 21 |
| Gonorrhoea | OR | NA | 3.88 | NA | 10.4 | 6.45 | NA | 9.11 |
| 95% CI | 1.71–8.83 | 4.32–25.0 | 2.32–17.9 | 3.40–24.4 | ||||
| p | 0.001 | < 0.001 | < 0.001 | < 0.001 | ||||
| n = 355 | n | 9 | 181 | 6 | 95 | 28 | 1 | 35 |
| Chlamydia | OR | NA | 3.95 | NA | 10.3 | 4.80 | NA | 7.70 |
| 95% CI | 1.73–9.02 | 4.39–24.3 | 1.78–13.0 | 2.52–23.5 | ||||
| p | < 0.001 | < 0.001 | 0.002 | < 0.001 | ||||
| n = 358 | n | 12 | 175 | 5 | 107 | 34 | 2 | 23 |
| HBV | OR | NA | 8.25 | NA | 27.7 | NA | NA | 18.2 |
| 95% CI | 2.90–23.5 | 7.80–98.3 | 3.69–89.9 | |||||
| p | < 0.001 | < 0.001 | < 0.001 | |||||
| n = 441 | n | 1 | 203 | 7 | 98 | 6 | 7 | 25 |
| HCV | OR | NA | 5.66 | NA | 16.3 | NA | NA | |
| 95% CI | 2.10–15.2 | 5.15–51.9 | ||||||
| p | 0.001 | < 0.001 | ||||||
| n = 431 | n | 8 | 204 | 7 | 115 | 3 | 0 | 13 |
NA: indicates that there were insufficient cases to permit estimation.
n: the number of samples for whom there was complete data.
We examined the possibility that the interaction between HPV and HIV might be a result of HIV-related immune suppression by examination of nadir and current (at the time of the operation) CD4+ T cell counts. The nadir count showed a negative significant association with HSIL (p = 0.027) whereas there was no significant association between HSIL and current CD4+ T cell counts (p = 0.73).
3.5. Control of effect modification and confounding factors
A variety of potential confounding factors or effect modifiers were examined for an association with HSIL (Table 6). Statistically significant associations were seen for increasing age (p = 0.011), male gender (p = 0.019), more than 50 lifetime number of sexual partners (p = 0.004), MSM (p < 0.001); site of surgery/biopsy (p < 0.001), and increasing number of operations (p < 0.001). Smoking history was not associated with HSIL.
Table 6.
Univariable analysis of HSIL in patients with warts – logistic regression analysis of potential covariates.
| Variable | Category | Odds ratio |
95% confidence interval |
p | |
|---|---|---|---|---|---|
| LCL | UCL | ||||
| Age | 1.018 | 1.004 | 1.033 | 0.011 | |
| Gender | Female | 1.00 (reference) | |||
| Male | 2.86 | 1.18 | 6.92 | 0.019 | |
| Lifetime number of sexual partners | 0–9 | 1.00 (reference) | |||
| More than 10 | 1.53 | 0.96 | 2.44 | 0.073 | |
| More than 50 | 1.98 | 1.25 | 3.15 | 0.004 | |
| Site of surgery/sample | Peri-anal only | 1.00 (reference) | |||
| Anal or anal & perianal | 3.20 | 1.88 | 5.43 | < 0.001 | |
| Increasing number of operations | 1.40 | 1.17 | 1.67 | < 0.001 | |
| Smoking status | Never smoked | 1.00 (reference) | |||
| Current smoker | 1.12 | 0.79 | 1.60 | 0.52 | |
| Ex-smoker | 1.14 | 0.63 | 2.08 | 0.66 | |
3.6. Estimation of the relative influence of each STI on the risk of HSIL
An investigation of the relative influence of the STIs on the risk of HSIL was conducted using boosted regression analysis adjusted for effect modification. This type of analysis can be conducted for a set of independent variables that are associated with each other, i.e. collinear. The results confirm the primary importance of hrHPV and the secondary influence of HIV. Additionally it shows that the remaining STIs are very similar to each other and clearly influential, all at between 7% and 10% (Table 7).
Table 7.
Results of boosted logistic regression analysis on odds ratio for HSIL.
| Influence of each variable (%) | |
|---|---|
| HPV | 35.8 |
| HIV | 13.9 |
| HSV 2 | 9.37 |
| Gonorrhoea | 9.06 |
| CHL | 8.47 |
| Syphilis | 8.45 |
| HBV | 7.93 |
| HCV | 7.00 |
A summarising analysis of HSIL risk using a generalised structural equation modelling (SEM) approach was conducted with all of the STIs and potential cofactors included as independent variables. The results are shown in Table 8. This analysis is based upon maximum likelihood estimation and so it can accommodate missing data and use all available observations. The analysis incorporates the cofactors as well as the STIs and it shows that the latent (i.e. unobserved) Cofactors variable comprised of: age, MSM status, male gender and lifetime number of sexual partners is associated with the latent STI variable.
Table 8.
Structural equation model (SEM) for HSIL.
| Variable | Contrast | OR | LCL95% | UCL95% | p | n |
|---|---|---|---|---|---|---|
| hrHPV | Yes vs. No | 1 | Reference | 460 | ||
| HIV | Yes vs. No | 5.31 | 2.00 | 14.1 | 0.001 | 640 |
| HSV 2 | Yes vs. No | 1.84 | 1.21 | 2.79 | 0.004 | 639 |
| Syphilis | Yes vs. No | 2.57 | 1.32 | 5.00 | 0.006 | 644 |
| Gonorrhoea | Yes vs. No | 2.11 | 1.33 | 3.36 | 0.002 | 645 |
| Chlamydia | Yes vs. No | 1.48 | 1.06 | 2.06 | 0.022 | 645 |
| HBV | Yes vs. No | 3.11 | 1.33 | 7.29 | 0.009 | 619 |
| HCV | Yes vs. No | 1.56 | 0.688 | 3.52 | 0.289 | 576 |
| Age | Standardised | 2.86 | 0.323 | 5.40 | 0.027 | 648 |
| Male gender | Yes vs. No | 4.66 | 2.12 | 10.3 | < 0.001 | 648 |
| MSM | Yes vs. No | 4.65 | 1.54 | 14.1 | 0.003 | 536 |
| Sex partners | < 50 | 1 | Reference | 533 | ||
| 50+ | 3.04 | 2.05 | 4.51 | < 0.001 | ||
| Cofactors→STI | Latent | 2.43 | 1.48 | 4.00 | < 0.001 | |
| STI→HSIL | Yes vs. No | 2.77 | 2.10 | 3.64 | < 0.001 | 644 |
4. Discussion
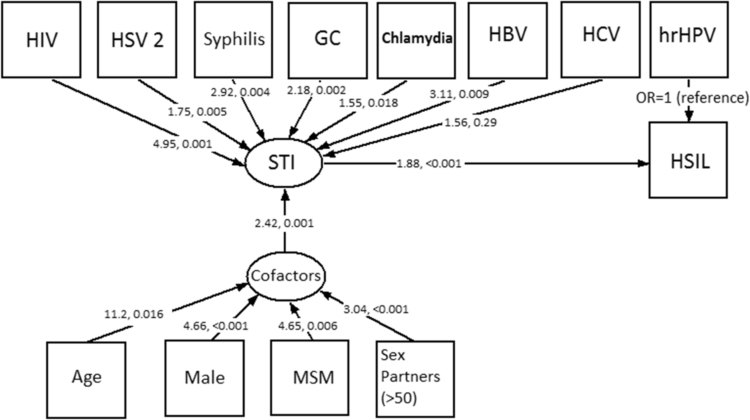
The summarising results of the study are illustrated in the path diagram (Fig. 1) which shows a hypothetical pattern of relationships of the variables which were included in the structural equation model of HSIL within this population. A strength of a SEM model is that observed variables which have been identified in the analysis can be combined into a representation of the underlying syndemic [56]. In our model we show the observed variables in boxes which are associated with underlying but unobservable latent variables (ovals). We represent the outcome HSIL as a direct consequence of hrHPV infection because it is a necessary and sufficient cause of HSIL with an adjusted odds ratio of 4.99. (This is the reference value for the other infections and cofactors.) We show other co-existing STIs which are amplifying the risk of HSIL, with different degree shown by the odds ratio adjusted for cofactors and for hrHPV: HIV OR = 5.31; HSV 2 OR = 1.84; Syphilis OR = 2.57; Gonorrhoea OR = 2.11; Chlamydia OR = 1.48; HBV OR = 3.11; HCV OR = 1.83. These act together with behavioural and demographic co-factors which our results suggest act as effect-modifiers for a further additional contribution to HSIL risk (see Table 8).
Fig. 1.
Path diagram showing the relationships between STIs, demographics and HSIL.
Squares indicate observed variables and ovals indicate unobserved latent variables. Arrows show hypothetical relationships indicated by this study. Values show the excess odds ratio above the hrHPV effect alone and p-values for each association indicated by an arrow.
Our analysis demonstrated that hrHPV alone was a sufficient cause of HSIL (OR = 4.99, 2.27–11.0, p < 0.001), but HIV alone was not, and the combination of HIV and hrHPV in the presence of warts substantially amplified the risk of HSIL (OR = 34.8, 95% CI: 12.3–98.5, p < 0.001). It should be noted that there were few patients that were HIV positive and hrHPV negative (n = 6). The rarity of such patients provides circumstantial support for the syndemic concept. How HIV and hrHPV interact at a cellular level is not clearly understood. One hypothesis is that advancing HIV immunosuppression allows reactivation of and increased replication of oncogenic HPV with subsequent epithelial abnormality development [44]. This hypothesis is supported by our observation of a significant association between HSIL and nadir CD4+ T cell counts in the HIV positive patients. Several studies have demonstrated that nadir CD4+ T cell counts are a correlate of residual immunodeficiency in HIV patients effectively treated with ART [57], [58].
The risk of HSIL was increased in both HIV-positive and HIV-negative individuals with a history of gonorrhoea in our study. Gonorrhoea has been linked to an increased risk of anal cancer and hence it is not surprising we have found an association with HSIL. The fact we were only able to use a history of gonorrhoea further strengthens the observation as it is likely some patients may have poor recall and any resulting misclassification bias would reduce our risk estimation towards the null value [59]. Gonorrhoea causes an inflammatory extracellular infection. In HIV patients with acute gonococcal infection HIV virus can be detected in urethral STIs, and the viral load in semen is reduced by treatment [60]. Of the STIs observed gonorrhoea is associated with the highest concentrations of HIV detected in semen [60]. It is plausible to assume increased HIV levels also occur in anal gonococcal infection. It is not known whether gonorrhoea or HIV infection may increase the HPV viral load in the anal canal, thus accelerating the effects of HPV infection. Various mechanisms for the interaction of gonorrhoea and HIV have been postulated, including increased local levels of HIV interacting with hrHPV. Gonococcal infection of the urinary tract has previously been demonstrated to increase the level of inflammatory cytokines and tumour necrosis factor [61] and this may be an alternative mechanism which could explain the observed findings in both HIV positive and negative men.
The finding of HSV 2 seropositivity in our study being associated with the risk of HSIL in the presence of warts is an interesting observation. HSV 2 is a known mutagen [62] and is recognised to cause micro-ulceration thus stimulating epithelial proliferation. Increased cell turnover is recognised to be a key factor for oncogenesis, with increased possibility for cell mutations and transformational events to occur within cells. HSV 2 has been linked to an increased risk of anal cancer among MSM, but not heterosexual men [63], and women [6]. In a study of archival tissue HSV 2 DNA was found in anal pre-cancer (3 of 4 specimens) and anal cancer specimens (5 of 15 specimens) and a role in disease progression has previously been proposed [13]. Very recent observations demonstrated that HSV 1 and 2 both down-regulate secretory leucocyte protease inhibitor (SPLI), the natural ligand for the HPV uptake receptor Annexin A2 S100A10 heterotetramer, and therefore HSV enables the uptake of HPV [64]. Low levels of SLPI are indeed associated with high-risk HPV in the development of AIN [65] and Head and Neck cancers [66], [67]. Interestingly SLPI is also known to block HIV in entering macrophages in the genital mucosa through the Annexin A2 S100A10 heterotetramer [68], [69]. The observed positive interaction of HPV with HIV and HSV 2 in the development of AIN fits mechanistically with the model that HSV 2 down-regulates the natural ligand of the HPV uptake receptor and one of the receptors HIV can use to infect cells in the genital mucosa. Therefore HSV 2 infection could amplify the effects of both HPV and HIV STIs and assist in the development of HSIL. Further studies on this intriguing topic are needed.
We did not find an association with current smoking and HSIL however other researchers have found an association with anal cancer [6], [19], [20], [63], [70], [71], [72]. As only two of our patients had anal cancer, to demonstrate an association of HSIL with cigarette smoking a longer duration of follow-up may be required.
Our research found an increased risk with both HBV infection and, to a lesser extent HCV. Similarly HIV and HCV co-infection have been found to be associated with increased progression of liver disease [73], so there may be some mechanism whereby hrHPV is also affected. It could also be postulated that with multiple viral STIs, the body is not able to effectively control them all at the same time, and viruses like hrHPV and HSV 2 could proliferate in these circumstances.
Daling et al., found women with a history of Chlamydia trachomatis infection had an elevated relative risk of anal cancer [6]. Conversely Chlamydia has been found more commonly amongst those without HPV DNA detection in a study by Kiviat [74]. More recent research has demonstrated recent or concurrent chlamydia was associated with hrHPV persistence in women [75], and may similarly occur in men, however this has not been demonstrated to date. As chlamydia is an intracellular infection the inflammatory reaction produced may also stimulate the immune system against hrHPV. Further studies to examine this hypothesis are required.
There is some research linking syphilis to cancers. A population based case-control study has found syphilis along with other factors was associated with an increased risk of anal cancer [76], and bladder cancer in another study [77]. A meta-analysis demonstrated an association between syphilis and gonorrhoea with prostatitis and prostate cancer [78]. Our data demonstrating an increased risk of HSIL with serological evidence of syphilis supports the role of syphilis in cancer development despite the mechanism not having been elucidated.
4.1. Study limitations
The limitations of our study are that despite data being collected prospectively at the time of the first patient operation either from the patient or from the clinical record, the evidence for gonorrhoea or chlamydia is based both on patient recall and clinical verification if possible and may be assessed with error. In addition many cases of anal gonorrhoea or chlamydia may go unnoticed because of a lack of symptoms or failure to present for testing. Additionally the infection site was not recorded; however as the majority of the patients were MSM it is reasonable to assume a significant proportion of the infection was rectal. The underestimation of both of these STIs is likely to reduce the risk of HSIL observed in this study. The possibility of misclassification of HSIL is exacerbated by the fact that none of the cases were confirmed by P16 staining because there were few cases which occurred after the publication of the LAST recommendations [53]. However misclassification bias is well understood to produce a bias towards the null hypothesis of no association [57]. As a result we may have underestimated the strength of the associations which we have found but we are most unlikely to have demonstrated spurious associations because of misclassification of HSIL (the outcome) or of HPV, HIV or other STIs (the exposures).
An additional limitation is that we do not know the time frames from when the STIs occurred or how many times an infection occurred. Hepatitis B virus infection status was based on the presence of HBV core antibodies and or surface antigen while HCV infection status was based on the presence of HCV antibodies (HCV RNA levels were not recorded), so some patients with HBV or HCV may have cleared their infection, thus diluting any observed association. An additional limitation for the estimation of risk associated with HBV and HCV is that there are relatively few patients with a history of these infections (32 HBV and 24 HCV).
We did not collect data on receptive anal intercourse which is a recognised risk factor for anal cancer [6]. We limited our data to a categorisation for men into heterosexual, bisexual, MSM. If it is valid to infer that receptive anal intercourse is very likely to occur for MSM patients the strong association provides an example of the co-integration of effects which is characteristic of a syndemic. An association with anal intercourse may be through transmission of an infective agent such as HPV, or alternatively some substance within semen [79]. Prostaglandins are found in high concentrations in semen and have been demonstrated to enhance tumour production, and further research remains to be done in this area [80].
4.2. Conclusions
The data demonstrates a complex series of syndemic [56] interactions with STIs, cofactors and anal warts to increase the risk of HSIL. Given the escalating epidemics of gonorrhoea and syphilis among MSM, and the recognised increased incidence of anal cancer there should be real concern about the syndemic interactions occurring within the MSM population to significantly further increase their risk of anal cancer. The data is interesting as each variable by itself did not produce an increased risk of HSIL except for hrHPV, but the more variables that were examined together the risk for HSIL increased especially for gonorrhoea, HSV 2, HBV and syphilis. Serology could be used to stratify men at increased risk of HSIL and used to triage patients for high resolution anoscopy. Conversely STI screening should be performed in anal cancer screening programs [81]. One of the strengths of our research is that all the patients had warts whereas in other studies if the patients did not have an anoscopy or genital examination that information would not be known.
The current use of PrEP (pre-exposure antiviral medication to prevent HIV infection) may have an unintended consequence of increasing anal cancer precursors and consequently anal cancer because of unsafe sex and increased STI transmission. Gonorrhoea and syphilis rates are increasing in MSM [82] and there are emerging serious concerns about the increasing levels of antimicrobial resistance, with the possibility of the infection being untreatable in the future [83]. In addition the increasing use of pre exposure antivirals to prevent HIV acquisition, whilst reducing the incidence of HIV has been reported in an meta-analysis to be associated with an increased incidence of gonorrhoea and syphilis [84]. Given the association we have found of gonorrhoea being associated with an increased risk of HSIL, we are concerned about the future risk of untreatable gonorrhoea further escalating the prevalence of HSIL and hence possibly further increasing the rates of anal cancer in men which is already perceived to be epidemic. Similarly warts are florid manifestations of increased cell turnover and may consequently accelerate HSIL because of increased cell turnover. The combination of multiple STIs appears to be one of the keys to unravelling the mysteries of cofactor interaction with hrHPV and is a complex issue. As developing gonorrhoea resistance is raising serious concerns [83] the combination of untreatable gonorrhoea and HSIL, possibly escalating the anal cancer epidemic in men poses a new problem.
If our results are confirmed in further studies there are public health policy implications that will need to be considered. These relate to programmes to encourage safe sexual behaviour and vaccination to prevent hrHPV especially in men.
Funding
W. Martin Kast holds the Walter A. Richter Cancer Research Chair. His HPV/HSV research is supported by National Institutes of Health grant number 2 R01 CA74397-17.
Statistical support for this work was provided by the Royal Perth Hospital Medical Research Foundation. (www.rphmrf.org.au)
Conflicts of interest
None.
Contributor Information
Jenny C. McCloskey, Email: jenny.mccloskey@health.wa.gov.au.
W. Martin Kast, Email: Martin.Kast@med.usc.edu.
James P. Flexman, Email: james.flexman@health.wa.gov.au.
Dugald McCallum, Email: dugald.mccallum@health.wa.gov.au.
Martyn A. French, Email: martyn.french@uwa.edu.au.
Michael Phillips, Email: michael.phillips@perkins.uwa.edu.au.
References
- 1.McCloskey J., Metcalf C., French M., Flexman J., Burke V., Beilin L. The frequency of high-grade intraepithelial neoplasia in anal/perianal warts is higher than previously recognised. Int. J. STD AIDS. 2007;18:538–542. doi: 10.1258/095646207781439694. [DOI] [PubMed] [Google Scholar]
- 2.McCloskey J., Phillips M., French M., Flexman J., McCallum D., Metcalf C. Update on the Royal Perth Hospital Anogenital Wart Database. In: Barros E., editor. HIV Infection- Impact Awareness and Social Implications of Living with HIV/AIDS. In Tech; 2011. pp. 81–90. [Google Scholar]
- 3.Patel P., Hanson D.L., Sullivan P.S., Novak R.M., Moorman A.C., Tong T.C. Incidence of types of cancer among HIV-Infected persons compared with the general population in the United States, 1992–2003. Ann. Intern. Med. 2008;148:728. doi: 10.7326/0003-4819-148-10-200805200-00005. (U29) [DOI] [PubMed] [Google Scholar]
- 4.Palefsky J., Holly E., Efirdc J., Da Costa M., Jay N., Berry J. Anal intraepithelial neoplasia in the highly active antiretroviral therapy era among HIV-positive men who have sex with men. AIDS. 2005;19:1407–1414. doi: 10.1097/01.aids.0000181012.62385.4a. [DOI] [PubMed] [Google Scholar]
- 5.de Pokomandy A., Rouleau D., Ghattas G., Trottier H., Vezina S., Cote P. HAART and progression to high-grade anal intraepithelial neoplasia in men who have sex with men and are infected with HIV. Clin. Infect. Dis. 2011;52:1174–1181. doi: 10.1093/cid/cir064. [DOI] [PubMed] [Google Scholar]
- 6.Daling J., Weiss N., Hislop G., Maden C., Coates R., Sherman K. Sexual practices, sexually transmitted diseases, and the incidence of anal cancer. N. Engl. J. Med. 1987;317:973–977. doi: 10.1056/NEJM198710153171601. [DOI] [PubMed] [Google Scholar]
- 7.Palefsky J.M., Holly E.A., Hogeboom C.J., Ralston M.L., DaCosta M.M., Botts R. Virologic, immunologic, and clinical parameters in the incidence and progression of anal squamous intraepithelial lesions in HIV-positive and HIV-negative homosexual men. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1998;17:314–319. doi: 10.1097/00042560-199804010-00004. [DOI] [PubMed] [Google Scholar]
- 8.Holly E., Whittemore A., Aston D., Ahn D., Nickoloff B., Kristiansen J. Anal cancer incidence: genital warts, anal fissure or fistula, hemorrhoids, and smoking. J. Natl. Cancer Inst. 1989;81:1726–1731. doi: 10.1093/jnci/81.22.1726. [DOI] [PubMed] [Google Scholar]
- 9.Hare M.J., Taylor‐Robinson D., Cooper P., Hare M.J., Taylor‐Robinson D., Cooper P. Evidence for an association between Chlamydia trachomatis and cervical intraepithelial neoplasia. BJOG: Int. J. Obstet. Gynaecol. 1982;89:489–492. doi: 10.1111/j.1471-0528.1982.tb03643.x. [DOI] [PubMed] [Google Scholar]
- 10.Jensen K.E., Thomsen L.T., Schmiedel S., Frederiksen K., Norrild B., van den Brule A. Chlamydia trachomatis and risk of cervical intraepithelial neoplasia grade 3 or worse in women with persistent human papillomavirus infection: a cohort study. Sex. Transm. Infect. 2014;90:550–555. doi: 10.1136/sextrans-2013-051431. [DOI] [PubMed] [Google Scholar]
- 11.Alemany L., Saunier M., Alvarado I., Quirós B., Salmeron J., Shin H.R. HPV DNA prevalence and type distribution in anal carcinomas worldwide. Int. J. Cancer. 2015:98–107. doi: 10.1002/ijc.28963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai A.C., Mendenhall E., Trostle J.A., Kawachi I. Co-occurring epidemics, syndemics, and population health. Lancet. 2017;389:978–982. doi: 10.1016/S0140-6736(17)30403-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palefsky J.M., Holly E.A., Gonzales J., Berline J., Ahn D.K., Greenspan J.S. Detection of human papillomavirus dna in anal intraepithelial neoplasia and anal cancer. Cancer Res. 1991;51:1014–1019. [PubMed] [Google Scholar]
- 14.Bjorge T., Engeland A., Luostarinen T., Mork J., Gislefoss R.E., Jellum E. Human papillomavirus infection as a risk factor for anal and perianal skin cancer in a prospective study. Br. J. Cancer. 2002;87:61–64. doi: 10.1038/sj.bjc.6600350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durante A.J., Williams A.B., Da Costa M., Darragh T.M., Khoshnood K., Palefsky J.M. Incidence of anal cytological abnormalities in a cohort of human immunodeficiency virus-infected women. Cancer Epidem. Biomark. 2003;12:638–642. [PubMed] [Google Scholar]
- 16.Holly E.A., Ralston M.L., Darragh T.M., Greenblatt R.M., Jay N., Palefsky J.M. Prevalence and risk factors for anal squamous intraepithelial lesions in women. J. Natl. Cancer Inst. 2001;93:843–849. doi: 10.1093/jnci/93.11.843. [DOI] [PubMed] [Google Scholar]
- 17.Frisch M., Glimelius B., Van den Brule J., Wohlfahrt J., Meijer C., Walboomers J. Sexually transmitted infection as a cause of anal cancer. N. Engl. J. Med. 1997;337:1350–1358. doi: 10.1056/NEJM199711063371904. [DOI] [PubMed] [Google Scholar]
- 18.Melbye M., Rabkin C., Frisch M., Biggar R. Changing patterns of anal cancer incidence in the United States, 1940–1989. Am. J. Epidemiol. 1994;139:772–780. doi: 10.1093/oxfordjournals.aje.a117073. [DOI] [PubMed] [Google Scholar]
- 19.Daling J.R., Sherman K.J., Hislop T.G., Maden C., Mandelson M.T., Beckman A.M. Cigarette smoking and the risk of anogenital cancer. Am. J. Epidemiol. 1992;135:180–189. doi: 10.1093/oxfordjournals.aje.a116270. [DOI] [PubMed] [Google Scholar]
- 20.Frisch M., Glimelius B., Wohlfahrt J., Adami H.O., Melbye M. Tobacco smoking as a risk factor in anal carcinoma: an antiestrogenic mechanism? J. Natl. Cancer Inst. 1999;91:708–715. doi: 10.1093/jnci/91.8.708. [DOI] [PubMed] [Google Scholar]
- 21.Frisch M., Smith E., Grulich A., Johansen C. Cancer in a population-based cohort of men and women in registered homosexual partnerships. Am. J. Epidemiol. 2003;157:966–972. doi: 10.1093/aje/kwg067. [DOI] [PubMed] [Google Scholar]
- 22.Palefsky J.M., Holly E.A., Ralston M.L., Jay N., Berry J.M., Darragh T.M. High incidence of anal high-grade squamous intra-epithelial lesions among HIV-positive and HIV-negative homosexual and bisexual men. AIDS. 1998;12:495–503. doi: 10.1097/00002030-199805000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Grulich A.E., Li Y.M., McDonald A., Correll P.K.L., Law M.G., Kaldor J.M. Rates of non-AIDS-defining infection before and cancers in people with HIV after AIDS diagnosis. AIDS. 2002;16:1155–1161. doi: 10.1097/00002030-200205240-00009. [DOI] [PubMed] [Google Scholar]
- 24.Stensen S., Kjaer S., Jensen S., Frederiksen K., Junge J., Iftner T. Factors associated with type-specific persistence of high-risk human papillomavirus infection: a population based study. Int. J. Cancer. 2016;138:361–368. doi: 10.1002/ijc.29719. [DOI] [PubMed] [Google Scholar]
- 25.Abbasakoor F., Boulos P.B. Anal intraepithelial neoplasia. Br. J. Surg. 2005;92:277–290. doi: 10.1002/bjs.4967. [DOI] [PubMed] [Google Scholar]
- 26.Palefsky J., Holly E., Ralston M., Da Costa M., Greenblatt R. Prevalence and risk factors for anal human papillomavirus infection in human immunodeficiency virus (HIV)-positive and high-risk HIV-negative women. J. Infect. Dis. 2001;183:383–391. doi: 10.1086/318071. [DOI] [PubMed] [Google Scholar]
- 27.Frisch M., Glimelius B., van den Brule A.J.C., Wohlfahrt J., Meijer C., Walboomers J.M.M. Benign anal lesions, inflammatory bowel disease and risk for high-risk human papillomavirus-positive and -negative anal carcinoma. Br. J. Cancer. 1998;78:1534–1538. doi: 10.1038/bjc.1998.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodman P., Halpert R.D. Invasive squamous-cell carcinoma of the anus arising in condyloma acuminatum - ct demonstration. Gastrointest. Radiol. 1991;16:267–270. doi: 10.1007/BF01887363. [DOI] [PubMed] [Google Scholar]
- 29.Lee S., McGregor D., Kuziez M. Malignant transformation of perianal condyloma acuminatum: a case report and review of the literature. Dis. Colon Rectum. 1981;24:462–467. doi: 10.1007/BF02626784. [DOI] [PubMed] [Google Scholar]
- 30.Siegel A. Malignant transformation of condyloma acuminatum - review of the literature and report of a case. Am. J. Surg. 1962;103:613–617. doi: 10.1016/0002-9610(62)90531-7. [DOI] [PubMed] [Google Scholar]
- 31.Ejeckam G.C., Idikio H.A., Nayak V., Gardiner J.P. Malignant transformation in an anal condyloma acuminatum. Can. J. Surg. 1983;26:170–173. [PubMed] [Google Scholar]
- 32.Gillat D., Teasdale C. Squamous cell carcinoma of the anus arising within condyloma acuminatum. Eur. J. Surg. Oncol. 1985;11:369–371. [PubMed] [Google Scholar]
- 33.Sturm J., Christenson C., Uecker J., Jr P.J. Squamous cell carcinoma of the anus arising in a giant condyloma acuminatum: a report of a case. Dis. Colon Rectum. 1975;18:147–151. doi: 10.1007/BF02587163. [DOI] [PubMed] [Google Scholar]
- 34.Chin-Hong P., Vittinghoff E., Cranston R., Browne L., Buchbinder S., Colfax G. Age-related prevalence of anal cancer precursors in homosexual men: the EXPLORE Study. J. Natl. Cancer Inst. 2005;97:896–905. doi: 10.1093/jnci/dji163. [DOI] [PubMed] [Google Scholar]
- 35.Johnson A.M., Mercer C.H., Erens B., Copas A.J., McManus S., Wellings K. Sexual behaviour in Britain: partnerships, practices, and HIV risk behaviours. Lancet. 2001;358:1835–1842. doi: 10.1016/S0140-6736(01)06883-0. [DOI] [PubMed] [Google Scholar]
- 36.Johnson L., Madeleine M., Newcomer L., Schwartz S., Daling J. Anal cancer incidence and survival: the surveillance, epidemiology, and end results experience, 1973–2000. Cancer. 2004;101:281–288. doi: 10.1002/cncr.20364. [DOI] [PubMed] [Google Scholar]
- 37.Ekstrand M.L., Stall R.D., Paul J.P., Osmond D.H., Coates T.J. Gay men report high rates of unprotected anal sex with partners of unknown or discordant HIV status. AIDS. 1999;13:1525–1533. doi: 10.1097/00002030-199908200-00013. [DOI] [PubMed] [Google Scholar]
- 38.Baggaley R.F., White R.G., Boily M.-C. HIV transmission risk through anal intercourse: systematic review, meta-analysis and implications for HIV prevention. Int. J. Epidemiol. 2010;39:1048–1063. doi: 10.1093/ije/dyq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Pokomandy A., Rouleau D., Ghattas G., Vezina S., Cote P., Macleod J. Prevalence, clearance, and incidence of anal human papillomavirus infection in HIV-infected men: the HIPVIRG cohort study. J. Infect. Dis. 2009;199:965–973. doi: 10.1086/597207. [DOI] [PubMed] [Google Scholar]
- 40.Machalek D.A., Poynten M., Jin F.Y., Fairley C.K., Farnsworth A., Garland S.M. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol. 2012;13:487–500. doi: 10.1016/S1470-2045(12)70080-3. [DOI] [PubMed] [Google Scholar]
- 41.Palefsky J., Holly E., Ralson M., Jay N. Prevalence and risk factors for human papillomavirus infection of the anal canal in human immunodeficiency virus HIV-positive and HIV-negative homosexual men. J. Infect. Dis. 1998;177:361–367. doi: 10.1086/514194. [DOI] [PubMed] [Google Scholar]
- 42.Goedert J., Coté T., Virgo P., Scoppa S., Kingma D., Gail M. Spectrum of AIDS-associated malignant disorders. Lancet. 1998;351:1833–1839. doi: 10.1016/s0140-6736(97)09028-4. [DOI] [PubMed] [Google Scholar]
- 43.Kiviat N.B., Critchlow C.W., Holmes K.K., Kuypers J., Sayer J., Dunphy C. Association of anal dysplasia and human papillomavirus with immunosuppression and HIV-infection among homosexual men. AIDS. 1993;7:43–49. doi: 10.1097/00002030-199301000-00007. [DOI] [PubMed] [Google Scholar]
- 44.Caussy D., Goedert J.J., Palefsky J., Gonzales J., Rabkin C.S., Digioia R.A. Interaction of human immunodeficiency and papilloma viruses - association with anal epithelial abnormality in homosexual men. Int. J. Cancer. 1990;46:214–219. doi: 10.1002/ijc.2910460212. [DOI] [PubMed] [Google Scholar]
- 45.Critchlow C.W., Holmes K.K., Wood R., Krueger L., Dunphy C., Vernon D.A. Association of human-immunodeficiency-virus and anal human papillomavirus infection among homosexual men. Arch. Intern. Med. 1992;152:1673–1676. [PubMed] [Google Scholar]
- 46.Palefsky J.M., Shiboski S., Moss A. Risk-factors for anal human papillomavirus infection and anal cytologic abnormalities in HIV-positive and HIV-negative homosexual men. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1994;7:599–606. [PubMed] [Google Scholar]
- 47.Palefsky J.M., Holly E.A., Ralston M.L., Arthur S.P., Jay N., Berry J.M. Anal squamous intraepithelial lesions in HIV-positive and HIV-negative homosexual and bisexual men - prevalence and risk factors. J. Acqir. Immune Defic. Syndr. Hum. Retrovirol. 1998;17:320–326. doi: 10.1097/00042560-199804010-00005. [DOI] [PubMed] [Google Scholar]
- 48.Engels E., R.J.B., Hall H. Cancer risk in people infected with human immunodeficiency virus in the United States. Int. J. Cancer. 2008;123:187–194. doi: 10.1002/ijc.23487. [DOI] [PubMed] [Google Scholar]
- 49.Heard I., Schmitz V., Costagliola D., Orth G., Kazatchkine M.D. Early regression of cervical lesions in HIV-seropositive women receiving highly active antiretroviral therapy. AIDS. 1998;12:1459–1464. doi: 10.1097/00002030-199812000-00007. [DOI] [PubMed] [Google Scholar]
- 50.Minkoff H., Ahdieh L., Massad L.S., Anastos K., Watts D.H., Melnick S. The effect of highly active antiretroviral therapy on cervical cytologic changes associated with oncogenic HPV among HIV-infected women. AIDS. 2001;15:2157–2164. doi: 10.1097/00002030-200111090-00011. [DOI] [PubMed] [Google Scholar]
- 51.Libois A., Feoli F., Nkuize M., Delforge M., Konopnicki D., Clumeck N. Prolonged antiretroviral therapy is associated with fewer anal high-grade squamous intraepithelial lesions in HIV-positive MSM in a cross-sectional study. Sex. Transm. Infect. 2017;93:15–17. doi: 10.1136/sextrans-2015-052444. [DOI] [PubMed] [Google Scholar]
- 52.Bloomberg M., Friss S., Munk C., Bautz A., Kjaer S. Genital warts and risk of cancer: danish study of nearly 50,000 patients with genital warts. JID. 2012;205:1544–1553. doi: 10.1093/infdis/jis228. [DOI] [PubMed] [Google Scholar]
- 53.Darragh T., Colgan T., Cox T., Heller D., Henry M., Luff R. The lower anogenital squamous terminology standardization project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Int. J. Gynecol. Pathol. 2013;32:76–115. doi: 10.1097/PGP.0b013e31826916c7. [DOI] [PubMed] [Google Scholar]
- 54.Friedman J., Hastie T., Tibshirani R. Additive logistic regression: a statistical view of boosting. Ann. Stat. 2000;28:337–407. [Google Scholar]
- 55.Little R. A test of missing completely at random for multivariate data with missing values. J. Am. Stat. Assoc. 1988;83:1198–1202. [Google Scholar]
- 56.Singer M.A., Bulled N.C., Ostrach B.D., Mendenhall E. Syndemics and the biosocial conception of health. Lancet. 2017;389:941–950. doi: 10.1016/S0140-6736(17)30003-X. [DOI] [PubMed] [Google Scholar]
- 57.Price P.F.S., Tan D.B., James I.R., Keane N.M., French M.A. Nadir CD4 T-cell counts continue to influence interferon-gamma responses in HIV patients who began antiretroviral treatment with advanced immunodeficiency. J. Acquir. Immune Defic. Syndr. 2008;49:462–464. doi: 10.1097/QAI.0b013e31817e637e. [DOI] [PubMed] [Google Scholar]
- 58.Siddique M.A.H.K., Dragileva E., Dondero M., Gebretsadik T., Shintani A., Peiperl L., Valentine F., Kalams S.A. Low CD4+ T cell nadir is an independent predictor of lower HIV-specific immune responses in chronically HIV-1-infected subjects receiving highly active antiretroviral therapy. J. Infect. Dis. 2006;194:661–665. doi: 10.1086/505913. [DOI] [PubMed] [Google Scholar]
- 59.Rothman K.J., Greenland S. Lash, editors. Modern Epidemiology. Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 60.Cohen M.S., Hoffman I.F., Royce R.A., Kazembe P., Dyer J.R., Daly C.C. Reduction of concentration of HIV-1, in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. Lancet. 1997;349:1868–1873. doi: 10.1016/s0140-6736(97)02190-9. [DOI] [PubMed] [Google Scholar]
- 61.Ramsey K.H., Schneider H., Cross A.S., Boslego J.W., Hoover D.L., Staley T.L. Inflammatory cytokines produced in response to experimental human gonorrhea. J. Infect. Dis. 1995;172:186–191. doi: 10.1093/infdis/172.1.186. [DOI] [PubMed] [Google Scholar]
- 62.Maitland N.J. The etiological relationship between herpes-simplex virus type-2 and carcinoma of the cervix - an unanswered or unanswerable question. Cancer Surv. 1988;7:457–467. [PubMed] [Google Scholar]
- 63.Daling J.R., Madeleine M., Johnson L., Schwartz S., Shera K., Wurscher M. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer. 2004;101:270–280. doi: 10.1002/cncr.20365. [DOI] [PubMed] [Google Scholar]
- 64.Skeate J.G., Porras T.B., Woodham A.W., Jang J.K., Taylor J.R., Brand H.E. HSV down-regulation of Secretory Leukocyte Protease Inhibitor enhances HPV16 infection of epithelial cells through the Annexin A2 heterotetramer. J. Gen. Virol. 2016;97:422–434. doi: 10.1099/jgv.0.000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nicol A.F., Brunette L.L., Nuovo G.J., Grinsztejn B., Friedman R.K., Veloso V.G. Secretory leukocyte protease inhibitor expression and high-risk HPV infection in anal lesions of HIV positive patients. J. Acquir. Immune Defic. Syndr. 2016;73:27–33. doi: 10.1097/QAI.0000000000001049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hoffmann M., Pfannenschmidt S., Hebebrand L., Görögh T., Halec G., Kahn T. The role of secretory leukocyte protease inhibitor in HPV infected head and neck cancer. Oncol. Rep. 2013;5:1962–1968. doi: 10.3892/or.2013.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cordes C., Hasler R., Werner C., Goroch T., Rocken C., Hebebrand L. The level of secretory leukocyte protease inhibitor is decreased in metastatic head and neck squamous cell carcinoma. Int. J. Oncol. 2011;39:185–191. doi: 10.3892/ijo.2011.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ma G., Greenwell-Wild T., Lei K. Secretory leukocyte protease inhibitor binds to annexin II, a cofactor for macrophage HIV-1 infection. J. Exp. Med. 2004;200:1337–1346. doi: 10.1084/jem.20041115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Woodham A.W., Skeate J.G., Sanna A.M., Taylor J.R., Da Silva D.M., Cannon P.M. HIV immune cell tropism and implications for treatment. AIDS Patient Care STDs. 2016;30:291–306. doi: 10.1089/apc.2016.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Daniell H.W. Causes of anal carcinoma. JAMA. 1985;254 358. [PubMed] [Google Scholar]
- 71.D'Souza G., Wiley D.J., Li X., Chmiel J.S., Margolick J.B., Cranston R.D. Incidence and epidemiology of anal cancer in the multicentre AIDS cohort study. J. Acquir. Immune Def. Syndr. 2008;48:491–499. doi: 10.1097/QAI.0b013e31817aebfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tseng H.F., Morgenstern H., Mack T.M., Peters R.K. Risk factors for anal cancer: results of a population-based case-control study. Cancer Causes Control. 2003;14:837–846. doi: 10.1023/b:caco.0000003837.10664.7f. [DOI] [PubMed] [Google Scholar]
- 73.Lee S., Watson M.W., Clark B., Flexman J.P., Cheng W., French M.A.H. Hepatitis C virus genotype and HIV coinfection affect cytokine mRNA levels in unstimulated PBMC but do not shift the T1/T2 balance. Immunol. Cell Biol. 2006;84:390–395. doi: 10.1111/j.1440-1711.2006.01451.x. [DOI] [PubMed] [Google Scholar]
- 74.Kiviat N., Rompalo A., Bowden R., Galloway D., Holmes K.K., Corey L. Anal human papillomavirus infection among human immunodeficiency virus-seropositive and virus-seronegative men. J. Infect. Dis. 1990;162:358–361. doi: 10.1093/infdis/162.2.358. [DOI] [PubMed] [Google Scholar]
- 75.Vielot N., Hudgens M., Mugo N., Chitwa M., Kimani J., Smith J. The role of Chlamydia trachomatis in high-risk human papillomavirus persistence among female sex workers in Nairobi, Kenya. Sex Transm. Dis. 2015;42:305–311. doi: 10.1097/OLQ.0000000000000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tseng H.-F., Morgenstern H., Mack T., Peters R. Risk factors for anal cancer: results of a population-based case-control study. Cancer Causes Control. 2003;14:837–846. doi: 10.1023/b:caco.0000003837.10664.7f. [DOI] [PubMed] [Google Scholar]
- 77.Mir M., Stephenson A., Grubb R., III, Black A., Kibel A., Izmirlian G. Predicting risk of bladder cancer using clinical and demographic information from prostate, lung, colorectal and ovarian cancer screening trial participants. Cancer Epidemiol. Biomark. Prev. 2013;22:2241–2249. doi: 10.1158/1055-9965.EPI-13-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dennis L.K., Lynch C.F., Torner J.C. Epidemiologic association between prostatitis and prostate cancer. Urology. 2002;60:78–83. doi: 10.1016/s0090-4295(02)01637-0. [DOI] [PubMed] [Google Scholar]
- 79.Kondlapoodi P. Anorectal cancer and homosexuality. JAMA. 1982;248:2114–2115. doi: 10.1001/jama.1982.03330170022015. [DOI] [PubMed] [Google Scholar]
- 80.Fischer S.M., Gleason G.L., Bohrman J.S., T.J.S. Prostaglandin enhancement of skin tumor initiation and promotion. Adv. Prostaglandin Thromboxane Res. 1980;6:517–522. [PubMed] [Google Scholar]
- 81.Fuchs W., Kreuter A., Hellmich M., Potthoff A., Swoboda J., Brockmeyer N. Asymptomatic anal sexually transmitted infections in HIV-positive men attending anal cancer screening. Br. J. Dermatol. 2015;174:831–838. doi: 10.1111/bjd.14288. [DOI] [PubMed] [Google Scholar]
- 82.Centers for Disease Control and Prevention . U.S. Department of Health and Human Services; Atlanta: 2016. Sexually Transmitted Disease Surveillance 2015. [Google Scholar]
- 83.Whiley D.M., Goire N., Lahra M.M., Donovan B., Limnios A.E., Nissen M.D. The ticking time bomb: escalating antibiotic resistance in Neisseria gonorrhoeae is a public health disaster in waiting. J. Antimicrob. Chemother. 2012;67:2059–2061. doi: 10.1093/jac/dks188. [DOI] [PubMed] [Google Scholar]
- 84.Kojima N., Joseph Davey D., Klausner J.D. Pre-exposure prophylaxis for HIV infection and new sexually transmitted infections among men who have sex with men. AIDS. 2016;30:2251–2252. doi: 10.1097/QAD.0000000000001185. [DOI] [PubMed] [Google Scholar]