Abstract
Background
Data regarding anal cutaneous HPV detection among HIV-positive and HIV-negative persons largely relies on studies among men who have sex with men in limited geographical settings. Understanding the distribution, determinants, and potential human health effects of anal cutaneous HPV types among men who have sex with women (MSW) is important.
Methods
Anal canal swab samples from 415 Russian MSW (384 HIV-negative and 31 HIV-positive) were tested for 43 β-HPVs and 29 γ-HPVs, using a multiplex PCR combined with Luminex technology.
Results
β-HPV was detected in 24.4% and γ-HPV in 15.9% of anal samples of all Russian MSW. In total, 34 β-HPV and 19 γ-HPV types were detected, with the most commonly detected β-HPV types being 110, 22 and 124 and the most common γ-HPV types being 95, 132 and 50. For both genera, being HIV-positive at the time of testing was a significant determinant of detection (74.2% for β-HPVs and 48.4% for γ-HPVs compared to 20.1% and 12.5% in HIV-negative MSW, respectively).
Conclusions
A wide spectrum and moderate prevalence of anal β-HPV and γ-HPV types was found in our MSW study sample, suggesting that routes other than penile-anal intercourse may be important in cutaneous HPV transmission.
Keywords: Anal cutaneous HPV, Beta-HPV, Gamma-HPV, HIV-negative MSW, Penile-anal, HPV transmission
Highlights
-
•
β and γ HPV types commonly colonize the anal canal of MSW, but their geographical variation in prevalence could be wide.
-
•
HIV-positive men were more likely to have both genera of HPV types detected.
-
•
Routes other than penile-anal intercourse may be important in cutaneous HPV transmission.
1. Introduction
Human papillomaviruses (HPV) are a broad and diverse group of viruses which include types that infect the human mucosa and those that are present at anatomical cutaneous sites [1]. Currently, 205 HPV types have been identified [2], with the majority belonging to the Alphapapillomavirus (α), Betapapillomavirus (β), and Gammapapillomavirus (γ) genera, depending on their epithelial tropism [3]. Mucosal high-risk HPVs belonging to the α-genus are known to be associated with the development of several types of cancer, including anogenital cancer [4]. There is some evidence of the oncogenic potential of non-α-HPV, cutaneous types based on their proposed and speculated role in skin cancer (for β-HPVs [5], [6], [7], [8] and γ-HPVs [9], [10], respectively). However, compared to α-HPVs, the distribution, etiology, and possible role of cutaneous types in the development of cancer is largely unknown.
Several recent studies among men who have sex with men (MSM) have described a high prevalence and broad spectrum of β- and γ-HPVs in the anal canal [11], [12], [13]. Relatively little, however, was known about the prevalence of cutaneous HPVs among men who have sex with women (MSW) until a recent pair of publications using data from the HPV in men (HIM) study [14], [15]. Data from this study not only suggested an equally high prevalence of β-HPV among both MSM and MSW, but their results also indicated a wide geographical variation in prevalence (65.6% in Mexico vs 48.6% in Brazil and 48.7% in the US) [15].
Further, much of the mechanistic hypotheses regarding cutaneous HPV transmission to genital sites are speculative, and are based on studies among MSM and HIV+ populations. Among MSMs, it is thought that infection primarily occurs mechanistically via penile-anal insertion, as evidence points to the transmission of cutaneous HPV between anatomical sites [14]. However, HIM's recent findings that MSWs are equally likely to harbour β-HPV, strengthened by the lack of association between β-HPV prevalence and sexual behaviours, suggest that alternative transmission routes may exist. Further, among HIV+ individuals, molecular mechanisms are thought to potentiate HPV penetration into the epithelium, although a larger body of evidence is needed to better understand this process [16].
The number of studies on anal cutaneous HPVs is remarkably limited, and information on the anal presence and determinants of HPVs in males, especially among non-MSM populations, remains especially unclear. Therefore, the aim of the current retrospective study is to evaluate the prevalence of anal β- and γ-HPVs in a large cohort of MSW from Russia, the first such known sample in the Eastern hemisphere.
2. Methods
2.1. Study population
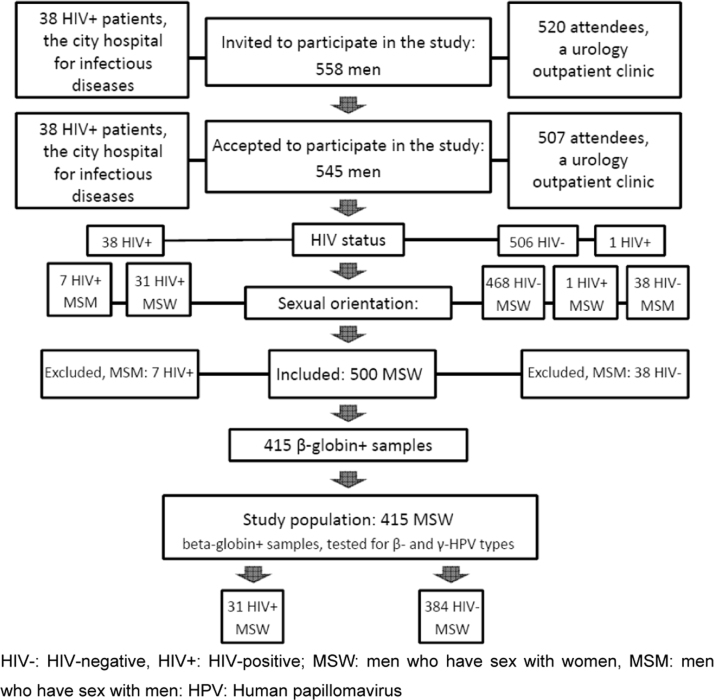
We retrospectively analysed 415 DNA-positive anal samples obtained from MSWs as part of two clinic-based studies conducted in St. Petersburg, Russia. The first [17] was a pilot study investigating rectal Chlamydia LGV infection performed in an infectious disease hospital providing treatment for HIV+ MSM and MSW, from December 2005 to January 2006. The second [18] was conducted among men seeking routine STI testing at the urology units of two university outpatient clinics, from February 2006 to February 2009. Out of 558 men who were originally invited to participate, 545 agreed (38 infectious disease hospital; 507 urology clinic) (Fig. 1). For the purposes of this study, 45 MSMs were excluded from analyses. Of the remaining 500 samples, 85 were found to be absent of β-globin, indicating no DNA was present in sample, leaving a total of 415 samples for analyses.
Fig. 1.
Flow chart of study population selection. HIV-: HIV-negative, HIV+: HIV-positive; MSW: men who have sex with women, MSM: men who have sex with men: HPV: Human papillomavirus.
Men at both the infectious disease clinic and the urology clinic who were at least 18 years old (verified by clinic staff upon appointment registration) and who reported no anorectal disorders were invited to participate in the study upon entering the clinic exam room, after which time a medical history and a standard physical examination were completed. MSW versus MSM status was determined for all participants through self-report of whether a man had had sex with another man during his lifetime (MSM) or not (MSW). Urological participants also completed a questionnaire concerning sexual behaviour (age at the time of sexual debut and number of lifetime sex partners); data on sexual behaviour were not available for infectious disease hospital participants.
HIV status was determined for infectious disease hospital patients by referring to the most recent test performed as part of their clinical care. For urology participants, a blood sample was taken for HIV testing at the time of enrollment. All participants having received an HIV+ test prior to this study were receiving HAART therapy at the time of enrollment.
All participants provided written informed consent, during which the anonymity of their data, especially in regards to its sexual nature, was stressed. The institutional review board of St. Petersburg Medical Academy of Postgraduate Studies approved the study.
DNA samples and questionnaire data were anonymized, and no study personnel besides the principle investigator had access to participants’ identifying information.
3. Collection of samples and DNA extraction
To sample the anal canal, a urethral brush wetted with phosphate buffered saline was inserted 1.5–2.0 centimetres from the anal verge of the canal and rotated 180° to the right and left. The swab was removed from the anal canal and then rinsed in 1000 µl of phosphate buffered saline in each of two separate tubes. The first sample was used for the detection of STIs, when applicable (MSM and HIV+ men). The second was placed in a refrigerator at 4 °C. At the end of the day, the samples were transferred to a −20 °C freezer and stored until HPV testing.
Using a 100 µl aliquot of the original anal sample, DNA was extracted in the laboratory of the Department of Laboratory Medicine at Karolinska Institutet (Stockholm, Sweden), using a freeze-thaw-boil procedure, as previously described [19]. The frozen DNA samples were shipped on dry ice to the laboratory of the Group of Infections and Cancer Biology at IARC (Lyon, France) for β- and γ-HPV-specific genotyping.
4. Detection and genotyping of HPV
Anal samples were tested for the presence of HPVs using type-specific polymerase chain reaction (PCR) bead-based multiplex genotyping (TS-MPG) assays that combine multiplex PCR and Luminex technology (Luminex Corp., Austin, TX, USA), as described elsewhere [15], [20], [21], [22], [23], [24]. The multiplex type-specific PCR method uses specific primers for the detection of 43 β-HPVs (species β−1: 5, 8, 12, 14, 19, 20, 21, 24, 25, 36, 47, 93; β−2: 9, 15, 17, 22, 23, 37, 38, 80, 100, 104, 107, 110, 111, 113, 120, 122, 145, 151; β−3: 49, 75, 76, 115; β−4: 92; β−5: 96, 150) and 29 γ-HPVs (species γ−1: 4, 65, 95; γ−2: 48; γ−3: 50; γ−4: 156; γ−5: 60, 88; γ−6: 101, 103, 108; γ−7: 109, 123, 134, 149; γ−8: 112, 119; γ−9: 116, 129; γ−10: 121, 130, 133; γ−11: 126; γ−12: 127, 132, 148; γ−13: 128; γ−14: 131; and HPV-SD2 [25]). HPV type species were classified according to de Villiers [3]. Two primers for the amplification of the β-globin gene were included to provide a positive control for the quality of the DNA in the sample [26]. Following multiplex PCR amplification, 10 µl of each reaction mixture was analysed by multiplex genotyping using the Luminex technology [20], [23].
5. Statistical analysis
In total, 545 out of 558 men who were invited (97.7%) agreed to participate (Fig. 1), including 38 HIV+ patients.
According to the questionnaire given, if a man reported having had sex (anal or oral) with other men during his lifetime he was categorized as MSM. If he reported having sex with only women, he was classified as MSW. Of those enrolled, 500 men (91.7%) reported being MSW and 45 (8.3%) MSM. To avoid the confounding effect of MSM versus MSW, MSMs (n=45) were excluded from the analyses. In addition, 85 men having β-globin-negative samples were excluded, resulting in 385 men in the present analysis; 31 HIV+ and 354 HIV-.
This analysis examined the prevalence of β- and γ-HPV types in a population of HIV+ and HIV- MSW, as well as associations between these types and sociodemographic characteristics. The proportions of β- and γ-HPVs were calculated overall and by HIV status. Odds Ratios (OR) and 95% confidence intervals (CI) were calculated using logistic regression to assess associations. Specifically, separate univariate logistic regression was used to evaluate the association between β- and γ-HPV and HIV status (positive versus negative). Univariate and multivariate logistic regression was also used to evaluate the association between β- and γ-HPV detection (separately) and age, age at sexual debut, and number of lifetime sexual partners (categorical) among HIV-negative participants. All analyses were completed using Stata versions 11.0 and 14.0 (StataCorp, College Station, TX, USA).
6. Results
Among the sample of 385 MSW, the median age was 31.5 years (range 18–60), the median age at sexual debut was 17.5 years (range 9–27, 61.8% between ages 16–19) and the median number of sexual partners was 15 (range 1–700).
The overall prevalence of β- and γ-HPVs was 24.4% (n=94) and 13.8% (n=53), respectively (Table 1). Among the 354 HIV- MSW, anal β-HPV positivity was 20.1% (n=71) and anal γ-HPV positivity was 10.7% (n=38) (Table 1). Among the 31 HIV+ MSW, β-HPV positivity was 74.2% (n=23) and γ-HPV positivity was 48.4% (n=15). Multiple β- infections were more prevalent in HIV+ than in HIV- MSW (56.5% vs 21.1%) (Table 1). Due to the lack of the sociodemographic (age) and sexual behaviour characteristics collected from HIV+ MSW, only univariate analyses are presented. β- and γ-HPV types were significantly more likely to be detected in the anal samples of HIV+ compared to HIV- MSW, with an odds ratio of 11.46 (95%CI: 4.92–26.69) and 7.80 (95%CI: 3.57–17.02) for β- and γ-HPV, respectively.
Table 1.
Univariate and multivariate associations between HIV status and likelihood of having any β- or γ-HPV detected in anal samples of n=385 MSW.
|
β-HPVa |
γ-HPVa |
|||||
|---|---|---|---|---|---|---|
| any | >1 | Unadjusted | any | >1 | Unadjusted | |
| β-HPV | β-HPVb | OR | γ-HPV | γ-HPVb | OR | |
| HIV status (%) | ||||||
| HIV- | 71/354 | 15/71 | ref | 38/354 | 8/38 | ref |
| (20.1) | (21.1) | (10.7) | (21.1) | |||
| HIV+ | 23/31 | 13/23 | 11.46 | 15/31 | 2/15 | 7.80 |
| (74.2) | (56.5) | (4.92–26.69) | (48.4) | (13.3) | (3.57–17.02) | |
| Total number (%) | ||||||
| 94/385 | 28/94 | 53/385 | 10/53 | |||
| (24.4) | (29.8) | (13.8) | (18.9) | |||
HIV-: HIV-negative; HIV+: HIV-positive
Includes participants who had at least one interpretable (β-globin positive) anal sample for any of the two HPV genus types tested (β-HPVs, and γ-HPVs)
Among participants who tested positive for that genera of HPV
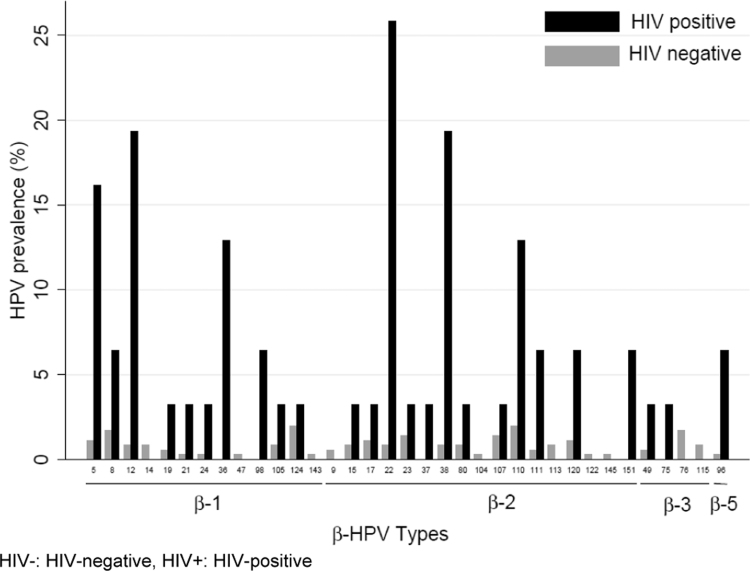
With regard to β-HPV, 34 out of 43 β-HPV types were detected among the MSW studied. The most frequently detected β-HPVs among HIV- MSW were HPV types 110 (2.0%), 124 (2.0%) and 22 (0.9%). Among HIV+ MSW, HPV types 22 (25.8%), 12 (19.4%), 38 (19.4%) and 5 (16.1%) were most frequently detected (Fig. 2). On the level of species, β2-HPV was most common among both HIV- and HIV+ MSW (Fig. 2).
Fig. 2.
Genotype-specific prevalence for β- HPVs by HIV status. HIV-: HIV-negative, HIV+: HIV-positive.
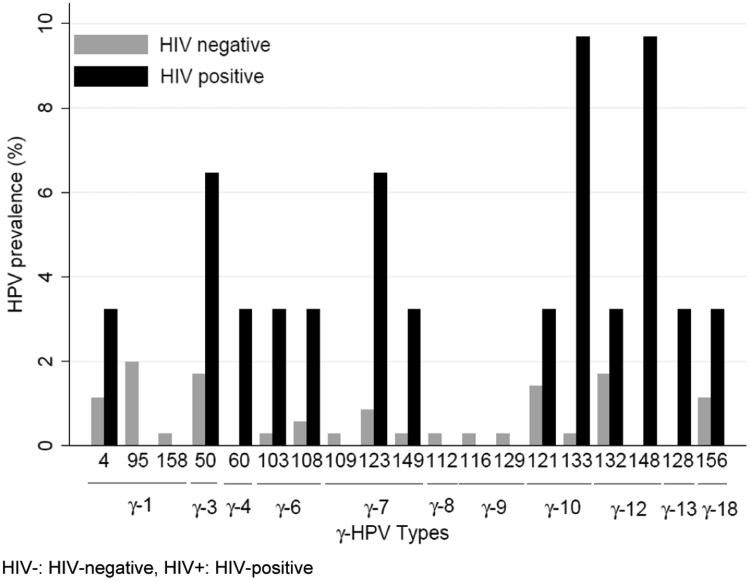
A total of 19 out of 29 γ-HPV types were detected. The most commonly detected γ-HPV types were 95, 132, 50 in HIV- MSW and types 148, 133, 123 and 50 in HIV+ MSW (Fig. 3). Among HIV- MSW, HPV species γ−1 was the most common among HIV- and γ-HPV species 10, 7, 6 and 12 were most common among HIV+. γ-HPV species 4 and 13 were found only among HIV+ men.
Fig. 3.
Genotype-specific prevalence for γ-HPVs by HIV status. HIV-: HIV-negative, HIV+: HIV-positive.
A multivariate analysis was conducted among the 384 HIV- MSW (Table 2). The adjusted ORs for the association between total number of lifetime female sexual partners and β-HPV positivity varied from 2.66 (6–10 partners; 95%CI: 1.00–7.03) to 4.54 (95%CI: 1.74–11.87). No significant association was seen between number of lifetime sexual partners and detection of γ-HPV (Table 2). In addition, later age at sexual debut was not significantly associated with anal β-HPV or γ-HPV infection.
Table 2.
Univariate and multivariate associations between sociodemographic and sexual behaviour risk factors and having β-HPVs or γ-HPVs detected of anal samples of n=354 HIV-negative MSW.
| Background characteristica,b | Total number (%) |
β-HPVa |
γ-HPVa |
||
|---|---|---|---|---|---|
| Unadjusted OR | Adjusted ORc | Unadjusted OR | Adjusted ORc | ||
| Age | |||||
| 18–24 | 71 (20.2) | 1 | 1 | 1 | 1 |
| 25–29 | 88 (25.1) | 2.26 (0.97–5.29) | 1.95 (0.82–4.64) | 0.46 (0.17–1.27) | 0.43 (0.15–1.20) |
| 30–34 | 92 (26.2) | 1.32 (0.54–3.22) | 1.04 (0.41–2.63) | 0.51 (0.19–1.35) | 0.47 (0.17–1.30) |
| 35–39 | 48 (13.7) | 2.02 (0.76–5.32) | 1.32 (0.47–3.74) | 0.77 (0.26–2.24) | 0.57 (0.18–1.80) |
| 40+ | 52 (14.8) | 2.03 (0.78–5.27) | 1.32 (0.48–3.62) | 0.70 (0.24–2.03) | 0.57 (0.18–1.80) |
| Age at sexual debut | |||||
| <16 | 62 (17.7) | 1 | 1 | 1 | 1 |
| 16–19 | 217 (61.8) | 1.47 (0.67–3.21) | 1.81 (0.80–4.11) | 0.96 (0.39–2.35) | 1.10 (0.43–2.81) |
| 20+ | 72 (20.5) | 1.79 (0.73–4.36) | 2.69 (1.00–7.25) | 0.83 (0.27–2.52) | 1.04 (0.31–3.54) |
| Number of lifetime sexual partners | |||||
| 0–5 | 76 (21.8) | 1 | 1 | 1 | 1 |
| 6–10 | 78 (22.4) | 2.35 (0.90–6.13) | 2.66 (1.00–7.03) | 0.97 (0.32–2.92) | 0.98 (0.32–2.99) |
| 11–20 | 73 (20.9) | 4.06 (1.60–10.27) | 4.54 (1.74–11.87) | 1.06 (0.35–3.19) | 1.10 (0.36–3.40) |
| >20 | 122 (35.0) | 2.80 (1.15–6.80) | 3.63 (1.40–9.41) | 1.60 (0.63–4.05) | 1.65 (0.59–4.58) |
Includes participants who had at least one interpretable (β-globin positive) anal sample for any of the two HPV genus types tested (β-HPV and γ-HPV gamma)
The following variables had missing values: age (4 missing); age at sexual debut (4 missing); number of lifetime sexual partners (6 missing)
Adjusted for age, age at sexual debut, and number of lifetime sex partners
7. Discussion
We found cutaneous β- and γ-HPV types to be relatively common in the anal canal of Russian MSW men (24% and 14%, respectively). Our findings follow those from a pair of publications on HPV prevalence in the HIM study across three countries in the Western hemisphere, Mexico, Brazil and the USA, reported β-HPV prevalences of 65.6%, 48.6%, and 48.7%, respectively [15] Findings of 66% and 27% β-HPV prevalence in Spain and Italy have also been reported [12], [13]. The current study is the largest known cohort of MSW from the Eastern hemisphere tested for a broad range of β- and γ- HPV types using technology similar to the aforementioned studies (Luminex). Taken together, these findings suggest a large variation in cutaneous HPV prevalence worldwide.
A low number of MSM enrolled in this study, and were therefore excluded from the main analyses. Discrimination against MSM populations in the country is a possibility, thus limiting their inclusion in large research efforts at present [27], [28]. However, data from other studies shows that HPVs were detected more often in MSM than MSW [11], [29]. These findings suggest that penile-anal sexual activity might indeed result in acquiring cutaneous HPV infections in some individuals, but the relatively common detection of these types in MSW does not allow this theory to entirely describe transmission.
Several hypotheses might explain transmission of cutaneous HPV types to the anal canal among MSW. Cutaneous HPV virions may be transmitted to the anal canal from other parts of the body that harbour active infections, as the presence of HPV DNA does not necessarily indicate the presence of infectious virus [30]. Indeed, cutaneous HPV is commensal by nature, and commonly detected in many regions of the body, including but not limited to the oral cavity [31] and gargles [32], nostril [32] and oesophageal [33] mucosa. Supportive of a role for non-sexual transmission routes, neither the HIM study [14], [15] nor our results found any strong associations between sexual activity and HPV detection. Another hypothesis is that HPV transmission could occur via sexual transmission but through routes apart from penile-anal, such as finger-genital transmission via self- or partner-initiated inoculation [15], [34], [35]. Unfortunately, men in our study were not asked to provide details concerning routine sexual practices that could involve anal touching (frequency of anal touching, use of sex toys, etc.). Future studies among MSM and MSW evaluating both non-sexual and sexual transmission routes including self-inoculation could be helpful in further establishing probable transmission routes.
Other limitations of our study do exist. For one, it is possible that men did not fully disclose sexual relations with other men, which could have effectively led to misclassification of MSM versus MSW. However, it is unlikely that this accounts for all MSW participants in whom HPV was detected. Further, due to the transient nature of HPV infection, it is likely that current infection status is due to recent sexual activity than lifetime sexual history. Therefore, it is possible among our study population that MSW versus MSM classification based on lifetime report of having ever had sex with another man was not entirely representative of recent sexual activity, leading to a potential loss of information among men who were MSM but whose recent behaviour was more similar to MSW activities, for example. Also, we were not able to collect data on key sociodemographic and sexual risk factors for HIV+ subjects, and were therefore limited in our ability to make any interpretations of adjusted risk among this population. Finally, the commensal nature of cutaneous HPV makes it inherently much more difficult to establish associations between its prevalence and important determinants compared to α-HPVs. Thus, we can only continue to accumulate evidence to build on this topic to move beyond this limitation.
Evidence regarding the relationship between anal cutaneous HPVs and HIV status is still building. In one prospective study, HIV+ status was significantly associated with a higher prevalence of anal β-HPV infection among the 135 Slovenian MSM [11]. In contrast, a study of 609 Italian MSM showed no significant association between β- and γ-HPV infection and HIV status, socio-demographic or sexual behavioral factors [12]. However, enrolled HIV+ were older than HIV- MSM and most of them were receiving combined antiretroviral therapy at the time of sampling [12]. In the current study, anal prevalence β- and γ-HPV detection was significantly higher in HIV+ than in HIV- men, although the low sample size of HIV+ is recognized as a limitation. Future studies with larger sample sizes may be able to strengthen the evidence base of the relationship between HIV and cutaneous HPV.
The current study provides additional evidence that cutaneous HPVs are a relatively common finding in the anal canal of MSW, favouring the hypothesis that transmission routes other than penile-anal exist for these types of HPV. Preliminary comparison with other recent findings indicates that it is possible that their prevalence in men worldwide could vary (Table 3). However, it must be acknowledged that direct comparisons between this and other studies should be regarded with caution as the laboratory techniques used may differ across studies as may do the choice of underlying population with regard to α-HPV infection status. Nevertheless, we believe that understanding the difference in prevalence across populations is the first step to understanding the etiology of cutaneous HPV infection. Understanding determinants of infection and high-risk groups is also potentially important information as we continue to refine our knowledge of the role of cutaneous HPVs in shared and distinct pathways for cancer pathogenesis [36], in particular through activation of DNA damage and TLR9 signalling pathways. Interestingly, β-HPV type 38 was recently discovered to the property of repressing the TLR9 signalling pathway in common with oncogenic viruses [8].
Table 3.
Reported varied worldwide prevalence for β- and γ-HPV types in the male anal canal.
| Study country | Study population | HPV genotyping, technique employed | Studied samples |
β-HPV types |
γ-HPV types |
||
|---|---|---|---|---|---|---|---|
| Overall prevalence | Most common | Overall prevalence | Most common | ||||
| Russia, this study | HIV- MSW | Luminex | 354 | 20.1 | 110, 124, 22 | 10.7 | 95, 132, 50 |
| HIV+ MSW | Luminex | 31 | 74.2 | 22, 12, 38 | 48.4 | 148, 133, 123 | |
| USA (15) | HIV- men | Luminex | 238 | 48.7 | 38, 21, 22 | ||
| Brazil (15) | HIV- men | Luminex | 241 | 48.6 | |||
| Mexico (15) | HIV- men | Luminex | 238 | 65.6 | |||
| Italy (12) | HIV- MSM | Luminex | 602 | 27.2 | 111, 120, 24 | ||
| HIV+ MSM | Luminex | 602 | 28.6 | 111, 120, 38 | |||
| HIV- MSM | Luminex | 597 | 27.9 | 121, 50, 133 | |||
| HIV+ MSM | Luminex | 597 | 33.5 | 121, 108, 132 | |||
| Spain (13) | HIV- MSM | Luminex | 153 | 59.1 | 38, 120 | 57.7 | 133, 121 |
| HIV+ MSM | Luminex | 66 | 65.6 | 12, 107 | 68.2 | 121, 50 | |
| Slovenia (11) | HIV- MSM | HPV Linear Array | 112 | 58.9 | 36, 38, 23, 24 | ||
| HIV+ MSM | HPV Linear Array | 23 | 95.7 | ||||
HIV-: HIV-negative; HIV+: HIV-positive
In summary, our findings indicate that β- and γ-HPV types may be relatively common in the anal canal of MSW men. Therefore, knowledge regarding β- and γ-HPV types and their distribution in diverse male populations is essential for developing a better understanding of the natural history, transmission dynamics, and the potential role of different HPV types as co-factors in the development of anogenital malignancies.
Funding and conflict of interests
The work reported in this paper was undertaken during the tenure of a Postdoctoral Fellowship from the International Agency for Research on Cancer, partially supported by the European Commission FP7 Marie Curie Actions – People – Co-funding of regional, national and international programmes (COFUND). Introduction to STI outpatient clinic work with specific attention for interviewing and sampling high-risk populations in Amsterdam, the Netherlands was supported through the UNESCO-American Society for Microbiology (Travel Award 2006 to V. Smelov). This work was supported in part by the Swedish Cancer Society (Scholarship 2011 to Vitaly Smelov). The funders had no role in study design, data collection and analysis.
Ethical approval
Ethical Committee of the Department of Clinical Investigations and Intellectual Property of St. Petersburg Medical Academy of Postgraduate Studies (North-Western State Medical University named after I.I. Mechnikov since 2011) under the Federal Agency of Public Health and Social Development of Roszdrav (Extract from Minutes No. 10 of SPbMAPS Ethical Committee meeting; date of approval: 10 November 2010).
Acknowledgments
The authors are grateful to the personnel of the Laboratory of Microbiology at the D.O. Ott Research Institute of Obstetrics and Gynecology, St. Petersburg State University Outpatient clinic and St. Petersburg City Infectious Diseases Hospital (all are in St. Petersburg, Russia), Laboratory of Immunogenetics at the VU University Medical Center (Amsterdam, the Netherlands) and the Department of Laboratory Medicine at the Karolinska Institutet (Stockholm, Sweden) for their dedicated technical and administrative support. The authors are grateful to Dr. Catherine Sauvaget (Screening Group, WHO/IARC, Lyon, France) for careful reading and comments. The authors are deeply grateful to Dr. Servaas Morré and Mrs. Jolijne Plejster (VU University Medical Center, Amsterdam) for the proper storing the samples and Prof. Joakim Dillner (Karolinska Institutet, Stockholm) for the support and organizing their shipments between the institutions.
References
- 1.Bzhalava D., Guan P., Franceschi S., Dillner J., Clifford G. A systematic review of the prevalence of mucosal and cutaneous human papillomavirus types. Virology. 2013;445(1–2):224–231. doi: 10.1016/j.virol.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 2.Reference clones at International HPV Reference Center [Internet]. [cited 2015 Jun 4]. Available from: 〈http://www.hpvcenter.se/html/refclones.html?%3C?Php%20echo%20time();%20?%3E〉.
- 3.de Villiers E.-M. Cross-roads in the classification of papillomaviruses. Virology. 2013;445(1–2):2–10. doi: 10.1016/j.virol.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 4.International Agency for Research on Cancer . IARC; Lyon: 2012. Monographs on the evaluation of carcinogenic risks to humans, volume 100 B, biological agents; p. 475. [Google Scholar]
- 5.C.M. Proby, C.A. Harwood, R.E. Neale, A.C. Green, S. Euvrard, L. Naldi, et al. A case-control study of betapapillomavirus infection and cutaneous squamous cell carcinoma in organ transplant recipients. Am J Transplant. 2011, vol. 11(7):pp. 1498–1508. [DOI] [PubMed]
- 6.Neale R.E., Weissenborn S., Abeni D., Bavinck J.N.B., Euvrard S., Feltkamp M.C.W. Human papillomavirus load in eyebrow hair follicles and risk of cutaneous squamous cell carcinoma. Cancer Epidemiol. Biomark. Prev. 2013;22(4):719–727. doi: 10.1158/1055-9965.EPI-12-0917-T. [DOI] [PubMed] [Google Scholar]
- 7.Struijk L., Bouwes Bavinck J.N., Wanningen P., van der Meijden E., Westendorp R.G.J., Ter Schegget J. Presence of human papillomavirus DNA in plucked eyebrow hairs is associated with a history of cutaneous squamous cell carcinoma. J. Invest Dermatol. 2003;121(6):1531–1535. doi: 10.1046/j.1523-1747.2003.12632.x. [DOI] [PubMed] [Google Scholar]
- 8.Pacini L., Savini C., Ghittoni R., Saidj D., Lamartine J., Hasan U.A. Downregulation of toll-like receptor 9 Expression by beta human papillomavirus 38 and implications for cell cycle control. J. Virol. 2015;89(22):11396–11405. doi: 10.1128/JVI.02151-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waterboer T., Abeni D., Sampogna F., Rother A., Masini C., Sehr P. Serological association of beta and gamma human papillomaviruses with squamous cell carcinoma of the skin. Br. J. Dermatol. 2008;159(2):457–459. doi: 10.1111/j.1365-2133.2008.08621.x. [DOI] [PubMed] [Google Scholar]
- 10.Paradisi A., Waterboer T., Sampogna F., Tabolli S., Simoni S., Pawlita M. Seropositivity for human papillomavirus and incidence of subsequent squamous cell and basal cell carcinomas of the skin in patients with a previous nonmelanoma skin cancer. Br. J. Dermatol. 2011;165(4):782–791. doi: 10.1111/j.1365-2133.2011.10403.x. [DOI] [PubMed] [Google Scholar]
- 11.Mlakar B., Kocjan B.J., Hošnjak L., Fujs Komloš K., Milošević M., Poljak M. Betapapillomaviruses in the anal canal of HIV positive and HIV negative men who have sex with men. J. Clin. Virol. 2014;61(2):237–241. doi: 10.1016/j.jcv.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Donà M.G., Gheit T., Latini A., Benevolo M., Torres M., Smelov V. Alpha, beta and gamma Human Papillomaviruses in the anal canal of HIV-infected and uninfected men who have sex with men. J. Infect. 2015;71(1):74–84. doi: 10.1016/j.jinf.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Torres M., Gheit T., McKay-Chopin S., Rodríguez C., Romero J.D., Filotico R. Prevalence of beta and gamma human papillomaviruses in the anal canal of men who have sex with men is influenced by HIV status. J. Clin. Virol. 2015;67:47–51. doi: 10.1016/j.jcv.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Sichero L., Nyitray A.G., Nunes E.M., Nepal B., Ferreira S., Sobrinho J.S. Diversity of human papillomavirus in the anal canal of men: the HIM study. Clin. Microbiol Infect. 2015 doi: 10.1016/j.cmi.2014.12.023. (Jan 14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nunes E.M., Sudenga S.L., Gheit T., Tommasino M., Baggio M.L., Ferreira S. Diversity of beta-papillomavirus at anogenital and oral anatomic sites of men: the HIM study. Virology. 2016;495:33–41. doi: 10.1016/j.virol.2016.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tugizov S.M., Herrera R., Chin-Hong P., Veluppillai P., Greenspan D., Michael Berry J. HIV-associated disruption of mucosal epithelium facilitates paracellular penetration by human papillomavirus. Virology. 2013;446(1–2):378–388. doi: 10.1016/j.virol.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smelov, Vitaly, Catsburg, Arnold, Shipitsyna, Elena, de Vries, Henry J.C., Shalepo, Kira, Gorelov, Andrey, et al. Chlamydia trachomatis infections in St. Petersburg, Russia: Preliminary serogroup distribution results in men. In: Chlamydial Infections: Proceedings of the Eleventh International Symposium on Human Chlamydial Infections. Niagara-on-the-Lake, Ontario, Canada: ed. / M. Chernesky; H. Caldwell; G. Christiansen. San Francisco, CA, 2006.
- 18.Smelov V., Eklund C., Bzhalava D., Novikov A., Dillner J. Expressed prostate secretions in the study of human papillomavirus epidemiology in the male. PloS One. 2013;8(6):e66630. doi: 10.1371/journal.pone.0066630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forslund O., Antonsson A., Edlund K., van den Brule A.J.C., Hansson B.-G., Meijer C.J.L.M. Population-based type-specific prevalence of high-risk human papillomavirus infection in middle-aged Swedish women. J. Med Virol. 2002;66(4):535–541. doi: 10.1002/jmv.2178. [DOI] [PubMed] [Google Scholar]
- 20.Schmitt M., Bravo I.G., Snijders P.J.F., Gissmann L., Pawlita M., Waterboer T. Bead-based multiplex genotyping of human papillomaviruses. J. Clin. Microbiol. 2006;44(2):504–512. doi: 10.1128/JCM.44.2.504-512.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gheit T., Billoud G., de Koning M.N.C., Gemignani F., Forslund O., Sylla B.S. Development of a sensitive and specific multiplex PCR method combined with DNA microarray primer extension to detect Betapapillomavirus types. J. Clin. Microbiol. 2007;45(8):2537–2544. doi: 10.1128/JCM.00747-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruer J.B., Pépin L., Gheit T., Vidal C., Kantelip B., Tommasino M. Detection of alpha- and beta-human papillomavirus (HPV) in cutaneous melanoma: a matched and controlled study using specific multiplex PCR combined with DNA microarray primer extension. Exp. Dermatol. 2009;18(10):857–862. doi: 10.1111/j.1600-0625.2009.00866.x. [DOI] [PubMed] [Google Scholar]
- 23.Schmitt M., Dondog B., Waterboer T., Pawlita M., Tommasino M., Gheit T. Abundance of multiple high-risk human papillomavirus (HPV) infections found in cervical cells analyzed by use of an ultrasensitive HPV genotyping assay. J. Clin. Microbiol. 2010;48(1):143–149. doi: 10.1128/JCM.00991-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hampras S.S., Giuliano A.R., Lin H.-Y., Fisher K.J., Abrahamsen M.E., Sirak B.A. Natural history of cutaneous human papillomavirus (HPV) infection in men: the HIM study. PloS One. 2014;9(9):e104843. doi: 10.1371/journal.pone.0104843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mokili J.L., Dutilh B.E., Lim Y.W., Schneider B.S., Taylor T., Haynes M.R. Identification of a novel human papillomavirus by metagenomic analysis of samples from patients with febrile respiratory illness. PloS One. 2013;8(3):e58404. doi: 10.1371/journal.pone.0058404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saiki R.K., Gelfand D.H., Stoffel S., Scharf S.J., Higuchi R., Horn G.T. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- 27.Men having sex with men in Eastern Europe: Implications of a hidden HIV epidemic. Regional analysis report. [Internet]. AIDS Alliance USAID; 2010. Available from: 〈http://www.aidstar-two.org/upload/MSM-Regional-Report_Final_November-2-2010.pdf〉.
- 28.Wirtz A.L., Zelaya C.E., Peryshkina A., Latkin C., Mogilnyi V., Galai N. Social and structural risks for HIV among migrant and immigrant men who have sex with men in Moscow, Russia: implications for prevention. AIDS Care. 2014;26(3):387–395. doi: 10.1080/09540121.2013.819407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nyitray A.G., Carvalho da Silva R.J., Baggio M.L., Smith D.’elle, Abrahamsen M., Papenfuss M. Six-month incidence, persistence, and factors associated with persistence of anal human papillomavirus in men: the HPV in men study. J. Infect. Dis. 2011;204(11):1711–1722. doi: 10.1093/infdis/jir637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smelov V., Eklund C., Arroyo Mühr L.S., Hultin E., Dillner J. Are human papillomavirus DNA prevalences providing high-flying estimates of infection? An international survey of HPV detection on environmental surfaces. Sex. Transm. Infect. 2013;89(8):627. doi: 10.1136/sextrans-2013-051280. [DOI] [PubMed] [Google Scholar]
- 31.Bottalico D., Chen Z., Dunne A., Ostoloza J., McKinney S., Sun C. The oral cavity contains abundant known and novel human papillomaviruses from the Betapapillomavirus and Gammapapillomavirus genera. J. Infect. Dis. 2011;204(5):787–792. doi: 10.1093/infdis/jir383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forslund O., Johansson H., Madsen K.G., Kofoed K. The nasal mucosa contains a large spectrum of human papillomavirus types from the Betapapillomavirus and Gammapapillomavirus genera. J. Infect. Dis. 2013;208(8):1335–1341. doi: 10.1093/infdis/jit326. [DOI] [PubMed] [Google Scholar]
- 33.Tornesello M.L., Monaco R., Nappi O., Buonaguro L., Buonaguro F.M. Detection of mucosal and cutaneous human papillomaviruses in oesophagitis, squamous cell carcinoma and adenocarcinoma of the oesophagus. J. Clin. Virol. 2009;45(1):28–33. doi: 10.1016/j.jcv.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 34.Sonnex C., Strauss S., Gray J.J. Detection of human papillomavirus DNA on the fingers of patients with genital warts. Sex. Transm. Infect. 1999;75(5):317–319. doi: 10.1136/sti.75.5.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hernandez B.Y., Wilkens L.R., Zhu X., Thompson P., McDuffie K., Shvetsov Y.B. Transmission of human papillomavirus in heterosexual couples. Emerg. Infect. Dis. 2008;14(6):888–894. doi: 10.3201/eid1406.070616.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galloway D.A., Laimins L.A. Human papillomaviruses: shared and distinct pathways for pathogenesis. Curr. Opin. Virol. 2015;14:87–92. doi: 10.1016/j.coviro.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]