Abstract
Background:
The aim of this review was to identify the mechanisms by which serotonin receptors involved at the central level are able to modulate the nociceptive response. Pain is a defense mechanism of the body that entails physio-logical, anatomical, neurochemical, and psychological changes, and is defined as an unpleasant sensory and emotional expe-rience with potential risk of tissue damage, comprising the leading cause of appointments with Physicians worldwide. Treatment for this symptom has generated several neuropharmacological lines of research, due to the different types of pain and the various drugs employed to treat this condition. Serotonin [5-HydroxyTryptamine (5-HT)] is a neurotransmitter with seven families (5-HT1–5-HT7) and approximately 15 receptor subtypes. Serotonin modulates neuronal activity; however, this neurotransmitter is related with a number of physiological processes, such as cardiovascular function, gastric motility, renal function, etc. On the other hand, several researches reported that serotonin modulates nociceptive response through 5-HT1, 5-HT2, 5-HT3, and 5-HT7 receptors in the Central Nervous System (CNS).
Method:
In this review, a search was conducted on PubMed, ProQuest, EBSCO, and the Science Citation Index for studies evaluating the effects of 5-HT1, 5-HT2, 5-HT3, and 5-HT7 receptors in the CNS on the modulation of different types of pain.
Conclusion
We concluded that 5-HT1, 5-HT2, 5-HT3, and 5-HT7 receptors in the CNS modulate the pain, but this depends on the distribution of the receptors, dose of agonists or antagonists, administration route, pain type and duration in order to inhibit, excite, or even maintain the nociceptive response.
Keywords: Pain, 5-HT1, 5-HT2, 5-HT3, 5-HT7, central nervous system
1. INTRODUCTION
Pain is a highly prevalent symptom in the majority of diseases and is the leading cause of visits to the doctor [1]. Although, at present and to the best of our knowledge, exact values are lacking, it is estimated that the prevalence of pain is 25–40% in communities, rising to 71-88% within cities. Pain, in itself, interferes with social, work, and professional activities, as well as mood and the periods of sleep and wakefulness of the patient, aspects that will definitely affect the quality of life of the person and their environment [2]. This represents an overload, not only for the health sector due to the burden of care obligation and the consumption of direct health resources (visits to Specialists, simple x-rays, resonances, drugs, physical therapy, etc.), but also for the economic and social sector because of its indirect impact on the number of casualties and work disabilities. We may be able to provide a general idea of the situation on analyzing the total work produced, but only for musculoskeletal diseases (one of the most common causes of chronic pain), which represent the third most common cause of illness in Mexico according to the World Health Organization in 2015.
The increasing use of aggressive treatment schemes based on combinations of radio- and concomitant chemotherapy, altered fractionation, and dose escalation, make pain a problem in daily clinical practice. However, it is important to note that in 40% of cases, adequate pain control is not achieved, indicating that the approach to a prevalent health problem is not much effective [1].
Many different neurotransmitter systems have been implicated in the transmission, processing, and control of pain [3]. Serotonin (5-HydroxyTryptamine, [5-HT]) is a widely distributed monoamine, in the periphery and in the Central Nervous System (CNS) and is involved in numerous physiological and behavioral disorders, such as major depression, anxiety, schizophrenia, mania, autism, obesity, pain, etc. 5-HT is synthesized from the amino acid L-tryptophan (from the diet) by sequential hydroxylation and decarboxylation. It is stored in presynaptic vesicles and released from nerve terminals during neuronal firing. Serotoninergic neurons at the CNS level are confined to the brainstem and are located in the raphe nuclei. The neurons project to the majority of the brain, including hippocampus, midbrain, prefrontal, parietal, and occipital cortical regions, cingulate cortex, thalamus, and cerebellum, whereas, 5-HT neurons in caudal raphe nuclei project to cerebellum and spinal cord [4].
It has been established that the descending 5-HT pathways exert an inhibitory (descending inhibition) or facilitatory (descending facilitation) influence on the spinal processing of nociceptive information, depending on acute or chronic pain states and the type of receptor acted upon [5-7]. Based on pharmacological, structural, and transductional characteristics, the 5-HT receptor family is divided into seven subfamilies (5-HT1–5-HT7), comprising 15 receptor subtypes, each of these corresponding to distinct genes. The involvement of the diverse receptor subtypes in pain neurotransmission remains largely unknown. Indeed, the use of relatively selective agonists and antagonists for these subtypes has led to inconsistent results due to poor selectivity of drugs and the diversity of experimental conditions [5, 8-10].
The peripheral pronociceptive role of 5-HT is well established to date; in contrast, its action at the spinal cord level and in supraspinal structures appears highly variable and remains a matter of debate [3]. The exact roles of 5-HT receptors involved in pain at the spinal cord are unelucidated. However, studies have revealed the presence of at least three families of 5-HT receptors in the spinal cord (5-HT1, 5-HT2, and 5-HT3), with varying affinity for 5-HT, and recently the 5-HT7 receptor has been postulated, which is also excitatory and which has been linked with, among other things, circadian rhythms, thermoregulation, and migraine [11].
Successful pain management therefore requires therapeutic strategies directed toward alleviating its affective attributes. The development of these strategies requires an understanding of the neurobiological mechanisms that modulate the affective dimension of pain. The main purpose of this review evaluated in detail the role of the 5-HT1, 5-HT2, 5-HT3, and 5-HT7 receptors as modulators of pain response in the CNS.
2. ACTIVITY OF SEROTONINERGIC RECEPTORS IN THE MODULATION OF PAIN 5-HT1
Serotonin is a neurotransmitter thought to be involved in multiple functions and that includes the modulation of the sensory, autonomic, motor systems, arterial pressure, sexual behavior, etc. 5-HT modulates spinal nociceptive transmission in a complex manner: it employs the involvement of multiple 5-HT receptor subtypes and their specific localization in the CNS [12]. The 5-HT1 Gi/-coupled receptor families are divided into A, B, C, D, E, and F subtypes [13-15]. 5-HT1 receptors are present in the whole spinal cord and in the grey matter in all the areas examined; the major class of 5-HT receptor found in the dorsal horn is the 5-HT1 family [16, 17]. Many subtypes of 5-HT1 receptors potentially contributing to medullospinal pain regulation; the rostroventral medial medulla provides the major 5-HT descending pathway to the spinal superficial dorsal horn, the initial relay point for nociceptive inputs into the CNS [3]. The 5HT1A receptor has been most studied as a modulator of pain, and it appears to play a modulatory role in nociception, it has a large distribution in the CNS rendering it possible for the actions of selective agonists or antagonist for this receptor to influence various spinal and supraspinal mechanisms that modulate pain processes. 5-HT1A receptor RNA messenger (mRNA) labeling was most pronounced in the olfactory bulb, anterior hippocampal rudiment, septum, hippocampus, entorhinal cortex, interpeduncular nucleus, thalamus, and in the medullary raphe nuclei, and is widely distributed in the spinal dorsal horn [18, 19]. 5-HT1A receptor appears to be present in primary afferent nociceptive fibres,, in the rostroventromedial medulla as well as in the dorsal horn of spinal cord, with 5-HT1A receptor highest density in lamina I and II [14, 20, 21]. Administration of 5-HT1A receptor agonists in the spinal cord has produced both pro- and antiallodynic effects [3]. The widespread presence of 5-HT1A receptors in the spinal cord and in dorsal and median raphe nuclei, as well as in cortical and limbic areas, suggests a possible involvement of these receptors in emotional states, cognition, and pain modulation [22]. Studies in nonhuman primates [23] and humans indicate that many areas involved in the mediation or modulation of pain, such as the raphe nucleus, amygdala, cingulate cortex, insula, and prefrontal cortex, possess a high density of 5-HT1A receptors [24-27]. In raphe nuclei, the 5-HT1A receptors are located in serotoninergic cell bodies and dendrites and function as somatodendritic autoreceptors [28]. Among the many types of serotonin receptors, the 5-HT1A receptor appears to be that which plays a significant role mediating regulatory effects of pain [29, 30]. EI Yassir et al. [31] and Zemlan et al. [32] showed that both 5-HT1A and 5-HT1B receptors were implicated in nociception at the dorsal-horn level. Indeed, 5-HT1A receptors appear to mimic the non-selective antinociceptive effects of serotonin, while 5-HT1B receptors mimic the selective effect.
Eide et al. [33] examined whether injection of 5-HT1A and 5-HT1B receptor agonists in mice had the ability to alter the tail-flick reflex, and whether effects on the reflex latency involve changes in tail skin temperature. These authors found that in mouse, both 5-HT1A and 5-HT1B receptor agonists inhibit the nociceptive tail-flick reflex when administered into the spinal subarachnoid space, and the effect does not depend on changes in tail skin temperature. Ali et al. [34] adopted a single route intrathecal (i.t.), microinjection in anesthetized rats, while recordings were carried out from dorsal horn neurons on drug application in both behavioral and electrophysiological studies; the authors showed that 5-HT increased nociceptive responses and it is suggested that this effect is associated with the activation of 5-HT1A receptors. Activity at 5-HT1B receptors has the effect of suppressing or reducing responsiveness. The increased responsiveness of dorsal-horn neurons to noxious stimulation associated with activity at 5-HT1A receptors may be associated either with increases in receptive field size, the promotion of spinal nocifensive reflexes, or facilitation of rostral transmission to specific brainstem sites. Moreover, the modulatory effects of 5-HT1B receptor activation on wide-dynamic-range neurons in the spinal cord were studied by Gjerstad et al., [35]; their results demonstrated that stimulation of the 5-HT1B receptors may exert both pro- and antinociceptive effects on wide-dynamic-range neurons in the dorsal horn after repeated electrical stimulation. Likewise, Zhang et al. [19] reported that the excitability of dorsal-horn neurons and the sensitivity of the neurons to i.t. 5-HT1A and 5-HT1B receptor agonists might increase on following the inflammation model. These authors employed an intraplantar (i.p.) injection of carrageenan, which is characterized by both rapid onset and resolution of the inflammation that causes restricted distribution of hyperalgesia.
Liu et al. [36] conducted a study to confirm which type of 5-HT receptor was involved in the descending pathway of antinociception from the brainstem to dorsal horn of the spinal cord in rats. They reported that the 5-HT1A receptor, not the 5-HT2 nor the 5-HT3 receptor, plays an important role in the descending pathway of antinociception from brainstem to spinal cord in intact rats, in rats with nerve injury, and in rats with inflammation. Hains et al. [37] performed a study to characterize the excitability of dorsal-horn neurons to 5-HT and to 5-HT1A and 5-HT3 receptor antagonists and agonists; high densities of 5-HT3 receptors are found in the substantia gelatinosa, at all levels of the spinal cord and electrophysiologic evidence demonstrates the plasticity of 5-HT systems after spinal cord injury. In addition, they indicate the importance of 5-HT modulation in the attenuation of ensuing chronic central pain.
Bonnefont et al. [38] made a study to investigate, according to the nature of the noxious stimulus, the manner in which the blockade of spinal 5-HT1A receptors could influence the antinociceptive actions of exogenous 5-HT, as well as of two analgesics involving endogenous 5-HT: Paracetamol and Venlafaxine. Their results showed that stimulation of the spinal 5-HT1A receptors could mediate a dual influence on the integration of nociceptive mechanisms and the stimulation of 5-HT1A receptors utilizing exogenous 5-HT or endogenous 5-HT mobilized by Paracetamol or Venlafaxine, which can elicit antinociception in the formalin test.
The role of medullary and spinal 5-HT1A receptors in the endogenous regulation of neuropathic hypersensitivity was studied by Wei et al. [39]; they concluded that administration of a selective 5-HT1A receptor antagonist, WAY-100635, into the rostroventromedial medulla or systemically produces selective attenuation of mechanical hypersensitivity in animals with experimental neuropathy and disinhibited descending pathways, this leading to the attenuation of hypersensitivity. Jeong et al. [12] examined the spinal actions of a range of 5-HT1 agonists, including Sumatriptan, on acute pain, plus their effect on afferent-evoked synaptic transmission into superficial dorsal-horn neurons. These authors concluded that at the cellular level, 5-HT1A, but not 5-HT1B, 5-HT1D, and 5-HT1F, receptor activation presynaptically inhibits primary afferent-evoked synaptic transmission in a subpopulation of lamina II superficial dorsal-horn neurons.
The findings displayed above raise the hypothesis that the 5-HT1A receptor inhibits the nociceptive transmission presynaptically, acting as an inhibitory autoreceptor, into the laminae I and II of the dorsal horn by an up-downregulation of 5-HT1A receptors in the rostro ventromedial medulla. In contrast, 5-HT1B receptor can act as pro and nociceptive facilitator.
2.1. 5-HT2
In rat brains, the highest levels of 5-HT2A receptors are found in the frontal cortex and other neocortical areas, claustrum, and olfactory tubercle [40] and exist in brainstem descending pain-modulation pathways, including nucleus raphe magnus, ventrolateral periaqueductal gray, and the spinal dorsal horn, reticular formation, central grey, thalamus, cerebral cortex, and limbic structures [41-42]. 5-HT2A receptors in the prefrontal cortex also play an important role in cognitive functions, such as working memory, conditioned avoidance, aversive classical conditioning, and visual discrimination [43]. Contrariwise, 5-HT2C receptor mRNA exhibits widespread distribution in the locus coeruleus, retrorubral area, substantia nigra pars compacta, ventral tegmental area, periaqueductal gray, basal nucleus, parabigeminal nucleus, and laterodorsal tegmental nucleus, and the 5-HT2C receptor is involved in serotoninergic control of the catecholaminergic and cholinergic areas.
Abbott et al. [44] conducted a study whose main purpose was to identify the receptor subtype mediating synergistic interaction between 5-HT and other inflammatory mediators; they found that 5-HT2A antagonists may be effective as peripherally acting analgesic agents and/or analgesic adjuncts. These analgesic actions would be expected to be specific to situations in which 5-HT release contributes to the generation of pain; similar results were obtained with the 5-HT3 agonist. 5-HT1 and 5-HT2C receptors have attracted interest in that they play a role in the control of mood, motor behavior, nociception, and endocrine secretion, because they are colocalized in individual neurons and, in functional models, they can modify each others’ actions. To help elucidate the functional role of peripheral 5-HT2A receptors, Van Steenwinckel et al. [42] investigated their localization in lumbar dorsal root ganglia employing immunocytochemistry; these authors found that the majority of 5-HT2A receptor immunoreactivity in lumbar dorsal root ganglia is localized in small- and medium-sized cell bodies, presumably nociceptive.
Millan et al. [45] reported that activation of 5-HT2C receptors enhances 5-HT1A receptor modulation in tail- flick model in rats, providing concrete support for the concept of interplay between 5-HT1A and 5-HT2C receptors in the expression of their functional actions. Obata et al. [8] examined the antiallodynic effect of i.t.-administered serotonin receptor agonists, including 5-HT1A, 5-HT1B, 5-HT2, and 5- HT3 receptor subtypes in an animal model utilizing spinal nerve ligation; they reported that administration of the 5-HT2 receptor agonist demonstrated dose-dependent antiallodynic actions with no associated motor weakness, this suggesting that the 5-HT2 receptor plays an essential role in spinal suppression of neuropathic pain. Activation of 5-HT2 receptors in dorsal horn in the spinal cord possesses antiallodynic action. The results are similar according to those of Sasaki et al. [9]; these authors determined the subtypes of the spinal 5-HT receptors involved in modulating nociceptive transmission in the formalin test, to better understand the pharmacological mechanisms of 5-HT-induced antinociception. Their results showed that DOI, a 5-HT2-receptor agonist (i.t.), caused a dose-dependent reduction in the number of flinches of the formalin-injected paw in both phases 1 and 2. Dorsal-horn 5-HT2 and 5-HT3 receptors, then, inhibit nociceptive transmission in response to chemical inflammatory stimuli.
The expression of 5-HT2A receptor mRNA in the lumbar spinal dorsal horn, the nucleus of raphe magnus, ventrolateral periaqueductal gray, and the dorsal raphe nucleus following carrageenan inflammation utilizing the in situ hybridization technique was evaluated by Zhang et al. [19]; these authors reported that one hour after Carrageenan injection, the expression of 5-HT2A receptor mRNA in ipsilateral dorsal horn, bilateral nucleus of raphe magnus, ventrolateral periaqueductal gray, and dorsal raphe nucleus was significantly increased. Obata et al. [46] investigated the possible involvement of other associated spinal receptor systems with respect to the antiallodynic effect of a 5-HT2 receptor agonist; they reported that muscarinic receptors may be involved in this effect. Doly et al. [47] analyzed the distribution of 5-HT2A receptor in rat spinal cord by immunocytochemistry employing an antibody directed against an N-terminal sequence of the receptor; these authors found that 5-HT2A receptors were widely distributed in the whole spinal cord, with particularly high expression in motoneuron groups, in the sympathetic preganglionic cell group, and in the dorsal horn, concluding that the 5-HT2A receptor localization is mainly postsynaptic.
Obata et al. [48] evaluated the antiallodynic effects of i.t. administration of 5-HT2C receptor agonists MK212, mCPP, and TFMPP, in a rat model of neuropathic pain induced by spinal nerve ligation; they concluded that i.t. administration of each 5-HT2C receptor agonist produced antiallodynic effects in a dose-dependent manner. These results suggest that stimulation of spinal 5-HT2C receptors produces antiallodynic effects via a different mechanism from that of 5-HT2A receptors. Nitada et al. [49] investigated the possible involvement of the 5-HT2A receptor in the pathogenesis of neuropathic pain using chronic constriction injury of the sciatic nerve in rats; their results indicated that the 5-HT2A receptor antagonist sarpogrelate, specifically ameliorated hyperalgesia without affecting the normal nociceptive reaction.
The role of peripheral serotonin 5HT2A and 5HT1A receptors on orofacial nocifensive behavioral activities evoked by the injection of formalin into the masseter muscle was evaluated in rat by Okamoto et al. [50]; they reported that local administration of the 5HT2A antagonist receptor, Ketanserin, but not of the 5HT1A antagonist receptor, Propranolol, into rat masseter muscle significantly reduced the orofacial nocifensive behavioral activity. Wei et al. [51] examined the effects of intraplantar (i.p.) administration of the 5-HT2A receptor antagonist, Ketanserin, on hyperalgesia, inflammation, and the expression of c-fos-like immunoreactivity in spinal cord dorsal horn in the carrageenan model of inflammation; these authors showed cellular evidence indicating that peripheral 5-HT2A receptors are involved in nociceptive processing in the CNS and that they are responsible for the production of neuronal activity at the level of spinal cord in an inflammatory pain model. In addition, the 5-HT2A receptor and 5-HT1B, 5-HT1D, 5-HT2C, 5-HT3, and 5-HT7 receptors are also expressed in primary afferent nociceptors. Sasaki et al. [10] in their study examined the effect of a selective 5-HT2A receptor antagonist, Sarpogrelate, on the hyperalgesia and allodynia induced by thermal injury in rats. They concluded that the antagonist blocks 5-HT2A receptors at primary afferent fiber terminals in the periphery to inhibit primary thermal hyperalgesia and secondary mechanical allodynia. Dorsal root ganglion neurons expressed 5-HT2A receptor mRNA, and stimulation of peripheral 5-HT2A receptors produced thermal hyperalgesia.
Kayser et al. [52] used mutant mice, which do not express 5-HT1A, 5-HT1B, 5-HT2A, or 5-HT3A receptors, as an indirect approach to further assess the respective roles of these receptors in the physiological control of nociceptive responses in a wide range of noxious mechanical and thermal stimuli and i.p. injection of formalin. 5-HT1B and, to a lesser degree 5-HT1A receptors, mediate an endogenous inhibitory control of nociception by 5-HT, whereas 5-HT2A and 5-HT3 receptors play a role in formalin-induced hyperalgesia in male mice and, compared with paired wild-type mice, 5-HT1A mutants exhibited increased sensitivity in the hot-plate test. Concomitant decrease of 5-HT-mediated pronociceptive influences can also be postulated as a result of 5-HT2A receptor downregulation.
5-HT2A receptor antagonists might aid in alleviating various kinds of neuropathic pain. Van Steenwinckel et al. [42] evaluated the effect of an epidural injection of MDL 11,939 (5-HT2A receptor antagonist) on the appearance of mechanical allodynia and hyperalgesia in model of peripheral neuropathy; they reported that there was significant upregulation of 5-HT2A receptor immunoreactivity in the lumbar dorsal horn and peripheral nociceptive cells after peripheral neuropathy treatment. The 5-HT2A receptor is involved in wide central sensitization of dorsal-horn neurons and in peripheral sensitization of nociceptive neurons. The effect of MDL 11,939 on the mechanical hypersensitivity induced by antineoplasic drug in rats was investigated by Thibault et al. [53], they reported that 5-HT2A receptors also play a pronociceptive role in the sensitization of peripheral nociceptors and in spinal nociceptive processing. Studies to clarify the mechanism of action of some drugs have been conducted. Xie et al. [41] reported that Tramadol treatment alters 5-HT2A receptor mRNA expression in brainstem nuclei and the spinal dorsal horn, which may partially mediate the analgesic effect of Tramadol.
Kupers et al. [43] investigated the role of the 5-HT2A receptor system in pain processing. They found that the 5-HT2A receptor plays a role in the processing of tonic heat pain, but not in the processing of short, phasic heat pain stimuli. 5-HT2A receptors in discrete brain areas are involved in the regulation of responses to tonic painful stimulation. The correlations observed in prefrontal and posterior cingulate cortices suggest a possible role with respect to cognitive evaluative appreciation and the emotional processing of pain (Fig. 1).
Fig. (1).
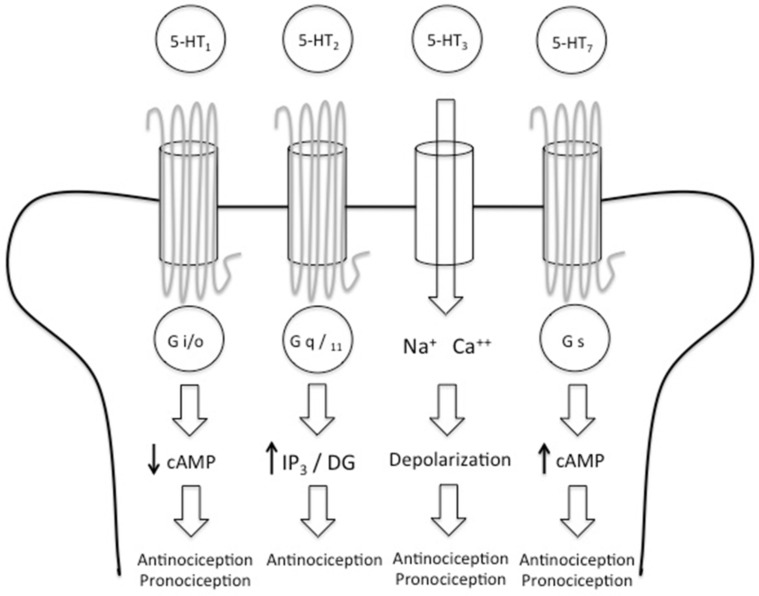
The 5-HT receptors are divided into 7 families (5-HT1, 5-HT2, 5-HT3, 5-HT4, 5-HT5, 5-HT6 and 5- HT7) that comprise 15 receptor subtypes. The receptors involved in the nociceptive pathway are 5-HT1, 5- HT2, 5-HT3 and 5-HT7. 5-HT1 is coupled to the G i/o protein and it reduces levels of cAMP generating anti and pronociceptive effects. 5-HT2 is coupled to the G q/11 protein and its activation leads to an increase of IP3 and DG levels, generating an antinociceptive effect. 5-HT3 is the only one that is not coupled to a G protein; instead this receptor is a ligand-gated cation channel, when activated 5-HT3 depolarizes the neuronal membrane and causes antinociception but also 5-HT3 can maintain the painful stimulus. 5.HT7 is coupled to G s protein, its activation generates an increase in the cAMP levels causing pro and antinociceptive effects.
2.2. 5-HT3
Kilpatrick et al. [54] found 5-HT3 binding sites in the brain for the first time, and now it is known that it participates in a wide variety of functions including, notably, pain modulation. The 5-HT3 receptor is a ligand-gated ion channel that, when activated, gives rise to fast, depolarizing responses in neurons [55]. Depolarization of both the fibers and the cell bodies of vagal afferents occurs in response to 5-HT3 receptor activation, activating a variety of second messenger signaling systems and, through these, indirectly regulating the function of ion channels involved in local, presynaptic control of neurotransmitter release [56, 57]. 5-HT3 receptor-mediated enhancement of 5-HT release has been reported in brain regions, for example, hippocampus, frontal cortex, hypothalamus, and raphe nucleus [58]. On the other hand, there is 5-HT3 receptor expression in the medial preoptic area, dorsal tegmental nucleus, trochlear nerve nucleus, and the facial nerve nucleus. The hybridization that exists within dorsal root ganglia support a role for 5-HT3 receptors in the modulation of activity in primary sensory afferent fibers and this is consistent with the role of 5-HT3 receptors in sensory processing [59]. Nayak et al. [60] reported that 5-HT3 serotonin receptors are present in isolated terminals from the corpus striatum, hippocampus, amygdala, and cerebellum of rat brain. 5-HT3 receptor activation produces, among other effects, postsynaptic depolarization of neurons throughout the nuclei in the striatum and, in addition, affects both spontaneous and evoked synaptic transmission [55]. A significant proportion of 5-HT3 binding sites (40%) are associated with the terminals of unmyelinated primary afferents, while others are found in intrinsic interneurons.
Comparison of the cellular population containing 5-HT3A and 5-HT3B mRNA subunits demonstrated that the population of peripheral neurons expressing the 5-HT3A subunit is larger than that expressing the 5-HT3B subunit [61]. No functional differences between the alternatively spliced 5-HT3A receptor subunits have been found to date; thus, is appears unlikely that alternative splicing of this subunit contributes to functional 5-HT3 receptor heterogeneity [62]. Morales et al. [63] reported the distribution in rat CNS of 5-HT3 receptor immunoreactivity; these authors found that it was present in the forebrain (isocortex, olfactory regions, hippocampal formation, and amygdala), brainstem (sensory and motor nuclei, and nuclei of the reticular formation), and spinal cord (dorsal and ventral horn). 5-HT3 innervation of the striatum modulates dopaminergic activity. Blandina et al. [64] indicated that at least part of this interaction occurs by means of the activation of a 5-HT3 receptor, thus presenting, to our knowledge, the first direct evidence of a functional role of a 5-HT3 receptor in brain.
Activation of 5-HT3 receptors produces a variety of effects, including membrane depolarization and increase in intracellular Ca2+, modulation of neurotransmitter release, excitation of central and peripheral neurons, and release of 5-HT from enterochromaffin cells of the small intestine [65]. 5-HT3 receptors may increase spontaneous release by producing localized depolarization of the presynaptic terminal, leading to an increased influx of Ca2+ through voltage-sensitive Ca2+ channels [55]. 5-HT3 receptors, in particular, are most closely related with the ACh receptor, and these receptors are present in the central and peripheral nervous systems [59]. Activation of the 5-HT3 receptor opens the cation channel of the 5-HT3 receptor, which becomes permeable, preferentially to Na+ and K+, but also to Ca2+ and other cations including the organic cation guanidinium [66]. 5-HT3 receptors are expressed in GABAergic interneurons; therefore, it is likely that 5-HT3 receptors are localized in GABAergic nerve terminals in the basolateral amygdala, modulating synaptic GABA transmission [67]. GABA antagonists block the action of 5-HT3. Tecott et al. [58] have shown that the distribution of the 5-HT3A subunit closely resembles that of 5-HT3-receptor binding sites; these authors suggest that the scattered distribution of the 5-HT3 receptor in forebrain may reflect expression in Gamma AminoButyric Acid (GABA)ergic interneurons. 5-HT3 receptors have been suggested to be involved in dopamine release, both in the striatum and in the nucleus accumbens [68]. In the spinal cord, 5-HT3 immunoreactivity was concentrated in the superficial layers of the dorsal horn in lamina I/III [69], where high densities of 5-HT3-receptor binding sites had previously been detected [56]. In addition to the postsynaptic localization of 5-HT3 receptors in interneurons, 5-HT3 receptors can also be found in presynaptic terminals [67].
Alhaider et al. [70] demonstrated that 5-HT3 receptors in intrinsic spinal cord neurons inhibit nociceptive spinal transmission in both behavioral (in mouse) and electrophysiological (in rat) tests. Glaum et al. [55] reported that the antinociceptive effects of 5-HT in both the tail-flick and hot-plate tests were blocked following i.t. application of 5-HT3 receptor antagonist ICS 205-930. 5-HT3 receptors are associated with the sensory endings of primary afferents, and there is good evidence that ICS 205-930 have antinociceptive actions at this site [71]. 5-HT3 receptors mediated serotonergic control of noxious transmission in the spinal cord. The responses of wide-dynamic-range (nociceptive) spinal dorsal-horn neurons to subcutaneous (s.c.) injection of formalin, and the electrically evoked responses of such neurons following intra plantar injection of carrageenan as inflammatory stimuli, was studied by Green et al. [72]. The authors’ results revealed that, in normal animals with no inflammation, blocking the 5-HT3 receptors exerted no significant effect on the electrically evoked responses of spinal dorsal-horn neurons. Moore and Weinreich [73] reported that 5-HT, a proinflammatory neurotransmitter, can activate 5-HT3 receptors to depolarize vagal afferent neurons, whereas 5-HT1 and 5-HT2 receptor subtypes bear highest affinity for the endogenous ligand and are thought to exert an overall antinociceptive action, and models of persistent pain have suggested a role for 5-HT3 receptor activation in the maintenance of pain [74]. 5-HT3 receptor could contribute to any central plasticity that accompanies this injury state, and the hyperalgesia and allodynia manifested after tissue injury involves different peripheral and/or central mechanisms [75].
Given that 5-HT3 receptor is a ligand gated ion channel, its pharmacology has been more studied than that of the other receptors. Camilleri and Boeckxstaens [76] reviewed a variety of studies regarding the treatment of abdominal pain in the irritable bowel syndrome (IBS) including 5-HT3 antagonists’ alosetron and ramosetron, concluding that they were effective in treating this type of pain.
5-HT facilitates persistent pain-like states via the activation of 5-HT3 receptors, most likely due to an increased, descending serotonergic drive from higher centers in the brain and, in particular, in the rostral ventromedial medulla [68]. At the spinal cord level, blocking 5-HT3 receptors reversed the increase in hypersensitivity induced by amygdaloidal administration of a low dose of glutamate. This finding suggests that spinal 5-HT3 receptors mediated the central nucleus of the amygdala (CEA)-induced increase of neuropathic hypersensitivity [77]. In summary, presynaptic 5-HT3 receptors increase neurotransmitter release whereas postsynaptic 5-HT3 receptors increase activity of both projection neurons and inhibitory interneurons.
2.3. 5-HT7
5-HT7 receptors comprise the most recently described members of the serotonin receptor family. An increasing number of studies have described the distribution of 5-HT7 receptor in rodents by utilizing immunohistochemical techniques [78-81]. These reports showed that the protein distribution is similar to that of the mRNA, with highest abundance in the thalamus, hypothalamus, and hippocampus [4, 82]. The 5-HT7A isoform predominates, followed by the 5-HT7B splice variant, while 5-HT7C and the 5-HT7D isoforms are least frequently expressed [83], the 5-HT7A receptor was the first splice variant cloned from human with a predicted length of 445 amino acids. In the spinal cord, 5-HT7 receptors were mainly found in the superficial laminae I and II of the dorsal horn, postsynaptically in local interneurons, and presynaptically in peptidergic fibers and in astrocytes [84]. Electron microscopic examination of the dorsal horn further revealed three main localizations: postsynaptic localization in peptidergic cell bodies and in numerous dendrites; presynaptic localization in unmyelinated and thin myelinated peptidergic fibers and in astrocytes [85], the pharmacological profile of 5-HT7 receptors is quite similar to that of the 5-HT1A receptors subtype [86].
Five major properties appear to define the 5-HT7 receptor and differentiate it from other 5-HT receptors as follows: limited sequence homology; presence and location of at least two introns; existence of an eighth hydrophobic domain; high-affinity binding of 5-HT, and positive coupling to adenylyl cyclase [87]. 5-HT7 receptors stimulate cAMP formation by activating adenylyl cyclases via a stimulatory Gs-protein, which also leads to Ras-dependent activation of the extracellular, signal-regulated kinases [88]. Activation of 5-HT7 receptors directly stimulates extracellular signal-regulated kinase in hippocampal neurons [89], an effect that can be of importance for hippocampal function and mood regulation [4]. 5-HT7 receptors appear to be mainly associated with limbic brain divisions receiving serotoninergic inputs (e.g., the hippocampus, amygdaloid complex, or mammillary nuclei). This suggests that 5-HT7 receptors are also involved in sleep induction and hypothermia, learning, mood, and in neuroendocrine or vegetative behaviors, and such observations were confirmed in a mouse strain with a disrupted 5-HT7 gene [86, 90]. Certain behavioral stimuli can trigger the electrical activity of the dorsal raphe nucleus, leading to 5-HT release and subsequent activation of 5-HT7 receptors in both the dorsal raphe nucleus and the medial raphe nucleus, which ultimately results in 5-HT release in the CNS [84].
Rocha-González et al. [91] conducted a study in which the main purpose was to determine the possible participation of local peripheral and spinal 5-HT7 receptors in formalin-induced nociception, employing electrophysiological, immunohistochemical, and behavioral data, the authors suggest a pronociceptive role for the 5-HT7 receptor in the dorsal horn of the spinal cord. Microinjection of formalin was preceded by either local or spinal administration of SB-269970 and/or 5-HT, both known for their antinociceptive activity, which significantly reduced formalin-induced flinching, while local 5-HT or 5-CT dose-dependently augmented the formalin-induced nociceptive behavior. On the other hand, the role of spinal 5-HT7 receptors in the antinociceptive effects of systemic morphine was elucidated in a study conducted by Drogul and Seyrek [92], these authors reported that systemically administered morphine activates the descending serotonergic pathways and that 5-HT7 receptors in the spinal cord play an important role in systemic morphine antinociception. Brenchat et al. [93] evaluated the potential role of the 5-HT7 receptor in nociception associated with a sensitizing stimulus in mice; intrinsic efficacy as an activator of human 5-HT7 receptors and the selectivity of 5-HT7 receptor agonists used were also investigated. Their results showed that 5-HT7 receptors participate in antinociceptive mechanisms and 5-HT7 receptor blockade by i.t. administration of SB-269970, inhibiting the antinociceptive effect of systemic morphine in the tail-flick test. The following year, Brenchat et al. [94] examined whether 5-HT7 receptors participates in some modulatory control of nerve injury-evoked mechanical hypersensitivity and thermal hyperalgesia in mice. These authors found a significant increase of 5-HT7 immunoreactivity in laminae I–II and III–V of the dorsal horn on the ipsilateral side of the spinal cord 11 days after nerve injury. In the case of 5-HT7 receptors, a recent study found that systemic administration of 5-HT7 receptor agonists reduced mechanical hypersensitivity in nerve-injured mice, suggesting that 5-HT7 receptors play an antinociceptive role. Studies suggest that spinal 5-HT7 receptors may play a pronociceptive, rather than an antinociceptive, role [82]. Likewise, systemic and spinal administration of the selective 5-HT7 receptor antagonist SB-269970 reduces the tactile allodynia induced by L5/L6 spinal nerve ligation, and 5-HT7 receptors possess a pronociceptive role in this type of pain spinal nerve ligation, which leads to a reduction in the level of 5-HT7 receptors [95].
The analgesic effect of morphine co-administered with the selective 5-HT7 receptor agonist E-55888, the antagonist SB-258719, or both, was evaluated by Brenchat et al. [96]. They reported on 5-HT7 receptors in opioid analgesia and pointed out a potential use of 5-HT7 receptor agonists as adjuvants of opioid analgesia; systemic administration of a selective 5-HT7 receptor agonist per se is not sufficient to reproduce the antinociception exerted by opioids in acute thermal nociceptive models. The respective roles of peripheral and spinal 5-HT7 receptors in the modulation of mechanical hypersensitivity were investigated under two different experimental pain conditions by Brenchat et al. [97]; they demonstrated that activation of 5-HT7 receptors exerts antinociceptive effects at the spinal cord level and pronociceptive effects at the periphery. A previous study at the light microscope level revealed that 5-HT7 receptors co-localize with GABA in neurons of the spinal-cord dorsal horn [93], and it has been reported that spinal GABAergic interneurons are involved in 5-HT7 receptor-mediated antinociception. Dogrul et al. [98] investigated the role of descending serotonergic pathways and spinal 5-HT7 receptors compared with 5-HT3 and 5-HT2A receptors in terms of the antinociceptive and antihyperalgesic effects of Paracetamol; they reported that activation of descending serotonergic pathways and spinal 5-HT7 receptors following systemic administration of Paracetamol produces antinociceptive and antihyperalgesic effects, and that the 5-HT7 receptor antagonist blocks the antinociceptive and antihyperalgesic effects of systemic Paracetamol, indicating a novel role of spinal 5-HT7 receptors in the mechanism of action of Paracetamol. The role of spinal 5-HT and 5-HT4/6/7 receptors in the long-term secondary mechanical allodynia and hyperalgesia induced by formalin in rat comprised a study conducted by Godínez-Chaparro et al. [99]; these authors showed that formalin activates a descending serotonergic system, which releases 5-HT at the spinal cord and contributes to the development and maintenance of secondary allodynia and hyperalgesia. Viguier et al. [100] investigated how 5-HT7 receptors contribute to neuropathic pain modulation by using potent 5-HT7 receptors antagonist (SB-269970) and/or agonists (MSD-5a, AS-19, E-55888) in rats with unilateral ligations of the sciatic nerve or the infraorbital nerve, reporting that 5-HT7 receptors mediated inhibitory control of the neuropathic pain underlying the excitation of GABAergic interneurons within the dorsal horn. Yang et al. [101] compared the role of 5-HT7 receptors and the influence of descending serotonergic modulation between formalin- and carrageenan-induced inflammatory pain; they concluded that activation of 5-HT7 receptors exerted a significant antinociceptive effect on formalin- induced pain, but no effect on carrageenan-induced pain, indicating differences in the involvement of 5-HT7 receptors according to the pain modality, (Fig. 2).
Fig. (2).
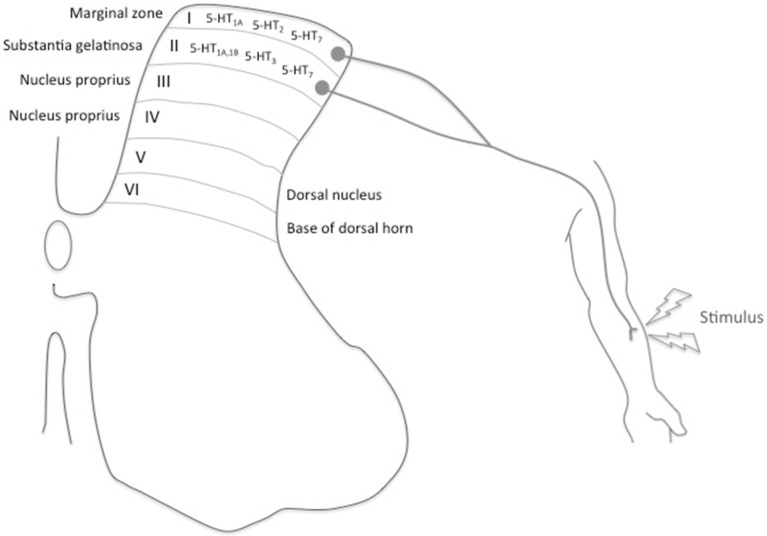
The dorsal horn is the area that receives the painful stimuli; it is divided into five Rexed laminae. The 5-HT1, 5-HT2, 5-HT3 and 5-HT7 receptors are mainly located in the lamina I and II. The receptor involved in antinociception (5-HT1A, 5-HT2 and 5-HT7) receptors are mainly expressed in the lamina I, also known as marginal zone. This lamina is mostly innervated by A-δ fibers. In the lamina II, substantia gelatinosa, the receptors involved in pronociception (5-HT1B, 5-HT3 and 5-HT7) are expressed. This lamina is innervated by C fibers.
3. DISCUSSION
Multiple 5-HT receptors exist in the central nervous system, and the serotonin in the raphe-spinal pathway has been implicated in playing an important role in analgesia. The main purpose of this review was to analyze the modulation of pain by 5-HT1, 5-HT2, 5-HT3, and 5-HT7 receptors at the central level. A total of 70 studies from different authors were evaluated, and we found certain differences and commonalties among these studies.
Several lines of evidence have implicated a role for the serotonin-containing component of the raphe-spinal system in modulating nociception. Medullary nucleus raphe magnus is thought to be a major source of the descending serotonin that contains fibers terminating in the spinal cord [102]. The physiological functions of the spinal cord and the impact of 5-HT on these are distributed in the following four areas: the first is the dorsal horn, which corresponds to primary relay of nociceptive inputs; the intermediolateral cell column from which originate the sympathetic preganglionic neurons is the second area, the third area is the central canal that might be involved in exchanges with the cerebrospinal fluid, and the last area is the ventral horn, which is implicated in motor functions [37]. Serotonin-containing axons descending from the brainstem are known to terminate in the ventral horn and in the intermediolateral column, as well as in the dorsal horn [103]. At the cellular level, 5-HT produces both pre- and postsynaptic inhibition and excitation within the spinal and trigeminal superficial dorsal horn [12, 104-108].
Different techniques, drugs, and pain models have been employed to determine the distribution of the different serotonergic receptors that are able to modulate the nociceptive response mediated by the descending system at the spinal cord and the presence of 5-HT1, 5-HT2, 5-HT3, and 5-HT7 receptors in different amounts and at different levels was reported with the majority of these studies implicating these receptors in pain inhibition. However, these studies mention the involvement of these receptors in hyperalgesia, or even in maintaining the painful stimulus. Furthermore, there have been different pain models that have attempted to explain the involvement of serotonergic receptors in the nociceptive response (the tail-flick test, the hot-plate test, the formalin test, the carrageenan test, etc.); these studies reported that the type of pain (acute pain, inflammatory pain, neuropathic pain, tonic pain, or chronic pain) determine which and how the serotoninergic receptors participate. Investigation with administration agonists or antagonists regarding 5-HT receptors showed that the anti- or hyperalgesic effect depends on the drug dose in all of the receptors. Finally, several authors have reported that the participation of serotonergic receptors depends on pain duration, and different serotonergic receptors may even participate in conjunction to inhibit, excite, or maintain the painful stimulus. In summary, there are important points to consider in order to understand how pain is modulated by serotonergic receptors in the central nervous system as follows: (i) the distribution of the different serotonergic receptors in the raphe-spinal pathway; (ii) the dose of agonists or antagonists on terms of the 5-HT receptors; (iii) the route of administration of agonists or antagonists with respect to the 5-HT receptors; (iv) the type of pain, and (v) pain duration. We think it necessary to engage in novel lines of investigation to clarify the involvement of 5-HT receptors in the modulation of pain considering the abovementioned points. The knowledge generated with future research could be used for generating new drugs and therapies to reduce pain and to improve the quality of life of patients.
CONCLUSION
We concluded that 5-HT1, 5-HT2, 5-HT3, and 5-HT7 receptors in the CNS intervene in the modulation of pain, but this modulation depends on the distribution of the receptors, the dose of agonists or antagonists, the route of administration, and the type and duration of pain required to inhibit, excite, or even maintain the nociceptive response.
Consent for Publication
Not applicable.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Imirizaldu M., Calvo J.L. Prevalencia y valoración del dolor. Revista ROL de Enfermería. 2009;32(6):414–420. [PubMed] [Google Scholar]
- 2.Molina J., Uribe A., Figueroa J. Dolor, calidad de vida y estado anímico relacionados con la salud de pacientes ancianos hospitalizados. Pensamientos en Psicología. 2013;11:43–53. [Google Scholar]
- 3.Millan M.J. Descending control of pain. Prog. Neurobiol. 2002;66(6):355–474. doi: 10.1016/s0301-0082(02)00009-6. [http://dx.doi.org/10.1016/S0301-0082(02)00009-6]. [PMID: 12034378]. [DOI] [PubMed] [Google Scholar]
- 4.Hedlund P.B., Sutcliffe J.G. Functional, molecular and pharmacological advances in 5-HT7 receptor research. Trends Pharmacol. Sci. 2004;25(9):481–486. doi: 10.1016/j.tips.2004.07.002. [http://dx.doi.org/10.1016/j.tips.2004. 07.002]. [PMID: 15559250]. [DOI] [PubMed] [Google Scholar]
- 5.Bardin L., Lavarenne J., Eschalier A. Serotonin receptor subtypes involved in the spinal antinociceptive effect of 5-HT in rats. Pain. 2000;86(1-2):11–18. doi: 10.1016/s0304-3959(99)00307-3. [http://dx.doi.org/10.1016/S0304-3959(99) 00307-3]. [PMID: 10779655]. [DOI] [PubMed] [Google Scholar]
- 6.Jeong C.Y., Choi J.I., Yoon M.H. Roles of serotonin receptor subtypes for the antinociception of 5-HT in the spinal cord of rats. Eur. J. Pharmacol. 2004;502(3):205–211. doi: 10.1016/j.ejphar.2004.08.048. [http://dx.doi.org/10. 1016/j.ejphar.2004.08.048]. [PMID: 15476746]. [DOI] [PubMed] [Google Scholar]
- 7.Dogrul A., Ossipov M.H., Porreca F. Differential mediation of descending pain facilitation and inhibition by spinal 5HT-3 and 5HT-7 receptors. Brain Res. 2009;1280:52–59. doi: 10.1016/j.brainres.2009.05.001. [http://dx. doi.org/10.1016/j.brainres.2009.05.001]. [PMID: 19427839]. [DOI] [PubMed] [Google Scholar]
- 8.Obata H., Saito S., Sasaki M., Ishizaki K., Goto F. Antiallodynic effect of intrathecally administered 5-HT(2) agonists in rats with nerve ligation. Pain. 2001;90(1-2):173–179. doi: 10.1016/s0304-3959(00)00401-2. [http://dx.doi. org/10.1016/S0304-3959(00)00401-2]. [PMID: 11166984]. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki M., Ishizaki K., Obata H., Goto F. Effects of 5-HT2 and 5-HT3 receptors on the modulation of nociceptive transmission in rat spinal cord according to the formalin test. Eur. J. Pharmacol. 2001;424(1):45–52. doi: 10.1016/s0014-2999(01)01117-7. [http://dx.doi.org/10.1016/S0014-2999(01) 01117-7]. [PMID: 11470259]. [DOI] [PubMed] [Google Scholar]
- 10.Sasaki M., Obata H., Kawahara K., Saito S., Goto F. Peripheral 5-HT2A receptor antagonism attenuates primary thermal hyperalgesia and secondary mechanical allodynia after thermal injury in rats. Pain. 2006;122(1-2):130–136. doi: 10.1016/j.pain.2006.01.021. [http://dx.doi.org/10.1016/ j.pain.2006.01.021]. [PMID: 16527395]. [DOI] [PubMed] [Google Scholar]
- 11.Leopoldo M., Lacivita E., Berardi F., Perrone R., Hedlund P.B. Serotonin 5-HT7 receptor agents: Structure-activity relationships and potential therapeutic applications in central nervous system disorders. Pharmacol. Ther. 2011;129(2):120–148. doi: 10.1016/j.pharmthera.2010.08.013. [http://dx.doi. org/10.1016/j.pharmthera.2010.08.013]. [PMID: 20923682]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeong H.J., Mitchell V.A., Vaughan C.W. Role of 5-HT(1) receptor subtypes in the modulation of pain and synaptic transmission in rat spinal superficial dorsal horn. Br. J. Pharmacol. 2012;165(6):1956–1965. doi: 10.1111/j.1476-5381.2011.01685.x. [http://dx.doi.org/10.1111/j.1476-5381.2011.01685.x]. [PMID: 21950560]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peroutka S.J. 5-Hydroxytryptamine receptor subtypes: molecular, biochemical and physiological characterization. Trends Neurosci. 1988;11(11):496–500. doi: 10.1016/0166-2236(88)90011-2. [http://dx.doi.org/10.1016/0166-2236(88) 90011-2]. [PMID: 2469177]. [DOI] [PubMed] [Google Scholar]
- 14.Zemlan F.P., Behbehani M.M., Murphy R.M. Serotonin receptor subtypes and the modulation of pain transmission. 1988. [DOI] [PubMed] [Google Scholar]
- 15.Barnes N.M., Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38(8):1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [http:// dx.doi.org/10.1016/S0028-3908(99)00010-6]. [PMID: 10462127]. [DOI] [PubMed] [Google Scholar]
- 16.Zemlan F.P., Schwab E.F. Characterization of a novel serotonin receptor subtype (5-HT1S) in rat CNS: interaction with a GTP binding protein. J. Neurochem. 1991;57(6):2092–2099. doi: 10.1111/j.1471-4159.1991.tb06427.x. [http:// dx.doi.org/10.1111/j.1471-4159.1991.tb06427.x]. [PMID: 1834802]. [DOI] [PubMed] [Google Scholar]
- 17.Murphy R.M., Zemlan F.P. Selective serotonin1A/1B agonists differentially affect spinal nociceptive reflexes. Neuropharmacology. 1990;29(5):463–468. doi: 10.1016/0028-3908(90)90168-q. [http://dx.doi.org/10.1016/0028-3908 (90)90168-Q]. [PMID: 2356002]. [DOI] [PubMed] [Google Scholar]
- 18.Wright D.E., Seroogy K.B., Lundgren K.H., Davis B.M., Jennes L. Comparative localization of serotonin1A, 1C, and 2 receptor subtype mRNAs in rat brain. J. Comp. Neurol. 1995;351(3):357–373. doi: 10.1002/cne.903510304. [http://dx.doi.org/10.1002/cne.903510304]. [PMID: 7706547]. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y.Q., Gao X., Ji G.C., Wu G.C. Expression of 5-HT2A receptor mRNA in rat spinal dorsal horn and some nuclei of brainstem after peripheral inflammation. Brain Res. 2001;900(1):146–151. doi: 10.1016/s0006-8993(01)02283-1. [http://dx.doi.org/10.1016/S0006-8993(01)02283-1]. [PMID: 11325358]. [DOI] [PubMed] [Google Scholar]
- 20.Marlier L., Teilhac J.R., Cerruti C., Privat A. Autoradiographic mapping of 5-HT1, 5-HT1A, 5-HT1B and 5-HT2 receptors in the rat spinal cord. Brain Res. 1991;550(1):15–23. doi: 10.1016/0006-8993(91)90400-p. [http://dx.doi.org/ 10.1016/0006-8993(91)90400-P]. [PMID: 1832328]. [DOI] [PubMed] [Google Scholar]
- 21.Laporte A.M., Fattaccini C.M., Lombard M.C., Chauveau J., Hamon M. Effects of dorsal rhizotomy and selective lesion of serotonergic and noradrenergic systems on 5-HT1A, 5-HT1B, and 5-HT3 receptors in the rat spinal cord. J. Neural Transm. (Vienna) 1995;100(3):207–223. doi: 10.1007/BF01276459. [http://dx.doi.org/10.1007/BF01276459]. [PMID: 8748667]. [DOI] [PubMed] [Google Scholar]
- 22.Hensler J.G., Kovachich G.B., Frazer A. A quantitative autoradiographic study of serotonin1A receptor regulation. Effect of 5,7-dihydroxytryptamine and antidepressant treatments. Neuropsychopharmacology. 1991;4(2):131–144. [PMID: 2025379]. [PubMed] [Google Scholar]
- 23.Azmitia E.C., Gannon P.J., Kheck N.M., Whitaker-Azmitia P.M. Cellular localization of the 5-HT1A receptor in primate brain neurons and glial cells. Neuropsychopharmacology. 1996;14(1):35–46. doi: 10.1016/S0893-133X(96)80057-1. [http://dx.doi.org/10.1016/S0893-133X(96)80057-1]. [PMID: 8719028]. [DOI] [PubMed] [Google Scholar]
- 24.Parsey R.V., Slifstein M., Hwang D.R., Abi-Dargham A., Simpson N., Mawlawi O., Guo N.N., Van Heertum R., Mann J.J., Laruelle M. Validation and reproducibility of measurement of 5-HT1A receptor parameters with [carbonyl-11C]WAY-100635 in humans: comparison of arterial and reference tisssue input functions. J. Cereb. Blood Flow Metab. 2000;20(7):1111–1133. doi: 10.1097/00004647-200007000-00011. [http://dx. doi.org/10.1097/00004647-200007000-00011]. [PMID: 10908045]. [DOI] [PubMed] [Google Scholar]
- 25.Rabiner E.A., Messa C., Sargent P.A., Husted-Kjaer K., Montgomery A., Lawrence A.D., Bench C.J., Gunn R.N., Cowen P., Grasby P.M. A database of [(11)C]WAY-100635 binding to 5-HT(1A) receptors in normal male volunteers: normative data and relationship to methodological, demographic, physiological, and behavioral variables. Neuroimage. 2002;15(3):620–632. doi: 10.1006/nimg.2001.0984. [http://dx.doi.org/10.1006/nimg.2001.0984]. [PMID: 11848705]. [DOI] [PubMed] [Google Scholar]
- 26.Hirvonen J., Kajander J., Allonen T., Oikonen V., Någren K., Hietala J. Measurement of serotonin 5-HT1A receptor binding using positron emission tomography and [carbonyl-(11)C]WAY-100635-considerations on the validity of cerebellum as a reference region. J. Cereb. Blood Flow Metab. 2007;27(1):185–195. doi: 10.1038/sj.jcbfm.9600326. [http://dx.doi.org/10.1038/sj.jcbfm.9600326]. [PMID: 16685258]. [DOI] [PubMed] [Google Scholar]
- 27.Martikainen I.K., Hirvonen J., Kajander J., Hagelberg N., Mansikka H., Någren K., Hietala J., Pertovaara A. Correlation of human cold pressor pain responses with 5-HT(1A) receptor binding in the brain. Brain Res. 2007;1172:21–31. doi: 10.1016/j.brainres.2007.07.036. [http://dx.doi.org/10. 1016/j.brainres.2007.07.036]. [PMID: 17803974]. [DOI] [PubMed] [Google Scholar]
- 28.Riad M., García S., Watkins K.C., Jodoin N., Doucet E., Langlois X., el Mestikawy S., Hamon M., Descarries L. Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J. Comp. Neurol. 2000;417(2):181–194. [http://dx.doi.org/10.1002/(SICI)1096-9861(20000207)417:2<181:AID-CNE4>3.0.CO;2-A]. [PMID: 10660896]. [PubMed] [Google Scholar]
- 29.Colpaert F.C. 5-HT(1A) receptor activation: new molecular and neuroadaptive mechanisms of pain relief. Curr. Opin. Investig. Drugs. 2006;7(1):40–47. [PMID: 16425670]. [PubMed] [Google Scholar]
- 30.Mico J.A., Berrocoso E., Ortega-Álvaro A., Gibert-Rahola J., Rojas-Corrales M.O. The role of 5-HT1A receptors in research strategy for extensive pain treatment. Curr. Top. Med. Chem. 2006;6(18):1997–2003. doi: 10.2174/156802606778522195. [http://dx.doi.org/10.2174/156802606778522195]. [PMID: 17017970]. [DOI] [PubMed] [Google Scholar]
- 31.El-yassir N., Fleetwood-Walkerm S.M., Mitchell R. Heterogeneous effects of serotonin in the dorsal horn of rat: the involvement of 5-HT~ receptor subtypes. Brain Res. 1988;456:147–158. doi: 10.1016/0006-8993(88)90356-3. [http://dx.doi.org/10.1016/0006-8993(88)90356-3]. [PMID: 2970278]. [DOI] [PubMed] [Google Scholar]
- 32.Zemlan F.P., Kow L.M., Pfaff D.W. Spinal serotonin (5-HT) receptor subtypes and nociception. J. Pharmacol. Exp. Ther. 1983;226(2):477–485. [PMID: 6308209]. [PubMed] [Google Scholar]
- 33.Eide P.K., Joly N.M., Hole K. The role of spinal cord 5-HT1A and 5-HT1B receptors in the modulation of a spinal nociceptive reflex. Brain Res. 1990;536(1-2):195–200. doi: 10.1016/0006-8993(90)90025-7. [http://dx.doi.org/10. 1016/0006-8993(90)90025-7]. [PMID: 2150769]. [DOI] [PubMed] [Google Scholar]
- 34.Ali Z., Wu G., Kozlov A., Barasi S. The actions of 5-HT1 agonists and antagonists on nociceptive processing in the rat spinal cord: results from behavioural and electrophysiological studies. Brain Res. 1994;661(1-2):83–90. doi: 10.1016/0006-8993(94)91184-3. [http://dx.doi.org/10.1016/0006-8993(94)91184-3]. [PMID: 7834389]. [DOI] [PubMed] [Google Scholar]
- 35.Gjerstad J., Tjølsen A., Hole K. A dual effect of 5-HT1B receptor stimulation on nociceptive dorsal horn neurones in rats. Eur. J. Pharmacol. 1997;335(2-3):127–132. doi: 10.1016/s0014-2999(97)01183-7. [http://dx.doi.org/10.1016/ S0014-2999(97)01183-7]. [PMID: 9369364]. [DOI] [PubMed] [Google Scholar]
- 36.Liu Z.Y., Zhuang D.B., Lunderberg T., Yu L.C. Involvement of 5-hydroxytryptamine(1A) receptors in the descending anti-nociceptive pathway from periaqueductal gray to the spinal dorsal horn in intact rats, rats with nerve injury and rats with inflammation. Neuroscience. 2002;112(2):399–407. doi: 10.1016/s0306-4522(02)00038-6. [http://dx.doi.org/ 10.1016/S0306-4522(02)00038-6]. [PMID: 12044457]. [DOI] [PubMed] [Google Scholar]
- 37.Hains B.C., Willis W.D., Hulsebosch C.E. Serotonin receptors 5-HT1A and 5-HT3 reduce hyperexcitability of dorsal horn neurons after chronic spinal cord hemisection injury in rat. Exp. Brain Res. 2003;149(2):174–186. doi: 10.1007/s00221-002-1352-x. [http://dx.doi.org/10.1007/s00221-002-1352-x]. [PMID: 12610685]. [DOI] [PubMed] [Google Scholar]
- 38.Bonnefont J., Chapuy E., Clottes E., Alloui A., Eschalier A. Spinal 5-HT1A receptors differentially influence nociceptive processing according to the nature of the noxious stimulus in rats: effect of WAY-100635 on the antinociceptive activities of paracetamol, venlafaxine and 5-HT. Pain. 2005;114(3):482–490. doi: 10.1016/j.pain.2005.01.019. [http://dx. doi.org/10.1016/j.pain.2005.01.019]. [PMID: 15777873]. [DOI] [PubMed] [Google Scholar]
- 39.Wei H., Pertovaara A. 5-HT(1A) receptors in endogenous regulation of neuropathic hypersensitivity in the rat. Eur. J. Pharmacol. 2006;535(1-3):157–165. doi: 10.1016/j.ejphar.2006.02.019. [http://dx.doi.org/10.1016/j.ejphar.2006. 02.019]. [PMID: 16545367]. [DOI] [PubMed] [Google Scholar]
- 40.Pazos A., Cortés R., Palacios J.M. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. II. Serotonin-2 receptors. Brain Res. 1985;346(2):231–249. doi: 10.1016/0006-8993(85)90857-1. [http://dx.doi.org/ 10.1016/0006-8993(85)90857-1]. [PMID: 4052777]. [DOI] [PubMed] [Google Scholar]
- 41.Xie H., Dong Z.Q., Ma F., Bauer W.R., Wang X., Wu G.C. Involvement of serotonin 2A receptors in the analgesic effect of tramadol in mono-arthritic rats. Brain Res. 2008;1210:76–83. doi: 10.1016/j.brainres.2008.02.049. [http:// dx.doi.org/10.1016/j.brainres.2008.02.049]. [PMID: 18417104]. [DOI] [PubMed] [Google Scholar]
- 42.Van Steenwinckel J., Noghero A., Thibault K., Brisorgueil M.J., Fischer J., Conrath M. The 5-HT2A receptor is mainly expressed in nociceptive sensory neurons in rat lumbar dorsal root ganglia. Neuroscience. 2009;161(3):838–846. doi: 10.1016/j.neuroscience.2009.03.087. [http://dx.doi.org/10.1016/ j.neuroscience.2009.03.087]. [PMID: 19362128]. [DOI] [PubMed] [Google Scholar]
- 43.Kupers R., Frokjaer V.G., Naert A., Christensen R., Budtz-Joergensen E., Kehlet H., Knudsen G.M. PET [18F]altanserin study of 5-HT2A receptor binding in the human brain and responses to painful heat stimulation. Neuroimage. 2009;44(3):1001–1007. doi: 10.1016/j.neuroimage.2008.10.011. [http://dx.doi.org/10.1016/j.neuroimage.2008.10.011]. [PMID: 19007894]. [DOI] [PubMed] [Google Scholar]
- 44.Abbott F.V., Hong Y., Blier P. Activation of 5-HT2A receptors potentiates pain produced by inflammatory mediators. Neuropharmacology. 1996;35(1):99–110. doi: 10.1016/0028-3908(95)00136-0. [http://dx.doi.org/10.1016/0028-3908(95)00136-0]. [PMID: 8684602]. [DOI] [PubMed] [Google Scholar]
- 45.Millan M.J., Girardon S., Bervoets K. 8-OH-DPAT-induced spontaneous tail-flicks in the rat are facilitated by the selective serotonin (5-HT)2C agonist, RO 60-0175: blockade of its actions by the novel 5-HT2C receptor antagonist SB 206,553. Neuropharmacology. 1997;36(4-5):743–745. doi: 10.1016/s0028-3908(97)00071-3. [http://dx.doi.org/10.1016/S0028-3908(97)00071-3]. [PMID: 9225301]. [DOI] [PubMed] [Google Scholar]
- 46.Obata H., Saito S., Sasaki M., Goto F. Possible involvement of a muscarinic receptor in the anti-allodynic action of a 5-HT2 receptor agonist in rats with nerve ligation injury. Brain Res. 2002;932(1-2):124–128. doi: 10.1016/s0006-8993(02)02288-6. [http://dx.doi.org/10.1016/S0006-8993(02)02288-6]. [PMID: 11911869]. [DOI] [PubMed] [Google Scholar]
- 47.Doly S., Madeira A., Fischer J., Brisorgueil M.J., Daval G., Bernard R., Vergé D., Conrath M. The 5-HT2A receptor is widely distributed in the rat spinal cord and mainly localized at the plasma membrane of postsynaptic neurons. J. Comp. Neurol. 2004;472(4):496–511. doi: 10.1002/cne.20082. [http://dx.doi.org/10.1002/cne.20082]. [PMID: 15065122]. [DOI] [PubMed] [Google Scholar]
- 48.Obata H., Saito S., Sakurazawa S., Sasaki M., Usui T., Goto F. Antiallodynic effects of intrathecally administered 5-HT(2C) receptor agonists in rats with nerve injury. Pain. 2004;108(1-2):163–169. doi: 10.1016/j.pain.2003.12.019. [http://dx.doi.org/10.1016/j.pain.2003.12.019]. [PMID: 15109520]. [DOI] [PubMed] [Google Scholar]
- 49.Nitanda A., Yasunami N., Tokumo K., Fujii H., Hirai T., Nishio H. Contribution of the peripheral 5-HT 2A receptor to mechanical hyperalgesia in a rat model of neuropathic pain. Neurochem. Int. 2005;47(6):394–400. doi: 10.1016/j.neuint.2005.06.002. [http://dx.doi.org/10.1016/j. neuint.2005.06.002]. [PMID: 16051396]. [DOI] [PubMed] [Google Scholar]
- 50.Okamoto K., Imbe H., Tashiro A., Kimura A., Donishi T., Tamai Y., Senba E. The role of peripheral 5HT2A and 5HT1A receptors on the orofacial formalin test in rats with persistent temporomandibular joint inflammation. Neuroscience. 2005;130(2):465–474. doi: 10.1016/j.neuroscience.2004.10.004. [http://dx.doi.org/10.1016/j.neuroscience.2004.10.004]. [PMID: 15664703]. [DOI] [PubMed] [Google Scholar]
- 51.Wei H., Chen Y., Hong Y. The contribution of peripheral 5-hydroxytryptamine A receptor to carrageenan-evoked hyperalgesia, inflammation and spinal Fos protein expression in the rat. Neuroscience. 2005;132(4):1073–1082. doi: 10.1016/j.neuroscience.2004.12.006. [http://dx.doi.org/10.1016/j. neuroscience.2004.12.006]. [PMID: 15857711]. [DOI] [PubMed] [Google Scholar]
- 52.Kayser V., Elfassi I.E., Aubel B., Melfort M., Julius D., Gingrich J.A., Hamon M., Bourgoin S. Mechanical, thermal and formalin-induced nociception is differentially altered in 5-HT1A-/-, 5-HT1B-/-, 5-HT2A-/-, 5-HT3A-/- and 5-HTT-/- knock-out male mice. Pain. 2007;130(3):235–248. doi: 10.1016/j.pain.2006.11.015. [http://dx.doi.org/10.1016/ j.pain.2006.11.015]. [PMID: 17250964]. [DOI] [PubMed] [Google Scholar]
- 53.Thibault K., Van Steenwinckel J., Brisorgueil M.J., Fischer J., Hamon M., Calvino B., Conrath M. Serotonin 5-HT2A receptor involvement and Fos expression at the spinal level in vincristine-induced neuropathy in the rat. Pain. 2008;140(2):305–322. doi: 10.1016/j.pain.2008.09.006. [http://dx.doi.org/10.1016/j.pain.2008.09.006]. [PMID: 18930597]. [DOI] [PubMed] [Google Scholar]
- 54.Kilpatrick G.J., Jones B.J., Tyers M.B. Identification and distribution of 5-HT3 receptors in rat brain using radioligand binding. Nature. 1987;330(6150):746–748. doi: 10.1038/330746a0. [http://dx.doi.org/10.1038/ 330746a0]. [PMID: 3696238]. [DOI] [PubMed] [Google Scholar]
- 55.Maricq A.V., Peterson A.S., Brake A.J., Myers R.M., Julius D. Primary structure and functional expression of the 5HT3 receptor, a serotonin-gated ion channel. Science. 1991;254(5030):432–437. doi: 10.1126/science.1718042. [http://dx.doi.org/10.1126/science.1718042]. [PMID: 1718042]. [DOI] [PubMed] [Google Scholar]
- 56.Glaum S.R., Brooks P.A., Spyer K.M., Miller R.J. 5-Hydroxytryptamine-3 receptors modulate synaptic activity in the rat nucleus tractus solitarius in vitro. Brain Res. 1992;589(1):62–68. doi: 10.1016/0006-8993(92)91162-8. [http://dx.doi.org/10.1016/0006-8993(92)91162-8]. [PMID: 1422823]. [DOI] [PubMed] [Google Scholar]
- 57.Miquel M.C., Emerit M.B., Nosjean A., Simon A., Rumajogee P., Brisorgueil M.J., Doucet E., Hamon M., Vergé D. Differential subcellular localization of the 5-HT3-As receptor subunit in the rat central nervous system. Eur. J. Neurosci. 2002;15(3):449–457. doi: 10.1046/j.0953-816x.2001.01872.x. [http://dx.doi.org/10.1046/j.0953-816x.2001.01872.x]. [PMID: 11876772]. [DOI] [PubMed] [Google Scholar]
- 58.van Hooft J.A., Vijverberg H.P. 5-HT(3) receptors and neurotransmitter release in the CNS: a nerve ending story? Trends Neurosci. 2000;23(12):605–610. doi: 10.1016/s0166-2236(00)01662-3. [http://dx.doi.org/10.1016/S0166-2236(00)01662-3]. [PMID: 11137150]. [DOI] [PubMed] [Google Scholar]
- 59.Tecott L.H., Maricq A.V., Julius D. Nervous system distribution of the serotonin 5-HT3 receptor mRNA. Proc. Natl. Acad. Sci. USA. 1993;90(4):1430–1434. doi: 10.1073/pnas.90.4.1430. [http://dx.doi.org/10.1073/pnas.90.4. 1430]. [PMID: 8434003]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nayak S.V., Rondé P., Spier A.D., Lummis S.C., Nichols R.A. Calcium changes induced by presynaptic 5-hydroxytryptamine-3 serotonin receptors on isolated terminals from various regions of the rat brain. Neuroscience. 1999;91(1):107–117. doi: 10.1016/s0306-4522(98)00520-x. [http://dx.doi. org/10.1016/S0306-4522(98)00520-X]. [PMID: 10336063]. [DOI] [PubMed] [Google Scholar]
- 61.Morales M., Wang S.D. Differential composition of 5-hydroxytryptamine3 receptors synthesized in the rat CNS and peripheral nervous system. J. Neurosci. 2002;22(15):6732–6741. doi: 10.1523/JNEUROSCI.22-15-06732.2002. [PMID: 12151552]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Hooft J.A., Yakel J.L. 5-HT3 receptors in the CNS: 3B or not 3B? Trends Pharmacol. Sci. 2003;24(4):157–160. doi: 10.1016/S0165-6147(03)00051-8. [http://dx.doi. org/10.1016/S0165-6147(03)00051-8]. [PMID: 12707000]. [DOI] [PubMed] [Google Scholar]
- 63.Morales M., Battenberg E., de Lecea L., Sanna P.P., Bloom F.E. Cellular and subcellular immunolocalization of the type 3 serotonin receptor in the rat central nervous system. Brain Res. Mol. Brain Res. 1996;36(2):251–260. doi: 10.1016/0169-328x(96)88406-3. [http://dx.doi.org/10.1016/0169-328X (96)88406-3]. [PMID: 8965645]. [DOI] [PubMed] [Google Scholar]
- 64.Blandina P., Goldfarb J., Green J.P. Activation of a 5-HT3 receptor releases dopamine from rat striatal slice. Eur. J. Pharmacol. 1988;155(3):349–350. doi: 10.1016/0014-2999(88)90528-6. [http://dx.doi.org/10.1016/0014-2999(88) 90528-6]. [PMID: 3234491]. [DOI] [PubMed] [Google Scholar]
- 65.Dubin A.E., Huvar R., D’Andrea M.R., Pyati J., Zhu J.Y., Joy K.C., Wilson S.J., Galindo J.E., Glass C.A., Luo L., Jackson M.R., Lovenberg T.W., Erlander M.G. The pharmacological and functional characteristics of the serotonin 5-HT(3A) receptor are specifically modified by a 5-HT(3B) receptor subunit. J. Biol. Chem. 1999;274(43):30799–30810. doi: 10.1074/jbc.274.43.30799. [http://dx.doi.org/10.1074/ jbc.274.43.30799]. [PMID: 10521471]. [DOI] [PubMed] [Google Scholar]
- 66.Brüss M., Barann M., Hayer-Zillgen M., Eucker T., Göthert M., Bönisch H. Modified 5-HT3A receptor function by co-expression of alternatively spliced human 5-HT3A receptor isoforms. Naunyn Schmiedebergs Arch. Pharmacol. 2000;362(4-5):392–401. doi: 10.1007/s002100000342. [http://dx.doi.org/10.1007/s002100000342]. [PMID: 11111833]. [DOI] [PubMed] [Google Scholar]
- 67.Koyama S., Matsumoto N., Kubo C., Akaike N. Presynaptic 5-HT3 receptor-mediated modulation of synaptic GABA release in the mechanically dissociated rat amygdala neurons. J. Physiol. 2000;529(Pt 2):373–383. doi: 10.1111/j.1469-7793.2000.00373.x. [http://dx.doi.org/10.1111/j.1469-7793. 2000.00373.x]. [PMID: 11101647]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chameau P., van Hooft J.A. Serotonin 5-HT(3) receptors in the central nervous system. Cell Tissue Res. 2006;326(2):573–581. doi: 10.1007/s00441-006-0255-8. [http://dx.doi.org/10.1007/s00441-006-0255-8]. [PMID: 16826372]. [DOI] [PubMed] [Google Scholar]
- 69.Asante C.O., Dickenson A.H. Descending serotonergic facilitation mediated by spinal 5-HT3 receptors engages spinal rapamycin-sensitive pathways in the rat. Neurosci. Lett. 2010;484(2):108–112. doi: 10.1016/j.neulet.2010.08.024. [http://dx.doi.org/10.1016/j.neulet.2010.08.024]. [PMID: 20709148]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alhaider A.A., Lei S.Z., Wilcox G.L. Spinal 5-HT3 receptor-mediated antinociception: possible release of GABA. J. Neurosci. 1991;11(7):1881–1888. doi: 10.1523/JNEUROSCI.11-07-01881.1991. [PMID: 2066767]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ali Z., Wu G., Kozlov A., Barasi S. The role of 5HT3 in nociceptive processing in the rat spinal cord: results from behavioural and electrophysiological studies. Neurosci. Lett. 1996;208(3):203–207. doi: 10.1016/0304-3940(95)12600-7. [http://dx.doi.org/10.1016/0304-3940(95)12600-7]. [PMID: 8733305]. [DOI] [PubMed] [Google Scholar]
- 72.Green G.M., Scarth J., Dickenson A. An excitatory role for 5-HT in spinal inflammatory nociceptive transmission; state-dependent actions via dorsal horn 5-HT(3) receptors in the anaesthetized rat. Pain. 2000;89(1):81–88. doi: 10.1016/S0304-3959(00)00346-8. [http://dx.doi.org/10.1016/S0304-3959 (00)00346-8]. [PMID: 11113296]. [DOI] [PubMed] [Google Scholar]
- 73.Moore K.A., Oh E.J., Weinreich D. 5-HT(3) receptors mediate inflammation-induced unmasking of functional tachykinin responses in vitro. J. Appl. Physiol. 2002;92(6):2529–2534. doi: 10.1152/japplphysiol.00974.2001. [http:// dx.doi.org/10.1152/japplphysiol.00974.2001]. [PMID: 12015369]. [DOI] [PubMed] [Google Scholar]
- 74.Oatway M.A., Chen Y., Weaver L.C. The 5-HT3 receptor facilitates at-level mechanical allodynia following spinal cord injury. Pain. 2004;110(1-2):259–268. doi: 10.1016/j.pain.2004.03.040. [http://dx.doi.org/10.1016/j.pain. 2004.03.040]. [PMID: 15275776]. [DOI] [PubMed] [Google Scholar]
- 75.Rahman W., Suzuki R., Rygh L.J., Dickenson A.H. Descending serotonergic facilitation mediated through rat spinal 5HT3 receptors is unaltered following carrageenan inflammation. Neurosci. Lett. 2004;361(1-3):229–231. doi: 10.1016/j.neulet.2003.12.069. [http://dx.doi.org/10.1016/j.neulet. 2003.12.069]. [PMID: 15135935]. [DOI] [PubMed] [Google Scholar]
- 76.Camilleri M., Boeckxstaens G. Dietary and pharmacological treatment of abdominal pain in IBS. Gut. 2017;66(5):966–974. doi: 10.1136/gutjnl-2016-313425. [http://dx.doi.org/10.1136/gutjnl-2016-313425]. [PMID: 28232472]. [DOI] [PubMed] [Google Scholar]
- 77.Sagalajev B., Bourbia N., Beloushko E., Wei H., Pertovaara A. Bidirectional amygdaloid control of neuropathic hypersensitivity mediated by descending serotonergic pathways acting on spinal 5-HT3 and 5-HT1A receptors. Behav. Brain Res. 2015;282:14–24. doi: 10.1016/j.bbr.2014.12.052. [http://dx.doi.org/10.1016/j.bbr.2014.12.052]. [PMID: 25557801]. [DOI] [PubMed] [Google Scholar]
- 78.Bickmeyer U., Heine M., Manzke T., Richter D.W. Differential modulation of I(h) by 5-HT receptors in mouse CA1 hippocampal neurons. Eur. J. Neurosci. 2002;16(2):209–218. doi: 10.1046/j.1460-9568.2002.02072.x. [http://dx.doi.org/ 10.1046/j.1460-9568.2002.02072.x]. [PMID: 12169103]. [DOI] [PubMed] [Google Scholar]
- 79.Belenky M.A., Pickard G.E. Subcellular distribution of 5-HT(1B) and 5-HT(7) receptors in the mouse suprachiasmatic nucleus. J. Comp. Neurol. 2001;432(3):371–388. doi: 10.1002/cne.1109. [http://dx.doi.org/10.1002/ cne.1109]. [PMID: 11246214]. [DOI] [PubMed] [Google Scholar]
- 80.Geurts F.J., De Schutter E., Timmermans J.P. Localization of 5-HT2A, 5-HT3, 5-HT5A and 5-HT7 receptor-like immunore-activity in the rat cerebellum. J. Chem. Neuroanat. 2002;24(1):65–74. doi: 10.1016/s0891-0618(02)00020-0. [http://dx.doi.org/10.1016/S0891-0618(02)00020-0]. [PMID: 12084412]. [DOI] [PubMed] [Google Scholar]
- 81.Muneoka K.T., Takigawa M. 5-Hydroxytryptamine7 (5-HT7) receptor immunoreactivity-positive ‘stigmoid body’-like structure in developing rat brains. Int. J. Dev. Neurosci. 2003;21(3):133–143. doi: 10.1016/s0736-5748(03)00029-7. [http://dx.doi.org/10.1016/S0736-5748(03)00029-7]. [PMID: 12711351]. [DOI] [PubMed] [Google Scholar]
- 82.Kvachnina E., Dumuis A., Wlodarczyk J., Renner U., Cochet M., Richter D.W., Ponimaskin E. Constitutive G s-mediated, but not G 12-mediated, activity of the 5-hydroxytryptamine 5-HT 7 (a) receptor is modulated by the palmitoylation of its C-terminal domain. Biochimica et Biophysica Acta (BBA)-. Mol. Cell Res. 2009;1793(11):1646–1655. doi: 10.1016/j.bbamcr.2009.08.008. [PMID: 19715731]. [DOI] [PubMed] [Google Scholar]
- 83.Vanhoenacker P., Haegeman G., Leysen J.E. 5-HT7 receptors: current knowledge and future prospects. Trends Pharmacol. Sci. 2000;21(2):70–77. doi: 10.1016/s0165-6147(99)01432-7. [http://dx.doi.org/10.1016/S0165-6147(99) 01432-7]. [PMID: 10664612]. [DOI] [PubMed] [Google Scholar]
- 84.Meuser T., Pietruck C., Gabriel A., Xie G.X., Lim K.J., Pierce Palmer P. 5-HT7 receptors are involved in mediating 5-HT-induced activation of rat primary afferent neurons. Life Sci. 2002;71(19):2279–2289. doi: 10.1016/s0024-3205(02)02011-8. [http://dx.doi.org/10.1016/S0024-3205(02) 02011-8]. [PMID: 12215375]. [DOI] [PubMed] [Google Scholar]
- 85.Matthys A., Haegeman G., Van Craenenbroeck K., Vanhoenacker P. Role of the 5-HT7 receptor in the central nervous system: from current status to future perspectives. Mol. Neurobiol. 2011;43(3):228–253. doi: 10.1007/s12035-011-8175-3. [http://dx.doi.org/10.1007/s12035-011-8175-3]. [PMID: 21424680]. [DOI] [PubMed] [Google Scholar]
- 86.Hoyer D., Clarke D.E., Fozard J.R., Hartig P.R., Martin G.R., Mylecharane E.J., Saxena P.R., Humphrey P.P. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol. Rev. 1994;46(2):157–203. [PMID: 7938165]. [PubMed] [Google Scholar]
- 87.Ruat M., Traiffort E., Leurs R., Tardivel-Lacombe J., Díaz J., Arrang J.M., Schwartz J.C. Molecular cloning, characterization, and localization of a high-affinity serotonin receptor (5-HT7) activating cAMP formation. Proc. Natl. Acad. Sci. USA. 1993;90(18):8547–8551. doi: 10.1073/pnas.90.18.8547. [http://dx.doi.org/10.1073/pnas.90.18.8547]. [PMID: 8397408]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Norum J.H., Hart K., Levy F.O. Ras-dependent ERK activation by the human G(s)-coupled serotonin receptors 5-HT4(b) and 5-HT7(a). J. Biol. Chem. 2003;278(5):3098–3104. doi: 10.1074/jbc.M206237200. [http://dx.doi. org/10.1074/jbc.M206237200]. [PMID: 12446729]. [DOI] [PubMed] [Google Scholar]
- 89.Errico M., Crozier R.A., Plummer M.R., Cowen D.S. 5-HT(7) receptors activate the mitogen activated protein kinase extracellular signal related kinase in cultured rat hippocampal neurons. Neuroscience. 2001;102(2):361–367. doi: 10.1016/s0306-4522(00)00460-7. [http://dx.doi.org/10.1016/S0306-4522(00)00460-7]. [PMID: 11166122]. [DOI] [PubMed] [Google Scholar]
- 90.Hedlund P.B., Danielson P.E., Thomas E.A., Slanina K., Carson M.J., Sutcliffe J.G. No hypothermic response to serotonin in 5-HT7 receptor knockout mice. Proc. Natl. Acad. Sci. USA. 2003;100(3):1375–1380. doi: 10.1073/pnas.0337340100. [http://dx.doi.org/10.1073/pnas.0337340100]. [PMID: 12529502]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rocha-González H.I., Meneses A., Carlton S.M., Granados-Soto V. Pronociceptive role of peripheral and spinal 5-HT7 receptors in the formalin test. Pain. 2005;117(1-2):182–192. doi: 10.1016/j.pain.2005.06.011. [http://dx.doi. org/10.1016/j.pain.2005.06.011]. [PMID: 16098671]. [DOI] [PubMed] [Google Scholar]
- 92.Dogrul A., Seyrek M. Systemic morphine produce antinociception mediated by spinal 5-HT7, but not 5-HT1A and 5-HT2 receptors in the spinal cord. Br. J. Pharmacol. 2006;149(5):498–505. doi: 10.1038/sj.bjp.0706854. [http://dx.doi.org/10.1038/sj.bjp.0706854]. [PMID: 16921395]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brenchat A., Romero L., García M., Pujol M., Burgueño J., Torrens A., Hamon M., Baeyens J.M., Buschmann H., Zamanillo D., Vela J.M. 5-HT7 receptor activation inhibits mechanical hypersensitivity secondary to capsaicin sensitization in mice. Pain. 2009;141(3):239–247. doi: 10.1016/j.pain.2008.11.009. [http://dx.doi.org/10.1016/ j.pain.2008.11.009]. [PMID: 19118950]. [DOI] [PubMed] [Google Scholar]
- 94.Brenchat A., Nadal X., Romero L., Ovalle S., Muro A., Sánchez-Arroyos R., Portillo-Salido E., Pujol M., Montero A., Codony X., Burgueño J., Zamanillo D., Hamon M., Maldonado R., Vela J.M. Pharmacological activation of 5-HT7 receptors reduces nerve injury-induced mechanical and thermal hypersensitivity. Pain. 2010;149(3):483–494. doi: 10.1016/j.pain.2010.03.007. [http://dx.doi.org/10.1016/j.pain. 2010.03.007]. [PMID: 20399562]. [DOI] [PubMed] [Google Scholar]
- 95.Amaya-Castellanos E., Pineda-Farías J.B., Castañeda-Corral G., Vidal-Cantú G.C., Murbartián J., Rocha-González H.I., Granados-Soto V. Blockade of 5-HT7 receptors reduces tactile allodynia in the rat. Pharmacol. Biochem. Behav. 2011;99(4):591–597. doi: 10.1016/j.pbb.2011.06.005. [http://dx.doi.org/10.1016/j.pbb.2011.06.005]. [PMID: 21693130]. [DOI] [PubMed] [Google Scholar]
- 96.Brenchat A., Ejarque M., Zamanillo D., Vela J.M., Romero L. Potentiation of morphine analgesia by adjuvant activation of 5-HT7 receptors. J. Pharmacol. Sci. 2011;116(4):388–391. doi: 10.1254/jphs.11039sc. [http://dx.doi. org/10.1254/jphs.11039SC]. [PMID: 21778664]. [DOI] [PubMed] [Google Scholar]
- 97.Brenchat A., Zamanillo D., Hamon M., Romero L., Vela J.M. Role of peripheral versus spinal 5-HT(7) receptors in the modulation of pain undersensitizing conditions. Eur. J. Pain. 2012;16(1):72–81. doi: 10.1016/j.ejpain.2011.07.004. [http://dx.doi.org/10.1016/j.ejpain.2011.07.004]. [PMID: 21843960]. [DOI] [PubMed] [Google Scholar]
- 98.Dogrul A., Seyrek M., Akgul E.O., Cayci T., Kahraman S., Bolay H. Systemic paracetamol-induced analgesic and antihyperalgesic effects through activation of descending serotonergic pathways involving spinal 5-HT7 receptors. Eur. J. Pharmacol. 2012;677(1-3):93–101. doi: 10.1016/j.ejphar.2011.12.016. [http://dx.doi.org/10.1016/j.ejphar.2011.12.016]. [PMID: 22206817]. [DOI] [PubMed] [Google Scholar]
- 99.Godínez-Chaparro B., López-Santillán F.J., Orduña P., Granados-Soto V. Secondary mechanical allodynia and hyperalgesia depend on descending facilitation mediated by spinal 5-HT4, 5-HT6 and 5-HT7 receptors. Neuroscience. 2012;222:379–391. doi: 10.1016/j.neuroscience.2012.07.008. [http://dx. doi.org/10.1016/j.neuroscience.2012.07.008]. [PMID: 22796074]. [DOI] [PubMed] [Google Scholar]
- 100.Viguier F., Michot B., Kayser V., Bernard J.F., Vela J.M., Hamon M., Bourgoin S. GABA, but not opioids, mediates the anti-hyperalgesic effects of 5-HT7 receptor activation in rats suffering from neuropathic pain. Neuropharmacology. 2012;63(6):1093–1106. doi: 10.1016/j.neuropharm.2012.07.023. [http://dx.doi.org/10.1016/j.neuropharm.2012.07.023]. [PMID: 22820553]. [DOI] [PubMed] [Google Scholar]
- 101.Yang J., Bae H.B., Ki H.G., Oh J.M., Kim W.M., Lee H.G., Choi J.I. Different role of spinal 5-HT (hydroxytryptamine) 7 receptors and descending serotonergic modulation in inflammatory pain induced in formalin and carrageenan rat models. Br. J. Anaesth. 2014;43(1):138–147. doi: 10.1093/bja/aet336. [DOI] [PubMed] [Google Scholar]
- 102.Bowker R.M., Westlund K.N., Sullivan M.C., Coulter J.D. Organization of descending serotonergic projections to the spinal cord. descending pathways to the spinal cord. Prog. Brain Res. 1982;57:239–265. doi: 10.1016/S0079-6123(08)64132-1. [http://dx.doi.org/10.1016/S0079-6123(08) 64132-1]. [DOI] [PubMed] [Google Scholar]
- 103.Belcher G., Ryall R.W., Schaffner R. The differential effects of 5-hydroxytryptamine, noradrenaline and raphe stimulation on nociceptive and non-nociceptive dorsal horn interneurones in the cat. Brain Res. 1978;151(2):307–321. doi: 10.1016/0006-8993(78)90887-9. [http://dx.doi.org/10.1016/ 0006-8993(78)90887-9]. [PMID: 679011]. [DOI] [PubMed] [Google Scholar]
- 104.Grudt T.J., Williams J.T., Travagli R.A. Inhibition by 5-hydroxytryptamine and noradrenaline in substantia gelatinosa of guinea-pig spinal trigeminal nucleus. J. Physiol. 1995;485(Pt 1):113–120. doi: 10.1113/jphysiol.1995.sp020716. [http://dx.doi.org/10.1113/jphysiol.1995.sp020716]. [PMID: 7658366]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hori Y., Endo K., Takahashi T. Long-lasting synaptic facilitation induced by serotonin in superficial dorsal horn neurones of the rat spinal cord. J. Physiol. 1996;492(Pt 3):867–876. doi: 10.1113/jphysiol.1996.sp021352. [http://dx.doi. org/10.1113/jphysiol.1996.sp021352]. [PMID: 8734996]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Travagli R.A., Williams J.T. Endogenous monoamines inhibit glutamate transmission in the spinal trigeminal nucleus of the guinea-pig. J. Physiol. 1996;491(Pt 1):177–185. doi: 10.1113/jphysiol.1996.sp021205. [http://dx.doi.org/ 10.1113/jphysiol.1996.sp021205]. [PMID: 9011609]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ito A., Kumamoto E., Takeda M., Shibata K., Sagai H., Yoshimura M. Mechanisms for ovariectomy-induced hyperalgesia and its relief by calcitonin: participation of 5-HT1A-like receptor on C-afferent terminals in substantia gelatinosa of the rat spinal cord. J. Neurosci. 2000;20(16):6302–6308. doi: 10.1523/JNEUROSCI.20-16-06302.2000. [PMID: 10934282]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lu Y., Perl E.R. Selective action of noradrenaline and serotonin on neurones of the spinal superficial dorsal horn in the rat. J. Physiol. 2007;582(Pt 1):127–136. doi: 10.1113/jphysiol.2007.131565. [http://dx.doi.org/10.1113/jphysiol.2007.131565]. [PMID: 17463043]. [DOI] [PMC free article] [PubMed] [Google Scholar]