Abstract
Background:
Chronic pain is a significant clinical problem and a very complex pathophysiological phenomenon. There is growing evidence that targeting the endocannabinoid system may be a useful approach to pain alleviation. Classically, the system includes G protein-coupled receptors of the CB1 and CB2 subtypes and their endogenous ligands. More recently, several subtypes of the large superfamily of cation TRP channels have been coined as “ionotropic cannabinoid receptors”, thus highlighting their role in cannabinoid signalling. Thus, the aim of this review was to explore the intimate connection between several “painful” TRP channels, endocannabinoids and nociceptive signalling.
Methods:
Research literature on this topic was critically reviewed allowing us not only summarize the existing evidence in this area of research, but also propose several possible cellular mechanisms linking nociceptive and cannabinoid signaling with TRP channels.
Results:
We begin with an overview of physiology of the endocannabinoid system and its major components, namely CB1 and CB2 G protein-coupled receptors, their two most studied endogenous ligands, anandamide and 2-AG, and several enzymes involved in endocannabinoid biosynthesis and degradation. The role of different endocannabinoids in the regulation of synaptic transmission is then discussed in detail. The connection between the endocannabinoid system and several TRP channels, especially TRPV1-4, TRPA1 and TRPM8, is then explored, while highlighting the role of these same channels in pain signalling.
Conclusion:
There is increasing evidence implicating several TRP subtypes not only as an integral part of the endocannabinoid system, but also as promising molecular targets for pain alleviation with the use of endo- and phytocannabinoids, especially when the function of these channels is upregulated under inflammatory conditions.
Keywords: Endocannabinoid, cannabinoid receptor, GABA receptor, TRP channel, calcium signalling, pain
1. Introduction
Persistent, chronic pain is a significant clinical problem and an extremely complex pathophysiological phenomenon that encompasses different pain conditions, symptoms and the whole hierarchy of intervening signal transduction pathways and mechanisms. Moreover, these ultimately culminate in pain sensation and experience, which, in turn, is also a complex pain perception related emotional state. It seems logical to try and break the vicious cycle between noxious stimuli, peripheral and central sensitization and enhanced psycho-emotional stress at the “gate”, where nociceptive signalling is initiated, yet the most efficient target in chronic pain treatment remains the opioid system of the brain. This system also controls reward and addictive behaviour; hence drugs acting at opioid receptors, opiates, are very addictive.
However, there is growing evidence that engaging the endocannabinoid system may be a useful complimentary (or even alternative) strategy, especially in refractory, difficult to treat cases of chronic pain [1-3]. The system includes neuroactive lipids and their receptors of the CB1 and CB2 subtypes, which are primarily found in the CNS and in the periphery, respectively [4]. Their activation likely acts in synergy with the opioid system, but CB receptors independently regulate a host of other processes implicated in chronic pain, most notably synthesis and release of pro-inflammatory mediators.
In this context, the action of lipids and cytokines on neuronal sensory Ca2+-permeable Transient Receptor Potential (TRP) channels is of particular interest, as many members of the large TRP superfamily of ion channels are increasingly implicated as important sensors of noxious stimuli [5-8]. Pharmacological targeting of these channels in pain may thus represent a useful strategy avoiding the wide-spread CNS side effects of the currently used drugs. By engaging the native protective cell signalling pathways, endocannabinoids (eCBs) as well as related phytocannabinoids can be promising candidates for the treatment of chronic pain since they possess low toxicity and low addiction potential. In this regard, inhibitors of enzymes (for example, fatty acid amide hydrolase (FAAH)) that hydrolyze eCBs are of particular interest [9].
Indeed, there is an enhanced thermal (to both cold and heat) and mechanical (to both touch and pressure) sensitivity in the chronic pain syndrome. Thus, the modulation of activity and properties (e.g. activation threshold, voltage dependence, polymodal regulation) of thermo- and mechanosensitive TRP channels can be of special interest in these conditions. It is also hardly coincidental that these same channels show distinct lipid-sensing properties, while various lipids strongly affect the sensitivity of TRPs to their natural activators. Here we aim to review the rapidly accumulating, but still remaining somewhat disparate, results indicating the connection between the endocannabinoid system and several thermo- and mechanosensitive TRP channels. We begin with a brief overview of physiological and pathophysiological roles of the endocannabinoid system and TRP channels in the nervous system, then discuss neuropharmacology of endo- and exogenous cannabinoids with reference to chronic pain conditions and other pathophysiological conditions, and summarise the current evidence for the action of these compounds on TRP channels, by focusing in particular on TRPA1, TRPV1-V4, and TRPM8 channels as some novel putative non CB1/CB2-receptors for cannabinoids. Finally, from this perspective we discuss possible cellular mechanisms linking nociceptive and cannabinoid signalling with TRP channels, and the promise of these groups of drugs and ion channels in the control of abnormal nociceptive signalling at the “gate”.
2. The Endocanabinoid system
Endocannabinoids modulate a variety of fundamental physiological processes. Changes of endocannabinoid signalling accompany many diseases and hence the endocannabinoid system is considered as a potentially important therapeutic target. In addition, cannabinoids are widely used and abused drugs with a significant cost to the society. Therefore, scientific advances in our understanding of the neurobiology of cannabinoid signalling may lead to better treatment and/or prevention of drug abuse.
2.1. Major Components of The Endocannabinoid System
Derivatives from the plant Cannabis sativa have been used for medicinal and recreational purposes for thousands of years. Nevertheless, our knowledge of the chemistry of cannabinoids and physiological aspects of their action is relatively recent. Only in 1964, when the structure of the most active ingredient Δ9-tetrahydrocannabinol (THC) that produces the primary psychoactive effects was determined by the Mechoulam's laboratory [10], 'the cannabinoid field was placed on a firm footing' [11]. This led to the synthesis of high-affinity cannabinoid ligands and eventually the identification of a brain cannabinoid receptor (CB1) [12, 13]. The second type of cannabinoid receptor (CB2) has been identified shortly afterwards [14]. Unlike CB1, which is abundantly expressed in several brain structures, CB2 receptors are predominantly localised in cells and tissues of the immune system. Over one hundred of other active cannabinoids have been identified in cannabis, classified as phytocannabinoids, such as the major phytocannabinoid cannabidiol (CBD) [3].
The discovery of cannabinoid receptors prompted a search for their endogenous ligands, the so-called endocannabinoids. In the 1990s, two endogenous brain lipids were identified as CB1 ligands: N-arachidonoylethanolamine (anandamide) [15, 16] and 2-arachidonylglycerol (2-AG) [15, 17, 18]. Other endogenous agonists of cannabinoid receptors, O-arachidonoyl ethanolamine (virodhamine) [19], N-arachidonoyl dopamine (NADA) [20] and 2-arachidonoyl glyceryl ether (noladin ether) [21], have later been discovered. These five best-characterised eCBs have the common 19-C backbone structure. Unlike classical neurotransmitters eCBs are not stored, instead, they are rapidly synthesized “on demand” and released by neurons in response to depolarization and consequent Ca2+ influx [22]. Since these molecules are hydrophobic (lipophilic), their action is thought to be limited mainly to local cell signalling in a paracrine or autocrine manner. Moreover, these lipid messengers are very short lived, as there exist powerful systems for their transport and biodegradation.
Two possible routes of 2-AG biosynthesis in neurons were considered [1]. Phospholipase C (PLC)-mediated hydrolysis of membrane phospholipids produces diacylglycerol (DAG), which may be subsequently converted to 2-AG by DAG lipase activity. Alternatively, phospholipase A1 (PLA1) may generate a lysophospholipid, which may be hydrolyzed to 2-AG by lyso-PLC. The fact that various structurally distinct inhibitors of PLC and DGL prevent 2-AG formation in cultures of cortical neurons indicates that the PLC/DGL pathway may play a primary role in this process [23]. Anandamide is thought to be produced via hydrolysis of the phospholipid precursor N-arachidonoyl phosphatidylethanol-amine (PE), catalyzed by a phospholipase D-type activity [1]. Finally, there are specific mechanisms for transport and degradation of eCBs accounting for termination of their action [1].
These, and more recent results (see [2, 24] for reviews) have shown the existence of an endocannabinoid signalling system, which includes:
1) two 7-transmembrane-domain G protein-coupled receptors (GPCRs) for THC - CB1 and CB2; 2) their two most studied endogenous ligands, the eCBs anandamide and 2-AG; 3) the five enzymes responsible for endocannabinoid biosynthesis (N-acyl-phosphatidyl- ethanolamine-selective phospholipase D (NAPE-PLD) and DGL α and β, for anandamide and 2-AG, respectively) and hydrolytic inactivation (FAAH and monoacylglycerol lipase, for anandamide and 2-AG, respectively) [24].
Currently, however, 'giving a definition of the complex endogenous signalling system known as the “endocannabinoid system” is becoming an increasingly difficult task' [24]. The reasons for the difficulty are discussed in details in the recent review [24]. Below we will mention just some of them. First, eCBs seem to target not only cannabinoid receptors. Among the potential targets are ligand-gated ion channels like 5-HT3, glycine and nicotinic acetylcholine receptors, cation channels of the TRP superfamily, such as TRPV1-4, TRPA1 or TRPM8, voltage-gated ion channels like T-type calcium channels or TASK potassium channels, and metabotropic receptors like GPR55 (for review see [4]). In this regard it is worth mentioning that TRPV1 receptors are suggested to be considered as ionotropic receptors for anandamide by some authors [25-28]. Second, a number of active at cannabinoid receptors novel endogenous lipids have been recently identified (for review see [29]). Moreover, the family of eCBs has recently grown to include a group of peptide ligands (so-called pepcans) acting as allosteric modulators of CB1 receptors [30-32]. Thus, even more targets of eCBs are likely to be identified in near future. Third, both anandamide and 2-AG have more than just one set of biosynthetic and degrading pathways and corresponding enzymes. A further complexity is that in some cases enzymes, which are “degrading” for eCBs, are “biosynthetic” for other mediators [24].
2.2. Physiological and Pathological Roles of the Endocannabinoid System: Focus on Synaptic Transmission and Pain
As judged from recreational and ancient medicinal use of Cannabis sativa, the roles of the endocannabinoid system should be predominantly related to: 1) cognition; 2) mood; 3) nociception and mechanisms counteracting inflammation; 4) regulation of food intake. While these roles have been confirmed with a variety of methods, it now appears that those are just a tip of an iceberg. The endocannabinoid system is involved in overwhelming variety of physiological processes – not only in the nervous, but also in the digestive, reproductive, respiratory and immune systems. Endocannabinoids enhance appetite, reduce pain, act as neuroprotectants and regulators of cytokine production and are somehow involved in the extinction of memories, to mention just a few of their effects.
Thus, it is not surprising that targeting the endocannabinoid system is suggested as a promising treatment opportunity for a number of pathological states. To mention just a few recent reviews, related to the nervous system only, these include anxiety and depressive symptoms [33, 34]; Alzheimer's disease [35]; schizophrenia [36-38]; pain and inflammation [9, 39, 40]; multiple sclerosis [41]; stroke and brain trauma [42]; Parkinson's disease [43]; epilepsy [44] and addiction [45].
Considering that CB1 receptors are predominantly localized on synaptic terminals, indicating their important role in synaptic transmission, that information from nociceptors to the brain is passed via synapses, and that many important principles of functioning of the endocannabinoid signalling system have been discovered as a result of studying its role in synaptic transmission we will start with a brief overview of the studies addressing physiological role of eCBs in modulation of synaptic transmission and principles of functioning of eCBs emerged from these studies. Namely, we will consider retrograde and nonretrograde modes of action of eCBs in information processing, differential roles of anandamide and 2-AG, crosstalk between CB receptors and TRPV1, and finally underestimated roles of CB2 in the central nervous system and CB1 in the periphery.
2.3. Regulation of Synaptic Transmission by Endo-cannabinoids
2.3.1. Endocannabinoids are Involved in Retrograde and Nonretrograde Signalling in Several Types of Synapses
Retrograde signalling means that eCBs are mobilized from postsynaptic neurons and activate presynaptic CB1 receptors, thus suppressing neurotransmitter release. The CB1 receptor is one of the most widely expressed GPCRs in the brain [46], and it is widely present both in the central and peripheral nervous system [47]. Within the neuron, CB1 receptors are often localized at axon terminals, and their activation leads to inhibition of transmitter release. The consequence is inhibition of neurotransmission via a presynaptic mechanism (see [48] for a review).
Perhaps one of the most exciting findings regarding cellular mechanisms of endocannabinoid action on synaptic transmission was the discovery of their role as retrograde messengers activating CB1 receptor [49, 50]. Important support for the role of eCBs as retrograde messengers came subsequently from the study of an electrophysiological phenomenon termed “depolarization-induced suppression of inhibition” (DSI), discovered initially in Purkinje neurons [51] in cerebellum and hippocampal pyramidal cells [52], but present also in neurons from neocortex (in several species) [53-55]. Postsynaptic spike firing or brief depolarization of the membrane of the postsynaptic neuron results in a transient (lasting about one minute) suppression of GABAergic synaptic transmission. Although DSI is triggered by a postsynaptic increase in intracellular free calcium ion concentration ([Ca2+]i), its localisation is presynaptic [56]. The role of eCBs as retrograde messengers in DSI was independently demonstrated by two groups [49, 50]. Specifically, it was shown that eCBs 'are released from postsynaptic neurons and travel backward across synapses, activating CB1 on presynaptic axons and suppressing neurotransmitter release' [11]. Moreover, a mechanistically similar phenomenon, depolarization-induced suppression of excitation (DSE), is also mediated by retrograde action of eCBs [57]. In addition, eCBs contribute to presynaptic forms of long-term depression (LTD) at both excitatory and inhibitory synapses [58-61]. These are now some of the best characterized retrograde messengers mediating multiple forms of short- and long-term synaptic plasticity [62-64]. There are important signal transduction differences in these forms of synaptic plasticity: short-term plasticity involves direct G protein-dependent inhibition of presynaptic Ca2+ influx through voltage-gated Ca2+ channels [57, 65], while long-term plasticity involves inhibition of adenylyl cyclase and the cAMP/PKA pathway [63, 66].
It is worth noting that besides CB1– dependent LTD of synaptic transmission, CB1– dependent long-term potentiation has recently been reported [67]. Indeed, it was shown that 2-AG-dependent retrograde signalling also mediates a sustained enhancement of glutamate release in the lateral perforant path, one of the two cortical inputs to the granule cells of the dentate gyrus. Induction of this form of long-term potentiation involved two types of glutamate receptors, changes in postsynaptic [Ca2+]i, and the postsynaptic enzyme that synthesizes 2-AG [67].
Nonretrograde signalling means that eCBs produced in postsynaptic neurons activate postsynaptic CB1 receptor or TRPV1 channels. Self-inhibition of neocortical GABAergic interneurons mediated by eCBs and CB1 receptor causing postsynaptic hyperpolarisation involves [Ca2+]i rise, production of 2-AG and coupling of CB1 receptor to a G protein-regulated inwardly rectifying K+ channel [68-70]. More recently, intracellular CB2 receptors were also found to mediate self-inhibition in prefrontal cortical pyramidal neurons [71]. This action is explained by functional coupling of CB2 receptors to Ca2+-activated chloride channels to decrease neuronal firing [72].
There is also growing evidence regarding underestimated role of central CB2 receptors. Thus, CB2 receptor mRNAs were found to be mostly expressed in a subset of excitatory and inhibitory neurons in the CA1, CA3 and dentate gyrus areas, but rarely in microglia [73]. Deletion of CB2 cannabinoid receptors impairs contextual long-term memory and enhances spatial working memory [74].
2.3.2. Different Endocannabinoids Play Distinct Roles in the Regulation of Synaptic Transmission
Among the first indications of distinct roles of anandamide and 2-AG was the discovery of a striking difference in localisation of two endocannabinoid-hydrolyzing enzymes, FAAH and monoglyceride lipase. Indeed, it was found that FAAH hydrolyzing anandamide is predominantly localized in somata and dendrites of principal cells, but not in interneurons [75]. It was located mostly on the membrane surface of intracellular organelles known to store Ca2+ (e.g. mitochondria, smooth endoplasmic reticulum), and less frequently on the somatic or dendritic plasma membrane [75]. In contrast, monoglyceride lipase, which catalyses 2-AG hydrolysis, is predominantly localized on axon terminals of granule cells, CA3 pyramidal cells and some interneurons [75]. These results strongly suggest that anandamide and 2-AG may have different functions. Convincing evidence for this has been reported at about the same time. It was demonstrated that inhibition of cyclooxygenase-2 (COX-2), but not FAAH, prolonged DSI in hippocampal pyramidal neurons [76]. Since COX-2 inhibition (affecting hydrolysis of both, 2-AG and anandamide) was effective, while inhibition of FAAH (selectively affecting anandamide hydrolysis) was not, it was concluded that 2-AG rather than anandamide mediated DSI in hippocampal neurons.
Since then, differential roles of anandamide and 2-AG in the regulation of synaptic transmission has been unequivocally demonstrated in a variety of preparations. Moreover, it is currently generally accepted that despite similarities in their chemical structure, 2-AG and anandamide synthesized and degraded by distinct enzymatic pathways, play fundamentally different physiological and pathophysiological roles [38, 77].
2.4. Interaction of Cannabinoid Receptors with other Proteins
Although several potentially interacting with CB1 proteins have previously been described [78, 79] relatively little is still known about other proteins, which may assemble with CB1 receptor in a multiprotein signaling complex. In this regard it is worth mentioning a recent finding identifying cannabinoids as key upstream regulators of the Wiskott-Aldrich syndrome protein-family verprolin-homologous protein 1 (WAVE1) complex via physical interactions within the CB1 receptor assembly [80]. It has been shown that the interactions play a fundamental role in actin dynamics and structural modulation in growth cones during development, as well as in activity-induced plasticity of dendritic spines [80]. Moreover, evidence has been provided that the interactions are important for inflammatory pain. Indeed, CB1 receptor agonists attenuated activity-dependent remodeling of dendritic spines in spinal cord neurons in vivo and suppressed inflammatory pain by regulating the WAVE1 complex [80].
Apart from previously reported desensitization, CB1 receptor sensitizes TRPV1 and promotes anandamide-responsiveness of TRPV1 in a major sub-population of capsaicin-sensitive primary sensory neurons, probably, again via a protein-protein interaction [81]. These results ‘indicate that increased tissue levels of anandamide, which seems to have limited TRPV1 desensitizing effect, could significantly reduce the potential analgesic efficacy of FAAH inhibitors. Therefore, in order to utilise the analgesic potential of endogenous anandamide fully in PSN, ways to interfere with the mechanisms, through which the CB1 receptor promotes anandamide-responsiveness and sensitization of TRPV1, must be identified’ [82].
Although not direct, but functionally very important interaction of CB1 and hyperpolarization-activated cyclic nucleotide-gated (HCN) channels has recently been reported to occur in pyramidal cells located in the superficial portion of the CA1 pyramidal cell layer (but absent from deep-layer cells) [83]. This interaction involves c-Jun-N-terminal kinases (JNKs), nitric oxide synthase, and intracellular cGMP [83]. These results raise an interesting question about possible presence of CB1- HCN pathway in peripheral sensory neurons and, thus, its potential role in chronic pain.
3. Relations between the endocannabinoid system and “painful” TRP channels
Although multiple physiological roles have been attributed to eCBs, as already discussed above, these natural neuroactive lipids have recently received most prominent attention as putative messengers for pain control, especially in the settings of persistent, chronic pain of both neuropathic and inflammatory origin [2, 84, 85]. At the same time, many members of the large TRP superfamily of cation channels are increasingly implicated as important sensors of noxious stimuli. There is also growing evidence indicating that, first, not all antinociceptive effects of cannabinoids are mediated exclusively by CB receptors, and, second, that several TRP subtypes may represent novel molecular targets for cannabinoids, as will be discussed in more detail later. These channels are sometimes even referred to as “ionotropic cannabinoid receptors” [86, 87] to distinguish them from the classical, G protein-coupled, or metabotropic CB1 and CB2 receptors. However, it is important to note that these same channels have many other primary physiological roles, for example in thermo- and mechanosensation, and although their cannabinoid sensitivity contributes to polymodal regulation of these TRPs it is unlikely to be the main factor of channel activation, at least under physiological conditions.
For the purposes of this review, of special interest are the effects on TRP channels of eCBs and phytocannabinoids. The latter group will be included in our discussion since most of phytocannabinoids are considered as non-psychoactive, and thus clinically useful drugs for pain management [88, 89]. Indeed, both eCBs and phytocannabinoids clearly are multiple-target drugs affecting various enzymes, receptors and ion channels, including TRPs.
3.1. “Painful” TRP Cation Channels
There is currently substantial evidence indicating multiple key roles of several TRP channels not only in detecting noxious stimuli and signalling within the pain system, but also in pain arising without a well-defined noxious input, e.g. when it outlasts the initial tissue damage and/or inflammation and becomes chronic. TRP channels are ubiquitous in sensory neurones, where they respond to mechanical, thermal and chemical stimuli and also interact with GPCR and cytokine receptors [90]. When their stimulation becomes excessive or, alternatively, their sensitivity to the stimuli becomes abnormally enhanced, activation of these channels causes painful sensations. The most obvious examples of such responses are those caused by compounds known to elicit unpleasant or painful sensations, such as cinnamaldehyde, mustard oil, gingerol, capsaicin and icilin-molecules now known as agonists of specific excitatory TRP subtypes, as well as thermal pain due to excessive cold or hot temperature mediated by thermosensitive TRPs.
The founding TRP member triggering much interest in the TRP-pain connection was identified by Julius and co-workers in 1997 as a polymodal sensor TRPV1, which is both the capsaicin receptor and heat-activated Ca2+-permeable channel [91]. This discovery was followed by the identification of the menthol and cold receptor TRPM8 in 2002 [92] and, somewhat later, noxious cold and pungent compounds (such as mustard oil) sensitive TRPA1 [93-95], which substantiated a more general role for TRP channels both in thermosensation and pain. Other TRPs widely discussed in connection with pain include TRPV2, TRPV3 and TRPV4 [5-8, 96, 97]. It is interesting to note that all these “painful” TRP channels physiologically are primarily thermosensitive channels (albeit they differ in the temperature range needed for their activation, from <17 OC for TRPA1 to >50 OC for TRPV2). However, it may be argued that thermal sensitivity is not necessarily the sole reason for their role in nociception [5]. In experimental models of pain, siRNA interference, antisense approach and knockout studies firmly confirmed the important roles of TRPV1, TRPV4 and TRPA1 channels in nociceptive signalling in vivo (reviewed in [5]).
TRPV2 knockout mice became available more recently, and they showed surprisingly normal heat responsiveness, thermal and mechanical nociception, including under inflammatory or neuropathic conditions [98]. With regard to TRPM8 the results are mixed – one study demonstrated no role of TRPM8 (unlike TRPA1) in cold hyperalgesia after spinal nerve injury [99], yet in chronic neuropathic pain TRPM8 activation produced analgesia [100]. Recently with the use of ablation of TRPV1 or TRPM8 expressing cells, significantly decreased responses to noxious heat and cold, respectively, have been demonstrated in mice [101].
There is also substantial evidence demonstrating that TRPV1 and TRPV4 channels are involved in inflammatory hyperalgesia, while more recently TRPC1 and TRPC6 have been implicated in mechanical hyperalgesia (reviewed in [6]). In addition, expressed in macrophages and microglia TRPM2, which is primarily a sensor for reactive oxygen species, contributes to the pathogenesis of inflammatory and neuropathic pain [102]. TRPM3, the most recently identified thermosensitive TRP, can detect noxious heat and is involved in the development of inflammatory heat hyperalgesia [103].
One subject that attracted particularly large body of research is TRP sensitization produced by inflammation mediators, such as ATP, the cytokines, prostaglandins, bradykinin and neuropeptides (e.g. substance P). The role of TRPV1 in inflammatory pain has been most extensively investigated. Mediators of inflammation and the second messengers they engage strongly sensitize TRPV1 thus causing thermal hyperalgesia. Signal transduction involves both protein kinase A (PKA) and protein kinase C (PKC) mediated phosphorylation of TRPV1 leading to increased channel translocation to the plasma membrane and enhanced channel activity, respectively (reviewed in [6]). Inhibition of the AKAP79/150 protein prevents TRPV1 sensitization, at least that caused by bradykinin and PGE2 and mediated by PKA [104].
The above discussed channels, TRPV1 and TRPA1 in particular, are thus believed to be very promising molecular targets for the next generation of analgesics, although the real success of such drugs is yet to be seen [7, 8]. Since multiple TRP subtypes are involved in pain transduction, the possibility of developing nonselective TRP antagonist as a useful strategy for pain treatment has been raised [8]. However, there is also valid concern that, giving the channels are widely expressed in the nervous system and on the periphery and that they can structurally and functionally interact with other TRP subtypes, such drugs (either subtype selective or nonselective) may have a number of side effects. One spectacular example of such problems is represented by the attempts to develop TRPV1 antagonists, which did not progress beyond testing in Phase II trials due to problems with the regulation of body temperature and detection of noxious heat [97]. Therefore, strategies aimed at preventing channel translocation to the plasma membrane or enhancement of channel sensitivity, rather than channel blockade, are also advocated [7]. As will be discussed in the following section, in some cases abrogation of channel sensitization can indeed be achieved through the action of cannabinoids.
3.2. Regulation of TRP Channels by Cannabinoids
Although regulation of several TRP members by cannabinoids is our main interest here, it should be noted that this represents only a subset of the very important and wide-spread TRP regulatory mechanisms involving lipids. Thus, there is an intricate voltage-lipid connection, especially relevant to gating of TRPV and TRPM channels [105, 106]. The action of various phosphoinositides, in particular phosphatidylinositol 4,5-bisphosphate (PIP2), has received much interest. Phospholipids turnover typically occurs subsequent to activation of many types of GPCR, some of which are intimately involved in nociceptive signalling (e.g. bradykinin and prostanoid receptors activated by prostaglandins).
Thus, activation of phospholipases can affect TRP channels. Indeed, TRPA1, TRPV3 and TRPV4 channels are activated by PIP2. TRPV1 channel is also affected by PIP2, there is controversy, however, as to whether it is inhibited [107] or activated [108, 109] by this phosphoinositid. TRPV4 and TRPM8 are directly activated by the products of the phospholipase A2 (PLA2) activity [110-112]. Depletion of PIP2 limits TRPM8 activity, thus representing a rather unique mechanism of channel desensitization following Ca2+ entry and activation of a Ca2+-sensitive PLC [113].
Therefore, regulation of various TRP channels by cannabinoids appears to be a part of the more general regulatory mechanisms via lipid signalling. Several specific examples will be discussed below, while summarising the findings in Table 1.
Table 1.
Summary of TRP channels regulated by cannabinoids.
| TRP Subtype | Relevant Cannabinoids | References |
|---|---|---|
| TRPV1 | anandamide, NADA, CBD, arachidonoyl-2-chloroethanolamine | [20, 114, 121, 123, 125] |
| TRPV2 | THC, CBD, CBN, CBG | [122, 129] |
| TRPV3 | CBD, THCV | [130] |
| TRPV4 | CBDV, THCV, anandamide (indirectly) | [112, 130] |
| TRPM8 | anandamide, NADA, CBD, CBD acid, THC, THC acid, CBG | [131, 132] |
| TRPA1 | CBD, CBD acid, THC, THC acid, CBC, CBG, Δ9-tetrahydrocannabiorcol (plant derivative of THC) | [94, 132, 136] |
3.2.1. TRPV1
In the study of the vasodilator action of anandamide it was identified as the first selective endogenous TRPV1 agonist, which activated vanilloid receptors on perivascular sensory nerves and caused release of calcitonin gene-related peptide (CGRP), an action not mimicked by other endogenous and synthetic CB1 and CB2 receptor agonists [114]. These findings have established a novel paradigm for the existence of non-CB1 or CB2 molecular targets of cannabinoids within the TRP superfamily of cation channels (reviewed in [115]. It should be noted that although anandamide and capsaicin have similar affinities for TRPV1, anandamide has considerably lower potency (reported EC50 values in the range 0.7-5 µM for anandamide [116] and 9.1 nM for capsaicin [117]). In acutely dissociated small dorsal root ganglion neurons, anandamide activates Ca2+ entry via TRPV1 in a manner that differs from that of capsaicin suggesting a primarily modulatory mode of action of anandamide on TRPV1 [118]. It also has relatively low intrinsic efficacy at the TRPV1 receptor in comparison to the CB1 receptor, and hence the relevance of anandamide activation of TRPV1 in the context of the above discussed endocannabinoid system functioning remains controversial. One needs to consider possible concentration- and time-dependent effects of anandamide on the two molecular targets, possible colocalisation of CB1 and TRPV1, as well as dynamic regulation of the efficacy and potency of anandamide at TRPV1 by multiple factors, as well as complex regulation of neuropeptide release by anandamide to fully understand these connections [116].
Since anandamide is low-potency partial TRPV1 agonist, its binding to TRPV1 should attenuate the action of a full agonist, consequently producing analgesic and anti-inflammatory effects. Another eCB, NADA, was later found in the brain and shown to be a potent (EC50 of about 50 nM) TRPV1 agonist [20]. Indeed, NADA evoked a TRPV1-mediated thermal hyperalgesia. Furthermore, NADA induced the release of substance P and CGRP from dorsal spinal cord slices and enhanced hippocampal paired-pulse depression, suggesting NADA/TRPV1-mediated role in synaptic plasticity [20]. Endocannabinoid- and TRPV1-mediated regulation of synaptic strength at central synapses was demonstrated in the dentate gyrus and the nucleus accumbens where TRPV1 activation by anandamide induced postsynaptic LTD [119, 120].
TRPV1 has also been shown to be a molecular target for CBD and its synthetic analogues [121, 122]. It stimulated TRPV1 with EC50 of 1-3.5 μM and efficacy similar to capsaicin. Interestingly, the effects of both TRPV1 agonists were not additive, while CBD desensitized TRPV1 to capsaicin. Another action of CBD is the inhibition of anandamide hydrolysis [121, 122]. In a rat model of acute inflammation, pharmacological evidence indicates that antihyperalgesic action of CBD is mediated via TRPV1, rather than CB1 or CB2 receptors [123].
One especially useful strategy for pain relief is based on TRP desensitization caused by agonists, exemplified by capsaicin action on TRPV1 [124], although administration of TRP agonists may initially cause discomfort and even acute pain. It is interesting to note in this connection that TRPV1-specific synthetic cannabinoid, arachidonoyl-2 chloroethanolamine, was found to desensitize TRPA1 channel and inhibit mustard oil-evoked responses in a TRPV1-dependent manner [125]. Alternatively, TRPA1-selective cannabinoids can desensitize TRPV1 via a Ca2+-dependent calcineurin pathway [126]. Additionally, there is now evidence that endocannabinoid 2-AG activates and desensitizes TRPV1 [127, 128].
3.2.2. TRPV2
Cannabinoids represent the sole class of naturally occurring TRPV2 modulators. In calcium mobilization and electrophysiological tests, CBD showed highest potency (EC50 of 3.7 μM), followed by THC (EC50 of 14 μM) and cannabinol (CBN) (EC50 of 77.7 μM) [129]. Functionally, CBD triggered release of CGRP from cultured rat dorsal root ganglion neurons, an effect not dependent on activation of a cannabinoid receptor or TRPV1.
In a systematic study of 11 pure cannabinoids and botanical extracts from Cannabis varieties De Petrocellis et al. (2011) [122] using HEK293 cells expressing rat TRPV2 found that some of these compounds strongly activated TRPV2 with a rank potency THC > CBD > cannabigivarin (CBGV) > cannabigerol (CBG) > tetrahydrocannabivarin (THCV) > cannabidivarin (CBDV) > CBN, whereas cannabinoic acids were inactive or less potent. Importantly, CBGV, THC, CBG, CBD and THCV induced TRPV2 desensitization in a dose-dependent manner with potency unrelated to the rank order of their potency for channel activation [122].
3.2.3. TRPV3 and TRPV4
De Petrocellis et al. (2012) [130] also found that CBD and THCV activated TRPV3 with high efficacy and potency (EC50 of 3.7 μM). CBDV and THCV activated TRPV4 with EC50 of 0.9-6.4 μM, whereas other cannabinoids (including CBGV, CBN and CBG) were significantly more efficacious at desensitizing this channel to TRPV4 agonist 4α-PDD than at activating it [130]. Moreover, the authors showed that cannabinoids can negatively regulate the expression of TRPV1-4 channels.
Anandamide can also cause robust activation of TRPV4, although in an indirect manner, through its metabolite arachidonic acid (AA) and the cytochrome P450 epoxygenase-dependent formation of epoxyeicosatrienoic acids [112].
3.2.4. TRPM8
Phytocannabinoids and cannabis extracts also exert some of their pharmacological actions by interacting with the cold and menthol receptor TRPM8. In HEK293 cells overexpressing TRPM8, anandamide and NADA inhibited TRPM8 at submicromolar concentrations. Activation of the co-expressed CB1 receptors also inhibited TRPM8 response to icilin [131]. Furthermore, of the six phytocannabinoids tested (CBD, CBD acid, THC, THC acid, cannabichromene (CBC), and CBG), all, with the exception of CBC, also inhibited menthol- and icilin-evoked [Ca2+]i increases at submicromolar concentrations [132]. CBG inhibited [Ca2+]i elevation in icilin-sensitive DRG neurons with potency (IC50 of 4.5 μM) similar to that of anandamide. Collectively, these findings implicate these compounds in the treatment of cancer [127, 133-135] and pain.
3.2.5. TRPA1
TRPA1 is not only cold receptor involved in acute noxious sensation and in cold allodynia induced by inflammation, nerve injury, and peripheral neuropathy [114], but also a highly promiscuous pharmacophore. Cannabinoids are no exception in case of TRPA1. The use of mustard oil, a well defined TRPA1 agonist, in animal models of acute pain and hyperalgesia prompted investigation of TRPA1 as yet another TRPA1 ionotropic cannabinoid receptor. Jordt et al. (2004) have found that THC excites sensory nerve fibres via TRPA1 [94]. All THC-sensitive sensory neurons from rat trigeminal ganglia also responded to mustard oil, and responses to both agonists could be blocked by ruthenium red.
In the above mentioned study of De Petrocellis et al. (2008) the authors also showed, in parallel with TRPM8, that all tested cannabinoids induced TRPA1-mediated [Ca2+]i rises with potency comparable to that of mustard oil isothiocyanate (the most potent being CBC with EC50 of 60 nM) [132].
One of the effects of TRPA1 activation is reduction of voltage-gated calcium and sodium currents in primary sensory neurons, and intrathecal injection of a non-electrophilic cannabinoid, THC, also produced TRPA1-dependent antinociception in the hot-plate test, similarly to the injected electrophilic TRPA1 activator cinnamaldehyde [136], thus suggesting that a TRPA1 agonistic approach could be useful for pain treatment. In this scenario, the initial activation of TRPA1 on sensory neurones by cannabinoids is followed by receptor desensitization, thus contributing to the anti-inflammatory and analgesic effects of these compounds [137]. Moreover, cannabinoid-induced activation of TRPA1 also induced desensitization of capsaicin-induced currents in cultured TG neurones, an effect lost in TRPA1-/- cells [137]. In this connection, it is interesting to note that non-steroidal anti-inflammatory drugs, such as the fenamate analogues, can also activate TRPA1 [138].
3.3. Possible Cellular Mechanisms Linking Nociceptive and Cannabinoid Signalling with TRP Channels
While the important roles of the cannabinoid system and several TRP channels in the pain control are well documented (Sections 2.2, 2.3 and 3.1, respectively), it seems a daunting task to place all the findings regarding regulation of TRP channels by cannabinoids (Section 3.2) within the framework of nociceptive and cannabinoid signalling. In general, activation of TRP channels causes three major cellular outcomes: membrane depolarisation, mainly due to Na+ influx, and consequently generation of action potentials in excitable cells, such as neurones; (2) [Ca2+]i rise due to Ca2+ influx; and (3) [Ca2+]i rise due to Ca2+ release from the intracellular stores as some TRPs are expressed in membranes of intracellular organelles, such as the endoplasmic reticulum, which can induce an additional Ca2+ influx via store-operated channels.
The [Ca2+]i rise has strong potential to contribute to antinociceptive events, e.g. by inactivation of voltage-gated Ca2+ channels or activation of Ca2+-dependent K+ and Cl- channels, modulation of neurotransmitter release, desensitization of some TRPs such as TRPV1, etc. However, activation of the excitatory TRP channels by cannabinoids (except TRPM8, which is commonly inhibited) seems at odds with the idea of their antinociceptive action. Yet it is hardly coincidental that all of the best characterised “painful” TRPs (Section 3.1) are affected by cannabinoids, as was summarised in Table 1.
Several mechanisms may explain this enigma. First, the agonistic action for pain treatment may be relevant whereby activation of TRPs in sensory neurones by cannabinoids is followed by receptor desensitization, as documented at least in case of TRPA1 [137]. Second, activation of TRPA1 can desensitize TRPV1, and vice versa, and such cross-desensitization of different TRPs may be exploited in pain control. Third, activation of TRPs can selectively affect synaptic strength at different types of synapses. In this regard, the following scenario can be considered as an example. Activation of mGlu1 receptors, playing key roles in the modulation of nociceptive processing in the thalamus [139], produces both eCBs and eicosanoid metabolites of AA, which both effectively activate TRPV1 receptors [114]. In turn, activation of TRPV1 located on presynaptic terminals induces LTD. Importantly, TRPV1 receptor activation selectively modifies synapses onto interneurons, whereas the neighboring excitatory synapses onto CA1 pyramidal cells are not affected [140]. Thus, overall effect of TRPV1 receptor activation at the network level (excitation or inhibition) may not be that obvious. Forth, as already discussed, cannabinoids appear to be low-potency partial TRP agonists (e.g. anandamide acting at TRPV1), in which case their binding to TRP channels should attenuate the action of a full agonist to produce analgesic effects. Finally, TRP agonists may be not only pro-, but also anti-inflammatory agents [141, 142], this way reducing inflammatory pain.
It is also notable that TRP channels are involved in direct protein-protein interaction, but this potential is not yet well explored and understood in the context of cannabinoid and nociceptive signalling. Sensitization of TRPV1 plays a key role under inflammatory conditions, while TRPV1 antagonists are of limited use due to their ability to induce hyperthermia. One recent study showed that GABAB1 receptor subunit inhibits TRPV1 sensitization. This action is independent of G protein signalling, but instead relies on close juxtaposition of the GABAB1 and TRPV1, and it does not disrupt TRPV1 functioning under normal conditions [143].
conclusion
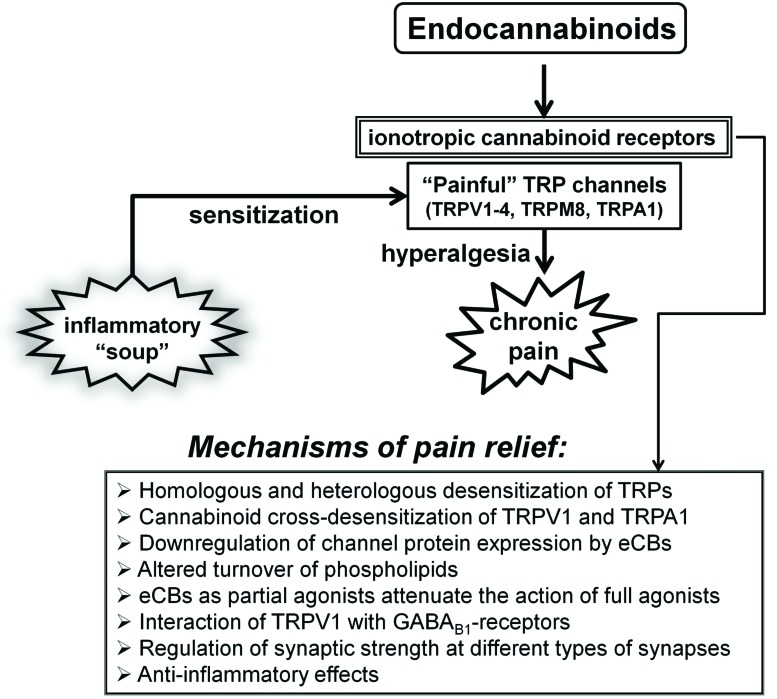
As summarised in this review, not only the endocannabinoid system plays important role in the control of nociceptive signalling among many other pivotal functions of this system, but also several TRP subtypes, which only few years ago were tentatively coined as “ionotropic cannabinoid receptors”, can now be firmly placed within the endocannabinoid system as its integral and important part (Fig. 1). It is fascinating that several TRPs having been most extensively studied in the transduction of noxious stimuli are also molecular targets for various cannabinoids. These include TRPV1-4, TRPA1 and TRPM8 proteins, all of which are also thermosensitive channels. This is hardly a casual relationship, and thus it is tempting to speculate that eCBs and phytocannabinoids exert their anti-nociceptive behaviour via their action on these, and possibly other TRPs, in addition to the better understood roles of CB1 and CB2 receptors, e.g. in the regulation of synaptic transmission. However, much caution is needed in the interpretation of still largely disparate results in this area of research. Not only cellular mechanisms linking nociceptive and cannabinoid signalling with TRP channels remain somewhat speculative, but also there is clearly a lack of specificity in the action of cannabinoids on TRP channels aggravated by the fact that the same TRP subtype can serve multiple purposes depending on mode of its activation and cell type, while different TRP proteins can form functional heteromultimers. Furthermore, the fact that many studies have been so far performed in heterologous expression systems or using isolated native cells highlights the urgent need for further studies to confirm the in vivo relevance of these effects of cannabinoids on TRP channels. It is also clear that in order to fully realise this presently emerging potential of cannabinoid/TRP signalling in pain control much better understanding of the various components of the endocannabinoid system itself and their interactions will also be required.
Fig. (1).
Schematic illustration of various interactions between the endocannabinoid signalling system and ‘painful’ TRP channels in pain control. Acidic ‘inflammatory soup' represents peptides, such as bradykinin and cytokines, various lipid messengers, such as prostaglandins, neurotransmitters (serotonin, and ATP) and neurotrophic factors, such as NGF. These various factors acting in synergy can strongly sensitize sensory nerve endings by altering the activation threshold and sensitivity of several TRPs to thermal and mechanical stimuli, thus leading to hyperalgesia. Several mechanisms that can, with the involvement of the endocannabinoid system, counteract these effects are listed below. See text for details of the mechanisms involved.
ACKNOWLEDGEMENTS
Declared none.
List of Abbreviations
- 2-AG
2-Arachidonoylglycerol
- AA
Arachidonic acid
- [Ca2+]i
Intracellular free calcium concentration
- CB1
Cannabinoid binding receptor-1
- CB2
Cannabinoid-binding receptor-2
- CBC
Cannabichromene
- CBD
Cannabidiol
- CBDV
Propyl analogue of CBD or cannabidivarin
- CBG
Cannabigerol
- CBGV
Propyl analogue of CBG or cannabigivarin
- CBN
Cannabinol
- CGRP
Calcitonin gene-related peptide
- COX-2
Cyclooxygenase-2
- DAG
Diacylglycerol
- DSE
Depolarization-induced suppression of excitation
- DSI
Depolarization-induced suppression of inhibition
- eCBs
Endocannabinoids
- FAAH
Fatty acid amide hydrolase
- GPCR
G protein-coupled receptor
- HCN
Hyperpolarization-activated cyclic nucleotide-gated channels
- LTD
Long-term depression
- NADA
N-arachidonoyl dopamine
- PIP2
Phosphatidylinositol 4,5-bisphosphate
- PKA
Protein kinase A
- PKC
Protein kinase C
- PLA1
Phospholipase A1
- PLA2
Phospholipase A2
- PLC
Phospholipase C
- THC
Δ9-Tetrahydrocannabinol
- THCV
Propyl analogue of THC or tetrahydrocannabivarin
- TRP
Transient receptor potential
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Freund T.F., Katona I., Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol. Rev. 2003;83(3):1017–1066. doi: 10.1152/physrev.00004.2003. [http://dx.doi.org/10.1152/physrev.00004.2003]. [PMID: 12843414]. [DOI] [PubMed] [Google Scholar]
- 2.Pacher P., Bátkai S., Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol. Rev. 2006;58(3):389–462. doi: 10.1124/pr.58.3.2. [http://dx.doi.org/10.1124/pr.58.3.2]. [PMID: 16968947]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borgelt L.M., Franson K.L., Nussbaum A.M., Wang G.S. The pharmacologic and clinical effects of medical cannabis. Pharmacotherapy. 2013;33(2):195–209. doi: 10.1002/phar.1187. [http://dx.doi.org/10.1002/ phar.1187]. [PMID: 23386598]. [DOI] [PubMed] [Google Scholar]
- 4.Pertwee R.G., Howlett A.C., Abood M.E., Alexander S.P., Di Marzo V., Elphick M.R., Greasley P.J., Hansen H.S., Kunos G., Mackie K., Mechoulam R., Ross R.A. International union of basic and clinical pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol. Rev. 2010;62(4):588–631. doi: 10.1124/pr.110.003004. [http://dx.doi.org/10.1124/pr.110.003004]. [PMID: 21079038]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cortright D.N., Krause J.E., Broom D.C. TRP channels and pain. Biochim. Biophys. Acta. 2007;1772(8):978–988. doi: 10.1016/j.bbadis.2007.03.003. [http://dx.doi.org/ 10.1016/j.bbadis.2007.03.003]. [PMID: 17467247]. [DOI] [PubMed] [Google Scholar]
- 6.Sexton J.E., Vernon J., Wood J.N. TRPs and pain. Handb. Exp. Pharmacol. 2014;223:873–897. doi: 10.1007/978-3-319-05161-1_6. [http://dx.doi.org/10.1007/978-3-319-05161-1_6]. [PMID: 24961972]. [DOI] [PubMed] [Google Scholar]
- 7.Sousa-Valente J., Andreou A.P., Urban L., Nagy I. Transient receptor potential ion channels in primary sensory neurons as targets for novel analgesics. Br. J. Pharmacol. 2014;171(10):2508–2527. doi: 10.1111/bph.12532. [http://dx.doi.org/10.1111/bph.12532]. [PMID: 24283624]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Premkumar L.S., Abooj M. TRP channels and analgesia. Life Sci. 2013;92(8-9):415–424. doi: 10.1016/j.lfs.2012.08.010. [http://dx.doi.org/10.1016/j.lfs.2012.08. 010]. [PMID: 22910182]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scarpelli R., Sasso O., Piomelli D. A double whammy: targeting both fatty acid amide hydrolase (FAAH) and cyclooxygenase (COX) to treat pain and inflammation. ChemMedChem. 2016;11(12):1242–1251. doi: 10.1002/cmdc.201500395. [http://dx.doi.org/10.1002/cmdc. 201500395]. [PMID: 26486424]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaoni Y., Mechoulam R. Isolation, structure and partial synthesis of an active constituent of hashish. J. Am. Chem. Soc. 1964;86:1646–1647. [http://dx.doi.org/10.1021/ja01062a046]. [Google Scholar]
- 11.Wilson R.I., Nicoll R.A. Endocannabinoid signaling in the brain. Science. 2002;296(5568):678–682. doi: 10.1126/science.1063545. [http://dx.doi.org/10.1126/ science.1063545]. [PMID: 11976437]. [DOI] [PubMed] [Google Scholar]
- 12.Matsuda L.A., Lolait S.J., Brownstein M.J., Young A.C., Bonner T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346(6284):561–564. doi: 10.1038/346561a0. [http://dx.doi.org/10.1038/346561a0]. [PMID: 2165569]. [DOI] [PubMed] [Google Scholar]
- 13.Gérard C., Mollereau C., Vassart G., Parmentier M. Nucleotide sequence of a human cannabinoid receptor cDNA. Nucleic Acids Res. 1990;18(23):7142. doi: 10.1093/nar/18.23.7142. [http://dx.doi.org/10.1093/nar/18.23.7142]. [PMID: 2263478]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munro S., Thomas K.L., Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365(6441):61–65. doi: 10.1038/365061a0. [http://dx.doi.org/10.1038/365061a0]. [PMID: 7689702]. [DOI] [PubMed] [Google Scholar]
- 15.Devane W.A., Hanus L., Breuer A., Pertwee R.G., Stevenson L.A., Griffin G., Gibson D., Mandelbaum A., Etinger A., Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258(5090):1946–1949. doi: 10.1126/science.1470919. [http://dx.doi.org/10.1126/science.1470919]. [PMID: 1470919]. [DOI] [PubMed] [Google Scholar]
- 16.Felder C.C., Briley E.M., Axelrod J., Simpson J.T., Mackie K., Devane W.A. Anandamide, an endogenous cannabimimetic eicosanoid, binds to the cloned human cannabinoid receptor and stimulates receptor-mediated signal transduction. Proc. Natl. Acad. Sci. USA. 1993;90(16):7656–7660. doi: 10.1073/pnas.90.16.7656. [http://dx.doi.org/10.1073/ pnas.90.16.7656]. [PMID: 8395053]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugiura T., Kondo S., Sukagawa A., Nakane S., Shinoda A., Itoh K., Yamashita A., Waku K. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 1995;215(1):89–97. doi: 10.1006/bbrc.1995.2437. [http://dx.doi. org/10.1006/bbrc.1995.2437]. [PMID: 7575630]. [DOI] [PubMed] [Google Scholar]
- 18.Mechoulam R., Ben-Shabat S., Hanus L., Ligumsky M., Kaminski N.E., Schatz A.R., Gopher A., Almog S., Martin B.R., Compton D.R. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995;50(1):83–90. doi: 10.1016/0006-2952(95)00109-d. [http://dx.doi.org/10.1016/0006-2952(95)00109-D]. [PMID: 7605349]. [DOI] [PubMed] [Google Scholar]
- 19.Porter A.C., Sauer J.M., Knierman M.D., Becker G.W., Berna M.J., Bao J., Nomikos G.G., Carter P., Bymaster F.P., Leese A.B., Felder C.C. Characterization of a novel endocannabinoid, virodhamine, with antagonist activity at the CB1 receptor. J. Pharmacol. Exp. Ther. 2002;301(3):1020–1024. doi: 10.1124/jpet.301.3.1020. [http://dx.doi.org/ 10.1124/jpet.301.3.1020]. [PMID: 12023533]. [DOI] [PubMed] [Google Scholar]
- 20.Huang S.M., Bisogno T., Trevisani M., Al-Hayani A., De Petrocellis L., Fezza F., Tognetto M., Petros T.J., Krey J.F., Chu C.J., Miller J.D., Davies S.N., Geppetti P., Walker J.M., Di Marzo V. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc. Natl. Acad. Sci. USA. 2002;99(12):8400–8405. doi: 10.1073/pnas.122196999. [http://dx.doi.org/ 10.1073/pnas.122196999]. [PMID: 12060783]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanus L., Abu-Lafi S., Fride E., Breuer A., Vogel Z., Shalev D.E., Kustanovich I., Mechoulam R. 2-arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc. Natl. Acad. Sci. USA. 2001;98(7):3662–3665. doi: 10.1073/pnas.061029898. [http://dx.doi.org/ 10.1073/pnas.061029898]. [PMID: 11259648]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Marzo V., Fontana A., Cadas H., Schinelli S., Cimino G., Schwartz J.C., Piomelli D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372(6507):686–691. doi: 10.1038/372686a0. [http://dx.doi.org/10.1038/372686a0]. [PMID: 7990962]. [DOI] [PubMed] [Google Scholar]
- 23.Stella N., Schweitzer P., Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388(6644):773–778. doi: 10.1038/42015. [http://dx.doi.org/10.1038/42015]. [PMID: 9285589]. [DOI] [PubMed] [Google Scholar]
- 24.Di Marzo V., Piscitelli F. The Endocannabinoid System and its Modulation by Phytocannabinoids. Neurotherapeutics. 2015;12(4):692–698. doi: 10.1007/s13311-015-0374-6. [http://dx.doi.org/10.1007/s13311-015-0374-6]. [PMID: 26271952]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Petrocellis L., Bisogno T., Davis J.B., Pertwee R.G., Di Marzo V. Overlap between the ligand recognition properties of the anandamide transporter and the VR1 vanilloid receptor: inhibitors of anandamide uptake with negligible capsaicin-like activity. FEBS Lett. 2000;483(1):52–56. doi: 10.1016/s0014-5793(00)02082-2. [http://dx.doi.org/10.1016/S0014-5793(00)02082-2]. [PMID: 11033355]. [DOI] [PubMed] [Google Scholar]
- 26.De Petrocellis L., Bisogno T., Maccarrone M., Davis J.B., Finazzi-Agro A., Di Marzo V. The activity of anandamide at vanilloid VR1 receptors requires facilitated transport across the cell membrane and is limited by intracellular metabolism. J. Biol. Chem. 2001;276(16):12856–12863. doi: 10.1074/jbc.M008555200. [http://dx.doi.org/10.1074/ jbc.M008555200]. [PMID: 11278420]. [DOI] [PubMed] [Google Scholar]
- 27.Smart D., Gunthorpe M.J., Jerman J.C., Nasir S., Gray J., Muir A.I., Chambers J.K., Randall A.D., Davis J.B. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1). Br. J. Pharmacol. 2000;129(2):227–230. doi: 10.1038/sj.bjp.0703050. [http://dx.doi. org/10.1038/sj.bjp.0703050]. [PMID: 10694225]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Di Marzo V., De Petrocellis L., Fezza F., Ligresti A., Bisogno T. Anandamide receptors. Prostaglandins Leukot. Essent. Fatty Acids. 2002;66(2-3):377–391. doi: 10.1054/plef.2001.0349. [http://dx.doi.org/10.1054/plef.2001. 0349]. [PMID: 12052051]. [DOI] [PubMed] [Google Scholar]
- 29.Pertwee R.G. Endocannabinoids and their Pharmacological actions. Handb. Exp. Pharmacol. 2015;231:1–37. doi: 10.1007/978-3-319-20825-1_1. [http://dx.doi.org/ 10.1007/978-3-319-20825-1_1]. [PMID: 26408156]. [DOI] [PubMed] [Google Scholar]
- 30.Bauer M., Chicca A., Tamborrini M., Eisen D., Lerner R., Lutz B., Poetz O., Pluschke G., Gertsch J. Identification and quantification of a new family of peptide endocannabinoids (Pepcans) showing negative allosteric modulation at CB1 receptors. J. Biol. Chem. 2012;287(44):36944–36967. doi: 10.1074/jbc.M112.382481. [http://dx.doi.org/10.1074/ jbc.M112.382481]. [PMID: 22952224]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hofer S.C., Ralvenius W.T., Gachet M.S., Fritschy J.M., Zeilhofer H.U., Gertsch J. Localization and production of peptide endocannabinoids in the rodent CNS and adrenal medulla. Neuropharmacology. 2015;98:78–89. doi: 10.1016/j.neuropharm.2015.03.021. [http://dx.doi.org/10.1016/ j.neuropharm.2015.03.021]. [PMID: 25839900]. [DOI] [PubMed] [Google Scholar]
- 32.Straiker A., Mitjavila J., Yin D., Gibson A., Mackie K. Aiming for allosterism: Evaluation of allosteric modulators of CB1 in a neuronal model. Pharmacol. Res. 2015;99:370–376. doi: 10.1016/j.phrs.2015.07.017. [http://dx. doi.org/10.1016/j.phrs.2015.07.017]. [PMID: 26211948]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fogaça M.V., Galve-Roperh I., Guimarães F.S., Campos A.C. Cannabinoids, neurogenesis and antidepressant drugs: Is there a link? Curr. Neuropharmacol. 2013;11(3):263–275. doi: 10.2174/1570159X11311030003. [http://dx.doi. org/10.2174/1570159X11311030003]. [PMID: 24179463]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogawa S., Kunugi H. Inhibitors of fatty acid amide hydrolase and monoacylglycerol lipase: New targets for future antidepressants. Curr. Neuropharmacol. 2015;13(6):760–775. doi: 10.2174/1570159X13666150612225212. [http://dx.doi. org/10.2174/1570159X13666150612225212]. [PMID: 26630956]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aso E., Ferrer I. CB2 cannabinoid receptor as potential target against alzheimer’s disease. Front. Neurosci. 2016;10:243. doi: 10.3389/fnins.2016.00243. [http://dx.doi.org/10.3389/fnins.2016.00243]. [PMID: 27303261]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leweke F.M., Mueller J.K., Lange B., Rohleder C. Therapeutic potential of cannabinoids in psychosis. Biol. Psychiatry. 2016;79(7):604–612. doi: 10.1016/j.biopsych.2015.11.018. [http://dx.doi.org/10.1016/j.biopsych.2015.11.018]. [PMID: 26852073]. [DOI] [PubMed] [Google Scholar]
- 37.Skosnik P.D., Cortes-Briones J.A., Hajós M. It’s all in the rhythm: The role of cannabinoids in neural oscillations and psychosis. Biol. Psychiatry. 2016;79(7):568–577. doi: 10.1016/j.biopsych.2015.12.011. [http://dx.doi.org/ 10.1016/j.biopsych.2015.12.011]. [PMID: 26850792]. [DOI] [PubMed] [Google Scholar]
- 38.Lu H.C., Mackie K. An Introduction to the Endogenous Cannabinoid System. Biol. Psychiatry. 2016;79(7):516–525. doi: 10.1016/j.biopsych.2015.07.028. [http://dx. doi.org/10.1016/j.biopsych.2015.07.028]. [PMID: 26698193]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piomelli D., Sasso O. Peripheral gating of pain signals by endogenous lipid mediators. Nat. Neurosci. 2014;17(2):164–174. doi: 10.1038/nn.3612. [http://dx.doi.org/10.1038/nn.3612]. [PMID: 24473264]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malek N., Starowicz K. Dual-acting compounds targeting endocannabinoid and endovanilloid systems-a novel treatment option for chronic pain management. Front. Pharmacol. 2016;7:257. doi: 10.3389/fphar.2016.00257. [http://dx.doi.org/10.3389/fphar.2016.00257]. [PMID: 27582708]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pryce G., Baker D. Endocannabinoids in multiple sclerosis and amyotrophic lateral sclerosis. Handb. Exp. Pharmacol. 2015;231:213–231. doi: 10.1007/978-3-319-20825-1_7. [http://dx.doi.org/10.1007/978-3-319-20825-1_7]. [PMID: 26408162]. [DOI] [PubMed] [Google Scholar]
- 42.Fernández-Ruiz J., Moro M.A., Martínez-Orgado J. Cannabinoids in neurodegenerative disorders and stroke/brain trauma: From preclinical models to clinical applications. Neurotherapeutics. 2015;12(4):793–806. doi: 10.1007/s13311-015-0381-7. [http://dx.doi.org/10.1007/s13311-015-0381-7]. [PMID: 26260390]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Navarro G., Morales P., Rodríguez-Cueto C., Fernández-Ruiz J., Jagerovic N., Franco R. Targeting cannabinoid CB2 receptors in the central nervous system. Medicinal chemistry approaches with focus on neurodegenerative disorders. Front. Neurosci. 2016;10:406. doi: 10.3389/fnins.2016.00406. [http://dx.doi.org/10.3389/fnins.2016.00406]. [PMID: 27679556]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soltesz I., Alger B.E., Kano M., Lee S.H., Lovinger D.M., Ohno-Shosaku T., Watanabe M. Weeding out bad waves: towards selective cannabinoid circuit control in epilepsy. Nat. Rev. Neurosci. 2015;16(5):264–277. doi: 10.1038/nrn3937. [http://dx.doi.org/10.1038/nrn3937]. [PMID: 25891509]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parsons L.H., Hurd Y.L. Endocannabinoid signalling in reward and addiction. Nat. Rev. Neurosci. 2015;16(10):579–594. doi: 10.1038/nrn4004. [http://dx.doi.org/10.1038/nrn4004]. [PMID: 26373473]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herkenham M., Lynn A.B., Little M.D., Johnson M.R., Melvin L.S., de Costa B.R., Rice K.C. Cannabinoid receptor localization in brain. Proc. Natl. Acad. Sci. USA. 1990;87(5):1932–1936. doi: 10.1073/pnas.87.5.1932. [http://dx.doi.org/10.1073/pnas.87.5.1932]. [PMID: 2308954]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu S.S., Mackie K. Distribution of the endocannabinoid system in the central nervous system. Handb. Exp. Pharmacol. 2015;231:59–93. doi: 10.1007/978-3-319-20825-1_3. [http://dx.doi.org/10.1007/978-3-319-20825-1_3]. [PMID: 26408158]. [DOI] [PubMed] [Google Scholar]
- 48.Szabo B., Schlicker E. Effects of cannabinoids on neurotransmission. Handb. Exp. Pharmacol. 2005;168(168):327–365. doi: 10.1007/3-540-26573-2_11. [http://dx. doi.org/10.1007/3-540-26573-2_11]. [PMID: 16596780]. [DOI] [PubMed] [Google Scholar]
- 49.Ohno-Shosaku T., Maejima T., Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29(3):729–738. doi: 10.1016/s0896-6273(01)00247-1. [http://dx.doi.org/10.1016/S0896-6273(01)00247-1]. [PMID: 11301031]. [DOI] [PubMed] [Google Scholar]
- 50.Wilson R.I., Nicoll R.A. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410(6828):588–592. doi: 10.1038/35069076. [http://dx.doi.org/10.1038/35069076]. [PMID: 11279497]. [DOI] [PubMed] [Google Scholar]
- 51.Llano I., Leresche N., Marty A. Calcium entry increases the sensitivity of cerebellar Purkinje cells to applied GABA and decreases inhibitory synaptic currents. Neuron. 1991;6(4):565–574. doi: 10.1016/0896-6273(91)90059-9. [http://dx.doi.org/10.1016/0896-6273(91)90059-9]. [PMID: 2015092]. [DOI] [PubMed] [Google Scholar]
- 52.Pitler T.A., Alger B.E. Postsynaptic spike firing reduces synaptic GABAA responses in hippocampal pyramidal cells. J. Neurosci. 1992;12(10):4122–4132. doi: 10.1523/JNEUROSCI.12-10-04122.1992. [PMID: 1403103]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kovacs F.E., Knop T., Urbanski M.J., Freiman I., Freiman T.M., Feuerstein T.J., Zentner J., Szabo B. Exogenous and endogenous cannabinoids suppress inhibitory neurotransmission in the human neocortex. Neuropsychopharmacology. 2012;37(5):1104–1114. doi: 10.1038/npp.2011.262. [http://dx.doi.org/10.1038/npp.2011.262]. [PMID: 22048459]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Storozhuk M.V., Ivanova S.Y., Piomelli D. Presence of depolarization-induced suppression of inhibition in a fraction of GABAergic synaptic connections in rat neocortical cultures. Neurosci. Behav. Physiol. 2006;36(7):709–713. doi: 10.1007/s11055-006-0077-x. [http://dx.doi.org/10.1007/ s11055-006-0077-x]. [PMID: 16841150]. [DOI] [PubMed] [Google Scholar]
- 55.Trettel J., Levine E.S. Endocannabinoids mediate rapid retrograde signaling at interneuron right-arrow pyramidal neuron synapses of the neocortex. J. Neurophysiol. 2003;89(4):2334–2338. doi: 10.1152/jn.01037.2002. [http://dx. doi.org/10.1152/jn.01037.2002]. [PMID: 12686587]. [DOI] [PubMed] [Google Scholar]
- 56.Alger B.E., Pitler T.A. Retrograde signaling at GABAA-receptor synapses in the mammalian CNS. Trends Neurosci. 1995;18(8):333–340. doi: 10.1016/0166-2236(95)93923-l. [http://dx.doi.org/10.1016/0166-2236(95)93923-L]. [PMID: 7482793]. [DOI] [PubMed] [Google Scholar]
- 57.Kreitzer A.C., Regehr W.G. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001;29(3):717–727. doi: 10.1016/s0896-6273(01)00246-x. [http://dx. doi.org/10.1016/S0896-6273(01)00246-X]. [PMID: 11301030]. [DOI] [PubMed] [Google Scholar]
- 58.Gerdeman G.L., Ronesi J., Lovinger D.M. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat. Neurosci. 2002;5(5):446–451. doi: 10.1038/nn832. [PMID: 11976704]. [DOI] [PubMed] [Google Scholar]
- 59.Robbe D., Kopf M., Remaury A., Bockaert J., Manzoni O.J. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc. Natl. Acad. Sci. USA. 2002;99(12):8384–8388. doi: 10.1073/pnas.122149199. [http://dx.doi.org/10.1073/pnas.122149199]. [PMID: 12060781]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chevaleyre V., Castillo P.E. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron. 2003;38(3):461–472. doi: 10.1016/s0896-6273(03)00235-6. [http://dx.doi.org/ 10.1016/S0896-6273(03)00235-6]. [PMID: 12741992]. [DOI] [PubMed] [Google Scholar]
- 61.Marsicano G., Wotjak C.T., Azad S.C., Bisogno T., Rammes G., Cascio M.G., Hermann H., Tang J., Hofmann C., Zieglgänsberger W., Di Marzo V., Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418(6897):530–534. doi: 10.1038/nature00839. [http://dx.doi.org/10.1038/nature00839]. [PMID: 12152079]. [DOI] [PubMed] [Google Scholar]
- 62.Regehr W.G., Carey M.R., Best A.R. Activity-dependent regulation of synapses by retrograde messengers. Neuron. 2009;63(2):154–170. doi: 10.1016/j.neuron.2009.06.021. [http://dx.doi.org/10.1016/j.neuron.2009.06.021]. [PMID: 19640475]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heifets B.D., Castillo P.E. Endocannabinoid signaling and long-term synaptic plasticity. Annu. Rev. Physiol. 2009;71:283–306. doi: 10.1146/annurev.physiol.010908.163149. [http://dx.doi.org/10.1146/annurev.physiol.010908.163149]. [PMID: 19575681]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kano M., Ohno-Shosaku T., Hashimotodani Y., Uchigashima M., Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol. Rev. 2009;89(1):309–380. doi: 10.1152/physrev.00019.2008. [http://dx.doi.org/ 10.1152/physrev.00019.2008]. [PMID: 19126760]. [DOI] [PubMed] [Google Scholar]
- 65.Wilson R.I., Kunos G., Nicoll R.A. Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron. 2001;31(3):453–462. doi: 10.1016/s0896-6273(01)00372-5. [http://dx.doi.org/10.1016/S0896-6273(01)00372-5]. [PMID: 11516401]. [DOI] [PubMed] [Google Scholar]
- 66.Chevaleyre V., Takahashi K.A., Castillo P.E. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu. Rev. Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [http://dx.doi.org/10.1146/annurev.neuro.29. 051605.112834]. [PMID: 16776579]. [DOI] [PubMed] [Google Scholar]
- 67.Wang W., Trieu B.H., Palmer L.C., Jia Y., Pham D.T., Jung K.M., Karsten C.A., Merrill C.B., Mackie K., Gall C.M., Piomelli D., Lynch G. A primary cortical input to hippocampus expresses a pathway-specific and endocannabinoid-dependent form of long-term potentiation. eNeuro. 2016;3(4):3. doi: 10.1523/ENEURO.0160-16.2016. [http://dx. doi.org/10.1523/ENEURO.0160-16.2016]. [PMID: 27517090]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bacci A., Huguenard J.R., Prince D.A. Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature. 2004;431(7006):312–316. doi: 10.1038/nature02913. [http://dx.doi.org/10. 1038/nature02913]. [PMID: 15372034]. [DOI] [PubMed] [Google Scholar]
- 69.Marinelli S., Pacioni S., Bisogno T., Di Marzo V., Prince D.A., Huguenard J.R., Bacci A. The endocannabinoid 2-arachidonoylglycerol is responsible for the slow self-inhibition in neocortical interneurons. J. Neurosci. 2008;28(50):13532–13541. doi: 10.1523/JNEUROSCI.0847-08.2008. [http://dx.doi.org/10.1523/JNEUROSCI.0847-08.2008]. [PMID: 19074027]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marinelli S., Pacioni S., Cannich A., Marsicano G., Bacci A. Self-modulation of neocortical pyramidal neurons by endocannabinoids. Nat. Neurosci. 2009;12(12):1488–1490. doi: 10.1038/nn.2430. [http://dx.doi.org/ 10.1038/nn.2430]. [PMID: 19915567]. [DOI] [PubMed] [Google Scholar]
- 71.den Boon F.S., Chameau P., Schaafsma-Zhao Q., van Aken W., Bari M., Oddi S., Kruse C.G., Maccarrone M., Wadman W.J., Werkman T.R. Excitability of prefrontal cortical pyramidal neurons is modulated by activation of intracellular type-2 cannabinoid receptors. Proc. Natl. Acad. Sci. USA. 2012;109(9):3534–3539. doi: 10.1073/pnas.1118167109. [http://dx.doi.org/10.1073/pnas.1118167109]. [PMID: 22331871]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Castillo P.E., Younts T.J., Chávez A.E., Hashimotodani Y. Endocannabinoid signaling and synaptic function. Neuron. 2012;76(1):70–81. doi: 10.1016/j.neuron.2012.09.020. [http://dx.doi.org/10.1016/j.neuron.2012.09.020]. [PMID: 23040807]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li Y., Kim J. Neuronal expression of CB2 cannabinoid receptor mRNAs in the mouse hippocampus. Neuroscience. 2015;311:253–267. doi: 10.1016/j.neuroscience.2015.10.041. [http://dx.doi.org/10.1016/j.neuroscience.2015.10.041]. [PMID: 26515747]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li Y., Kim J. CB2 cannabinoid receptor knockout in mice impairs contextual long-term memory and enhances spatial working memory. 2016. [DOI] [PMC free article] [PubMed]
- 75.Gulyas A.I., Cravatt B.F., Bracey M.H., Dinh T.P., Piomelli D., Boscia F., Freund T.F. Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur. J. Neurosci. 2004;20(2):441–458. doi: 10.1111/j.1460-9568.2004.03428.x. [http://dx.doi.org/10.1111/j.1460-9568.2004. 03428.x]. [PMID: 15233753]. [DOI] [PubMed] [Google Scholar]
- 76.Kim J., Alger B.E. Inhibition of cyclooxygenase-2 potentiates retrograde endocannabinoid effects in hippocampus. Nat. Neurosci. 2004;7(7):697–698. doi: 10.1038/nn1262. [http://dx.doi.org/10.1038/nn1262]. [PMID: 15184902]. [DOI] [PubMed] [Google Scholar]
- 77.Di Marzo V., De Petrocellis L. Why do cannabinoid receptors have more than one endogenous ligand? 2012. [DOI] [PMC free article] [PubMed]
- 78.Howlett A.C., Blume L.C., Dalton G.D. CB(1) cannabinoid receptors and their associated proteins. Curr. Med. Chem. 2010;17(14):1382–1393. doi: 10.2174/092986710790980023. [http://dx.doi.org/10.2174/092986710790980023]. [PMID: 20166926]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smith T.H., Sim-Selley L.J., Selley D.E. Cannabinoid CB1 receptor-interacting proteins: novel targets for central nervous system drug discovery? Br. J. Pharmacol. 2010;160(3):454–466. doi: 10.1111/j.1476-5381.2010.00777.x. [http:// dx.doi.org/10.1111/j.1476-5381.2010.00777.x]. [PMID: 20590557]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Njoo C., Agarwal N., Lutz B., Kuner R. The cannabinoid receptor CB1 interacts with the WAVE1 complex and plays a role in actin dynamics and structural plasticity in neurons. PLoS Biol. 2015;13(10):e1002286. doi: 10.1371/journal.pbio.1002286. [http://dx.doi.org/10.1371/journal.pbio. 1002286]. [PMID: 26496209]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hermann H., De Petrocellis L., Bisogno T., Schiano Moriello A., Lutz B., Di Marzo V. Dual effect of cannabinoid CB1 receptor stimulation on a vanilloid VR1 receptor-mediated response. Cell. Mol. Life Sci. 2003;60(3):607–616. doi: 10.1007/s000180300052. [http://dx.doi.org/10.1007/ s000180300052]. [PMID: 12737320]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen J., Varga A., Selvarajah S., Jenes A., Dienes B., Sousa-Valente J., Kulik A., Veress G., Brain S.D., Baker D., Urban L., Mackie K., Nagy I. Spatial distribution of the cannabinoid type 1 and capsaicin receptors may contribute to the complexity of their crosstalk. Sci. Rep. 2016;6:33307. doi: 10.1038/srep33307. [http://dx.doi.org/ 10.1038/srep33307]. [PMID: 27653550]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maroso M., Szabo G.G., Kim H.K., Alexander A., Bui A.D., Lee S.H., Lutz B., Soltesz I. Cannabinoid control of learning and memory through HCN channels. Neuron. 2016;89(5):1059–1073. doi: 10.1016/j.neuron.2016.01.023. [http://dx.doi.org/10.1016/j.neuron.2016.01.023]. [PMID: 26898775]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Manzanares J., Julian M., Carrascosa A. Role of the cannabinoid system in pain control and therapeutic implications for the management of acute and chronic pain episodes. Curr. Neuropharmacol. 2006;4(3):239–257. doi: 10.2174/157015906778019527. [http://dx.doi.org/10.2174/ 157015906778019527]. [PMID: 18615144]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rahn E.J., Hohmann A.G. Cannabinoids as pharmacotherapies for neuropathic pain: from the bench to the bedside. Neurotherapeutics. 2009;6(4):713–737. doi: 10.1016/j.nurt.2009.08.002. [http://dx.doi.org/10.1016/j.nurt.2009. 08.002]. [PMID: 19789075]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Akopian A.N., Ruparel N.B., Jeske N.A., Patwardhan A., Hargreaves K.M. Role of ionotropic cannabinoid receptors in peripheral antinociception and antihyperalgesia. Trends Pharmacol. Sci. 2009;30(2):79–84. doi: 10.1016/j.tips.2008.10.008. [http://dx.doi.org/10.1016/j.tips.2008.10.008]. [PMID: 19070372]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Caterina M.J. TRP channel cannabinoid receptors in skin sensation, homeostasis, and inflammation. ACS Chem. Neurosci. 2014;5(11):1107–1116. doi: 10.1021/cn5000919. [http://dx.doi.org/10.1021/cn5000919]. [PMID: 24915599]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Di Marzo V. Phytocannabinoids: a growing family of plant natural products with increasing pharmacological and clinical importance. Planta Med. 2016;81:S1–S381. [Google Scholar]
- 89.Ligresti A., De Petrocellis L., Di Marzo V. From phytocannabinoids to cannabinoid receptors and endocannabinoids: Pleiotropic physiological and pathological roles through complex pharmacology. Physiol. Rev. 2016;96(4):1593–1659. doi: 10.1152/physrev.00002.2016. [http://dx.doi.org/ 10.1152/physrev.00002.2016]. [PMID: 27630175]. [DOI] [PubMed] [Google Scholar]
- 90.Blackshaw L.A. Transient receptor potential cation channels in visceral sensory pathways. Br. J. Pharmacol. 2014;171(10):2528–2536. doi: 10.1111/bph.12641. [http://dx.doi.org/10.1111/bph.12641]. [PMID: 24641218]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Caterina M.J., Schumacher M.A., Tominaga M., Rosen T.A., Levine J.D., Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–824. doi: 10.1038/39807. [http://dx.doi.org/10.1038/39807]. [PMID: 9349813]. [DOI] [PubMed] [Google Scholar]
- 92.McKemy D.D., Neuhausser W.M., Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416(6876):52–58. doi: 10.1038/nature719. [http://dx.doi.org/10.1038/ nature719]. [PMID: 11882888]. [DOI] [PubMed] [Google Scholar]
- 93.Bandell M., Story G.M., Hwang S.W., Viswanath V., Eid S.R., Petrus M.J., Earley T.J., Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41(6):849–857. doi: 10.1016/s0896-6273(04)00150-3. [http://dx.doi.org/10.1016/S0896-6273 (04)00150-3]. [PMID: 15046718]. [DOI] [PubMed] [Google Scholar]
- 94.Jordt S.E., Bautista D.M., Chuang H.H., McKemy D.D., Zygmunt P.M., Högestätt E.D., Meng I.D., Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427(6971):260–265. doi: 10.1038/nature02282. [http://dx. doi.org/10.1038/nature02282]. [PMID: 14712238]. [DOI] [PubMed] [Google Scholar]
- 95.Story G.M., Peier A.M., Reeve A.J., Eid S.R., Mosbacher J., Hricik T.R., Earley T.J., Hergarden A.C., Andersson D.A., Hwang S.W., McIntyre P., Jegla T., Bevan S., Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112(6):819–829. doi: 10.1016/s0092-8674(03)00158-2. [http:// dx.doi.org/10.1016/S0092-8674(03)00158-2]. [PMID: 12654248]. [DOI] [PubMed] [Google Scholar]
- 96.Kaneko Y., Szallasi A. Transient receptor potential (TRP) channels: a clinical perspective. Br. J. Pharmacol. 2014;171(10):2474–2507. doi: 10.1111/bph.12414. [http://dx.doi.org/10.1111/bph.12414]. [PMID: 24102319]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Moran M.M., McAlexander M.A., Bíró T., Szallasi A. Transient receptor potential channels as therapeutic targets. Nat. Rev. Drug Discov. 2011;10(8):601–620. doi: 10.1038/nrd3456. [http://dx.doi.org/10.1038/nrd3456]. [PMID: 21804597]. [DOI] [PubMed] [Google Scholar]
- 98.Park U., Vastani N., Guan Y., Raja S.N., Koltzenburg M., Caterina M.J. TRP vanilloid 2 knock-out mice are susceptible to perinatal lethality but display normal thermal and mechanical nociception. J. Neurosci. 2011;31(32):11425–11436. doi: 10.1523/JNEUROSCI.1384-09.2011. [http://dx.doi. org/10.1523/JNEUROSCI.1384-09.2011]. [PMID: 21832173]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Katsura H., Obata K., Mizushima T., Yamanaka H., Kobayashi K., Dai Y., Fukuoka T., Tokunaga A., Sakagami M., Noguchi K. Antisense knock down of TRPA1, but not TRPM8, alleviates cold hyperalgesia after spinal nerve ligation in rats. Exp. Neurol. 2006;200(1):112–123. doi: 10.1016/j.expneurol.2006.01.031. [http://dx.doi.org/10.1016/j.expneurol.2006. 01.031]. [PMID: 16546170]. [DOI] [PubMed] [Google Scholar]
- 100.Proudfoot C.J., Garry E.M., Cottrell D.F., Rosie R., Anderson H., Robertson D.C., Fleetwood-Walker S.M., Mitchell R. Analgesia mediated by the TRPM8 cold receptor in chronic neuropathic pain. Curr. Biol. 2006;16(16):1591–1605. doi: 10.1016/j.cub.2006.07.061. [http://dx.doi.org/10. 1016/j.cub.2006.07.061]. [PMID: 16920620]. [DOI] [PubMed] [Google Scholar]
- 101.Pogorzala L.A., Mishra S.K., Hoon M.A. The cellular code for mammalian thermosensation. J. Neurosci. 2013;33(13):5533–5541. doi: 10.1523/JNEUROSCI.5788-12.2013. [http://dx.doi.org/10.1523/JNEUROSCI.5788-12.2013]. [PMID: 23536068]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Haraguchi K., Kawamoto A., Isami K., Maeda S., Kusano A., Asakura K., Shirakawa H., Mori Y., Nakagawa T., Kaneko S. TRPM2 contributes to inflammatory and neuropathic pain through the aggravation of pronociceptive inflammatory responses in mice. J. Neurosci. 2012;32(11):3931–3941. doi: 10.1523/JNEUROSCI.4703-11.2012. [http://dx.doi.org/10.1523/ JNEUROSCI.4703-11.2012]. [PMID: 22423113]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vriens J., Owsianik G., Hofmann T., Philipp S.E., Stab J., Chen X., Benoit M., Xue F., Janssens A., Kerselaers S., Oberwinkler J., Vennekens R., Gudermann T., Nilius B., Voets T. TRPM3 is a nociceptor channel involved in the detection of noxious heat. Neuron. 2011;70(3):482–494. doi: 10.1016/j.neuron.2011.02.051. [http://dx.doi.org/10. 1016/j.neuron.2011.02.051]. [PMID: 21555074]. [DOI] [PubMed] [Google Scholar]
- 104.Zhang X., Li L., McNaughton P.A. Proinflammatory mediators modulate the heat-activated ion channel TRPV1 via the scaffolding protein AKAP79/150. Neuron. 2008;59(3):450–461. doi: 10.1016/j.neuron.2008.05.015. [http://dx. doi.org/10.1016/j.neuron.2008.05.015]. [PMID: 18701070]. [DOI] [PubMed] [Google Scholar]
- 105.Nilius B., Mahieu F., Karashima Y., Voets T. Regulation of TRP channels: a voltage-lipid connection. Biochem. Soc. Trans. 2007;35(Pt 1):105–108. doi: 10.1042/BST0350105. [http://dx.doi.org/10.1042/BST0350105]. [PMID: 17233613]. [DOI] [PubMed] [Google Scholar]
- 106.Voets T., Nilius B. Modulation of TRPs by PIPs. J. Physiol. 2007;582(Pt 3):939–944. doi: 10.1113/jphysiol.2007.132522. [http://dx.doi.org/10.1113/jphysiol.2007. 132522]. [PMID: 17395625]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cao E., Cordero-Morales J.F., Liu B., Qin F., Julius D. TRPV1 channels are intrinsically heat sensitive and negatively regulated by phosphoinositide lipids. Neuron. 2013;77(4):667–679. doi: 10.1016/j.neuron.2012.12.016. [http:// dx.doi.org/10.1016/j.neuron.2012.12.016]. [PMID: 23439120]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lukacs V., Thyagarajan B., Varnai P., Balla A., Balla T. ; Rohacs T. Dual regulation of TRPV1 by phosphoinositides. J. Neurosci. 2007;27(26):7070–7080. doi: 10.1523/JNEUROSCI.1866-07.2007. [http://dx.doi.org/10.1523/ JNEUROSCI.1866-07.2007]. [PMID: 17596456]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ufret-Vincenty C.A., Klein R.M., Hua L., Angueyra J., Gordon S.E. Localization of the PIP2 sensor of TRPV1 ion channels. J. Biol. Chem. 2011;286(11):9688–9698. doi: 10.1074/jbc.M110.192526. [http://dx.doi.org/10.1074/ jbc.M110.192526]. [PMID: 21224382]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vanden Abeele F., Zholos A., Bidaux G., Shuba Y., Thebault S., Beck B., Flourakis M., Panchin Y., Skryma R., Prevarskaya N. Ca2+-independent phospholipase A2-dependent gating of TRPM8 by lysophospholipids. J. Biol. Chem. 2006;281(52):40174–40182. doi: 10.1074/jbc.M605779200. [http://dx.doi.org/10.1074/jbc.M605779200]. [PMID: 17082190]. [DOI] [PubMed] [Google Scholar]
- 111.Andersson D.A., Nash M., Bevan S. Modulation of the cold-activated channel TRPM8 by lysophospholipids and polyunsaturated fatty acids. J. Neurosci. 2007;27(12):3347–3355. doi: 10.1523/JNEUROSCI.4846-06.2007. [http:// dx.doi.org/10.1523/JNEUROSCI.4846-06.2007]. [PMID: 17376995]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Watanabe H., Vriens J., Prenen J., Droogmans G., Voets T., Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424(6947):434–438. doi: 10.1038/nature01807. [http://dx.doi.org/10.1038/nature01807]. [PMID: 12879072]. [DOI] [PubMed] [Google Scholar]
- 113.Rohács T., Lopes C.M., Michailidis I., Logothetis D.E. PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat. Neurosci. 2005;8(5):626–634. doi: 10.1038/nn1451. [http://dx.doi.org/10.1038/nn1451]. [PMID: 15852009]. [DOI] [PubMed] [Google Scholar]
- 114.Zygmunt P.M., Petersson J., Andersson D.A., Chuang H., Sørgård M., Di Marzo V., Julius D., Högestätt E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400(6743):452–457. doi: 10.1038/22761. [http://dx.doi.org/10. 1038/22761]. [PMID: 10440374]. [DOI] [PubMed] [Google Scholar]
- 115.De Petrocellis L., Di Marzo V. Role of endocannabinoids and endovanilloids in Ca2+ signalling. Cell Calcium. 2009;45(6):611–624. doi: 10.1016/j.ceca.2009.03.003. [http://dx.doi.org/10.1016/j.ceca.2009.03.003]. [PMID: 19356798]. [DOI] [PubMed] [Google Scholar]
- 116.Ross R.A. Anandamide and vanilloid TRPV1 receptors. Br. J. Pharmacol. 2003;140(5):790–801. doi: 10.1038/sj.bjp.0705467. [http://dx.doi.org/10.1038/ sj.bjp.0705467]. [PMID: 14517174]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Correll C.C., Phelps P.T., Anthes J.C., Umland S., Greenfeder S. Cloning and pharmacological characterization of mouse TRPV1. Neurosci. Lett. 2004;370(1):55–60. doi: 10.1016/j.neulet.2004.07.058. [http://dx.doi.org/10.1016/ j.neulet.2004.07.058]. [PMID: 15489017]. [DOI] [PubMed] [Google Scholar]
- 118.Fischbach T., Greffrath W., Nawrath H., Treede R.D. Effects of anandamide and noxious heat on intracellular calcium concentration in nociceptive drg neurons of rats. J. Neurophysiol. 2007;98(2):929–938. doi: 10.1152/jn.01096.2006. [http://dx.doi.org/10.1152/jn.01096.2006]. [PMID: 17581853]. [DOI] [PubMed] [Google Scholar]
- 119.Chávez A.E., Chiu C.Q., Castillo P.E. TRPV1 activation by endogenous anandamide triggers postsynaptic long-term depression in dentate gyrus. Nat. Neurosci. 2010;13(12):1511–1518. doi: 10.1038/nn.2684. [http://dx.doi.org/10.1038/nn.2684]. [PMID: 21076423]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Grueter B.A., Brasnjo G., Malenka R.C. Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nat. Neurosci. 2010;13(12):1519–1525. doi: 10.1038/nn.2685. [http://dx.doi. org/10.1038/nn.2685]. [PMID: 21076424]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bisogno T., Hanus L., De Petrocellis L., Tchilibon S., Ponde D.E., Brandi I., Moriello A.S., Davis J.B., Mechoulam R., Di Marzo V. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 2001;134(4):845–852. doi: 10.1038/sj.bjp.0704327. [http://dx.doi.org/10.1038/sj.bjp.0704327]. [PMID: 11606325]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.De Petrocellis L., Ligresti A., Moriello A.S., Allarà M., Bisogno T., Petrosino S., Stott C.G., Di Marzo V. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 2011;163(7):1479–1494. doi: 10.1111/j.1476-5381.2010.01166.x. [http://dx.doi.org/10.1111/j.1476-5381.2010.01166.x]. [PMID: 21175579]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Costa B., Giagnoni G., Franke C., Trovato A.E., Colleoni M. Vanilloid TRPV1 receptor mediates the antihyperalgesic effect of the nonpsychoactive cannabinoid, cannabidiol, in a rat model of acute inflammation. Br. J. Pharmacol. 2004;143(2):247–250. doi: 10.1038/sj.bjp.0705920. [http://dx.doi.org/10.1038/sj.bjp.0705920]. [PMID: 15313881]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jara-Oseguera A., Simon S.A., Rosenbaum T. TRPV1: on the road to pain relief. Curr. Mol. Pharmacol. 2008;1(3):255–269. doi: 10.2174/1874467210801030255. [http://dx.doi.org/10.2174/1874467210801030255]. [PMID: 20021438]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ruparel N.B., Patwardhan A.M., Akopian A.N., Hargreaves K.M. Desensitization of transient receptor potential ankyrin 1 (TRPA1) by the TRP vanilloid 1-selective cannabinoid arachidonoyl-2 chloroethanolamine. Mol. Pharmacol. 2011;80(1):117–123. doi: 10.1124/mol.110.068940. [http://dx.doi.org/10.1124/mol.110.068940]. [PMID: 21441412]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Patwardhan A.M., Jeske N.A., Price T.J., Gamper N., Akopian A.N., Hargreaves K.M. The cannabinoid WIN 55,212-2 inhibits transient receptor potential vanilloid 1 (TRPV1) and evokes peripheral antihyperalgesia via calcineurin. Proc. Natl. Acad. Sci. USA. 2006;103(30):11393–11398. doi: 10.1073/pnas.0603861103. [http://dx.doi.org/10.1073/pnas. 0603861103]. [PMID: 16849427]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Petrosino S., Schiano Moriello A., Cerrato S., Fusco M., Puigdemont A., De Petrocellis L., Di Marzo V. The anti-inflammatory mediator palmitoylethanolamide enhances the levels of 2-arachidonoyl-glycerol and potentiates its actions at TRPV1 cation channels. Br. J. Pharmacol. 2016;173(7):1154–1162. doi: 10.1111/bph.13084. [http://dx.doi.org/10.1111/bph.13084]. [PMID: 25598150]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zygmunt P.M., Ermund A., Movahed P., Andersson D.A., Simonsen C., Jönsson B.A., Blomgren A., Birnir B., Bevan S., Eschalier A., Mallet C., Gomis A., Högestätt E.D. Monoacylglycerols activate TRPV1--a link between phospholipase C and TRPV1. PLoS One. 2013;8(12):e81618. doi: 10.1371/journal.pone.0081618. [http://dx.doi.org/10. 1371/journal.pone.0081618]. [PMID: 24312564]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Qin N., Neeper M.P., Liu Y., Hutchinson T.L., Lubin M.L., Flores C.M. TRPV2 is activated by cannabidiol and mediates CGRP release in cultured rat dorsal root ganglion neurons. J. Neurosci. 2008;28(24):6231–6238. doi: 10.1523/JNEUROSCI.0504-08.2008. [http://dx.doi.org/10.1523/ JNEUROSCI.0504-08.2008]. [PMID: 18550765]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.De Petrocellis L., Orlando P., Moriello A.S., Aviello G., Stott C., Izzo A.A., Di Marzo V. Cannabinoid actions at TRPV channels: effects on TRPV3 and TRPV4 and their potential relevance to gastrointestinal inflammation. Acta Physiol. (Oxf.) 2012;204(2):255–266. doi: 10.1111/j.1748-1716.2011.02338.x. [http://dx.doi.org/10.1111/j.1748-1716.2011.02338.x]. [PMID: 21726418]. [DOI] [PubMed] [Google Scholar]
- 131.De Petrocellis L., Starowicz K., Moriello A.S., Vivese M., Orlando P., Di Marzo V. Regulation of transient receptor potential channels of melastatin type 8 (TRPM8): effect of cAMP, cannabinoid CB(1) receptors and endovanilloids. Exp. Cell Res. 2007;313(9):1911–1920. doi: 10.1016/j.yexcr.2007.01.008. [http://dx.doi.org/10.1016/j.yexcr.2007.01.008]. [PMID: 17428469]. [DOI] [PubMed] [Google Scholar]
- 132.De Petrocellis L., Vellani V., Schiano-Moriello A., Marini P., Magherini P.C., Orlando P., Di Marzo V. Plant-derived cannabinoids modulate the activity of transient receptor potential channels of ankyrin type-1 and melastatin type-8. J. Pharmacol. Exp. Ther. 2008;325(3):1007–1015. doi: 10.1124/jpet.107.134809. [http://dx.doi.org/10.1124/jpet.107.134809]. [PMID: 18354058]. [DOI] [PubMed] [Google Scholar]
- 133.Borrelli F., Pagano E., Romano B., Panzera S., Maiello F., Coppola D., De Petrocellis L., Buono L., Orlando P., Izzo A.A. Colon carcinogenesis is inhibited by the TRPM8 antagonist cannabigerol, a Cannabis-derived non-psychotropic cannabinoid. Carcinogenesis. 2014;35(12):2787–2797. doi: 10.1093/carcin/bgu205. [http://dx.doi.org/10.1093/ carcin/bgu205]. [PMID: 25269802]. [DOI] [PubMed] [Google Scholar]
- 134.De Petrocellis L., Ligresti A., Schiano M.A., Iappelli M., Verde R., Stott C.G., Cristino L., Orlando P., Di Marzo V. Non-THC cannabinoids inhibit prostate carcinoma growth in vitro and in vivo: pro-apoptotic effects and underlying mechanisms. Br. J. Pharmacol. 2013;168(1):79–102. doi: 10.1111/j.1476-5381.2012.02027.x. [http://dx.doi.org/10.1111/j.1476-5381. 2012.02027.x]. [PMID: 22594963]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ligresti A., Moriello A.S., Starowicz K., Matias I., Pisanti S., De Petrocellis L., Laezza C., Portella G., Bifulco M., Di Marzo V. Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J. Pharmacol. Exp. Ther. 2006;318(3):1375–1387. doi: 10.1124/jpet.106.105247. [http://dx.doi.org/10.1124/ jpet.106.105247]. [PMID: 16728591]. [DOI] [PubMed] [Google Scholar]
- 136.Andersson D.A., Gentry C., Alenmyr L., Killander D., Lewis S.E., Andersson A., Bucher B., Galzi J.L., Sterner O., Bevan S., Högestätt E.D., Zygmunt P.M. TRPA1 mediates spinal antinociception induced by acetaminophen and the cannabinoid Δ(9)-tetrahydrocannabiorcol. Nat. Commun. 2011;2:551. doi: 10.1038/ncomms1559. [http://dx. doi.org/10.1038/ncomms1559]. [PMID: 22109525]. [DOI] [PubMed] [Google Scholar]
- 137.Akopian A.N., Ruparel N.B., Patwardhan A., Hargreaves K.M. Cannabinoids desensitize capsaicin and mustard oil responses in sensory neurons via TRPA1 activation. J. Neurosci. 2008;28(5):1064–1075. doi: 10.1523/JNEUROSCI.1565-06.2008. [http://dx.doi.org/10.1523/JNEUROSCI.1565-06.2008]. [PMID: 18234885]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zygmunt P.M., Högestätt E.D. TRPA1. Handb. Exp. Pharmacol. 2014;222:583–630. doi: 10.1007/978-3-642-54215-2_23. [http://dx.doi.org/10.1007/978-3-642-54215-2_23]. [PMID: 24756722]. [DOI] [PubMed] [Google Scholar]
- 139.Salt T.E., Jones H.E., Copeland C.S., Sillito A.M. Function of mGlu1 receptors in the modulation of nociceptive processing in the thalamus. Neuropharmacology. 2014;79:405–411. doi: 10.1016/j.neuropharm.2013.12.016. [http://dx.doi. org/10.1016/j.neuropharm.2013.12.016]. [PMID: 24373900]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gibson H.E., Edwards J.G., Page R.S., Van Hook M.J., Kauer J.A. TRPV1 channels mediate long-term depression at synapses on hippocampal interneurons. Neuron. 2008;57(5):746–759. doi: 10.1016/j.neuron.2007.12.027. [http:// dx.doi.org/10.1016/j.neuron.2007.12.027]. [PMID: 18341994]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Radresa O., Paré M., Albert J.S. Multiple roles of transient receptor potential (TRP) channels in inflammatory conditions and current status of drug development. Curr. Top. Med. Chem. 2013;13(3):367–385. doi: 10.2174/1568026611313030012. [http://dx.doi.org/10.2174/1568026611313030012]. [PMID: 23432066]. [DOI] [PubMed] [Google Scholar]
- 142.Tsuji F., Murai M., Oki K., Seki I., Ueda K., Inoue H., Nagelkerken L., Sasano M., Aono H. Transient receptor potential vanilloid 1 agonists as candidates for anti-inflammatory and immunomodulatory agents. Eur. J. Pharmacol. 2010;627(1-3):332–339. doi: 10.1016/j.ejphar.2009.10.044. [http://dx.doi.org/10.1016/j.ejphar.2009.10.044]. [PMID: 19878665]. [DOI] [PubMed] [Google Scholar]
- 143.Hanack C., Moroni M., Lima W.C., Wende H., Kirchner M., Adelfinger L., Schrenk-Siemens K., Tappe-Theodor A., Wetzel C., Kuich P.H., Gassmann M., Roggenkamp D., Bettler B., Lewin G.R., Selbach M., Siemens J. GABA blocks pathological but not acute TRPV1 pain signals. Cell. 2015;160(4):759–770. doi: 10.1016/j.cell.2015.01.022. [http://dx.doi.org/10.1016/j.cell.2015.01.022]. [PMID: 25679765]. [DOI] [PubMed] [Google Scholar]