Abstract
Objective
To evaluate the acceptability and performance of cervical cancer (CC) screening using visual inspection with acetic acid (VIA) integrated into a rural immunization clinic in Uganda.
Methods/materials
We conducted a cross-sectional pilot study in rural Uganda. We explored associations between women's characteristics and acceptance of VIA testing. We collected samples for Papanicolaou (Pap) smear testing in a random subset of women and used results from this test as a comparator for assessing VIA performance.
Results
We enrolled 625 women of whom 571 (91.4%) accepted and 54 (8.6%) refused CC screening. In the univariate model, age (Odds Ratio (OR)=1.10; p-value<0.001) and employment status (OR 2.00; p-value=0.019) were significantly associated with acceptance of VIA screening. In the multivariate model, no characteristic was independently associated with acceptance of VIA screening after adjusting for other factors. Compared to reference Pap smear, CC screening with VIA had a sensitivity of 50% and a specificity of 97.7%.
Conclusions
CC screening with VIA is highly acceptable in the setting of rural immunization clinics in Uganda. Studies to assess which screening method would be the most effective and cost-effective are needed before stakeholders can consider adopting screening programs at scale.
1. Introduction
Cervical cancer (CC), the third most common cancer in women, causes over 270,000 deaths worldwide each year [1]. In Uganda, CC is the most common cancer among women, with an age adjusted incidence of 47.5 per 100,000 population, one of the highest in the world [2]. CC is associated with significant morbidity and substantial social and economic consequences for families and communities [3].
CC is preventable by primary prevention using human papillomavirus (HPV) vaccination or secondary prevention through screening, early detection, and treatment of pre-cancerous lesions before progression into invasive cancer. The HPV vaccine has only recently been introduced in Uganda [4], while millions of Ugandan women have already been infected with HPV [5], [6], [7]. Additionally, CC screening programs in Uganda and other low-income countries have been limited to pilot projects and other sub-national programs in hospitals and health centers [8].
Several methods exist for CC screening: the Papanicolaou test (Pap smear), visual inspection of the cervix with acetic acid (VIA) or Lugol's iodine (VILI), cervicography and HPV testing, although cervicography is no longer recommended by the World Health Organization (WHO) or any other guidelines. VIA is highly effective as a primary screening tool [9] and is affordable [9], [10].
Earlier studies of CC screening in Uganda have shown negative attitudes by health workers towards CC screening, which may have limited referral for screening [11]. Studies also have demonstrated that limited knowledge by the general public about CC, poverty, and unfriendly health workers were potential barriers to the success of emerging screening programs [12]. Recent studies have found that more screening programs are emerging, especially in urban settings [13], [14], [15], [16], [17], using innovative approaches such as involvement of male partners [13], pocket-sized colposcopes [18], integration of screening services with more pervasive HIV care [19], and self-sampling by women [20], [21], [22]. However, challenges for wider use of CC screening remain, including embarrassment associated with screening procedures [23] and perceived low quality of health services [17].
Although a recent study suggests that CC screening with visual inspection is highly acceptable to women in the urban hospital setting [24], we did not find existing evidence that CC screening with VIA was acceptable to rural populations. Building off of prior successes integrating CC screening into HIV care, we sought to assess the potential utility of adding CC screening to existing clinical services for childhood immunizations since women routinely visit health facilities for their children's immunization and could be offered CC screening during the same visit. In this paper, we report the results of a study in which we assessed the acceptability and performance of CC screening using VIA in the setting of an immunization clinic in rural Uganda where screening is currently non-existent.
2. Materials and methods
We conducted a cross-sectional survey of women who were offered CC screening at Luweero Health Center IV (HC-IV) between July 2014 and May 2015. The health center is located in rural central Uganda, approximately 75 km from Uganda's capital Kampala. HC-IVs are rural health centers designed to provide primary health services and a limited number of advanced care procedures in rural communities. Together with the district health team (DHT) we purposively selected Luweero HC IV to be the implementing health facility for this study because it served a large catchment population and had nurses with training to conduct CC screening. We sought and received ethical approval for this study from the Joint Clinical Research Center Institutional Review Board and the Uganda National Council of Science and Technology.
Before the pilot study, we sought and received support from the Uganda Ministry of Health and Luweero District authorities. We conducted a four-day CC screening with VIA training with 15 nurse midwives at the HC-IV facilitated by experts from the Uganda Cancer Institute. We provided nurse midwives with a VIA screening protocol to use in the study. We also conducted a series of campaigns and demand creation activities—radio advertisements and campaigns during immunization outreaches—which culminated in a project launch ceremony that was attended by stakeholders including the Ministry of Health and the WHO office in Uganda.
We invited all women who attended the Luweero HC-IV immunization clinic during the study period to participate in the study. We excluded pregnant women, women with CC, women menstruating at time of recruitment, and women who gave birth during the previous 6 weeks.
After entering the immunization clinic, we invited women to a health education session in which a nurse midwife educated about immunization, CC burden, risk, and potential impact on women's health. The nurse midwife also explained the VIA screening process and then asked women to volunteer for the procedure. We asked women who refused screening to participate in an exit survey in which we collected data on demographic and clinical characteristics, as well as reasons for refusal.
We administered informed consent to women who accepted screening, followed by VIA screening according to the protocol. Following completion of the VIA screening, we administered the exit survey, and asked about the testing experience instead of reasons for refusal. We allowed women to choose whether they wanted to undergo screening before or after immunization activities.
We organized and facilitated the referral of women who had abnormal results from VIA screening to the Mulago National Referral Hospital for colposcopy and additional treatment. We also organized and facilitated the referral of women identified to have other co-morbidities, such as infections, to the nearest appropriate health facility.
We collected data from the paper-based exit surveys, double-entered it in Epidata 3.1, and performed cross check. We combined this survey dataset with clinical data from VIA and Pap smear screening. To evaluate factors associated with women's acceptability of VIA, we performed descriptive analyses of patient characteristics stratified by women who agreed to or refused screening. We used Student's t-test for differences in means and Chi-square test for differences in proportions to determine if any of the differences was statistically significant. We used univariate logistic regression to identify independent associations between women's characteristics and a binary outcome variable for acceptance of VIA screening. We used multivariate logistic regression with robust estimation to identify associations between women's characteristics and acceptance of VIA screening, when adjusted for covariates. All analyses were conducted in Stata 12.0.
3. Results
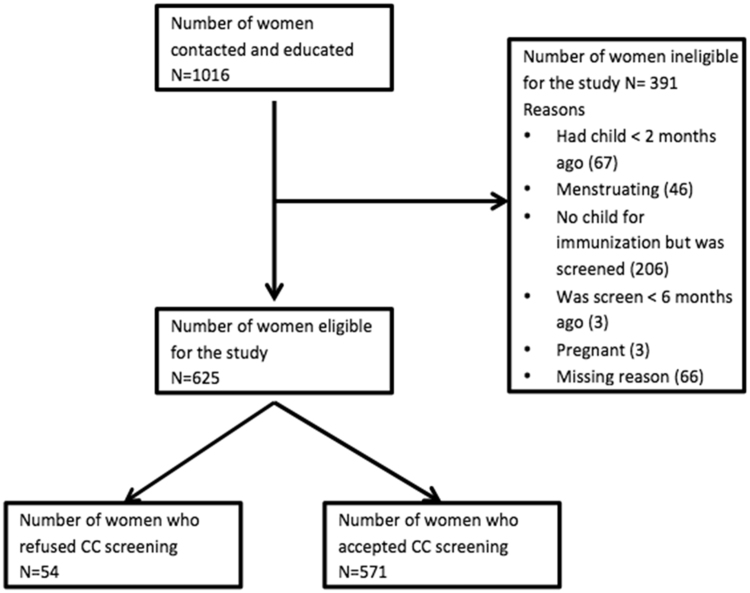
During the study period, we approached 1016 women for participation in the study. Of these, 625 women met the inclusion criteria for our study. Reasons for exclusion included: had children less than 2 months ago, was menstruating during recruitment, was screened less than 6 months ago, and was pregnant. 571 out of 625 eligible women (91.4%) accepted VIA screening and 54 (8.6%) refused. Fig. 1 describes the flow of the study population. Table 1 shows demographics of the study participants stratified by acceptance of VIA screening. Women who refused screening were significantly younger (Mean age 23 years vs. 27 years, P-value = 0.001), more likely to be single (17% vs. 11%, P-value = 0.029), and more likely to be employed (46% vs. 30%, P-value = 0.017). There were no statistically significant differences in other demographic characteristics.
Fig. 1.
Flow diagram of study population.
Table 1.
Demographic characteristics of the study population by acceptance of visual inspection with acetic acid (VIA) screening for cervical cancer.
| Characteristic | Overall (N = 625) | Accepted VIA (n = 571) | Refused VIA (n = 54) | P-value |
|---|---|---|---|---|
| Age, Mean (SD) | 26.2 (6.8) | 26.5 (6.8) | 22.8 (4.9) | 0.0001 |
| Marital status, n (%) | 0.029 | |||
| Single | 71 (11.4) | 62 (10.9) | 9 (16.7) | |
| Married | 532 (85.1) | 491 (86.0) | 41 (75.9) | |
| Divorced and others | 22 (3.5) | 18 (3.1) | 4 (7.4) | |
| Highest education, n (%) | 0.180 | |||
| None or primary | 277 (44.3) | 259 (45.3) | 18 (33.3) | |
| Secondary | 320 (51.2) | 286 (50.1) | 34 (63.0) | |
| Post-secondary | 28 (4.5) | 26 (4.6) | 2 (3.7) | |
| Religion, n (%) | 0.742 | |||
| Christian | 445 (71.2) | 409 (71.6) | 36 (66.7) | |
| Muslim | 141 (22.6) | 127 (22.2) | 14 (25.9) | |
| Other | 39 (6.2) | 35 (6.1) | 4 (7.4) | |
| Employed, n (%) | 199 (31.8) | 174 (30.5) | 25 (46.3) | 0.017 |
| Monthly earnings, Mean (SD) | $55.8 ($58.8) | $57.7 ($60.8) | $42.0 ($38.7) | 0.2416 |
| Transport cost to health center, mean (SD) | $0.59 ($0.79) | $0.60 ($0.80) | $0.49 ($0.68) | 0.3422 |
| Spending at health center, mean (SD) | $0.34 ($0.50) | $0.35 ($0.52) | $0.28 ($0.31) | 0.3255 |
Of the 571 women who accepted screening, 100% said they would undergo future screening and nearly all said they would recommend screening to others (Table 2). Women who accepted screening reported a variety of clinical characteristics. 42 women (7.5%) reported that they were HIV-positive (Table 2).
Table 2.
Clinical and other characteristics of women who accepted screening.
| Characteristics | Value |
|---|---|
| Parity, mean (SD) | 3.0 (2.1) |
| Gravidity, mean (SD) | 3.1 (2.2) |
| Self-reported HIV positive, n (%) | 42 (7.5) |
| Cervicitis | 12 (2.1) |
| Vaginal candidiasis | 26 (4.6) |
| Urinary tract infection | 4 (0.7) |
| Pelvic inflammatory disease | 16 (2.8) |
| Abnormal vaginal discharge | 14 (2.5) |
| Tumor | 1 (0.2) |
| Post-coital bleeding | 1 (0.2) |
| Would undergo future screening, n (%) | 571 (100) |
| Would recommend screening to others, n (%) | 570 (99.8) |
Of the 54 women who refused VIA screening, 26 (48.2%) refused because of fear of finding that they had CC, 15 (27.8%) because they had no time to undergo screening, 10 (18.5%) because of anticipated pain from the procedure, and 3 (5.6%) because they were feeling unwell on the day of recruitment.
Table 3 presents the unadjusted and adjusted odds ratios obtained from logistic regression for the association between different demographic characteristics and acceptance of VIA screening. In the univariable model, age and employment status were significantly associated with acceptance of VIA screening. However, in the multivariate model that adjusted for confounding covariates, none of the participant characteristics was significantly associated with acceptance of VIA screening.
Table 3.
Univariate and multivariate logistic regression results showing the relationship between the characteristics of women and acceptance of VIA screening.
| Characteristic | Crude OR | p-value | Adjusted OR | p-value |
|---|---|---|---|---|
| Age | 1.10 | < 0.001 | 1.10 | 0.179 |
| Marital status | ||||
| Married vs. single | 1.70 | 0.159 | 1.30 | 0.661 |
| Divorced vs. single | 0.65 | 0.518 | 0.48 | 0.509 |
| Highest level of education | ||||
| Secondary vs. none or primary | 0.58 | 0.078 | 0.41 | 0.179 |
| Post-secondary vs. none or primary | 0.90 | 0.896 | 0.68 | 0.777 |
| Religion | ||||
| Muslim vs. Christian | 0.80 | 0.497 | 0.47 | 0.156 |
| Other vs. Christian | 0.77 | 0.639 | 1.10 | 0.948 |
| Employed vs. not employed | 2.00 | 0.019 | 1.00 | 0.344 |
| Monthly earnings | 1.00 | 0.171 | 2.30 | 0.331 |
| Transport costs to health center | 1.30 | 0.416 | 2.10 | 0.573 |
Employment status was omitted due to multicollinearity.
4. Discussion
In this study, we found high acceptance of CC screening using VIA by women in a rural immunization clinic in central Uganda. Acceptance was robust across demographic characteristics of women in the study.
Our study adds to the body of literature on cervical cancer screening acceptability in Uganda. A previous study in an urban, tertiary hospital setting in Uganda found CC screening with VIA or VIA/VILI was widely acceptable [24]. Another Uganda-based study evaluated the acceptability of vaginal and self-sampling with the human papilloma virus (HPV) DNA testing method and found similarly high acceptance rates. Combined with our findings, there is growing evidence for broad acceptability of services related to CC screening using any method in both rural and urban settings [22].
The majority of women who refused VIA screening reported fears of potentially learning they had cervical cancer or lack of time as reasons for avoiding screening. Future CC policies and programs should address women's perceptions and fears of screening to improve uptake. Additionally, programs might consider identifying health systems inefficiencies that could reduce the time burden associated with screening to make screening faster and more manageable for women. However, given the generally robust acceptability of CC screening, future local and national screening programs hold great potential for increasing coverage rates and creating long-term positive impact on health outcomes.
The rate of positive VIA tests may suggest a low incidence of CC in this population. This may be because mothers tend to be young in the routine immunization setting. The mean age of childbearing in Uganda is 28 years [25], which is at the low end of recommended age for CC screening (25–49) in Uganda [26]. The low rate of positive VIA tests may also be due to inadequate training of nurses that conducted VIA screening, inadequate quality control, or inadequate preparation of the acetic acid.
Despite the low incidence, CC screening in this setting is still likely to be cost-effective because the service can leverage existing clinical resources. Future studies might assess the marginal cost of CC screening in this setting and the cost-effectiveness measured as cost per screened woman, cost per case of cancer found, cost per life-year saved, and cost per disability-adjusted life-year averted. The cost-effectiveness of CC screening is also influenced by the uptake of treatment if screened positive. Future studies should also evaluate the uptake of CC treatment among women that screened positive and factors that may influence uptake.
While integrating screening into rural immunization clinics is highly acceptable, extra efforts are needed to increase the actual uptake of screening. Research suggests that slightly more than 50% of children in Uganda are fully immunized, and partial immunization rates range from 24% to 89%. This might limit the number of women in the catchment population that would receive screening in immunization clinics [27]. Outreach campaigns that aim to simultaneously increase demand for immunization and screening could augment coverage rates for both prevention programs.
Furthermore, women participated in our study may not be representative of all women in rural Uganda. Prior research suggests that women who immunize their children in Uganda tend to have higher levels of education than those who do not [27]; 56% our study participants had at least secondary education. More targeted efforts may be required to reach women with lower levels of education who might be less likely to enter an immunization clinic setting.
The study was conducted in rural health centers that are designed to provide primary health services and a limited number of advanced care procedures in rural communities. Such health centers are unlikely to provide CC treatments for women that are screened positive by VIA. In this study, we organized and facilitated the referral of women who had abnormal results from VIA screening to Mulago National Referral Hospital for colposcopy and other treatments. For future implementation, before screening programs in rural immunization clinics can be scaled up, those clinics’ and nearby health centers’ capacity for CC treatment needs to be considered.
5. Conclusions
Cervical cancer screening is highly acceptable and potentially scalable in the setting of rural immunization clinics in Uganda. Studies to assess the performance of VIA screening compared to the gold standard and the cost-effectiveness of CC screening methods needed before stakeholders and governments can consider adapting screening programs in rural immunization clinics at scale.
Authors’ contributions
All authors have made substantive intellectual contribution to study design, analysis, and interpretation of data. All authors have been involved in drafting the manuscript or revising it. All authors have given final approval of the version to be published and have agreed to be accountable for all aspects of the work.
Funding
This study is funded by Grand Challenges Canada.
Conflict of interest
The study authors have no financial or non-financial competing interests to declare.
Acknowledgements
The authors would like to thank Dr. Agaba Byamukama and the staff at Luweero HC IV for their contribution to this study.
References
- 1.Arbyn M., Castellsague X., de Sanjose S., Bruni L., Saraiya M., Bray F. Worldwide burden of cervical cancer in 2008. Ann. Oncol. 2011;22(12):2675–2686. doi: 10.1093/annonc/mdr015. [DOI] [PubMed] [Google Scholar]
- 2.Wabinga H.R., Parkin D.M., Wabwire-Mangen F., Nambooze S. Trends in cancer incidence in Kyadondo County, Uganda, 1960–1997. Br. J. Cancer. 2000;82(9):1585–1592. doi: 10.1054/bjoc.1999.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sherris J., Wittet S., Kleine A., Sellors J., Luciani S., Sankaranarayanan R. Evidence-based, alternative cervical cancer screening approaches in low-resource settings. Int. Perspect. Sex Reprod. Health. 2009;35(3):147–154. doi: 10.1363/ifpp.35.147.09. [DOI] [PubMed] [Google Scholar]
- 4.Gulland A. Uganda launches HPV vaccination programme to fight its commonest cancer. BMJ. 2012;345:e6055. doi: 10.1136/bmj.e6055. [DOI] [PubMed] [Google Scholar]
- 5.De Vuyst H., Alemany L., Lacey C., Chibwesha C.J., Sahasrabuddhe V., Banura C. The burden of human papillomavirus infections and related diseases in sub-Saharan Africa. Vaccine. 2013;31(Suppl. 5):SF32–SF46. doi: 10.1016/j.vaccine.2012.07.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asiimwe S., Whalen C.C., Tisch D.J., Tumwesigye E., Sethi A.K. Prevalence and predictors of high-risk human papillomavirus infection in a population-based sample of women in rural Uganda. Int. J. STD AIDS. 2008;19(9):605–610. doi: 10.1258/ijsa.2008.008025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paul P., Winkler J.L., Bartolini R.M., Penny M.E., Huong T.T., Nga le T. Screen-and-treat approach to cervical cancer prevention using visual inspection with acetic acid and cryotherapy: experiences, perceptions, and beliefs from demonstration projects in Peru, Uganda, and Vietnam. Oncologist. 2013;18(12):1278–1284. doi: 10.1634/theoncologist.2013-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim J.J., Campos H.G., O'Shea M., Diaz M., Mutyaba I. Model-based impact and cost-effectiveness of cervical cancer prevention in sub-Saharan Africa. Vaccine. 2013;31(Suppl. 5):SF60–SF72. doi: 10.1016/j.vaccine.2012.07.093. [DOI] [PubMed] [Google Scholar]
- 9.De Vuyst H., Claeys P., Njiru S., Muchiri L., Steyaert S., De Sutter P. Comparison of pap smear, visual inspection with acetic acid, human papillomavirus DNA-PCR testing and cervicography. Int. J. Gynaecol. Obstet. 2005;89(2):120–126. doi: 10.1016/j.ijgo.2005.01.035. [DOI] [PubMed] [Google Scholar]
- 10.Mvundura M., Tsu V. Estimating the costs of cervical cancer screening in high-burden sub-Saharan African countries. Int. J. Gynaecol. Obstet. 2014;126(2):151–155. doi: 10.1016/j.ijgo.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 11.Mutyaba T., Mmiro F.A., Weiderpass E. Knowledge, attitudes and practices on cervical cancer screening among the medical workers of Mulago Hospital, Uganda. BMC Med. Educ. 2006;6:13. doi: 10.1186/1472-6920-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mutyaba T., Faxelid E., Mirembe F., Weiderpass E. Influences on uptake of reproductive health services in Nsangi community of Uganda and their implications for cervical cancer screening. Reprod. Health. 2007;4:4. doi: 10.1186/1742-4755-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mutyaba T., Mirembe F., Sandin S., Weiderpass E. Male partner involvement in reducing loss to follow-up after cervical cancer screening in Uganda. Int. J. Gynaecol. Obstet. 2009;107(2):103–106. doi: 10.1016/j.ijgo.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 14.Mutyaba T., Mirembe F., Sandin S., Weiderpass E. Evaluation of 'see-see and treat' strategy and role of HIV on cervical cancer prevention in Uganda. Reprod. Health. 2010;7:4. doi: 10.1186/1742-4755-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaccarella S., Lortet-Tieulent J., Plummer M., Franceschi S., Bray F. Worldwide trends in cervical cancer incidence: impact of screening against changes in disease risk factors. Eur. J. Cancer. 2013;49(15):3262–3273. doi: 10.1016/j.ejca.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 16.Mwaka A.D., Wabinga H.R., Mayanja-Kizza H. Mind the gaps: a qualitative study of perceptions of healthcare professionals on challenges and proposed remedies for cervical cancer help-seeking in post conflict northern Uganda. BMC Fam. Pract. 2013;14:193. doi: 10.1186/1471-2296-14-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osingada C.P., Ninsiima G., Chalo R.N., Muliira J.K., Ngabirano T. Determinants of uptake of cervical cancer screening services at a no-cost reproductive health clinic managed by nurse-midwives. Cancer Nurs. 2014 doi: 10.1097/NCC.0000000000000156. [DOI] [PubMed] [Google Scholar]
- 18.Osingada C.P., Ninsiima G., Chalo R.N., Muliira J.K., Ngabirano T. Agreement of colposcope and gynocular in assessment of cervical lesions by swede score: a randomized, crossover pilot trial. J. Low Genit. Tract. Dis. 2013;17(4):372–377. doi: 10.1097/LGT.0b013e31827ba7c5. [DOI] [PubMed] [Google Scholar]
- 19.Kumakech E., Andersson S., Wabinga H., Berggren V. Integration of HIV and cervical cancer screening perceptions of healthcare providers and policy makers in Uganda. BMC Public Health. 2014;14:810. doi: 10.1186/1471-2458-14-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogilvie G.S., Mitchell S., Sekikubo M., Biryabarema C., Byamugisha J., Jeronimo J. Results of a community-based cervical cancer screening pilot project using human papillomavirus self-sampling in Kampala, Uganda. Int. J. Gynaecol. Obstet. 2013;122(2):118–123. doi: 10.1016/j.ijgo.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 21.Ogilvie G.S., Mitchell S., Sekikubo M., Biryabarema C., Byamugisha J., Jeronimo J. A multicountry evaluation of careHPV testing, visual inspection with acetic acid, and papanicolaou testing for the detection of cervical cancer. Int. J. Gynecol. Cancer. 2014;24(3):576–585. doi: 10.1097/IGC.0000000000000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bansil P., Wittet S., Lim J.L., Winkler J.L., Paul P., Jeronimo J. Acceptability of self-collection sampling for HPV-DNA testing in low-resource settings: a mixed methods approach. BMC Public Health. 2014;14:596. doi: 10.1186/1471-2458-14-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teng F.F., Mitchell S.M., Sekikubo M., Biryabarema C., Byamugisha J.K., Steinberg M. Understanding the role of embarrassment in gynaecological screening: a qualitative study from the ASPIRE cervical cancer screening project in Uganda. BMJ Open. 2014;4(4):e004783. doi: 10.1136/bmjopen-2014-004783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teng F.F., Mitchell S.M., Sekikubo M., Biryabarema C., Byamugisha J.K., Steinberg M. Acceptability of cervical cancer screening via visual inspection with acetic acid or Lugol's iodine at Mulago Hospital, Uganda. Int. J. Gynaecol. Obstet. 2012;119(3):262–265. doi: 10.1016/j.ijgo.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 25.World Population Prospects, The 2015 Revision, United Nations Department of Economic and Social Affairs, Population Division. (Accessed on 12 January 2015 at 〈http://esa.un.org/unpd/wpp/DVD/〉).
- 26.Ministry of Health, Strategic Plan for Cervical Cancer Prevention and Control in Uganda: 2010–2014, April 2010, Kampala, Uganda. 〈http://www.rho.org/files/PATH_Uganda_cxca_strat_plan_2010-2014.pdf〉.
- 27.Bbaale E. Factors influencing childhood immunization in Uganda. J. Health, Popul. Nutr. 2013;31(1):118–129. doi: 10.3329/jhpn.v31i1.14756. [DOI] [PMC free article] [PubMed] [Google Scholar]