Abstract
Background
Classically the oxidative stress and more recently inflammatory processes have been identified as the major causes of brain aging. Oxidative stress and inflammation affect each other, but there is more information about the effects of oxidative stress on aging than regarding the contribution of inflammation on it.
Methods
In the intense research for methods to delay or mitigate the effects of aging, are interesting polyphenols, natural molecules synthesized by plants (e.g. resveratrol). Their antioxidant and anti-inflammatory properties make them useful molecules in the prevention of aging.
Results
The antiaging effects of polyphenols could be due to several related mechanisms, among which are the prevention of oxidative stress, SIRT1 activation and inflammaging modulation, via regulation of some signaling pathways, such as NF-κB.
Conclusion
In this review, we describe the positive effects of polyphenols on the prevention of the changes that occur during aging in the brain and their consequences on cognition, emphasizing the possible modulation of inflammaging by polyphenols through a SIRT1-mediated mechanism.
Keywords: Polyphenols, aging, brain, oxidative stress, inflammation, SIRT1, NF-κB
1. INTRODUCTION
The development of medicine and technology has helped to increase the proportion of the aging population worldwide. This demographic context has created the need to better understand the changes that occur in the brain during aging and to find strategies to prevent, delay, or mitigate their consequences. Regarding this, nowadays age-related cognitive decline and development of dementia have been one of the most pressing health issues. Therefore in order to improve senior citizens’ lives, a growing body of research is devoted to the identification of the mechanisms that both produce and moderate the aging process [1]. Molecular mechanisms involved in the aging process are not yet well known, but oxidative stress [2] and activation of inflammation [3, 4] have been identified as leading causes. In this regard, polyphenols may be key molecules contributing to the prevention of brain aging due to their antioxidant [5, 6], and anti-inflammatory properties [7]. Polyphenols constitute one of the most numerous and ubiquitously distributed group of plant secondary metabolites and are generally involved in defense against stress, such as ultraviolet radiation, aggression by pathogens, or draught [8-10]. They are present in all plants that are commonly consumed in a healthy balance diet as i.e. in the Mediterranean diet, including grains, legumes, fruits, vegetables, extra virgin olive oil, red wine and tea [9-11]. Continuing research highlights the dynamic capacity of polyphenols to protect against age-associated disorders through a variety of important mechanisms. Numerous lines of evidence suggest that dietary polyphenols such as resveratrol and flavonoids have the capacity to mitigate age-associated cellular damage due to their antioxidant capacity, their ability to activate the antioxidant defenses [5, 6], and their antiinflammatory capacity [12, 13]. The last includes the inhibition of signaling pathways related with the activation of inflammation such as nuclear factor-kappa B (NF-κB), the modulation of several cell survival/cell-cycle genes [14-17], and activation of deacetylase enzymes like sirtuin 1 (SIRT1) [18, 19]. However, more studies are needed to deeply understand the mechanism insight of the beneficial effects of polyphenols on brain aging. Accordingly, this review will discuss the protective effects of polyphenols on the changes that occur during aging in the brain, analyzing the action mechanisms involved.
2. Structure, classes and food sources of polyphenols
Several thousand molecules having a polyphenol structure (i.e., several hydroxyl groups on aromatic rings) have been identified in higher plants, and several hundred are found in edible plants [20]. All plant phenolic compounds arise from a common intermediate, phenylalanine, or a close precursor, shikimic acid. Primarily they occur in conjugated forms with one or more sugar residues linked to hydroxyl groups, although direct linkages of the sugar (polysaccharide or monosaccharide) to an aromatic carbon also exist. Association with other compounds, like carboxylic and organic acids, amines, lipids, and connection with other phenol is also common [21]. Depending on the number of their phenol rings and the structural elements that bind these rings to one another, polyphenols are classified into the following groups: stilbenes, flavonoids, phenolic acids, lignans and others [11, 22].
Table 1 shows different polyphenols which have been found to have beneficial effects in aging; most of them belong to flavonoid class which are the most abundant polyphenols in foods [20]. Although the most studied and considered as prototypical polyphenol with anti-aging effects is resveratrol, which belongs to stilbenes class. Stilbenes contain two phenyl moieties connected by a two-carbon methylene bridge. The resveratrol is found largely in grapes and red fruits [8, 23]. A product of grapes, the red wine also, contains significant amount of resveratrol [20]. Resveratrol has been studied as a possible treatment for several age related diseases such as the Alzheimer’s disease since a few years [24, 25], in the prevention of aging process, and to prolong lifespan [26, 27]. Flavonoids comprise the most abundant group of polyphenols in foods, although each of them are generally present at relatively low concentrations [20]. This group has a common basic structure consisting of a 15-carbon skeleton that is organized in two aromatic rings interlinked by a third carbon chain [8]. Among the most common flavonoids with antiaging effects are quercetin, naringenin, catechins and kaempferol, present in onions, citrics, and vegetables in general [20, 28-30]. Several reports support the concept that flavonoid intake inhibits certain biochemical processes of brain aging, and might thus prevent to some extent the decline of cognitive functions during aging, as well as the development of neurodegenerative diseases [31]. Silymarin is a flavolignan which possesses wide range of mechanisms as antioxidant and anti-inflammatory, elevating some neurotransmitters concentration in brain and having antidepressant effect in animal models [32]. It is abundant in milk thistle (extract from Cardus marianum) where other lignans are also present [33]. Lignans are diphenolic compounds that contain a 2,3-dibenzylbutane structure that is formed by the dimerization of two cinnamic acid residues. Lignans show a huge structural diversity and are also found in flax, sesame, many grains [29], roots, rhizomes, stems, leaves, seeds, and fruits [34], in olive oil [35], among others. Phenolic acids are found abundantly in foods (wine, red fruits, onions, black radish, and coffee, among others) and divided into two classes: derivatives of benzoic acid and derivatives of cinnamic acid. The more common consist of p-coumaric, caffeic, ferulic and sinapic acids [36], which have been pointed out to have neurotherapeutic effects [37].
Table 1.
Main groups of polyphenols that have been pointed out for exercising antiaging effects, and some examples of representative compounds of each group. Polyphenols are structurally characterized by having several aromatic rings connected to hydroxyl groups.
| STILBENES Resveratrol | (3,4′,5-Trihydroxy-trans-stilbene, 5-((1E)-2-(4-hydroxyphenyl)ethenyl)-1,3-benzenediol) |
| FLAVONOIDS Quercetin | (2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-4H-1-benzopyran-4-one, 3,3′,4′,5,6-Pentahydroxyflavone, Quercetin-3-O-rhamnoside) |
| Naringenin | ((±)-Naringenin, (±)-2,3-Dihydro-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one, 4′,5,7-Trihydroxyflavanone) |
|
FLAVOLIGNAN Silymarin |
((2R,3S)-3,5,7-Trihydroxy-2-((2R)-2-(4-hydroxy-3-methoxyphenyl)-3-(hydroxymethyl)-2,3-dihydro-1,4-benzodioxin-6-yl)-2,3-dihydro-4H-chromen-4-one) |
3. Bioavailability and Effects of polyphe-nols on brain
Although, most polyphenols seem to present really low bioavailability from oral administration, many evidences show beneficial health effect through this administration route. The main problem explaining this low bioavailability seems to be its rapid metabolism, although a still controversial aspect is whether their metabolites maintain the therapeutic properties. In addition, it has been reported that some polyphenols are retained in neural tissue, reaching higher concentration than in plasma (for review see [38, 39]). Much of the relevance of polyphenols in protecting the brain aging is due to their ability to cross the blood brain barrier, due to their lipophilic nature [40-44]. Polyphenols affect a wide range of mechanisms in the brain, that help to protect against aging, improving cognition, exploratory behavior, spatial learning and memory [19, 45-47]. Therefore, polyphenols contribute to maintain mental health, as long as they reduce the risk of dementia [48] and prevent the onset from neurodegenerative diseases [9, 10, 49]. Polyphenols help to maintain the cerebral mass [50] and mitochondrial integrity as it was demonstrated after the oral administration of resveratrol for 28 days in rats [51]. It was also described that chronic treatment with polyphenols prevents the descent in the major neurotransmitters (serotonin, dopamine and noradrenalin), that occurs normally as a consequence of aging; this is the case of the polyphenol resveratrol [19, 47]. Moreover, flavonoids like quercetin inhibit enzymes such as monoamine oxidase (MAO), having antidepressant effects [52]. Polyphenols also favor the activation of some antiaging proteins, as it is the case of SIRT1 [53] which affects synaptic plasticity and memory (see below). The mechanism inside this set of brain effects can be related with the antioxidant and anti-inflammatory properties of polyphenols. As antioxidants, polyphenols protect lipids, proteins, carbohydrates and DNA from oxidative damage [6, 54, 55], and they also induce increased levels of antioxidant defense systems such as the enzyme glutathione peroxidase, ascorbic acid and, superoxide dismutase [56, 57]. On the other hand, polyphenols also have the ability to suppress neuroinflammation [58]. It has been shown in a series of studies in vitro and in vivo that polyphenols have potential to inhibit neuroinflammation through attenuating the activation of intracellular signaling pathways like MAPK and NF-kB [59-63].
4. Effects of polyphenols on oxidative stress in brain aging
The accumulation of diverse detrimental changes in the cells and tissues in ageing results in a progressive loss of physiological integrity, leading to impaired function and increased vulnerability to death. This deterioration is the primary risk factor for major human age-related diseases, as it is the case of some neurodegenerative diseases. There are 10 hallmarks that represent common denominators of aging in different organisms, with special emphasis on mammalian aging. These hallmarks are: genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication [64]. However, among many theories purposed for explaining the mechanism insight aging, free radical/oxidative stress theory is one of the most accepted [2]. A certain amount of oxidative damage takes place even under normal conditions; however, the rate of this damage increases during the aging process as the efficiency of antioxidative and repair mechanisms decreases [65, 66]. In this way, the process of brain aging has been associated with a progressive imbalance between antioxidant defense and the concentration of intracellular reactive oxygen species (ROS) [67, 68]. Consequently, the cognitive decline associated with aging correlates with a decrease in concentration of antioxidants in serum [69] and brain [70].
An antioxidant compound may be defined as any substance that retards, prevents, or eliminates oxidative damage caused by ROS in a target molecule [5]. Oxidative stress theory postulation has led to an increased research on the antioxidants’ role in the prevention of aging. Although there is no consensus about the antioxidants effectiveness in vivo and much less about their mechanism of action during aging in the body; for example, there are studies indicating that dietary antioxidants reduce cognitive impairment preventing oxidative damage in the brain of aged rats [71], but also they could suppress the expression of some genes related with brain aging in mice [72]. In any case, several studies suggest that antioxidants such as vitamin B, E, the ω-3 fatty acids [73] and polyphenols can prevent the cognitive and motor decline, reducing the risk of neurodegenerative diseases [19, 47, 74-76].
Interestingly, in comparison with other antioxidants, polyphenols have the ability to exert numerous ROS-scavenging independent actions. In this sense, polyphenols can act as antioxidants by directly inhibiting or quenching ROS due to the presence of benzene ring-bound hydroxyl groups that are capable of donating either one hydrogen atom or a single electron to the reactive species [77, 78]. A phenoxyl radical is generated which in turn can react with a second radical, forming a stable quinone structure [78]. Besides, some polyphenols are also able to reduce ROS levels by directly inhibiting the major ROS-forming enzymes including monoamine oxidase or xanthine oxidase [77]. Polyphenols have additional abilities, they can also chelate iron and copper ions rendering them inactive to participate in free radical generating reactions [8], with important consequences on the prevention of neurodegenerative diseases. Regarding this, it has been found that polyphenols prevent metal deposition, regulate redox metal homeostasis, and prevent neurotoxicity, acting as potential therapeutic agents for dementia, Alzheimer’s [79], and Parkinson’s diseases [80]. On the other hand, polyphenols can act as antioxidants indirectly, by modulating several signaling cascades including the Nrf2 and NF-κB or via modulation of the expression of microRNAs; leading to an induction of the expression of the antioxidant and detoxifying enzymes, but also elevating the intracellular glutathion levels [81-83]. Moreover, polyphenols are now recognized as molecules able to modulate pathways that regulate mitochondrial biogenesis (i.e., inducing SIRT1), mitochondrial membrane potential (i.e., mitochondrial permeability transition pore opening and uncoupling effects), the components of mitochondrial electron transport chain (i.e., modulating complexes I to V activity) and ATP synthesis [77]. It has also been demonstrated that polyphenols modulate the intra-mitochondrial oxidative status (i.e., inhibiting/inducing ROS formation/removal enzymes), and ultimately mitochondrially-triggered cell death (i.e., modulating intrinsic-apoptosis) [77].
5. Effects of polyphenols on inflammag-ing: The role of SIRT1
The word “inflammaging” was coined by Franceschi in 2000 [84], which refers to an exaggerated response of the immune system against inflammatory stimuli in brain during aging. Inflammaging has been postulated lately as one of the main characteristics of the brain aging process [3, 4]. Neuroinflammation associated with aging can result from many causes; some of them are: the accumulation of damage in tissues (due in part to oxidative damage) [85, 86]; the exaggerated response of both the innate and adaptive immune system against pathogens and dysfunctional cells [87]; the tendency of senescent cells to secrete proinflammatory cytokines [3,4]; and deregulation of autophagy immune system, through over activation of mTOR, which in turn generates defective proteins accumulation [88, 89]. These alterations cause activation of the inflammasome and other proinflammatory signaling pathways such as the MAPK [61, 90-92], and the NF-kB signaling pathways [92]; but also PI3K/Akt/mTOR pathway, which besides regulating autophagy, interacts with the cited proinflammatory pathways [93, 94]. Once these pathways are activated, cytokines production increases such as IL-1β, TNF-α, interferons and prostaglandins [3, 4]. All these components contribute significantly to cognitive and motor decline in brain aging [95]. The prolongation of this state has many brain consequences such as structural changes in front and temporal areas [96, 97], impaired synthesis of catecholamines and serotonin [19, 47, 76, 98-101], and synaptic deterioration [102], among others. Accordingly, it has been observed in young rats that overproduction of cytokines in key brain regions like the hippocampus causes premature aging and impaired memory [86]. Thus, the set of changes that occur during inflammatory processes in brain contribute to aging process and additionally can contribute to the development of neurodegenerative diseases such as Alzheimer [103], schizophrenia [104], Parkinson's, and multiple sclerosis, among others [105, 106].
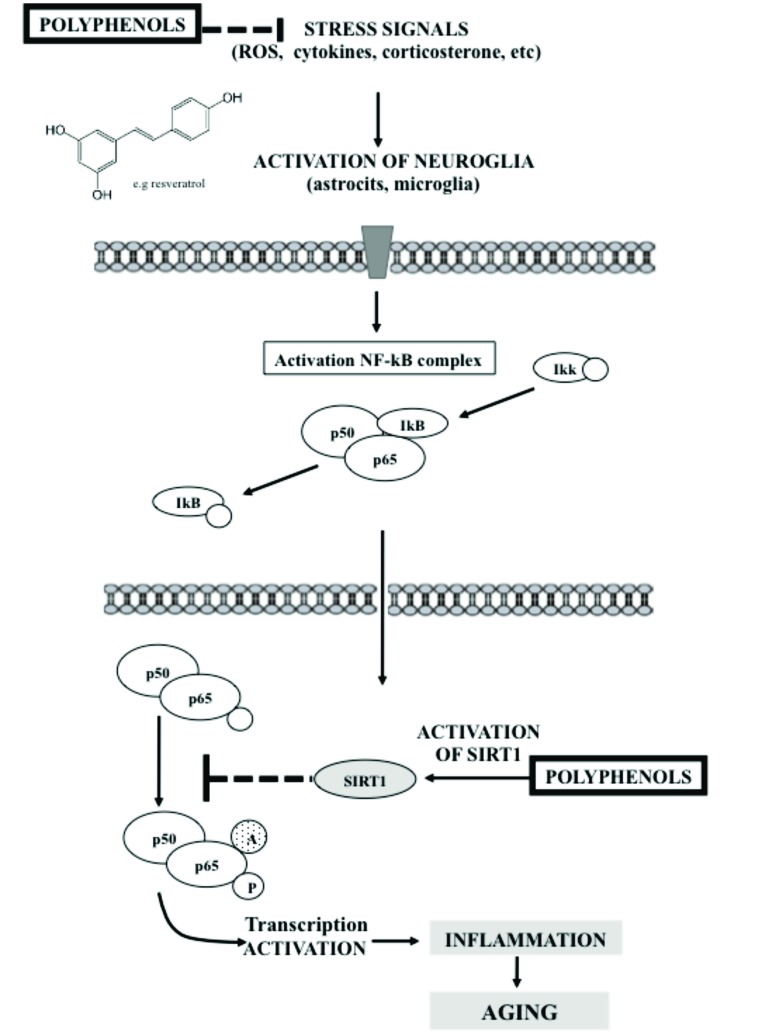
Considering neuroinflammation as a key factor in the process of brain aging, many of the anti-aging strategies are oriented towards the prevention or attenuation of this proinflammatory state. In this sense, it has been pointed out that polyphenols exercising anti-aging effects also modulate the brain’s immune system [92]. For example, it has been found that diets enriched with resveratrol or flavonoids reduce neuroinflammation, by decreasing cytokines production (such as IL-1β in the hippocampus of older rodents) with an impact on cognitive processes improvement [92, 107-109]. Regarding this, the modulation of NF-κB (which in turn can be mediated by the SIRT1, among other mechanisms) has been postulated as important molecular mechanism in the prevention of the aging effects by polyphenols [110, 111] (Fig. 1).
Fig. (1).
Scheme of the effect of polyphenols on SIRT1 and NF-κB signaling pathway involved in neuroinflammation. Stress signals as cytokines and ROS activate astrocytes and microglia, stimulating NF-κB signaling pathway, which leads to transcription of proinflammatory genes. This cascade of intracellular events results in increased cytokine production, ROS generation, inflammation, tissue damage and aging. Polyphenols (i.e resveratrol) can reduce the level of stress signals such as ROS, by avoiding oxidative stress, and citokines by activating and protecting SIRT1, which in turns cause a suppression of inflammation. SIRT1 directly inhibits NF-κB (by deacetylating the RelA/p65 subunit at lysine 310); preventing transcription activation of proinflammatory genes.
SIRT1 are histone and non-histone deacetylase enzymes responsible for regulating physiological and metabolic responses to stress signals, playing a critical role in cell survival [112-114]. SIRT1 also participates in the conservation of the cellular glucose homeostasis [115-117], which altogether favors the longevity of the organism [18, 118] and protects against aging [119, 120]. Even more, SIRT1 directly protect against oxidative stress and modulate inflammatory responses (see Fig. 1), preventing the onset of neurological diseases [121-125]. Moreover, it has been demonstrated that SIRT1 levels are reduced in hippocampus of old rats which contributes to brain ageing [126], progression of many inflammatory diseases [127], and cognitive impairment [128]. In addition, SIRT1 also appear to contribute to the development of neurodegenerative diseases as Alzheimer's or Parkinson [119, 120, 129, 130]. Therefore, these constitute a possible target for treating these diseases. Therefore, molecules that modulate the SIRT1 expression may represent a promise in preventing hallmarks of aging [131]. The mechanisms responsible for the decline of SIRT1 associated with aging are still unknown, although one of the main causes could be oxidative damage [128]. It has been reported that polyphenols can activate SIRT1 through an allosteric mechanism common to chemically diverse SIRT1 activators, but this effect has been only demonstrated in vitro [132, 133]. Polyphenols also induce SIRT1 overexpression contributing to protect cells against oxidative stress [53, 134-136]. The reason why polyphenols increase SIRT1 level in vivo is not well known, but could be related to their antioxidant effect, since oxidative stress reduces SIRT1 mRNA level [137]. Cysteine residues from SIRT1 are vulnerable to oxidation which affects both the activity of SIRT1 and its degradation by the proteosomes [138, 139]. Furthermore, SIRT1 overexpression is directly involved in the modulation of neuroinflammation in aging process by deacetylating non-histone proteins [140]. It has been demonstrated that SIRT1 deacetylated lysine 310 of RelA/p65 subunit of NF-kB, a critical subunit for activation of transcription of proinflammatory genes, triggers inflammatory processes [110]. This NF-kB signaling pathway is the prototypical one involved in inflammaging [3, 4, 110, 141]. In the brain, this process is mainly related to glia cells, where the expression of cytokines is promoted [142, 143]; but also synaptic plasticity in neurons is affected, contributing to memory process [107, 144, 145]. NF-κB consists of a heterodimeric complex of p50/p52 and p65 proteins. In the cytoplasm, NF-κB heterodimer joins the inhibitory protein IκB and thus the entire complex is inactive [146]. ROS and other proinflammatory molecules activate protein kinase that phosphorylates IkB, which releases the complex of p50/p65 [147], allowing it to translocate to the nucleus where it can act as a transcription factor to bind DNA at specific promoter regions [148, 149]. The transcriptional activation domain of NF-κB is in the p65 subunit [142, 150, 151]. This p65 subunit is also modulated by posttranslational modifications such as phosphorylation at serines (276, 311, 529 and 536) and acetylation at lysines 310 [110, 151], 122, 123, 218 and 221 [149, 152, 153]. The over activation of this NF-κB signaling pathway is one of the transcriptional signs of aging process [141, 154]. In this way, it has been demonstrated that the conditional expression of an inhibitor of NF-κB in aged skin of transgenic mice causes phenotypic rejuvenation of this tissue [141]. Similarly, genetic and pharmacological inhibition of NF-κB signaling pathway prevents age associated characteristics in different models of accelerated aging mice [155, 156]. It has also been pointed out that the acetylation of lysine 310 of RelA/p65 NF-κB subunit increases the duration and effectiveness of the NF-κB activation, generating increased inflammation [151] (Fig. 1). Yeung et al., in 2004 [110] showed that SIRT1 deacetylase enzyme can interact with RelA/p65 protein complex NF-κB, deacetylating lysine 310 of RelA/p65 NF-kB subunit, and inhibiting transcription of proinflammatory genes [92, 110, 140, 141, 157-159]. Additionally, other study reinforced the idea that SIRT1 deacetylate NF-κB, since during HIV-1 studies, Kwon et al., 2008 [157] demonstrated that the viral protein Tat binds to SIRT1, inhibiting its activity, thereby preventing NF-κB deacetylation; thus triggering the immune system activation. Together these observations support the idea that inflammatory responses and aging processes can be aggravated by enhancing the activation of NF-κB, suggesting that SIRT1 could promote longevity by inhibiting activation of NF-κB [110, 141]. In this way, old rats fed diet rich in polyphenol also showed reduced expression of NF-κB in the hippocampus, striatum and frontal cortex together with an improvement in cognitive abilities [160]. In this regard, longevity factors, such as SIRT1 and their activators (i.e polyphenols) could regulate the efficiency of NF-κB signaling [3, 4]. Similar results have been shown in cancer studies, where resveratrol has been shown to exert antitumor actions through NF-κB inhibition [161-163]. Not only resveratrol, but also different flavonoid mixtures have been shown to induce SIRT1-mediated NF-κB inhibition even in brain after oral administration [164-167]. Similarly, reductions have been described in NF-κB levels by naringenin, silybin, or quercetin, even in vivo after oral administration [168-172]. As it is schematized in Fig. (1), polyphenols can activate SIRT1, since they may protect SIRT1 against oxidative stress effects, helping to avoid neurodegeneration and cognitive impairment associated with ageing [173]. This is very important in the brain since it has been shown that SIRT1 regulate energy metabolism, axonal growth, dendrite formation, neuronal plasticity, neuronal survival against stress, and suppress inflammation by NF-κB modulation [18, 92, 174], as has been pointed out in models of chronic inflammatory diseases [19, 110, 175-178]. Therefore, the activation of SIRT1 by polyphenol treatments may be helpful in the prevention of brain aging. However, many questions regarding doses, safety, tolerance and efficacy of polyphenol treatments in elderly health people are still unanswered due to the lack in clinical studies on conditions of normal aging.
Conclusion
In summary, brain aging is a physiological process which is caused by a set of mechanisms, with a predominant importance of oxidative stress and neuroinflammation, which in turn influence each other and generate a general state that contributes to cognitive impairment. Nowadays, research is going on to explore novel antiaging molecules including polyphenols which are expected to be useful as adjuvant therapy against aging symptoms by their neuroprotective properties. The available literature seems to suggest that polyphenols, found in fruits and vegetables, may be useful in providing protection against aging due to their antioxidant and anti-inflammatory properties. Polyphenols protect SIRT1 against oxidative damage which in turn modulates the activation of NF-κB signaling pathway in brain regions critical for accurate functionality of cognitive processes. In vitro and in vivo studies have open the doors to the consideration of polyphenols as antiaging molecules. However, open questions hamper the clinical use of these natural compounds in normal aging process, because there is a lack in clinical studies in conditions of normal aging; and also low bioavailability is reported for polyphenols in general, mainly through oral administration. Moreover, studies for the risk assessment and safety evaluation to determine undesirable effects of polyphenols should also be necessary. Therefore, further research focusing on human clinical trials of individual polyphenols and their combinations should be carried out in order to clarify the specific role of these compounds as antiaging brain molecules.
Consent for Publication
Not applicable.
ACKNOWLEDGEMENTS
Financial contribution: Universitat de les Illes Balears (UIB), Govern Balear (ECT 025 09), Pont La Caixa-UIB Program (7/2014) and SAF2014-55903-R (MINECO, Madrid, Spain). F. Sarubbo was supported by a UIB contract.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Wimo A., Winblad B., Jönsson L. The worldwide societal costs of dementia: Estimates for 2009. Alzheimers Dement. 2010;6(2):98–103. doi: 10.1016/j.jalz.2010.01.010. [http://dx.doi.org/10.1016/j.jalz.2010.01.010]. [PMID: 20298969]. [DOI] [PubMed] [Google Scholar]
- 2.Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11(3):298–300. doi: 10.1093/geronj/11.3.298. [http://dx.doi.org/ 10.1093/geronj/11.3.298]. [PMID: 13332224]. [DOI] [PubMed] [Google Scholar]
- 3.Salminen A., Kauppinen A., Suuronen T., Kaarniranta K. SIRT1 longevity factor suppresses NF-kappaB -driven immune responses: regulation of aging via NF-kappaB acetylation? BioEssays. 2008;30(10):939–942. doi: 10.1002/bies.20799. [http://dx.doi.org/10.1002/bies.20799]. [PMID: 18800364]. [DOI] [PubMed] [Google Scholar]
- 4.Salminen A., Ojala J., Huuskonen J., Kauppinen A., Suuronen T., Kaarniranta K. Interaction of aging-associated signaling cascades: inhibition of NF-kappaB signaling by longevity factors FoxOs and SIRT1. Cell. Mol. Life Sci. 2008;65(7-8):1049–1058. doi: 10.1007/s00018-008-7461-3. [http://dx.doi.org/10.1007/s00018-008-7461-3]. [PMID: 18193389]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halliwell B., Zentella A., Gomez E., Kershenobich D. Antioxidants and human disease: a general introduction. Nutr. Rev. 1997;55(1 Pt 2):S44–S49. doi: 10.1111/j.1753-4887.1997.tb06100.x. [PMID: 9155225]. [DOI] [PubMed] [Google Scholar]
- 6.Khurana S., Venkataraman K., Hollingsworth A., Piche M., Tai T.C. Polyphenols: benefits to the cardiovascular system in health and in aging. Nutrients. 2013;5(10):3779–3827. doi: 10.3390/nu5103779. [http://dx.doi.org/ 10.3390/nu5103779]. [PMID: 24077237]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tangney C.C., Rasmussen H.E. Polyphenols, inflammation, and cardiovascular disease. Curr. Atheroscler. Rep. 2013;15(5):324. doi: 10.1007/s11883-013-0324-x. [http://dx.doi.org/10.1007/s11883-013-0324-x]. [PMID: 23512608]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pandey K.B., Rizvi S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009;2(5):270–278. doi: 10.4161/oxim.2.5.9498. [http://dx.doi.org/10.4161/oxim.2.5.9498]. [PMID: 20716914]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scalbert A., Johnson I.T., Saltmarsh M. Polyphenols: antioxidants and beyond. Am. J. Clin. Nutr. 2005;81(1) Suppl.:215S–217S. doi: 10.1093/ajcn/81.1.215S. [PMID: 15640483]. [DOI] [PubMed] [Google Scholar]
- 10.Scalbert A., Manach C., Morand C., Rémésy C., Jiménez L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005;45(4):287–306. doi: 10.1080/1040869059096. [http://dx.doi.org/10.1080/ 1040869059096]. [PMID: 16047496]. [DOI] [PubMed] [Google Scholar]
- 11.Spencer J.P., Abd El Mohsen M.M., Minihane A.M., Mathers J.C. Biomarkers of the intake of dietary polyphenols: strengths, limitations and application in nutrition research. Br. J. Nutr. 2008;99(1):12–22. doi: 10.1017/S0007114507798938. [http://dx.doi.org/10.1017/S0007114507798938]. [PMID: 17666146]. [DOI] [PubMed] [Google Scholar]
- 12.Rahman I., Biswas S.K., Kirkham P.A. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem. Pharmacol. 2006;72(11):1439–1452. doi: 10.1016/j.bcp.2006.07.004. [http://dx.doi.org/10.1016/j.bcp. 2006.07.004]. [PMID: 16920072]. [DOI] [PubMed] [Google Scholar]
- 13.Elumalai P., Lakshmi S. Role of Quercetin benefits in neurodegeneration. Adv. Neurobiol. 2016;12:229–245. doi: 10.1007/978-3-319-28383-8_12. [http://dx.doi. org/10.1007/978-3-319-28383-8_12]. [PMID: 27651256]. [DOI] [PubMed] [Google Scholar]
- 14.Williams R.J., Spencer J.P., Rice-Evans C. Flavonoids: antioxidants or signalling molecules? Free Radic. Biol. Med. 2004;36(7):838–849. doi: 10.1016/j.freeradbiomed.2004.01.001. [http://dx.doi.org/10.1016/j.freeradbiomed.2004.01.001]. [PMID: 15019969]. [DOI] [PubMed] [Google Scholar]
- 15.Yoon J.H., Baek S.J. Molecular targets of dietary polyphenols with anti-inflammatory properties. Yonsei Med. J. 2005;46(5):585–596. doi: 10.3349/ymj.2005.46.5.585. [http://dx.doi.org/10.3349/ymj.2005.46.5.585]. [PMID: 16259055]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim H.P., Son K.H., Chang H.W., Kang S.S. Anti-inflammatory plant flavonoids and cellular action mechanisms. J. Pharmacol. Sci. 2004;96(3):229–245. doi: 10.1254/jphs.crj04003x. [http://dx.doi.org/10.1254/jphs.CRJ04003X]. [PMID: 15539763]. [DOI] [PubMed] [Google Scholar]
- 17.Stangl V., Dreger H., Stangl K., Lorenz M. Molecular targets of tea polyphenols in the cardiovascular system. Cardiovasc. Res. 2007;73(2):348–358. doi: 10.1016/j.cardiores.2006.08.022. [http://dx.doi.org/10.1016/j.cardiores.2006. 08.022]. [PMID: 17020753]. [DOI] [PubMed] [Google Scholar]
- 18.Chung S., Yao H., Caito S., Hwang J.W., Arunachalam G., Rahman I. Regulation of SIRT1 in cellular functions: role of polyphenols. Arch. Biochem. Biophys. 2010;501(1):79–90. doi: 10.1016/j.abb.2010.05.003. [http:// dx.doi.org/10.1016/j.abb.2010.05.003]. [PMID: 20450879]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarubbo F. Estrategias neuroprotectoras en el envejecimiento cerebral. 2016 http://hdl.handle.net/10803/385717
- 20.Manach C., Scalbert A., Morand C., Rémésy C., Jiménez L. Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr. 2004;79(5):727–747. doi: 10.1093/ajcn/79.5.727. [PMID: 15113710]. [DOI] [PubMed] [Google Scholar]
- 21.Kondratyuk T.P., Pezzuto J.M. Natural product polyphenols of relevance to human health. Arch. Physiol. Biochem. 2004;42(s1):46–63. [http://dx.doi.org/10.1080/13880200490893519]. [Google Scholar]
- 22.Beckman C.H. Phenolic-storing cells: keys to programmed cell death and periderm formation in wilt disease resistance and in general defence responses in plants? Physiol. Mol. Plant Pathol. 2000;57(3):101–110. [http://dx.doi.org/10.1006/pmpp.2000.0287]. [Google Scholar]
- 23.Sun H-Y., Xiao C-F., Cai Y-C., Chen Y., Wei W., Liu X-K., Lv Z-L., Zou Y. Efficient synthesis of natural polyphenolic stilbenes: resveratrol, piceatannol and oxyresveratrol. Chem. Pharm. Bull. (Tokyo) 2010;58(11):1492–1496. doi: 10.1248/cpb.58.1492. [http://dx.doi.org/10.1248/ cpb.58.1492]. [PMID: 21048342]. [DOI] [PubMed] [Google Scholar]
- 24.Franco R., Cedazo-Minguez A. Successful therapies for Alzheimer’s disease: why so many in animal models and none in humans? Front. Pharmacol. 2014;5:146. doi: 10.3389/fphar.2014.00146. [http://dx.doi.org/10.3389/ fphar.2014.00146]. [PMID: 25009496]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen T., Wang X.N., Lou H.X. Natural stilbenes: an overview. Nat. Prod. Rep. 2009;26(7):916–935. doi: 10.1039/b905960a. [http://dx.doi.org/10.1039/ b905960a]. [PMID: 19554241]. [DOI] [PubMed] [Google Scholar]
- 26.Queen B.L., Tollefsbol T.O. Polyphenols and aging. Curr. Aging Sci. 2010;3(1):34–42. doi: 10.2174/1874609811003010034. [http://dx.doi.org/10.2174/1874609811003010034]. [PMID: 20298168]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baur J.A., Sinclair D.A. Therapeutic potential of resveratrol: the in vivo evidence. Nat. Rev. Drug Discov. 2006;5(6):493–506. doi: 10.1038/nrd2060. [http://dx.doi.org/10.1038/nrd2060]. [PMID: 16732220]. [DOI] [PubMed] [Google Scholar]
- 28.Russo M., Spagnuolo C., Tedesco I., Bilotto S., Russo G.L. The flavonoid quercetin in disease prevention and therapy: facts and fancies. Biochem. Pharmacol. 2012;83(1):6–15. doi: 10.1016/j.bcp.2011.08.010. [http://dx.doi. org/10.1016/j.bcp.2011.08.010]. [PMID: 21856292]. [DOI] [PubMed] [Google Scholar]
- 29.Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients. 2010;2(12):1231–1246. doi: 10.3390/nu2121231. [http://dx.doi.org/10.3390/ nu2121231]. [PMID: 22254006]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goufo P., Trindade H. Rice antioxidants: phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, γ-oryzanol, and phytic acid. Food Sci. Nutr. 2014;2(2):75–104. doi: 10.1002/fsn3.86. [http://dx.doi.org/10.1002/fsn3.86]. [PMID: 24804068]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmitt-Schillig S., Schaffer S., Weber C.C., Eckert G.P., Müller W.E. Flavonoids and the aging brain. J. Physiol. Pharmacol. 2005;56(Suppl. 1):23–36. [PMID: 15800383]. [PubMed] [Google Scholar]
- 32.Karimi G. Evaluation of antidepressant effect of ethanolic and aqueous extracts of Silybum marianum L. seed in mice. Faslnamah-i Giyahan-i Daruyi. 2007;6:38–43. [Google Scholar]
- 33.Heinonen S., Nurmi T., Liukkonen K., Poutanen K., Wähälä K., Deyama T., Nishibe S., Adlercreutz H. In vitro metabolism of plant lignans: new precursors of mammalian lignans enterolactone and enterodiol. J. Agric. Food Chem. 2001;49(7):3178–3186. doi: 10.1021/jf010038a. [http://dx.doi.org/10.1021/jf010038a]. [PMID: 11453749]. [DOI] [PubMed] [Google Scholar]
- 34.Saleem M., Kim H.J., Ali M.S., Lee Y.S. An update on bioactive plant lignans. Nat. Prod. Rep. 2005;22(6):696–716. doi: 10.1039/b514045p. [http://dx. doi.org/10.1039/b514045p]. [PMID: 16311631]. [DOI] [PubMed] [Google Scholar]
- 35.Owen R.W., Mier W., Giacosa A., Hull W.E., Spiegelhalder B., Bartsch H. Identification of lignans as major components in the phenolic fraction of olive oil. Clin. Chem. 2000;46(7):976–988. [PMID: 10894841]. [PubMed] [Google Scholar]
- 36.Shahidi F., Naczk M. Food Phenolics: Sources, Chemistry, Effects, Applications. Food Chem. 1996;57(3):481–482. [Google Scholar]
- 37.Suksrichavalit T., Prachayasittikul S., Isarankura C., Ayudhya N., Prachayasittik V. Synthesis of a “clickable” Angiopep-conjugated p-coumaric acid for brain-targeted delivery. J. Mater. Sci. 2014;49(23):8204–8213. [http://dx.doi.org/10.1007/s10853-014-8529-0]. [Google Scholar]
- 38.D’Archivio M., Filesi C., Varì R., Scazzocchio B., Masella R. Bioavailability of the polyphenols: status and controversies. Int. J. Mol. Sci. 2010;11(4):1321–1342. doi: 10.3390/ijms11041321. [http://dx.doi.org/10.3390/ ijms11041321]. [PMID: 20480022]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Almeida S., Alves M.G., Sousa M., Oliveira P.F., Silva B.M. Are polyphenols strong dietary agents against neurotoxicity and neurodegeneration? Neurotox. Res. 2016;30(3):345–366. doi: 10.1007/s12640-015-9590-4. [http:// dx.doi.org/10.1007/s12640-015-9590-4]. [PMID: 26745969]. [DOI] [PubMed] [Google Scholar]
- 40.Abbott N.J., Patabendige A.A., Dolman D.E., Yusof S.R., Begley D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010;37(1):13–25. doi: 10.1016/j.nbd.2009.07.030. [http://dx.doi.org/10.1016/ j.nbd.2009.07.030]. [PMID: 19664713]. [DOI] [PubMed] [Google Scholar]
- 41.Abbott N.J. Blood-brain barrier structure and function and the challenges for CNS drug delivery. J. Inherit. Metab. Dis. 2013;36(3):437–449. doi: 10.1007/s10545-013-9608-0. [http://dx.doi.org/10.1007/s10545-013-9608-0]. [PMID: 23609350]. [DOI] [PubMed] [Google Scholar]
- 42.Moriya J., Chen R., Yamakawa J., Sasaki K., Ishigaki Y., Takahashi T. Resveratrol improves hippocampal atrophy in chronic fatigue mice by enhancing neurogenesis and inhibiting apoptosis of granular cells. Biol. Pharm. Bull. 2011;34(3):354–359. doi: 10.1248/bpb.34.354. [http://dx.doi.org/10.1248/bpb.34.354]. [PMID: 21372384]. [DOI] [PubMed] [Google Scholar]
- 43.Narita K., Hisamoto M., Okuda T., Takeda S. Differential neuroprotective activity of two different grape seed extracts. PLoS One. 2011;6(1):e14575. doi: 10.1371/journal.pone.0014575. [http://dx.doi.org/10.1371/journal.pone. 0014575]. [PMID: 21283677]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Z., Zhuang C., Sheng S., Shao L., Zhao W., Zhao S. Overexpression of a resveratrol synthase gene (PcRS) from Polygonum cuspidatum in transgenic Arabidopsis causes the accumulation of trans-piceid with antifungal activity. Plant Cell Rep. 2011;30(11):2027–2036. doi: 10.1007/s00299-011-1110-2. [http://dx.doi.org/10.1007/s00299-011-1110-2]. [PMID: 21717185]. [DOI] [PubMed] [Google Scholar]
- 45.Liu J., Yu H., Ning X. Effect of quercetin on chronic enhancement of spatial learning and memory of mice. Sci. China C Life Sci. 2006;49(6):583–590. doi: 10.1007/s11427-006-2037-7. [http://dx.doi.org/10.1007/s11427-006-2037-7]. [PMID: 17312997]. [DOI] [PubMed] [Google Scholar]
- 46.Ansari M.A., Abdul H.M., Joshi G., Opii W.O., Butterfield D.A. Protective effect of quercetin in primary neurons against Abeta(1-42): relevance to Alzheimer’s disease. J. Nutr. Biochem. 2009;20(4):269–275. doi: 10.1016/j.jnutbio.2008.03.002. [http://dx.doi.org/10.1016/j.jnutbio.2008.03. 002]. [PMID: 18602817]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sarubbo F., Ramis M.R., Aparicio S., Ruiz L., Esteban S., Miralles A., Moranta D. Improving effect of chronic resveratrol treatment on central monoamine synthesis and cognition in aged rats. Age (Dordr.) 2015;37(3):9777. doi: 10.1007/s11357-015-9777-x. [http://dx.doi.org/10.1007/ s11357-015-9777-x]. [PMID: 25895558]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Truelsen T., Thudium D., Grønbaek M., Copenhagen T., Heart C. Amount and type of alcohol and risk of dementia: the Copenhagen City Heart Study. Neurology. 2002;59(9):1313–1319. doi: 10.1212/01.wnl.0000031421.50369.e7. [http://dx.doi.org/10.1212/01.WNL.0000031421.50369.E7]. [PMID: 12427876]. [DOI] [PubMed] [Google Scholar]
- 49.Gomez-Pinilla F., Nguyen T.T. Natural mood foods: the actions of polyphenols against psychiatric and cognitive disorders. Nutr. Neurosci. 2012;15(3):127–133. doi: 10.1179/1476830511Y.0000000035. [http://dx.doi.org/10.1179/ 1476830511Y.0000000035]. [PMID: 22334236]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smoliga J.M., Baur J.A., Hausenblas H.A. Resveratrol and health--a comprehensive review of human clinical trials. Mol. Nutr. Food Res. 2011;55(8):1129–1141. doi: 10.1002/mnfr.201100143. [http://dx.doi.org/10.1002/ mnfr.201100143]. [PMID: 21688389]. [DOI] [PubMed] [Google Scholar]
- 51.Yang T., Wang L., Zhu M., Zhang L., Yan L. Properties and molecular mechanisms of resveratrol: a review. Pharmazie. 2015;70(8):501–506. [PMID: 26380517]. [PubMed] [Google Scholar]
- 52.Bandaruk Y., Mukai R., Kawamura T., Nemoto H., Terao J. Evaluation of the inhibitory effects of quercetin-related flavonoids and tea catechins on the monoamine oxidase-A reaction in mouse brain mitochondria. J. Agric. Food Chem. 2012;60(41):10270–10277. doi: 10.1021/jf303055b. [http://dx.doi.org/10.1021/jf303055b]. [PMID: 23009399]. [DOI] [PubMed] [Google Scholar]
- 53.Davis J.M., Murphy E.A., Carmichael M.D. Effects of the dietary flavonoid quercetin upon performance and health. Curr. Sports Med. Rep. 2009;8(4):206–213. doi: 10.1249/JSR.0b013e3181ae8959. [http://dx.doi.org/10.1249/JSR. 0b013e3181ae8959]. [PMID: 19584608]. [DOI] [PubMed] [Google Scholar]
- 54.Cirillo G., Curcio M., Vittorio O., Iemma F., Restuccia D., Spizzirri U., Puoci F., Picci N. Polyphenol conjugates and human health: a perspective review. 2014. [DOI] [PubMed]
- 55.Karimi G., Vahabzadeh M., Lari P., Rashedinia M., Moshiri M. “Silymarin”, a promising pharmacological agent for treatment of diseases. Iran. J. Basic Med. Sci. 2011;14(4):308–317. [PMID: 23492971]. [PMC free article] [PubMed] [Google Scholar]
- 56.de Groot H., Rauen U. Tissue injury by reactive oxygen species and the protective effects of flavonoids. Fundam. Clin. Pharmacol. 1998;12(3):249–255. doi: 10.1111/j.1472-8206.1998.tb00951.x. [http://dx.doi.org/10.1111/j.1472-8206.1998. tb00951.x]. [PMID: 9646056]. [DOI] [PubMed] [Google Scholar]
- 57.Nencini C., Giorgi G., Micheli L. Protective effect of silymarin on oxidative stress in rat brain. Phytomedicine. 2007;14(2-3):129–135. doi: 10.1016/j.phymed.2006.02.005. [http://dx.doi.org/10.1016/j.phymed.2006.02.005]. [PMID: 16638633]. [DOI] [PubMed] [Google Scholar]
- 58.Venigalla M., Gyengesi E., Sharman M., Münch G. Novel promising therapeutics against chronic neuroinflammation and neurodegeneration in Alzheimer’s disease. Neurochem. Int. 2015 doi: 10.1016/j.neuint.2015.10.011. [PMID: 26529297]. [DOI] [PubMed] [Google Scholar]
- 59.Rice-Evans C.A., Miller N.J., Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996;20(7):933–956. doi: 10.1016/0891-5849(95)02227-9. [http://dx.doi.org/10.1016/0891-5849(95)02227-9]. [PMID: 8743980]. [DOI] [PubMed] [Google Scholar]
- 60.Santangelo C., Varì R., Scazzocchio B., Di Benedetto R., Filesi C., Masella R. Polyphenols, intracellular signalling and inflammation. Ann. Ist. Super. Sanita. 2007;43(4):394–405. [PMID: 18209273]. [PubMed] [Google Scholar]
- 61.Spencer J.P. Flavonoids and brain health: multiple effects underpinned by common mechanisms. Genes Nutr. 2009;4(4):243–250. doi: 10.1007/s12263-009-0136-3. [http://dx.doi.org/10.1007/s12263-009-0136-3]. [PMID: 19685255]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spencer J.P. The impact of flavonoids on memory: physiological and molecular considerations. Chem. Soc. Rev. 2009;38(4):1152–1161. doi: 10.1039/b800422f. [http://dx.doi.org/10.1039/b800422f]. [PMID: 19421586]. [DOI] [PubMed] [Google Scholar]
- 63.Spencer J.P. The impact of fruit flavonoids on memory and cognition. Br. J. Nutr. 2010;104(Suppl. 3):S40–S47. doi: 10.1017/S0007114510003934. [http://dx.doi.org/ 10.1017/S0007114510003934]. [PMID: 20955649]. [DOI] [PubMed] [Google Scholar]
- 64.López-Otín C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [http://dx.doi.org/10.1016/j.cell.2013.05.039]. [PMID: 23746838]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rizvi S.I., Maurya P.K. Alterations in antioxidant enzymes during aging in humans. Mol. Biotechnol. 2007;37(1):58–61. doi: 10.1007/s12033-007-0048-7. [http://dx. doi.org/10.1007/s12033-007-0048-7]. [PMID: 17914165]. [DOI] [PubMed] [Google Scholar]
- 66.Rizvi S.I., Maurya P.K. Markers of oxidative stress in erythrocytes during aging in humans. Ann. N. Y. Acad. Sci. 2007;1100:373–382. doi: 10.1196/annals.1395.041. [http://dx.doi.org/10.1196/annals.1395.041]. [PMID: 17460201]. [DOI] [PubMed] [Google Scholar]
- 67.Reiter R.J. Melatonin, active oxygen species and neurological damage. Drug News Perspect. 1998;11(5):291–296. doi: 10.1358/dnp.1998.11.5.863675. [http://dx. doi.org/10.1358/dnp.1998.11.5.863675]. [PMID: 15616649]. [DOI] [PubMed] [Google Scholar]
- 68.Reiter R.J. Oxidative damage in the central nervous system: protection by melatonin. Prog. Neurobiol. 1998;56(3):359–384. doi: 10.1016/s0301-0082(98)00052-5. [http://dx.doi.org/10.1016/S0301-0082(98)00052-5]. [PMID: 9770244]. [DOI] [PubMed] [Google Scholar]
- 69.Rinaldi P., Polidori M.C., Metastasio A., Mariani E., Mattioli P., Cherubini A., Catani M., Cecchetti R., Senin U., Mecocci P. Plasma antioxidants are similarly depleted in mild cognitive impairment and in Alzheimer’s disease. Neurobiol. Aging. 2003;24(7):915–919. doi: 10.1016/s0197-4580(03)00031-9. [http://dx.doi.org/10.1016/S0197-4580(03)00031-9]. [PMID: 12928050]. [DOI] [PubMed] [Google Scholar]
- 70.Berr C., Balansard B., Arnaud J., Roussel A.M., Alpérovitch A. Cognitive decline is associated with systemic oxidative stress: the EVA study. Etude du Vieillissement Artériel. J. Am. Geriatr. Soc. 2000;48(10):1285–1291. doi: 10.1111/j.1532-5415.2000.tb02603.x. [http://dx.doi.org/10.1111/j.1532-5415. 2000.tb02603.x]. [PMID: 11037017]. [DOI] [PubMed] [Google Scholar]
- 71.Lau F.C., Shukitt-Hale B., Joseph J.A. The beneficial effects of fruit polyphenols on brain aging. Neurobiol. Aging. 2005;26(Suppl. 1):128–132. doi: 10.1016/j.neurobiolaging.2005.08.007. [http://dx.doi.org/10.1016/j.neurobiolaging. 2005.08.007]. [PMID: 16194581]. [DOI] [PubMed] [Google Scholar]
- 72.Liu J., Killilea D., Ames B. Age-associated mitochondrial oxidative decay: improvement of carnitine acetyltransferase substrate-binding affinity and activity in brain by feeding old rats acetyl-L- carnitine and/or R.-alpha -lipoic acid. Proc. Natl Acad. 2002;99:1876–1881. doi: 10.1073/pnas.261709098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Coley N., Vaurs C., Andrieu S. Nutrition and cognition in aging adults. Clin. Geriatr. Med. 2015;31(3):453–464. doi: 10.1016/j.cger.2015.04.008. [http://dx.doi. org/10.1016/j.cger.2015.04.008]. [PMID: 26195103]. [DOI] [PubMed] [Google Scholar]
- 74.Corredor C.A. Metabolismo, nutrición y shock. 2006. p. 293. [Google Scholar]
- 75.Joseph J., Cole G., Head E., Ingram D. Nutrition, brain aging, and neurodegeneration. J. Neurosci. 2009;29(41):12795–12801. doi: 10.1523/JNEUROSCI.3520-09.2009. [http://dx.doi.org/10.1523/JNEUROSCI.3520-09.2009]. [PMID: 19828791]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ramis M., Sarubbo F., Terrasa J., Moranta D., Aparicio S., Miralles A., Esteban S. Chronic α-tocopherol increases central monoamines synthesis and improves cognitive and motor abilities in old rats. Rejuvenation Res. 2015 doi: 10.1089/rej.2015.1685. [PMID: 26414867]. [DOI] [PubMed] [Google Scholar]
- 77.Sandoval-Acuña C., Ferreira J., Speisky H. Polyphenols and mitochondria: an update on their increasingly emerging ROS-scavenging independent actions. Arch. Biochem. Biophys. 2014;559:75–90. doi: 10.1016/j.abb.2014.05.017. [http://dx.doi.org/10.1016/j.abb.2014.05.017]. [PMID: 24875147]. [DOI] [PubMed] [Google Scholar]
- 78.Hollman P.C., Cassidy A., Comte B., Heinonen M., Richelle M., Richling E., Serafini M., Scalbert A., Sies H., Vidry S. The biological relevance of direct antioxidant effects of polyphenols for cardiovascular health in humans is not established. J. Nutr. 2011;141(5):989S–1009S. doi: 10.3945/jn.110.131490. [http://dx.doi.org/10.3945/jn.110.131490]. [PMID: 21451125]. [DOI] [PubMed] [Google Scholar]
- 79.Lakey-Beitia J., Berrocal R., Rao K.S., Durant A.A. Polyphenols as therapeutic molecules in Alzheimer’s disease through modulating amyloid pathways. Mol. Neurobiol. 2015;51(2):466–479. doi: 10.1007/s12035-014-8722-9. [http://dx.doi.org/10.1007/s12035-014-8722-9]. [PMID: 24826916]. [DOI] [PubMed] [Google Scholar]
- 80.Schapira A.H. Etiology of Parkinson’s disease. Neurology. 2006;66(10) Suppl. 4:S10–S23. doi: 10.1212/wnl.66.10_suppl_4.s10. [http://dx.doi.org/10.1212/WNL.66.10_ suppl_4.S10]. [PMID: 16717248]. [DOI] [PubMed] [Google Scholar]
- 81.Bohn T. Dietary factors affecting polyphenol bioavailability. Nutr. Rev. 2014;72(7):429–452. doi: 10.1111/nure.12114. [http://dx.doi.org/10.1111/nure.12114]. [PMID: 24828476]. [DOI] [PubMed] [Google Scholar]
- 82.Curti V., Capelli E., Boschi F., Nabavi S.F., Bongiorno A.I., Habtemariam S., Nabavi S.M., Daglia M. Modulation of human miR-17-3p expression by methyl 3-O-methyl gallate as explanation of its in vivo protective activities. Mol. Nutr. Food Res. 2014;58(9):1776–1784. doi: 10.1002/mnfr.201400007. [http://dx.doi.org/10.1002/mnfr.201400007]. [PMID: 24975036]. [DOI] [PubMed] [Google Scholar]
- 83.Tsuji P.A., Stephenson K.K., Wade K.L., Liu H., Fahey J.W. Structure-activity analysis of flavonoids: direct and indirect antioxidant, and antiinflammatory potencies and toxicities. Nutr. Cancer. 2013;65(7):1014–1025. doi: 10.1080/01635581.2013.809127. [http://dx.doi.org/10.1080/ 01635581.2013.809127]. [PMID: 24087992]. [DOI] [PubMed] [Google Scholar]
- 84.Franceschi C., Bonafè M., Valensin S., Olivieri F., De Luca M., Ottaviani E., De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [http://dx.doi.org/10.1111/j.1749-6632.2000.tb06651.x]. [PMID: 10911963]. [DOI] [PubMed] [Google Scholar]
- 85.Martinon F. Signaling by ROS drives inflammasome activation. Eur. J. Immunol. 2010;40(3):616–619. doi: 10.1002/eji.200940168. [http://dx.doi.org/10.1002/ eji.200940168]. [PMID: 20201014]. [DOI] [PubMed] [Google Scholar]
- 86.Barrientos R.M., Kitt M.M., Watkins L.R., Maier S.F. Neuroinflammation in the normal aging hippocampus. Neuroscience. 2015;309:84–99. doi: 10.1016/j.neuroscience.2015.03.007. [http://dx.doi.org/10.1016/j.neuroscience.2015.03.007]. [PMID: 25772789]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Deeks S.G. HIV infection, inflammation, immunosenescence, and aging. Annu. Rev. Med. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756. [http://dx.doi.org/10. 1146/annurev-med-042909-093756]. [PMID: 21090961]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pallauf K., Rimbach G. Autophagy, polyphenols and healthy ageing. Ageing Res. Rev. 2013;12(1):237–252. doi: 10.1016/j.arr.2012.03.008. [http://dx.doi.org/ 10.1016/j.arr.2012.03.008]. [PMID: 22504405]. [DOI] [PubMed] [Google Scholar]
- 89.Yang F., Chu X., Yin M., Liu X., Yuan H., Niu Y., Fu L. mTOR and autophagy in normal brain aging and caloric restriction ameliorating age-related cognition deficits. Behav. Brain Res. 2014;264:82–90. doi: 10.1016/j.bbr.2014.02.005. [http://dx.doi.org/10.1016/j.bbr.2014.02.005]. [PMID: 24525424]. [DOI] [PubMed] [Google Scholar]
- 90.Bhat N.R., Zhang P., Lee J.C., Hogan E.L. Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. J. Neurosci. 1998;18(5):1633–1641. doi: 10.1523/JNEUROSCI.18-05-01633.1998. [PMID: 9464988]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Culbert A.A., Skaper S.D., Howlett D.R., Evans N.A., Facci L., Soden P.E., Seymour Z.M., Guillot F., Gaestel M., Richardson J.C. MAPK-activated protein kinase 2 deficiency in microglia inhibits pro-inflammatory mediator release and resultant neurotoxicity. Relevance to neuroinflammation in a transgenic mouse model of Alzheimer disease. J. Biol. Chem. 2006;281(33):23658–23667. doi: 10.1074/jbc.M513646200. [http://dx.doi.org/10.1074/jbc.M513646200]. [PMID: 16774924]. [DOI] [PubMed] [Google Scholar]
- 92.Spencer J.P., Vafeiadou K., Williams R.J., Vauzour D., Spencer J., Vafeiadou K., Williams R., Vauzour D. Neuroinflammation: modulation by flavonoids and mechanisms of action. Mol. Aspects Med. 2012;33(1):83–97. doi: 10.1016/j.mam.2011.10.016. [http://dx.doi.org/10.1016/j.mam.2011. 10.016]. [PMID: 22107709]. [DOI] [PubMed] [Google Scholar]
- 93.Song K., Wang H., Krebs T.L., Danielpour D. Novel roles of Akt and mTOR in suppressing TGF-β/ALK5-mediated Smad3 activation. EMBO J. 2006;25(1):58–69. doi: 10.1038/sj.emboj.7600917. [http://dx.doi.org/10.1038/ sj.emboj.7600917]. [PMID: 16362038]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Han S., Yun Y. NF-κB/STAT3/PI3K signaling crosstalk in iMycEμ B lymphoma. Mol. Cancer. 2010;9:97. doi: 10.1186/1476-4598-9-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kreutzberg G.W. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19(8):312–318. doi: 10.1016/0166-2236(96)10049-7. [http://dx.doi.org/ 10.1016/0166-2236(96)10049-7]. [PMID: 8843599]. [DOI] [PubMed] [Google Scholar]
- 96.Ownby R.L. Neuroinflammation and cognitive aging. Curr. Psychiatry Rep. 2010;12(1):39–45. doi: 10.1007/s11920-009-0082-1. [http://dx.doi.org/10.1007/ s11920-009-0082-1]. [PMID: 20425309]. [DOI] [PubMed] [Google Scholar]
- 97.Barrientos R.M., Frank M.G., Watkins L.R., Maier S.F. Aging-related changes in neuroimmune-endocrine function: implications for hippocampal-dependent cognition. Horm. Behav. 2012;62(3):219–227. doi: 10.1016/j.yhbeh.2012.02.010. [http://dx.doi.org/10.1016/j.yhbeh.2012.02.010]. [PMID: 22370243]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.García-Bueno B., Leza J.C. Mecanismos inflamatorios/ antiinflamatorios en el cerebro tras la exposición a estrés. Rev. Neurol. 2008;46(11):675–683. [PMID: 18509827]. [PubMed] [Google Scholar]
- 99.Esteban S., Garau C., Aparicio S., Moranta D., Barceló P., Fiol M., Rial R. Chronic melatonin treatment and its precursor L-tryptophan im-prove the monoaminergic neurotransmission and related behavior in the aged rat brain. 2010. [DOI] [PubMed]
- 100.Esteban S., Garau C., Aparicio S., Moranta D., Barceló P., Ramis M., Tresguerres J.A., Rial R. Improving effects of long-term growth hormone treatment on monoaminergic neurotransmission and related behavioral tests in aged rats. Rejuvenation Res. 2010;13(6):707–716. doi: 10.1089/rej.2010.1053. [http://dx.doi.org/10.1089/rej.2010.1053]. [PMID: 21208059]. [DOI] [PubMed] [Google Scholar]
- 101.Moranta D., Barceló P., Aparicio S., Garau C., Sarubbo F., Ramis M., Nicolau C., Esteban S. Intake of melatonin increases tryptophan hydroxylase type 1 activity in aged rats: Preliminary study. Exp. Gerontol. 2014;49(1):1–4. doi: 10.1016/j.exger.2013.10.012. [http://dx.doi.org/10.1016/ j.exger.2013.10.012]. [PMID: 24189046]. [DOI] [PubMed] [Google Scholar]
- 102.Bliss T.V., Collingridge G.L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361(6407):31–39. doi: 10.1038/361031a0. [http://dx.doi.org/10.1038/361031a0]. [PMID: 8421494]. [DOI] [PubMed] [Google Scholar]
- 103.Liu L., Chan C. The role of inflammasome in Alzheimer’s disease. Ageing Res. Rev. 2014;15:6–15. doi: 10.1016/j.arr.2013.12.007. [http://dx.doi.org/10.1016/ j.arr.2013.12.007]. [PMID: 24561250]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wonodi I., Schwarcz R. Cortical kynurenine pathway metabolism: a novel target for cognitive enhancement in Schizophrenia. Schizophr. Bull. 2010;36(2):211–218. doi: 10.1093/schbul/sbq002. [http://dx.doi.org/10.1093/ schbul/sbq002]. [PMID: 20147364]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Simi A., Lerouet D., Pinteaux E., Brough D. Mechanisms of regulation for interleukin-1β in neurodegenerative disease. Neuropharmacology. 2007;52(8):1563–1569. doi: 10.1016/j.neuropharm.2007.02.011. [http://dx.doi.org/10.1016/ j.neuropharm.2007.02.011]. [PMID: 17428507]. [DOI] [PubMed] [Google Scholar]
- 106.Van Eldik L.J., Thompson W.L., Ralay Ranaivo H., Behanna H.A., Martin W.D. Glia proinflammatory cytokine upregulation as a therapeutic target for neurodegenerative diseases: function-based and target-based discovery approaches. Int. Rev. Neurobiol. 2007;82:277–296. doi: 10.1016/S0074-7742(07)82015-0. [http://dx.doi.org/10.1016/S0074-7742(07)82015-0]. [PMID: 17678967]. [DOI] [PubMed] [Google Scholar]
- 107.Williams C.M., El Mohsen M.A., Vauzour D., Rendeiro C., Butler L.T., Ellis J.A., Whiteman M., Spencer J.P. Blueberry-induced changes in spatial working memory correlate with changes in hippocampal CREB phosphorylation and brain-derived neurotrophic factor (BDNF) levels. Free Radic. Biol. Med. 2008;45(3):295–305. doi: 10.1016/j.freeradbiomed.2008.04.008. [http://dx.doi.org/10.1016/j.freeradbiomed.2008. 04.008]. [PMID: 18457678]. [DOI] [PubMed] [Google Scholar]
- 108.Abraham J., Johnson R.W. Consuming a diet supplemented with resveratrol reduced infection-related neuroinflammation and deficits in working memory in aged mice. Rejuvenation Res. 2009;12(6):445–453. doi: 10.1089/rej.2009.0888. [http://dx.doi.org/10.1089/rej.2009.0888]. [PMID: 20041738]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Flowers A., Lee J.Y., Acosta S., Hudson C., Small B., Sanberg C.D., Bickford P.C. NT-020 treatment reduces inflammation and augments Nrf-2 and Wnt signaling in aged rats. J. Neuroinflammation. 2015;12(1):174. doi: 10.1186/s12974-015-0395-4. [http://dx.doi.org/10.1186/s12974-015-0395-4]. [PMID: 26376629]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yeung F., Hoberg J.E., Ramsey C.S., Keller M.D., Jones D.R., Frye R.A., Mayo M.W. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23(12):2369–2380. doi: 10.1038/sj.emboj.7600244. [http://dx.doi.org/10.1038/sj.emboj. 7600244]. [PMID: 15152190]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wenzel U. Nutrition, sirtuins and aging. Genes Nutr. 2006;1(2):85–93. doi: 10.1007/BF02829950. [http://dx.doi.org/10.1007/BF02829950]. [PMID: 18850202]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brunet A. Science. 2004;303(5666):2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 113.Yamamoto H., Schoonjans K., Auwerx J. Sirtuin functions in health and disease. Mol. Endocrinol. 2007;21(8):1745–1755. doi: 10.1210/me.2007-0079. [http://dx.doi.org/10.1210/me.2007-0079]. [PMID: 17456799]. [DOI] [PubMed] [Google Scholar]
- 114.Horio Y., Hayashi T., Kuno A., Kunimoto R. Cellular and molecular effects of sirtuins in health and disease. Clin. Sci. 2011;121(5):191–203. doi: 10.1042/CS20100587. [http://dx.doi.org/10.1042/CS20100587]. [PMID: 21599635]. [DOI] [PubMed] [Google Scholar]
- 115.Kawahara T.L., Michishita E., Adler A.S., Damian M., Berber E., Lin M., McCord R.A., Ongaigui K.C., Boxer L.D., Chang H.Y., Chua K.F. SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaB-dependent gene expression and organismal life span. Cell. 2009;136(1):62–74. doi: 10.1016/j.cell.2008.10.052. [http://dx.doi.org/10.1016/j.cell.2008. 10.052]. [PMID: 19135889]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kanfi Y., Peshti V., Gil R., Naiman S., Nahum L., Levin E., Kronfeld-Schor N., Cohen H.Y. SIRT6 protects against pathological damage caused by diet-induced obesity. Aging Cell. 2010;9(2):162–173. doi: 10.1111/j.1474-9726.2009.00544.x. [http://dx.doi.org/10.1111/j.1474-9726.2009.00544.x]. [PMID: 20047575]. [DOI] [PubMed] [Google Scholar]
- 117.Zhong L., D’Urso A., Toiber D., Sebastian C., Henry R.E., Vadysirisack D.D., Guimaraes A., Marinelli B., Wikstrom J.D., Nir T., Clish C.B., Vaitheesvaran B., Iliopoulos O., Kurland I., Dor Y., Weissleder R., Shirihai O.S., Ellisen L.W., Espinosa J.M., Mostoslavsky R. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1α. Cell. 2010;140(2):280–293. doi: 10.1016/j.cell.2009.12.041. [http://dx.doi.org/10.1016/j.cell.2009.12.041]. [PMID: 20141841]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Duan W. Sirtuins: from metabolic regulation to brain aging. Front. Aging Neurosci. 2013;5(36):5–36. doi: 10.3389/fnagi.2013.00036. [http://dx.doi.org/10.3389/ fnagi.2013.00036]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Qin W., Yang T., Ho L., Zhao Z., Wang J., Chen L., Zhao W., Thiyagarajan M., MacGrogan D., Rodgers J.T., Puigserver P., Sadoshima J., Deng H., Pedrini S., Gandy S., Sauve A.A., Pasinetti G.M. Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J. Biol. Chem. 2006;281(31):21745–21754. doi: 10.1074/jbc.M602909200. [http://dx.doi.org/10.1074/jbc.M602909200]. [PMID: 16751189]. [DOI] [PubMed] [Google Scholar]
- 120.Jiang M., Wang J., Fu J., Du L., Jeong H., West T., Xiang L., Peng Q., Hou Z., Cai H., Seredenina T., Arbez N., Zhu S., Sommers K., Qian J., Zhang J., Mori S., Yang X.W., Tamashiro K.L., Aja S., Moran T.H., Luthi-Carter R., Martin B., Maudsley S., Mattson M.P., Cichewicz R.H., Ross C.A., Holtzman D.M., Krainc D., Duan W. Neuroprotective role of Sirt1 in mammalian models of Huntington’s disease through activation of multiple Sirt1 targets. Nat. Med. 2011;18(1):153–158. doi: 10.1038/nm.2558. [http://dx. doi.org/10.1038/nm.2558]. [PMID: 22179319]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang F., Wang S., Gan L., Vosler P.S., Gao Y., Zigmond M.J., Chen J. Protective effects and mechanisms of sirtuins in the nervous system. Prog. Neurobiol. 2011;95(3):373–395. doi: 10.1016/j.pneurobio.2011.09.001. [http:// dx.doi.org/10.1016/j.pneurobio.2011.09.001]. [PMID: 21930182]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang H.N., Li L., Gao P., Chen H.Z., Zhang R., Wei Y.S., Liu D.P., Liang C.C. Involvement of the p65/RelA subunit of NF-kappaB in TNF-α-induced SIRT1 expression in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2010;397(3):569–575. doi: 10.1016/j.bbrc.2010.05.160. [http://dx.doi.org/10.1016/j.bbrc.2010.05.160]. [PMID: 20617556]. [DOI] [PubMed] [Google Scholar]
- 123.Zhang Z., Lowry S.F., Guarente L., Haimovich B. Roles of SIRT1 in the acute and restorative phases following induction of inflammation. J. Biol. Chem. 2010;285(53):41391–41401. doi: 10.1074/jbc.M110.174482. [http://dx.doi.org/10.1074/jbc.M110.174482]. [PMID: 20966076]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gillum M.P., Kotas M.E., Erion D.M., Kursawe R., Chatterjee P., Nead K.T., Muise E.S., Hsiao J.J., Frederick D.W., Yonemitsu S., Banks A.S., Qiang L., Bhanot S., Olefsky J.M., Sears D.D., Caprio S., Shulman G.I. SirT1 regulates adipose tissue inflammation. Diabetes. 2011;60(12):3235–3245. doi: 10.2337/db11-0616. [http://dx.doi.org/ 10.2337/db11-0616]. [PMID: 22110092]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yao H., Chung S., Hwang J.W., Rajendrasozhan S., Sundar I.K., Dean D.A., McBurney M.W., Guarente L., Gu W., Rönty M., Kinnula V.L., Rahman I. SIRT1 protects against emphysema via FOXO3-mediated reduction of premature senescence in mice. J. Clin. Invest. 2012;122(6):2032–2045. doi: 10.1172/JCI60132. [http://dx.doi.org/10. 1172/JCI60132]. [PMID: 22546858]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Quintas A., de Solís A.J., Díez-Guerra F.J., Carrascosa J.M., Bogónez E. Age-associated decrease of SIRT1 expression in rat hippocampus: prevention by late onset caloric restriction. Exp. Gerontol. 2012;47(2):198–201. doi: 10.1016/j.exger.2011.11.010. [http://dx.doi.org/10.1016/j.exger. 2011.11.010]. [PMID: 22143179]. [DOI] [PubMed] [Google Scholar]
- 127.Michán S., Li Y., Chou M.M., Parrella E., Ge H., Long J.M., Allard J.S., Lewis K., Miller M., Xu W., Mervis R.F., Chen J., Guerin K.I., Smith L.E., McBurney M.W., Sinclair D.A., Baudry M., de Cabo R., Longo V.D. SIRT1 is essential for normal cognitive function and synaptic plasticity. J. Neurosci. 2010;30(29):9695–9707. doi: 10.1523/JNEUROSCI.0027-10.2010. [http://dx.doi.org/10.1523/JNEUROSCI.0027-10.2010]. [PMID: 20660252]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wu A., Ying Z., Gomez-Pinilla F. Oxidative stress modulates Sir2alpha in rat hippocampus and cerebral cortex. Eur. J. Neurosci. 2006;23(10):2573–2580. doi: 10.1111/j.1460-9568.2006.04807.x. [http://dx.doi.org/10.1111/j.1460-9568.2006.04807.x]. [PMID: 16817860]. [DOI] [PubMed] [Google Scholar]
- 129.Herskovits A.Z., Guarente L. SIRT1 in neurodevelopment and brain senescence. Neuron. 2014;81(3):471–483. doi: 10.1016/j.neuron.2014.01.028. [http://dx.doi. org/10.1016/j.neuron.2014.01.028]. [PMID: 24507186]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Herskovits A.Z., Guarente L. Sirtuin deacetylases in neurodegenerative diseases of aging. Cell Res. 2013;23(6):746–758. doi: 10.1038/cr.2013.70. [http://dx.doi.org/10.1038/cr.2013.70]. [PMID: 23689277]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hall J.A., Dominy J.E., Lee Y., Puigserver P. The sirtuin family’s role in aging and age-associated pathologies. J. Clin. Invest. 2013;123(3):973–979. doi: 10.1172/JCI64094. [http://dx.doi.org/10.1172/JCI64094]. [PMID: 23454760]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Howitz K.T., Bitterman K.J., Cohen H.Y., Lamming D.W., Lavu S., Wood J.G., Zipkin R.E., Chung P., Kisielewski A., Zhang L.L., Scherer B., Sinclair D.A. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425(6954):191–196. doi: 10.1038/nature01960. [http://dx.doi.org/10.1038/nature01960]. [PMID: 12939617]. [DOI] [PubMed] [Google Scholar]
- 133.Hubbard B., Gomes A., Dai H., Li J., Case A., Considine T., Riera T., Lee J. Evidence for a common mechanism of SIRT1 regulation by allo-steric activators. Science (80-.) 2013;339(6124):1216–1219. doi: 10.1126/science.1231097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mannari C., Bertelli A.A., Stiaccini G., Giovannini L. Wine, sirtuins and nephroprotection: not only resveratrol. Med. Hypotheses. 2010;75(6):636–638. doi: 10.1016/j.mehy.2010.08.004. [http://dx.doi.org/10.1016/j.mehy.2010. 08.004]. [PMID: 20932649]. [DOI] [PubMed] [Google Scholar]
- 135.Cohen H., Miller C., Bitterman K., Wall N., Hekking B., Kessler B., Howitz K., Gorospe M., de Cabo R., Sinclair D. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305(5682):390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- 136.Bhullar K., Hubbard B. Lifespan and healthspan extension by resveratrol. 2015. [DOI] [PubMed]
- 137.Yamakuchi M., Ferlito M., Lowenstein C.J. miR-34a repression of SIRT1 regulates apoptosis. Proc. Natl. Acad. Sci. USA. 2008;105(36):13421–13426. doi: 10.1073/pnas.0801613105. [http://dx.doi.org/10.1073/pnas.0801613105]. [PMID: 18755897]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cai W., Ramdas M., Zhu L., Chen X., Striker G.E., Vlassara H. Oral advanced glycation endproducts (AGEs) promote insulin resistance and diabetes by depleting the antioxidant defenses AGE receptor-1 and sirtuin 1. Proc. Natl. Acad. Sci. USA. 2012;109(39):15888–15893. doi: 10.1073/pnas.1205847109. [http://dx.doi.org/10.1073/pnas.1205847109]. [PMID: 22908267]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Furukawa A., Tada-Oikawa S., Kawanishi S., Oikawa S. H2O2 accelerates cellular senescence by accumulation of acetylated p53 via decrease in the function of SIRT1 by NAD+ depletion. Cell. Physiol. Biochem. 2007;20(1-4):45–54. doi: 10.1159/000104152. [http://dx.doi.org/10. 1159/000104152]. [PMID: 17595514]. [DOI] [PubMed] [Google Scholar]
- 140.Xie J., Zhang X., Zhang L. Negative regulation of inflammation by SIRT1. Pharmacol. Res. 2013;67(1):60–67. doi: 10.1016/j.phrs.2012.10.010. [http://dx. doi.org/10.1016/j.phrs.2012.10.010]. [PMID: 23098819]. [DOI] [PubMed] [Google Scholar]
- 141.Adler A.S., Sinha S., Kawahara T.L., Zhang J.Y., Segal E., Chang H.Y. Motif module map reveals enforcement of aging by continual NF-kappaB activity. Genes Dev. 2007;21(24):3244–3257. doi: 10.1101/gad.1588507. [http://dx.doi.org/10.1101/gad.1588507]. [PMID: 18055696]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kaltschmidt C., Kaltschmidt B., Neumann H., Wekerle H., Baeuerle P.A. Constitutive NF-κ B activity in neurons. Mol. Cell. Biol. 1994;14(6):3981–3992. doi: 10.1128/mcb.14.6.3981. [http://dx.doi.org/10.1128/MCB.14. 6.3981]. [PMID: 8196637]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Meberg P.J., Kinney W.R., Valcourt E.G., Routtenberg A. Gene expression of the transcription factor NF-κ B in hippocampus: regulation by synaptic activity. Brain Res. Mol. Brain Res. 1996;38(2):179–190. doi: 10.1016/0169-328x(95)00229-l. [http://dx.doi.org/10.1016/0169-328X(95)00229-L]. [PMID: 8793106]. [DOI] [PubMed] [Google Scholar]
- 144.Jana M., Dasgupta S., Liu X., Pahan K. Regulation of tumor necrosis factor-alpha expression by CD40 ligation in BV-2 microglial cells. J. Neurochem. 2002;80(1):197–206. doi: 10.1046/j.0022-3042.2001.00691.x. [http://dx.doi. org/10.1046/j.0022-3042.2001.00691.x]. [PMID: 11796758]. [DOI] [PubMed] [Google Scholar]
- 145.Nakajima K., Matsushita Y., Tohyama Y., Kohsaka S., Kurihara T. Differential suppression of endotoxin-inducible inflammatory cytokines by nuclear factor kappa B (NFkappaB) inhibitor in rat microglia. Neurosci. Lett. 2006;401(3):199–202. doi: 10.1016/j.neulet.2006.03.014. [http://dx. doi.org/10.1016/j.neulet.2006.03.014]. [PMID: 16580131]. [DOI] [PubMed] [Google Scholar]
- 146.Baldwin A.S., Jr The NF-κ B and I κ B proteins: new discoveries and insights. Annu. Rev. Immunol. 1996;14(14):649–683. doi: 10.1146/annurev.immunol.14.1.649. [http:// dx.doi.org/10.1146/annurev.immunol.14.1.649]. [PMID: 8717528]. [DOI] [PubMed] [Google Scholar]
- 147.Ghosh S., Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl.):S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [http://dx.doi.org/10.1016/ S0092-8674(02)00703-1]. [PMID: 11983155]. [DOI] [PubMed] [Google Scholar]
- 148.Siebenlist U., Franzoso G., Brown K. Structure, regulation and function of NF-κ B. Annu. Rev. Cell Biol. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [http://dx.doi.org/10.1146/annurev.cb.10.110194.002201]. [PMID: 7888182]. [DOI] [PubMed] [Google Scholar]
- 149.Oeckinghaus A., Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009;1(4):a000034. doi: 10.1101/cshperspect.a000034. [http://dx.doi.org/10.1101/cshperspect.a000034]. [PMID: 20066092]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kaltschmidt B., Widera D., Kaltschmidt C. Signaling via NF-κB in the nervous system.Biochim. Biophys. Acta - Mol. Cell Res. 2005;1745(3):287–299. doi: 10.1016/j.bbamcr.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 151.Chen L.F., Greene W.C. Regulation of distinct biological activities of the NF-kappaB transcription factor complex by acetylation. J. Mol. Med. (Berl.) 2003;81(9):549–557. doi: 10.1007/s00109-003-0469-0. [http://dx.doi.org/10. 1007/s00109-003-0469-0]. [PMID: 12920522]. [DOI] [PubMed] [Google Scholar]
- 152.Schmitz M.L., Mattioli I., Buss H., Kracht M. NF-kappaB: a multifaceted transcription factor regulated at several levels. ChemBioChem. 2004;5(10):1348–1358. doi: 10.1002/cbic.200400144. [http://dx.doi.org/10. 1002/cbic.200400144]. [PMID: 15457532]. [DOI] [PubMed] [Google Scholar]
- 153.Perkins N.D. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat. Rev. Mol. Cell Biol. 2007;8(1):49–62. doi: 10.1038/nrm2083. [http://dx.doi.org/10.1038/nrm2083]. [PMID: 17183360]. [DOI] [PubMed] [Google Scholar]
- 154.Quivy V., Van Lint C. Regulation at multiple levels of NF-kappaB-mediated transactivation by protein acetylation. Biochem. Pharmacol. 2004;68(6):1221–1229. doi: 10.1016/j.bcp.2004.05.039. [http://dx.doi.org/10.1016/ j.bcp.2004.05.039]. [PMID: 15313420]. [DOI] [PubMed] [Google Scholar]
- 155.Osorio F.G., Bárcena C., Soria-Valles C., Ramsay A.J., de Carlos F., Cobo J., Fueyo A., Freije J.M., López-Otín C. Nuclear lamina defects cause ATM-dependent NF-κB activation and link accelerated aging to a systemic inflammatory response. Genes Dev. 2012;26(20):2311–2324. doi: 10.1101/gad.197954.112. [http://dx.doi.org/10.1101/gad. 197954.112]. [PMID: 23019125]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tilstra J.S., Robinson A.R., Wang J., Gregg S.Q., Clauson C.L., Reay D.P., Nasto L.A., St Croix C.M., Usas A., Vo N., Huard J., Clemens P.R., Stolz D.B., Guttridge D.C., Watkins S.C., Garinis G.A., Wang Y., Niedernhofer L.J., Robbins P.D. NF-κB inhibition delays DNA damage-induced senescence and aging in mice. J. Clin. Invest. 2012;122(7):2601–2612. doi: 10.1172/JCI45785. [http://dx.doi.org/ 10.1172/JCI45785]. [PMID: 22706308]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kwon H.S., Brent M.M., Getachew R., Jayakumar P., Chen L.F., Schnolzer M., McBurney M.W., Marmorstein R., Greene W.C., Ott M. Human immunodeficiency virus type 1 Tat protein inhibits the SIRT1 deacetylase and induces T cell hyperactivation. Cell Host Microbe. 2008;3(3):158–167. doi: 10.1016/j.chom.2008.02.002. [http://dx.doi.org/10.1016/ j.chom.2008.02.002]. [PMID: 18329615]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chen J., Zhou Y., Mueller-Steiner S., Chen L.F., Kwon H., Yi S., Mucke L., Gan L. SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J. Biol. Chem. 2005;280(48):40364–40374. doi: 10.1074/jbc.M509329200. [http://dx.doi.org/10. 1074/jbc.M509329200]. [PMID: 16183991]. [DOI] [PubMed] [Google Scholar]
- 159.Kauppinen A., Suuronen T., Ojala J., Kaarniranta K., Salminen A. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell. Signal. 2013;25(10):1939–1948. doi: 10.1016/j.cellsig.2013.06.007. [http://dx.doi.org/10.1016/j.cellsig.2013.06. 007]. [PMID: 23770291]. [DOI] [PubMed] [Google Scholar]
- 160.Goyarzu P., Malin D.H., Lau F.C., Taglialatela G., Moon W.D., Jennings R., Moy E., Moy D., Lippold S., Shukitt-Hale B. [DOI] [PubMed]; Joseph J.A. Blueberry supplemented diet: effects on object recognition memory and nuclear factor-kappa B levels in aged rats. Nutr. Neurosci. 2004;7(2):75–83. doi: 10.1080/10284150410001710410. [http://dx.doi.org/10.1080/ 10284150410001710410]. [PMID: 15279493]. [DOI] [PubMed] [Google Scholar]
- 161.Jang M., Cai L., Udeani G., Slowing K., Thomas C., Beecher C., Fong H., Farnsworth N., Kinghorn A., Mehta R., Moon R., Pezzuto J. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275(5297):218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 162.Holmes-McNary M., Baldwin A.S., Jr Chemopreventive properties of trans-resveratrol are associated with inhibition of activation of the IkappaB kinase. Cancer Res. 2000;60(13):3477–3483. [PMID: 10910059]. [PubMed] [Google Scholar]
- 163.Manna S.K., Mukhopadhyay A., Aggarwal B.B. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-κ B, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J. Immunol. 2000;164(12):6509–6519. doi: 10.4049/jimmunol.164.12.6509. [http://dx.doi.org/10.4049/jimmunol. 164.12.6509]. [PMID: 10843709]. [DOI] [PubMed] [Google Scholar]
- 164.Shukitt-Hale B., Lau F.C., Carey A.N., Galli R.L., Spangler E.L., Ingram D.K., Joseph J.A. Blueberry polyphenols attenuate kainic acid-induced decrements in cognition and alter inflammatory gene expression in rat hippocampus. Nutr. Neurosci. 2008;11(4):172–182. doi: 10.1179/147683008X301487. [http://dx.doi.org/10.1179/147683008X301487]. [PMID: 18681986]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Risitano R., Currò M., Cirmi S., Ferlazzo N., Campiglia P., Caccamo D., Ientile R., Navarra M. Flavonoid fraction of Bergamot juice reduces LPS-induced inflammatory response through SIRT1-mediated NF-κB inhibition in THP-1 monocytes. PLoS One. 2014;9(9):e107431. doi: 10.1371/journal.pone.0107431. [http://dx.doi.org/10.1371/journal.pone. 0107431]. [PMID: 25260046]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Duarte D.A., Rosales M.A., Papadimitriou A., Silva K.C., Amancio V.H., Mendonça J.N., Lopes N.P., de Faria J.B., de Faria J.M. Polyphenol-enriched cocoa protects the diabetic retina from glial reaction through the sirtuin pathway. J. Nutr. Biochem. 2015;26(1):64–74. doi: 10.1016/j.jnutbio.2014.09.003. [http://dx.doi.org/10.1016/j.jnutbio.2014.09. 003]. [PMID: 25448608]. [DOI] [PubMed] [Google Scholar]
- 167.Zhang S., Qi Y., Xu Y., Han X., Peng J., Liu K., Sun C.K. Protective effect of flavonoid-rich extract from Rosa laevigata Michx on cerebral ischemia-reperfusion injury through suppression of apoptosis and inflammation. Neurochem. Int. 2013;63(5):522–532. doi: 10.1016/j.neuint.2013.08.008. [http://dx.doi.org/10.1016/j.neuint.2013.08.008]. [PMID: 24012531]. [DOI] [PubMed] [Google Scholar]
- 168.Raza S.S., Khan M.M., Ahmad A., Ashafaq M., Islam F., Wagner A.P., Safhi M.M., Islam F. Neuroprotective effect of naringenin is mediated through suppression of NF-κB signaling pathway in experimental stroke. Neuroscience. 2013;230:157–171. doi: 10.1016/j.neuroscience.2012.10.041. [http://dx.doi.org/10.1016/j.neuroscience.2012.10.041]. [PMID: 23103795]. [DOI] [PubMed] [Google Scholar]
- 169.Bai X., Zhang X., Chen L., Zhang J., Zhang L., Zhao X., Zhao T., Zhao Y. Protective effect of naringenin in experimental ischemic stroke: down-regulated NOD2, RIP2, NF-κB, MMP-9 and up-regulated claudin-5 expression. Neurochem. Res. 2014;39(8):1405–1415. doi: 10.1007/s11064-014-1326-y. [http://dx.doi.org/10.1007/s11064-014-1326-y]. [PMID: 24842554]. [DOI] [PubMed] [Google Scholar]
- 170.Song X., Zhou B., Cui L., Lei D., Zhang P., Yao G., Xia M., Hayashi T., Hattori S., Ushiki-Kaku Y., Tashiro S.I., Onodera S., Ikejima T. Silibinin ameliorates Aβ25-35-induced memory deficits in rats by modulating autophagy and attenuating neuroinflammation as well as oxidative stress. Neurochem. Res. 2017;42(4):1073–1083. doi: 10.1007/s11064-016-2141-4. [http://dx.doi.org/10.1007/s11064-016-2141-4]. [PMID: 28004303]. [DOI] [PubMed] [Google Scholar]
- 171.Wang Q., Zou L., Liu W., Hao W., Tashiro S., Onodera S., Ikejima T. Inhibiting NF-κB activation and ROS production are involved in the mechanism of silibinin’s protection against D-galactose-induced senescence. Pharmacol. Biochem. Behav. 2011;98(1):140–149. doi: 10.1016/j.pbb.2010.12.006. [http://dx.doi.org/10.1016/j.pbb.2010.12.006]. [PMID: 21167197]. [DOI] [PubMed] [Google Scholar]
- 172.Suganthy N., Devi K.P., Nabavi S.F., Braidy N., Nabavi S.M. Bioactive effects of quercetin in the central nervous system: Focusing on the mechanisms of actions. Biomed. Pharmacother. 2016;84:892–908. doi: 10.1016/j.biopha.2016.10.011. [http://dx.doi.org/10.1016/j.biopha.2016.10.011]. [PMID: 27756054]. [DOI] [PubMed] [Google Scholar]
- 173.Ng F., Wijaya L., Tang B.L. SIRT1 in the brain-connections with aging-associated disorders and lifespan. Front. Cell. Neurosci. 2015;9(March):64. doi: 10.3389/fncel.2015.00064. [PMID: 25805970]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Labinskyy N., Csiszar A., Veress G., Stef G., Pacher P., Oroszi G., Wu J., Ungvari Z. Vascular dysfunction in aging: potential effects of resveratrol, an anti-inflammatory phytoestrogen. Curr. Med. Chem. 2006;13(9):989–996. doi: 10.2174/092986706776360987. [http://dx.doi.org/10.2174/ 092986706776360987]. [PMID: 16611080]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Birrell M.A., McCluskie K., Wong S., Donnelly L.E., Barnes P.J., Belvisi M.G. Resveratrol, an extract of red wine, inhibits lipopolysaccharide induced airway neutrophilia and inflammatory mediators through an NF-kappaB-independent mechanism. FASEB J. 2005;19(7):840–841. doi: 10.1096/fj.04-2691fje. [PMID: 15734790]. [DOI] [PubMed] [Google Scholar]
- 176.Milne J.C., Lambert P.D., Schenk S., Carney D.P., Smith J.J., Gagne D.J., Jin L., Boss O., Perni R.B., Vu C.B., Bemis J.E., Xie R., Disch J.S., Ng P.Y., Nunes J.J., Lynch A.V., Yang H., Galonek H., Israelian K., Choy W., Iffland A., Lavu S., Medvedik O., Sinclair D.A., Olefsky J.M., Jirousek M.R., Elliott P.J., Westphal C.H. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450(7170):712–716. doi: 10.1038/nature06261. [http://dx.doi.org/10.1038/nature06261]. [PMID: 18046409]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Milne J.C., Denu J.M. The Sirtuin family: therapeutic targets to treat diseases of aging. Curr. Opin. Chem. Biol. 2008;12(1):11–17. doi: 10.1016/j.cbpa.2008.01.019. [http://dx.doi.org/10.1016/j.cbpa.2008.01.019]. [PMID: 18282481]. [DOI] [PubMed] [Google Scholar]
- 178.Rajendrasozhan S., Yang S.R., Edirisinghe I., Yao H., Adenuga D., Rahman I. Deacetylases and NF-kappaB in redox regulation of cigarette smoke-induced lung inflammation: epigenetics in pathogenesis of COPD. Antioxid. Redox Signal. 2008;10(4):799–811. doi: 10.1089/ars.2007.1938. [http://dx.doi.org/10.1089/ars.2007.1938] [PMID: 18220485] [DOI] [PMC free article] [PubMed] [Google Scholar]