Abstract
Persistent infection with human papillomavirus (HPV) is a key factor in the development of precancerous lesions and invasive cervical cancer. Prophylactic vaccines to immunize against HPV are an effective approach to reducing HPV related disease burden. In this study, we investigated the immunogenicity and dosage effect of a trivalent HPV 16/18/58 vaccine (3vHPV) produced in Escherichia coli (E.coli), with Gardasil quadrivalent vaccine (4vHPV, Merck & Co.) as a positive control. Sera collected from rhesus macaques vaccinated with three dosage formulations of 3vHPV (termed low-, mid-, and high-dosage formulations, respectively), and the 4vHPV vaccine were analyzed by both Pseudovirus-Based Neutralization Assay (PBNA) and Enzyme-Linked Immunosorbent Assay (ELISA). Strong immune responses against HPV 16/18/58 were successfully elicited, and dosage-dependence was observed, with likely occurrence of immune interference between different L1-VLP antigens. HPV 16/18 specific neutralizing antibody (nAb) and total immunoglobulin G (IgG) antibody responses in rhesus macaques receiving 3vHPV at the three dosages tested were generally non-inferior to those observed in rhesus macaques receiving 4vHPV throughout the study period. Particularly, HPV 18 nAb titers induced by the mid-dosage formulation that contained the same amounts of HPV 16/18 L1-VLPs as Gardasil 4vHPV were between 7.3 to 12.7-fold higher compared to the positive control arm from weeks 24–64. The durability of antibody responses specific to HPV 16/18 elicited by 3vHPV vaccines was also shown to be non-inferior to that associated with Gardasil 4vHPV.
Abbreviations: HPV, human papillomavirus; 3vHPV, trivalent HPV 16/18/58 vaccine; 4vHPV, Gardasil quadrivalent vaccine; PBNA, Pseudovirus-Based Neutralization Assay; ELISA, Enzyme-Linked Immunosorbent Assay; nAb, neutralizing antibody; AH, Aluminum Hydroxide; DMEM, Dulbecco's Modified Eagle's Medium; PBS, Phosphate-buffered saline; GFP, green fluorescent protein; GMT, Geometric Mean Titer; Ig, Immunoglobulin; HCP, Host cell protein
Keywords: Human papillomavirus, HPV 16/18/58, GMTs, Trivalent, Immunogenicity
1. Introduction
Cervical cancer has become a major public health concern worldwide, more than 90% cases of which are caused by human papillomavirus (HPV) infection. As the second most common gynecological malignancy throughout the world, cervical cancer is diagnosed among about half a million women annually, with over 50% of the cohort ending up dead [1]. Although Papanicolaou testing may be a well-established strategy for reducing mortality from cervical neoplasia, it is of no use to the prevention of HPV infection or development of precancerous lesions. It would be wise, however, to utilize a vaccine that is capable of blocking HPV infection to prevent the initiation of the malignant disease process.
HPVs are non-enveloped, epitheliotropic, and double-stranded circular DNA viruses. More than 170 different HPV types have been identified, which are divided into two groups: low-risk HPV types and high-risk HPV types [2], [3]. While the high-risk types, for instance, HPV 16, 18, and 58, can cause dysplasia that may further progress to cancer, infection with low-risk types tends to lead to genital warts, cervical dysplasia, but seldom results in cancer [4], [5]. Currently available prophylactic HPV vaccines mainly target high-risk types, particularly the HPV 16 and 18 [6], [7], [8]. Though rarely found elsewhere worldwide, HPV 58 ranks third among HPV types associated with cervical cancer cases reported in Korea, Japan, and China [7], [9]. The larger share of disease burden derived from HPV 58 infection in East Asia may reflect differences in host genetics, as well as the oncogenicity of circulating variants [9], [10]. Therefore, the development of next-generation HPV vaccines for East Asia should factor in the unique pattern of epidemic HPV 58 prevalence [9].
At present there are three HPV prophylactic vaccines on the market: Cervarix by GlaxoSmithKline (UK), Gardasil and Gardasil9 by Merck & Co. (USA). Cervarix is produced in insect cells [11], while for both Gardasil HPV vaccines, the L1-VLPs are produced in Saccharomyces cerevisiae [12]. These three HPV vaccines are proved highly effective in protecting against HPV related infection and diseases [13], [14], [15]. Regarding the worldwide uptake of HPV vaccines, developed countries like Canada, New Zealand, and the U.S. were the first to include HPV vaccines in national immunization plans [16], [17]. However, when it comes to the affordability of these vaccines for developing countries which have seen near 80% of the global cervical cancer cases but lack effective Pap screening programs, the cost associated with production and storage of these HPV vaccines poses a huge challenge to the successful immunization of women in those resource-limited regions. Hence novel, inexpensive prophylactic HPV vaccine production platforms are favorably needed.
We are currently investigating a trivalent HPV vaccine that consists of a mixture of three HPV type L1-VLPs composed of the L1 major capsid proteins of HPV 16, 18 and 58 recombinantly expressed in E.coli. The trivalent HPV 16/18/58 (3vHPV) vaccine is formulated with aluminum hydroxide (AH). It shares with Gardasil and Cervarix two identical oncogenic HPV types, HPV 16 and 18, which combined are responsible for approximately 70% of HPV related cancer cases and 5% of worldwide cancer cases [18], [19]. On top of that, the 3vHPV also targets HPV 58, as is the case for Gardasil9. The immune responses induced by the 3vHPV vaccine in rhesus macaques were measured by use of ELISA (total IgG antibody) and PBNA (neutralizing antibody), and Gardasil 4vHPV was employed as the positive control. The results presented here show that the immunogenicity and durability of 3vHPV is comparable to that of Gardasil 4vHPV at the same dosage. Encouragingly, given the remarkably low cost and simplicity of production of HPV vaccine using E.coli expression system, in combination with immunogenic non-inferiority to Gardasil 4vHPV, our 3vHPV vaccine will contribute to the broader implementation of HPV vaccination in developing countries.
2. Materials and methods
2.1. Preparation and characterization of 3vHPV vaccine L1-VLPs
The L1 major capsid proteins of HPV 16, 18 and 58 were all expressed as GST-L1 fusions in E.coli. Expression constructs were designed according to our pending patent applications (Application No.: CN201410683185.0, CN201410672158.3, and CN201410672161.5). Briefly, truncation of the N-terminal 5 residues and C-terminal 29 residues was made to the coding sequence for HPV 16 L1 protein (HPV 16 L1-ΔN5ΔC29). Similar deletions were also present in the coding sequences for HPV 18 L1 (HPV 18 L1-ΔN5ΔC30) and HPV 58 L1 (HPV 58 L1-ΔN5ΔC23). All three truncated constructs were amplified by polymerase chain reaction from the corresponding codon-optimized HPV full-length L1 clones, and subcloned into expression vector pGEX-2T (GE healthcare) using the BamH I and Xho I restriction sties. Protein expression and purification of the truncated L1 proteins were carried out using a similar protocol described previously [20], [21], [22]. Briefly, protein expression was induced overnight at room temperature with 0.2 mM isopropyl-β-D-thiogalactopyranoside. Cell lysates after sonication were sequentially subjected to purification by affinity chromatography, anion exchange chromatography, and gel-filtration chromatography. GST tag was removed via cleavage by recombinant GST-Human rhinovirus 3 C protease (produced in-house) following affinity chromatography. Identity and purity of L1 proteins were confirmed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE).
The purified L1-pentamers were assembled in vitro into HPV L1-VLPs in acetic acid-sodium acetate buffer, which were further purified by gel-filtration chromatography using a Superdex 200 10/300 GL Chromatographic Separation Column (GE healthcare), and subsequently analyzed by High Performance Liquid Chromatography (HPLC) using a TSK-GELG5000 column (Tosoh, Japan). The size distributions of the three HPV L1-VLPs were characterized by Dynamic Light Scattering (DLS) using a Nano Zeta-sizer (Malvern Instruments Ltd).
Residual host cell protein (HCP) content was determined using a quantitative anti-E.coli HCP ELISA kit (Cygnus Technologies). Residual DNA content determination was carried out using DNA probe hybridization technique with DIG High Prime DNA Labeling and Detection Starter Kit I from Roche. Endotoxin content was measured using Gel-Clot Limit Test. All other putative process-related impurities were also quantitatively analyzed.
2.2. Vaccine formulations and study design
The trivalent vaccine, 3vHPV (Lot No.20140501), contained a mixture of three in vitro assembled HPV 16/18/58 L1-VLPs adjuvanted with 500 μg of aluminum hydroxide (AH) in 500 μl of acetic acid-sodium acetate buffer. In this study, 3 doses of three 3vHPV dosage formulations containing 20 μg/10 μg/10 μg, 40 μg/20 μg/20 μg, and 60 μg/30 μg/30 μg of HPV 16/18/58 L1-VLPs, respectively (termed low-, mid-, and high-dosage formulations accordingly), were administered to groups of female rhesus macaques (n=5) aged 3–5 via intramuscular injection in a 0, 4, and 24 week regimen. Gardasil (Lot No. J007501, Merck &Co.) was chosen as the positive control, which contained the same amounts of HPV 16/18 L1-VLPs as mid-dosage 3vHPV formulation but less adjuvant (225 μg of aluminum hydroxyphosphate sulfate per dose). The negative control, Alhydrogel 2% (Lot No. 4879, Brenntag) was only formulated with an amount of AH equal to that of 3vHPV and visually indistinguishable from vaccine. Composition information of the experimental vaccines and controls are summarized in Table 1. Serum samples were collected from all vaccinated rhesus macaques at weeks 0, 2, 4, 6, 8, 12, 16, 20, 24, 26, 28, 32, 36, 40, 44, 48, 52, 56, 60, and 64 to measure HPV type-specific antibody responses.
Table 1.
Antigen and adjuvant composition of vaccines administered.
| L1-VLP amount for each HPV type per dose |
|||||
|---|---|---|---|---|---|
| Group | Vaccine | HPV 16 | HPV 18 | HPV 58 | Adjuvant amount per dose |
| 3vHPV high-dosage | 3vHPV | 60 μg | 30 μg | 30 μg | 500 μg |
| 3vHPV mid-dosage | 3vHPV | 40 μg | 20 μg | 20 μg | 500 μg |
| 3vHPV low-dosage | 3vHPV | 20 μg | 10 μg | 10 μg | 500 μg |
| Positive control | Gardasil | 40 μg | 20 μg | – | 225 μg |
| Negative control | Alhydrogel 2% | – | – | – | 500 μg |
2.3. Ethics statement
All experiments involving rhesus macaques were carried out in strict accordance with the administrative rules and regulations by the Academy of Military Medical Sciences regarding the use of Laboratory Animals. Prior approval for the study was granted by the Institutional Animal Care and Use Committee of Academy of Military Medical Sciences. Rhesus macaques were randomly assigned to groups based on the sequential selection from an inventory.
2.4. Generation of HPV pseudoviruses
HPV 16/18/58 pseudoviruses were generated by cotransfection of human 293FT cells (Invitrogen) as previously described [23], [24], [25], [26], with minor modifications. Briefly, for HPV 16, 20 million 293FT cells were plated 16 h before cotransfection with 60 μg of plasmid p16L1L2 containing codon-optimized HPV 16 capsid genes, L1 and L2, plus 60 μg of pseudogenome plasmid pEF-GFP using 150 μl of Lipo2000 transfection reagent (Invitrogen). About 10 h later, the culture media, Dulbecco's Modified Eagle's Medium (DMEM), were replaced with DMEM supplemented with 10% fetal bovine serum, 1% L-Glutamine (Invitrogen), 1% non-essential amino acids and 1% penicillin/streptomycin (Invitrogen) (Complete Medium). Cells were digested with 0.05% trypsin for 10 min 3 days post transfection, and harvested afterwards. The obtained cell pellets were subjected to two rinses with phosphate-buffered saline (PBS), and then resuspended in PBS-Mg (PBS supplemented with 9.5 mM MgCl2), followed by the addition of Brij58 and Benzonase (Sigma) at a final concentration of 0.5% each, as well as Plasmid-Safe ATP-dependent DNase (Epicentre) at a final concentration of 0.2%. After incubation at 37 ℃ for 24 h, the concentration of NaCl was adjusted to 850 mM before placing the mixture at 4 ℃ for 15 min and subsequently clarifying by centrifugation at 5000g for 10 min. The supernatant was diluted 100-fold with ice-cold Complete Medium aforementioned, and then aliquoted into 1.5 ml sterile centrifuge tubes, which were subsequently stored at −80 °C for further assay. The same protocol was also applied to the production of HPV 18 and 58 pseudovirues with plasmids p18L1L2 and p58L1L2 (L1 and L2 genes condon-optimized), respectively.
2.5. The Pseudovirus-Based Neutralization Assay (PBNA)
The PBNA was adapted from a previously described experimental setup [23], [27]. Briefly, human 293FT cells were preplated in 96-well flat-bottom plates (Corning Inc.) with Complete Medium at a cell density of 15,000 cells/well. Plates were then incubated at 37 ℃ and 5% CO2 for at least 5 h before adding the diluted serum samples.
Sera collected from rhesus macaques in the experimental and control arms were initially diluted 20-fold with Complete Medium, followed by 4-fold serial dilutions in 96-well round-bottom plates (Corning Inc.), with the final volume of each dilution being 100 μl. Each serum dilution was tested in duplicate. One hundred μl of HPV pseudovirus stock was added to each well containing a serum sample, followed by incubation at 4 ℃ for 1 h. These mixed samples were then transferred to monolayers of 293FT cells, and continued to be incubated at 37 ℃ and 5% CO2 for 3 days, after which cell counting based on GFP fluorescence in each sample was conducted with a SpectraMax i3-Minimax reader (Molecular Devices) set at 10 ms/well using excitation/emission wavelengths of 460/535 nm, respectively. Serum neutralization titers were calculated with the Reed-Muench Method and defined as the reciprocals of the highest serum dilutions that caused at least a 50% reduction in cell infection when compared to the pseudovirus-only control that consisted of 100 μl of each of Complete Medium and pseudovirus stock. Neutralizing antibody responses with geometric mean titers (GMTs) above a cutoff value of 40 were considered positive for all three HPV types (HPV 16/18/58).
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
HPV 16/18/58 specific IgG antibodies were determined by ELISA using HPV 16/18/58 L1-VLPs, respectively, as coating antigens. The methodology has been described previously [28], [29], with minor modifications. Briefly, 96-well flat-bottom plates (Corning Inc.) were coated overnight at 4 ℃ with 0.3 μg/0.3 μg/0.2 μg of HPV 16/18/58 L1-VLPs respectively per well, followed by three washes with PBS supplemented with 0.05% Tween 20 (Merck & Co.) (PBS/T). The plates were then incubated for 1 h at 37 ℃ with blocking solution (2% bovine serum albumin in PBS/T). Starting at a dilution of 1:500, two-fold serial dilutions of each serum sample were assayed. One hundred μl of diluted sera were added to the VLP-coated plates before incubation for 1.5 h at room temperature. Inclusion of 100 μl of Rabbit Anti-Monkey IgG antibody conjugated with horseradish peroxidase (Bs-0335R, Bioss Inc.) into each reaction mix was carried out subsequently. After a further incubation at room temperature for 1 h, the plates were washed 5 times with PBS/T. Reactions were terminated with 100 μl of 1 M sulfuric acid per well after staining the plates with TMB Substrate Solution (Thermo Fisher Scientific) at room temperature for 15 min in the dark. Absorbance at 450 nm was measured using a Model 680 microplate reader (Bio-Rad). Endpoint IgG titers were expressed as the reciprocals of the highest serum dilutions which resulted in an absorbance value twice that derived from serum samples taken from the negative control group.
2.7. Statistical analyses
Antibody titers obtained from the experimental sera collected at the indicated time points were compiled and log10-transformed with Graphpad Prism (Version 6.01, Graphpad Software, Inc.). Statistical analyses of log10-transformed nAb and IgG titers were performed using a two-sided Mann-Whitney U test in SPSS (Version 22.0, IBM SPSS Software), with P-values reported. A P-value less than 0.05 is considered statistically significant.
3. Results
3.1. HPV 16/18/58 L1-VLP characterization
All purification products, namely the HPV 16/18/58 L1-pentamers, were analyzed by SDS-PAGE. The sizes of HPV 16/18/58 L1 proteins shown by SDS-PAGE were consistent with the corresponding theoretical molecular masses (52.4 KD, 52.6 KD, and 52.9 KD, respectively) (data not shown). The purity of all three HPV L1-pentamers after purification was calculated to be over 95% using UVP Labworks (Version 4.6, Media Cybernetics) based on SDS-PAGE result.
HPV 16/18/58 L1-VLPs, assembled from the respective L1-pentamers, all accounted for near 100% of the assayed sample contents, with basically no residual L1-pentamers detected, according to HPLC. Particle size distributions of each HPV L1-VLPs were investigated by DLS. HPV 16 L1-VLPs were demonstrated to have particles of sizes ranging from 24.51 to 91.20 nm, with a reported Z-average size of 49.03 nm. For HPV 18 L1-VLPs, particle diameters ranged from 28.32 to 141.60 nm, and the reported Z-average size was 60.62 nm. HPV 58 L1-VLPs were shown to have a Z-average size of 49.82 nm, with diameters spanning from 28.21 to 91.28 nm. The polydispersity indexes given by DLS for all three HPV L1-VLPs were lower than 0.05, indicating highly monodisperse size distributions (www.biophysics.bioc.cam.ac.uk/wp-content/uploads/2011/02/DLS_Terms_defined_Malvern.pdf).
The levels of residual HCP (<0.001%), residual DNA (<10 ng/dose), endotoxin (<5 EU/dose), and other process-related impurities were all within the acceptable ranges specified by China Food and Drug Administration. An Investigational New Drug application for clinical trial development with the trivalent HPV 16/18/58 vaccine has been submitted to the CFDA (Application No. CXSL1500033).
3.2. Successful elicitation of strong immune responses by the 3vHPV vaccines
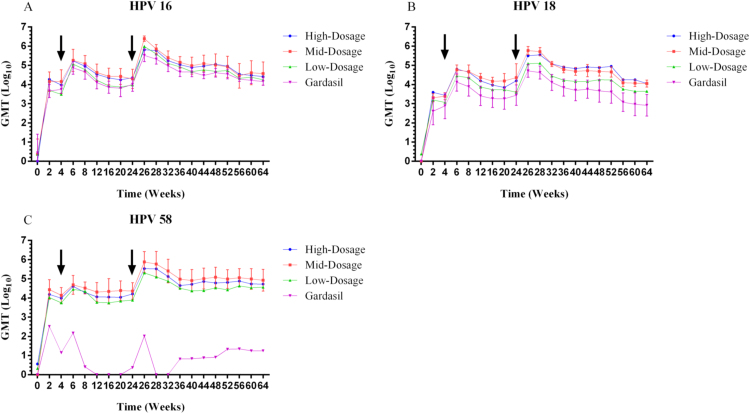
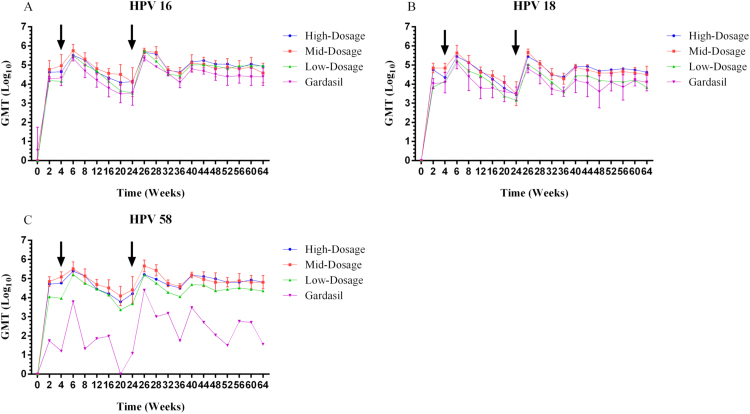
The overall kinetics of antibody responses induced by the three 3vHPV formulations generally followed a similar “Prime-Boost” vaccination immune response trend for HPV 16/18/58 over the study period (Figs. 1 and 2). Administration of 3vHPV formulations at week 0 elicited significant immune responses, with nAb GMTs and total IgG GMTs for HPV 16/18/58 increasing dramatically to some 103–104 and 104–105, respectively, within the first 2 weeks of vaccination. Following a booster shot at week 4, antibody responses against all three HPV types were enhanced and reached local peak titers at week 6. From weeks 6–24, both nAb and total IgG antibody titers underwent declines to different degrees for HPV 16, 18, and 58. A third vaccination at week 24 again moderately boosted both nAb and total IgG titers for all three HPV types. The antibody responses peaked at the highest levels at week 26 (two weeks after the final vaccination), fell slightly thereafter and reached a plateau through week 64. The “Prime-Boost” vaccination schedule in rhesus macaques demonstrated successful elicitation of durable immune responses against HPV 16, 18, and 58 with the investigational 3vHPV vaccines.
Fig. 1.
HPV 16, 18 and 58 type-specific nAb GMTs (log10-transformed) for the 3vHPV vaccine at three different dosages (high-, mid-, and low-dosage) compared to the commercial Gardasil 4vHPV vaccine (positive control). Sera from rhesus macaques immunized with the three experimental vaccine formulations and controls were collected at the indicated time points and tested for nAb titers for HPV 16, 18 and 58 using PBNA. Responses are reported as the geometric means of endpoint dilution titers (n=5 animals per group) for HPV 16, 18 and 58. Error bars represent the standard error of log10-transformed titers. For the purpose of clear illustration, only the error bars of representative mid-dosage 3vHPV formulation (for HPV 16/18/58) and Gardasil 4vHPV (for HPV 16/18) are depicted. Arrows indicate vaccination boosts at weeks 4 and 24. The figure was prepared using Graphpad Prism (Version 6.01).
Fig. 2.
HPV 16, 18 and 58 type-specific total IgG GMTs (log10-transformed) for the 3vHPV vaccine at three different dosages (high-, mid-, and low-dosage) compared to the commercial Gardasil 4vHPV vaccine (positive control). Sera from rhesus macaques immunized with the three experimental vaccine formulations and controls were collected at the indicated time points, and tested for HPV 16, 18 and 58 L1-VLP specific total IgG antibody titers using ELISA. Responses are reported as the geometric means of endpoint dilution titers (n=5 animals per group) for HPV 16, 18 and 58. Error bars represent the standard error of log10-transformed titers. For the purpose of clear illustration, only the error bars of representative mid-dosage 3vHPV formulation (for HPV 16/18/58) and Gardasil 4vHPV (for HPV 16/18) are depicted. Arrows indicate vaccination boosts at weeks 4 and 24. The figure was prepared using Graphpad Prism (Version 6.01).
3.3. Dosage-dependence observed and likely occurrence of immune interference
Dosage effect due to the difference in antigen contents was analyzed using two-sided Mann-Whitney U tests. As shown in Fig. 1, although generally mid-dosage 3vHPV induced slightly higher levels of nAbs for HPV 16 and 58 than high-dosage formulation at each sampling time point, statistically the difference was not significant (P-values=0.534 and 0.123, respectively). It was a mixed trend for HPV 18 where during the first 32 weeks, nAb responses induced by mid-dosage 3vHPV were a bit higher compared to those by high-dosage; thereafter, high-dosage associated nAb responses rose above those elicited by mid-dosage formulation till week 64. Yet overall HPV 18 specific nAb titers induced by mid- and high-dosage 3vHPV presented no significant difference (P-value=0.715). Numerically lower nAb GMTs were observed with low-dosage formulation when compared to mid- or high-dosage formulations, with a significant difference seen between low- and mid-dosage for HPV 18 (P-value=0.040) and 58 (P-value=0.023). Comparison of total IgG GMTs against HPV 16, 18, and 58 also revealed no significant difference between mid- and high-dosage formulations (P-values=0.903, 0.818 and 0.560, respectively) throughout the study period, while IgG GMTs observed in low-dosage group were generally inferior in comparison with mid-dosage group, statistically significant for HPV 18 (P-value=0.005) and 58 (P-value=0.001). Taken together, dosage-dependence was observed, yet immune interference probably occurred when comparing the nAb GMTs associated with the three study dosages of 3vHPV.
3.4. Immunogenicity of 3vHPV comparable to that of Gardasil 4vHPV
As shown in Fig. 1A and B, it is evident that the 3vHPV vaccine was immunogenically comparable, even arguably superior to the positive control, Gardasil 4vHPV, in terms of nAb GMTs for HPV 16/18 at each indicated time point. Stronger HPV 16/18 specific nAb responses were observed for mid-dosage 3vHPV versus 4vHPV from weeks 0 through 64, and Mann-Whitney U tests revealed statistically significant P-values of 0.042 for HPV 16 and <0.001 for HPV 18. Peak nAb titers for HPV 16 and 18 solicited by mid-dosage 3vHPV were 6.6 and 10.3-fold higher than those observed in the 4vHPV group at week 26, respectively (Table 2). In the persistence phase at week 64, mid-dosage 3vHPV induced anti-HPV 16 and 18 nAb titers 1.6 and 12.7-fold, respectively, higher than those measured in the 4vHPV group (Table 2). Even the low-dosage 3vHPV that contained half the amounts of HPV 16 and 18 antigens exhibited immunogenicity comparable (P-value=0.443 for HPV 16) or statistically superior (P-value=0.025 for HPV 18) to Gardasil. Plus, the duration of nAb responses specific to HPV 18 for 3vHPV vaccines appeared superior to that associated with Gardasil (Fig. 1B) as of week 64.
Table 2.
Fold difference in Geometric Mean Titers of a trivalent HPV 16/18/58 vaccine (mid-dosage) over Gardasil quadrivalent vaccine.
| Sera sampling time from vaccinated rhesus macaques (weeks), i.e. post dose 3 |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HPV type | 24 | 26 | 28 | 32 | 36 | 40 | 44 | 48 | 52 | 56 | 60 | 64 | |
| nAb titer | 16 | 1.9 | 7.6 | 3.4 | 2.7 | 3.1 | 2.1 | 4.2 | 2.5 | 2.4 | 1.5 | 2.5 | 2.6 |
| 18 | 8.3 | 11.3 | 12.3 | 9.1 | 8.8 | 9.8 | 9.3 | 10.5 | 11.1 | 10.2 | 12.8 | 13.7 | |
| IgG titer | 16 | 3.6 | 2.4 | 5.7 | 1.5 | 3.1 | 2.1 | 2.2 | 2.0 | 3.1 | 2.3 | 3.1 | 1.6 |
| 18 | 1.0 | 7.1 | 4.3 | 6.4 | 4.8 | 4.8 | 4.8 | 9.5 | 3.0 | 6.5 | 2.4 | 2.5 | |
HPV 16 and 18 specific IgG peak titers at week 26 and titers 40 weeks post dose 3 were consistently higher in the mid-dosage 3vHPV group than in the 4vHPV group (Fig. 2A and B), correlating well with the PBNA results. Mann-Whitney U tests of log10-transformed antibody titers of the mid-dosage group versus the 4vHPV group from weeks 0–64 revealed statistically significant P-values of 0.003 and 0.001, respectively, for HPV 16 and 18.
For HPV 58, a genotype absent from Gardasil 4vHPV, nAb and total IgG antibody responses were still observed after each vaccination (Figs. 1C and 2C), albeit much weaker than those elicited by 3vHPV vaccine candidates during the entire experimental process.
4. Discussion
While the investigational 3vHPV vaccine induced strong immune responses against all three HPV types, they all likely suffered from immune interference, albeit to different degrees, as indicated by comparing HPV type-specific nAb titers elicited by the three 3vHPV dosages tested. Despite no direct comparison of nAb levels induced by the 3vHPV vaccine to those by the corresponding monovalent vaccines in this study due to the research cost associated with rhesus macaques, a previous study showed the occurrence of immune interference among HPV 16, 18, and 58 in Balb/c mice model [30].
Mid-dosage 3vHPV formulation was capable of eliciting HPV 16/18 specific antibody responses non-inferior to those induced by Gardasil 4vHPV containing the same amounts of HPV 16/18 L1-VLPs in rhesus macaques over the entire sampling period. Even with a low-dosage formulation containing only half the amounts of HPV 16/18 antigens, the observed HPV 16 and 18 specific GMTs were generally above those induced by Gardasil 4vHPV. It is factually difficult to pinpoint the underlying cause for these observations, though a possible explanation would be the differences in the production and purification processes, as well as the adjuvants used between the studied vaccine and positive control. The 3vHPV vaccine was produced in E.coli and formulated with 500 μg of AH per dose, as opposed to yeast and 225 μg of amorphous aluminum hydroxyphospate sulfate per dose for Gardasil 4vHPV [12], [13].
It should be noted that, despite not containing HPV 58 L1-VLPs, Gardasil 4vHPV was still able to elicit weak HPV 58 specific antibody responses, consistent with a previous report [31]. Cervarix bivalent HPV vaccine, which is also devoid of HPV 58, was demonstrated to be able to evoke low cross-neutralizing antibody response against HPV 58, too [31], [32], [33], [34]. This effect could largely arise from the existence of cross-neutralizing linear epitopes or possible conserved epitopes shared among HPV types [35], [36].
Currently available HPV prophylactic vaccines, including Cervarix bivalent, Gardasil and Gardasil9, are all non-infectious subunit vaccines. Production systems employed for the manufacture of Cervarix and Gardasil vaccines are insect cells and yeast [11], [12], respectively, both of which are eukaryotic expression systems involving relatively laborious operations, lengthy production cycle, and high production cost. The trivalent HPV vaccine investigated here is recombinantly expressed in E.coli, indicating lower manufacturing cost and shorter production cycle. As with Cervarix and Gardasil 4vHPV vaccines, the investigational 3vHPV targets HPV 16 and 18. In addition, like Gardasil9, it is also able to induce significant antibody responses against HPV 58, an HPV strain that is the third most prevalent in East Asia [37], [38]. Inclusion of HPV 58 in the development of next-generation HPV vaccines, as suggested by Chan [9], is much desired in East Asia. Therefore, our effort to develop the cost-effective trivalent HPV 16/18/58 vaccine would undoubtedly contribute to this endeavor.
This study showed that a novel trivalent HPV 16/18/58 vaccine adjuvanted with aluminum hydroxide elicited robust and durable immune responses against all three vaccine HPV types in rhesus macaques. Immunogenic non-inferiority with respect to HPV 16 and 18 for the 3vHPV vaccine was observed in comparison with Gardasil 4vHPV. Given the low production cost and simplicity of manufacturing associated with E.coli expression system, this vaccine holds promise as a widely accessible vaccine in the prevention of HPV epidemics for women in resource-limited regions.
Conflict of interest statement
All authors except Y.F. Qiu were employees of Beijing Health Guard Biotechnology Inc. when this study was performed and potentially own stock or hold stock options in the Company. Y.F. Qiu was an employee of Laboratory Animal Centre of Academy of Military Medical Sciences, Beijing, China. Health Guard is currently developing a trivalent HPV 16/18/58 vaccine, and was the primary source of funding for this study.
Author Contributions
Y.J. Liu and H.J. Zhang designed the study; F. Yin, Y.J. Wang, N. Chen, Y.F. Qiu, Y. Wang, M. Yan, and J.P. Chen performed the experiments; D.Q. Jiang and N. Chen wrote the paper. All authors read and approved the final manuscript.
Acknowledgements
Special thanks go to Professor Xiaojiang Chen at the University of Southern California for his critical comments on the manuscript. This work was also supported by the Beijing Major Science & Technology Project (grant number D090507043409008) and a Special Technology Innovation Funding Project from Beijing Economic-Technological Developmental Area (grant number JSYF2010150).
Contributor Information
Haijiang Zhang, Email: hj.zhang@bj-klws.com.
Yongjiang Liu, Email: yj.liu@bj-klws.com.
References
- 1.Eiben G.L., da Silva D.M., Fausch S.C., Le Poole I.C., Nishimura M.I., Kast W.M. Cervical cancer vaccines: recent advances in HPV research. Viral Immunol. 2003;16:111–121. doi: 10.1089/088282403322017866. [DOI] [PubMed] [Google Scholar]
- 2.Dell G., Gaston K. Human papillomaviruses and their role in cervical cancer. Cell Mol. Life Sci. 2001;58:1923–1942. doi: 10.1007/PL00000827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Villiers E.M. Cross-roads in the classification of papillomaviruses. Virology. 2013;445:2–10. doi: 10.1016/j.virol.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 4.Brentjens M.H., Yeung-Yue K.A., Lee P.C., Tyring S.K. Human papillomavirus: a review. Dermatol. Clin. 2002;20:315–331. doi: 10.1016/s0733-8635(01)00028-6. [DOI] [PubMed] [Google Scholar]
- 5.Brendle S.A., Bywaters S.M., Christensen N.D. Pathogenesis of infection by human papillomavirus. Curr. Probl. Dermatol. 2014;45:47–57. doi: 10.1159/000355963. [DOI] [PubMed] [Google Scholar]
- 6.Daftarian P., Mansour M., Benoit A.C., Pohajdak B., Hoskin D.W., Brown R.G. Eradication of established HPV 16-expressing tumors by a single administration of a vaccine composed of a liposome-encapsulated CTL-T helper fusion peptide in a water-in-oil emulsion. Vaccine. 2006;24:5235–5244. doi: 10.1016/j.vaccine.2006.03.079. [DOI] [PubMed] [Google Scholar]
- 7.Cho H., Lee H.J., Heo Y.K., Cho Y., Gwon Y.D., Kim M.G. Immunogenicity of a trivalent human papillomavirus L1 DNA-encapsidated, non-replicable baculovirus nanovaccine. PLoS One. 2014;9:e95961. doi: 10.1371/journal.pone.0095961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ault K.A., Giuliano A.R., Edwards R.P., Tamms G., Kim L.L., Smith J.F. A phase I study to evaluate a human papillomavirus (HPV) type 18 L1 VLP vaccine. Vaccine. 2004;22:3004–3007. doi: 10.1016/j.vaccine.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 9.Chan P.K. Human papillomavirus type 58: the unique role in cervical cancers in east Asia. Cell Biosci. 2012;2:17–19. doi: 10.1186/2045-3701-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bae J.H., Cheung J.L., Lee S.J., Luk A.C., Tong S.Y., Chan P.K. Distribution of human papillomavirus type 58 variants in progression of cervical dysplasia in Korean women. J. Microbiol. Biotechnol. 2009;19:1051–1054. doi: 10.4014/jmb.0812.679. [DOI] [PubMed] [Google Scholar]
- 11.Inglis S., Shaw A., Koenig S. Chapter 11: hpv vaccines: commercial research & development. Vaccine. 2006;24:99–105. doi: 10.1016/j.vaccine.2006.05.119. [DOI] [PubMed] [Google Scholar]
- 12.Shi L., Sings H.I., Bryan J.T., Wang B., Wang Y., Mach H. GARDASIL: prophylactic human papillomavirus vaccine development--from bench top to bed-side. Clin. Pharm. Ther. 2007;81:259–264. doi: 10.1038/sj.clpt.6100055. [DOI] [PubMed] [Google Scholar]
- 13.Luxembourg A., Brown D., Bouchard C., Giuliano A.R., Iversen O.E., Joura E.A. Phase II studies to select the formulation of a multivalent HPV L1 virus-like particle (VLP) vaccine. Hum. Vaccin. Immunother. 2015;11:1313–1322. doi: 10.1080/21645515.2015.1012010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leung T.F., Liu A.P., Lim F.S., Thollot F., Oh H.M., Lee B.W. Comparative immunogenicity and safety of human papillomavirus (HPV)−16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine administered according to 2- and 3-dose schedules in girls aged 9-14 years: results to month 12 from a randomized trial. Hum. Vaccin. Immunother. 2015;11:1689–1702. doi: 10.1080/21645515.2015.1050570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schiller J.T., Castellsague X., Garland S.M. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine. 2012;30:123–138. doi: 10.1016/j.vaccine.2012.04.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barr E., Sings H.L. Prophylactic HPV vaccines: new interventions for cancer control. Vaccine. 2008;26:6244–6257. doi: 10.1016/j.vaccine.2008.07.056. [DOI] [PubMed] [Google Scholar]
- 17.Gattoc L., Nair N., Ault K. Human papillomavirus vaccination: current indications and future directions. Obstet. Gynecol. Clin. North Am. 2013;40:177–197. doi: 10.1016/j.ogc.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forman D., de Martel C., Lacey C.J., Soerjomataram I., Lortet-Tieulent J., Bruni L. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30:12–23. doi: 10.1016/j.vaccine.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 19.de Martel C., Ferlay J., Franceschi S., Vignat J., Bray F., Forman D. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607–615. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 20.Chen X.S., Garcea R.L., Goldberg I., Casini G., Harrison S.C. Structure of small virus-like particles assembled from the L1 protein of human papillomavirus 16. Mol. Cell. 2000;5:557–567. doi: 10.1016/s1097-2765(00)80449-9. [DOI] [PubMed] [Google Scholar]
- 21.Bishop B., Dasgupta J., Chen X.S. Structure-based engineering of papillomavirus major capsid l1: controlling particle assembly. Virol. J. 2007;4:3. doi: 10.1186/1743-422X-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen X.S., Casini G., Harrison S.C., Garcea R.L. Papillomavirus capsid protein expression in Escherichia coli: purification and assembly of HPV11 and HPV16 L1. J. Mol. Biol. 2001;307:173–182. doi: 10.1006/jmbi.2000.4464. [DOI] [PubMed] [Google Scholar]
- 23.Lu W.X., Cheng T., Li S.W., Pan H.R., Shen W.T., Chen Y.X. Establishment and application of human papillomavirus type 16 pseudovirions neutralization assay. Sheng wu Gong cheng xue bao = Chin. J. Biotechnol. 2006;22:990–995. [PubMed] [Google Scholar]
- 24.Buck C.B., Thompson C.D. Production of papillomavirus-based gene transfer vectors. Current protocols in cell biology / editorial board. Juan S Bonifacino [Et. al]. 2007 doi: 10.1002/0471143030.cb2601s37. [DOI] [PubMed] [Google Scholar]
- 25.Buck C.B., Pastrana D.V., Lowy D.R., Schiller J.T. Efficient intracellular assembly of papillomaviral vectors. J. Virol. 2004;78:751–757. doi: 10.1128/JVI.78.2.751-757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buck C.B., Pastrana D.V., Lowy D.R., Schiller J.T. Generation of HPV pseudovirions using transfection and their use in neutralization assays. Methods Mol. Med. 2005;119:445–462. doi: 10.1385/1-59259-982-6:445. [DOI] [PubMed] [Google Scholar]
- 27.Pastrana D.V., Buck C.B., Pang Y.Y., Thompson C.D., Castle P.E., FitzGerald P.C. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology. 2004;321:205–216. doi: 10.1016/j.virol.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 28.Dessy F.J., Giannini S.L., Bougelet C.A., Kemp T.J., David M.P., Poncelet S.M. Correlation between direct ELISA, single epitope-based inhibition ELISA and Pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Hum. Vaccin. 2008;4:425–434. doi: 10.4161/hv.4.6.6912. [DOI] [PubMed] [Google Scholar]
- 29.Zhao H., Lin Z.J., Huang S.J., Li J., Liu X.H., Guo M. Correlation between ELISA and pseudovirion-based neutralisation assay for detecting antibodies against human papillomavirus acquired by natural infection or by vaccination. Hum. Vaccin. Immunother. 2014;10:740–746. doi: 10.4161/hv.27619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang T., Xu Y., Qiao L., Wang Y., Wu X., Fan D. Trivalent Human Papillomavirus (HPV) VLP vaccine covering HPV type 58 can elicit high level of humoral immunity but also induce immune interference among component types. Vaccine. 2010;28:3479–3487. doi: 10.1016/j.vaccine.2010.02.057. [DOI] [PubMed] [Google Scholar]
- 31.Draper E., Bissett S.L., Howell-Jones R., Waight P., Soldan K., Jit M. A randomized, observer-blinded immunogenicity trial of Cervarix((R)) and Gardasil((R)) human papillomavirus vaccines in 12-15 year old girls. PLoS One. 2013;18:e61825. doi: 10.1371/journal.pone.0061825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bissett S.L., Draper E., Myers R.E., Godi A., Beddows S. Cross-neutralizing antibodies elicited by the Cervarix(R) human papillomavirus vaccine display a range of alpha-9 inter-type specificities. Vaccine. 2014;32:1139–1146. doi: 10.1016/j.vaccine.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Draper E., Bissett S.L., Howell-Jones R., Edwards D., Munslow G., Soldan K. Neutralization of non-vaccine human papillomavirus pseudoviruses from the A7 and A9 species groups by bivalent HPV vaccine sera. Vaccine. 2011;29:8585–8590. doi: 10.1016/j.vaccine.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kemp T.J., Hildesheim A., Safaeian M., Dauner J.G., Pan Y., Porras C. HPV16/18 L1 VLP vaccine induces cross-neutralizing antibodies that may mediate cross-protection. Vaccine. 2011;29:2011–2014. doi: 10.1016/j.vaccine.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin B., Wang X., Zhang Y., Cheng H., Hu L. Preliminary research of induction of the multiple HPV antibody by HPV L1 type conserved sequence aimed at human papillomavirus major protein. Sheng Wu Yi Xue Gong. Cheng Xue Za Zhi. 2011;28:982–987. [PubMed] [Google Scholar]
- 36.Combita A.L., Touze A., Bousarghin L., Christensen N.D., Coursaget P. Identification of two cross-neutralizing linear epitopes within the L1 major capsid protein of human papillomaviruses. J. Virol. 2002;76:6480–6486. doi: 10.1128/JVI.76.13.6480-6486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clifford G., Franceschi S., Diaz M., Munoz N., Villa L.L. Chapter 3: hpv type-distribution in women with and without cervical neoplastic diseases. Vaccine. 2006;24:26–34. doi: 10.1016/j.vaccine.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 38.Kwag H.L., Kim H.J., Chang D.Y., Kim H.J. The production and immunogenicity of human papillomavirus type 58 virus-like particles produced in Saccharomyces cerevisiae. J. Microbiol. 2012;50:813–820. doi: 10.1007/s12275-012-2292-1. [DOI] [PubMed] [Google Scholar]