Abstract
We have previously described a hominid-specific long non-coding RNA, MORT (also known as ZNF667-AS1, Gene ID: 100128252), which is expressed in all normal cell types, but epigenetically silenced during cancer-associated immortalization of human mammary epithelial cells. Initial analysis of The Cancer Genome Atlas (TCGA) showed that 15 of 17 cancer types, which represent the 10 most common cancers in women and men, display DNA methylation associated MORT silencing in a large fraction of their tumors. In this study we analyzed MORT expression and DNA methylation state in the remaining 16 TCGA cancer types not previously reported. Seven of the 16 cancer types showed DNA methylation linked MORT silencing in a large fraction of their tumors. These are carcinomas (cervical cancer, and cancers of esophagus, stomach, and bile duct), and the non-epithelial tumors mesothelioma, sarcoma, and uterine carcinosarcoma. Together with the findings from our previous report, MORT expression is silenced by aberrant DNA methylation in 22 of 33 of TCGA cancer types. These 22 cancers include most carcinoma types, blood derived cancers and sarcomas. In conclusion, results suggest that the MORT gene is one of the most common epigenetic aberrations seen in human cancer. Coupled with the timing of MORT gene silencing during in vitro epithelial cell immortalization and its occurrence early in the temporal arc of human carcinogenesis, this provides strong circumstantial evidence for a tumor suppressor role for MORT.
Keywords: DNA Methylation, Gene Silencing, lncRNA, ncRNA, lincRNA, MORT, ZNF667-AS1, Epigenetics
Introduction
MORT was originally found as a transcript silenced during in vitro immortalization of human mammary epithelial cells 1. Like a significant majority of lncRNAs, MORT’s molecular function remains enigmatic. The MORT gene is specific to higher primates, is expressed in all normal human cell types, and MORT RNA is located predominantly in the cytoplasm 1. Analysis of MORT expression and the DNA methylation state of its promoter in 17 cancer types from The Cancer Genome Atlas (TCGA) 2, which represent the 10 most frequent cancers in males and females, showed MORT is epigenetically silenced in 15 of 17 these cancers 1. Based on the data from the original in vitro study 1, we predicted epigenetic MORT silencing occurs early in human carcinogenesis and therefore could be seen in premalignant lesions, such as ductal carcinoma in situ of the breast and colonic adenomas. We used data from clinical samples from published genomic data sets 3– 8 to address this possibility, and indeed, MORT loss occurs prior to or at the stage of pre-malignancy and not thereafter 9. Taken together these facts suggest that MORT transcript has a tumor suppressive role and is not simply an epigenetic “passenger error.”
Since our previous analysis of MORT in TCGA datasets was not exhaustive and only reported on 17 out of 33 TCGA cancer types, the goal of this short study was to extend our earlier work and complete the analysis of MORT DNA methylation associated gene silencing in the final 16 TCGA cancer types.
Methods
We integrated the MORT expression level and the DNA methylation state of its promoter region using TCGA data as described before 1. The Illumina HiSeq RNA-seq and HumanMethylation450 DNA methylation data for samples of 16 TCGA cancer types listed in Table 1 were downloaded from the GDC data portal. The data were analyzed in the R programming environment, version 3.4.2 10. The mean RNA-Seq rpkm values for the two exons constituting the MORT RNA were plotted against the mean DNA methylation beta value of the 7 CpGs from the MORT promoter region for the individual samples of each cancer type. The Spearman correlation coefficient rho between the MORT RNA level and the DNA methylation of MORT promoter was calculated using the function cor.test.
Table 1. The 16 TCGA cancer types analyzed in this study.
The numbers of primary tumor and normal samples for which both the MORT RNA expression and the MORT promoter DNA methylation data were available are listed. *DNA methylation data from HumanMethylation27 platform that covers 2 CpGs out of 7 CpGs covered by HumanMethylation450 were used.
| TCGA Cancer Type Name | Abbreviation | Tumor
samples |
Normal
samples |
|---|---|---|---|
| adrenocortical carcinoma | ACC | 79 | 0 |
| cervical squamous cell carcinoma and
endocervical adenocarcinoma |
CESC | 304 | 3 |
| cholangiocarcinoma | CHOL | 36 | 9 |
| esophageal carcinoma | ESCA | 184 | 9 |
| glioblastoma multiforme | GBM | 51 | 1 |
| kidney chromophobe | KICH | 66 | 0 |
| brain lower grade glioma | LGG | 516 | 0 |
| mesothelioma | MESO | 87 | 0 |
| ovarian serous cystadenocarcinoma | OV * | 295 | 0 |
| pheochromocytoma and paraganglioma | PCPG | 179 | 3 |
| sarcoma | SARC | 259 | 0 |
| stomach adenocarcinoma | STAD | 373 | 0 |
| testicular germ cell tumors | TGCT | 150 | 0 |
| thymoma | THYM | 120 | 2 |
| uterine carcinosarcoma | UCS | 57 | 0 |
| uveal melanoma | UVM | 80 | 0 |
Results and discussion
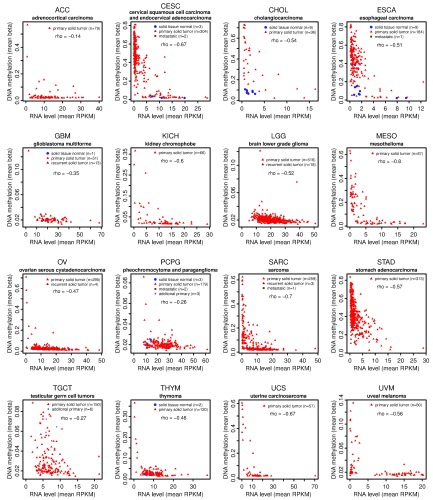
Seven of sixteen analyzed cancer types (CESC, CHOL, ESCA, MESO, SARC, STAD, and UCS) show strong MORT silencing by DNA methylation ( Figure 1). The negative correlation rho between MORT expression and DNA methylation in these cancers is below -0.5; the DNA methylation level in some tumor samples of these cancers exceeds 0.5 beta (> 50% DNA methylation), and a large fraction of the tumor samples in these cancer types have very low to no MORT expression level ( Figure 1). The correlation of MORT expression and promoter DNA methylation in the remaining nine cancer types is also negative; however, the maximum level of the DNA methylation of MORT promoter in some of these cancers is either very low (UVM), or a very few tumor samples have MORT silenced (ACC, KICH, OV, and THYM), and some of the cancer types (GBM, LGG, PCPG, and TGCT) do not appear to display MORT gene silencing ( Figure 1).
Figure 1. Integration of the MORT expression and the MORT promoter DNA methylation TCGA data for 16 tumor types.
The x-axis shows the MORT expression level according to RNA-seq and y-axis shows the level of MORT promoter DNA methylation according to Illumina HumanMethylation450 microarray. The correlation coefficient rho between the MORT expression and the DNA methylation of MORT promoter for each tumor type is displayed. The OV has a very low number (10) of samples analyzed by the HumanMethylation450 platform, therefore the data from the HumanMethylation27 platform that covers 2 CpGs out of 7 CpGs covered by HumanMethylation450 were used.
The analysis presented shows DNA methylation associated MORT gene silencing in 7 of 16 TCGA cancer types. Compared to the 17 TCGA cancer types presented in our original study, 1 most the 16 cancer types presented here lack their respective normal tissues samples and some of them have lower amounts of tumor samples ( Table 1). Nevertheless, the distribution of MORT expression and DNA methylation data in tumor samples clearly indicates MORT silencing in multiple cancer types ( Figure 1).
Cervical tumors (CESC) have high proportion of MORT silencing ( Figure 1); more than 75% of 304 cervical tumor samples have MORT promoter DNA hypermethylated and MORT silenced. Using TCGA data, a recent study found MORT downregulated in cervical cancer 11, but surprisingly did not report on or hypothesize potential mechanisms for this transcriptional repression. Here we confirm and extend their initial analysis of MORT silencing in cervical cancer and show further that this silencing is strongly linked to aberrant DNA methylation of the MORT promoter.
Combined together with the findings from our previous report 1, Table 2 shows MORT is silenced by DNA methylation in a super majority of TCGA cancer types (22 of 33). MORT loss occurs predominantly due to epigenetic silencing and increased DNA methylation of its promoter in breast cancer 9. This could likely be extended to all 22 cancer types with the high fraction of MORT negative samples and the high correlation between MORT RNA level and MORT promoter DNA methylation, where MORT likely plays a tumor suppressive role. The other 11 cancer types, with a little to no MORT silencing, might have tumor suppressive pathway, where MORT is involved, interrupted elsewhere and/or MORT may play some additional vital role in tissues these tumors originate from - e.g. prostate, thyroid, brain, testes, or ovary - since these tissues typically have the highest levels of MORT RNA 1.
Table 2. Summary of MORT silencing in all 33 TCGA cancer types.
The cancer types with MORT silencing in a large fraction of tumor samples are indicated. Results from this study are indicated (*), results from our previous report (ref 1) are indicated (**).
| Abbreviation | TCGA cancer type name |
MORT
silencing |
|---|---|---|
| ACC | adrenocortical carcinoma | No * |
| BLCA | bladder urothelial carcinoma | Yes ** |
| BRCA | breast invasive carcinoma | Yes ** |
| CESC | cervical squamous cell carcinoma
and endocervical adenocarcinoma |
Yes * |
| CHOL | cholangiocarcinoma | Yes * |
| COAD | colon adenocarcinoma | Yes ** |
| DLBC | lymphoid neoplasm diffuse large
b-cell lymphoma |
Yes ** |
| ESCA | esophageal carcinoma | Yes * |
| GBM | glioblastoma multiforme | No * |
| HNSC | head and neck squamous cell
carcinoma |
Yes ** |
| KICH | kidney chromophobe | No * |
| KIRC | kidney renal clear cell
carcinoma |
Yes ** |
| KIRP | kidney renal papillary cell carcinoma | Yes ** |
| LAML | acute myeloid leukemia | Yes ** |
| LGG | brain lower grade glioma | No * |
| LIHC | liver hepatocellular carcinoma | Yes ** |
| LUAD | lung adenocarcinoma | Yes ** |
| LUSC | lung squamous cell carcinoma | Yes ** |
| MESO | mesothelioma | Yes * |
| OV | ovarian serous
cystadenocarcinoma |
No * |
| PAAD | pancreatic adenocarcinoma | Yes ** |
| PCPG | pheochromocytoma and
paraganglioma |
No * |
| PRAD | prostate adenocarcinoma | No ** |
| READ | rectum adenocarcinoma | Yes ** |
| SARC | sarcoma | Yes * |
| SKCM | skin cutaneous melanoma | Yes ** |
| STAD | stomach adenocarcinoma | Yes * |
| TGCT | testicular germ cell tumors | No * |
| THCA | thyroid carcinoma | No ** |
| THYM | thymoma | No * |
| UCEC | uterine corpus endometrial
carcinoma |
Yes ** |
| UCS | uterine carcinosarcoma | Yes * |
| UVM | uveal melanoma | No * |
In summary, our findings show that the MORT gene is one of the most common epigenetic aberrations seen in human cancer. Coupled together with MORT silencing occurring early in the temporal arc of human carcinogenesis it strongly supports a tumor suppressive role for MORT.
Data availability
Illumina HiSeq RNA-seq and HumanMethylation450 DNA methylation data for TCGA cancer types used in the present study can be downloaded from the GDC data portal.
Acknowledgments
The results shown here are based upon data generated by the TCGA Research Network: http://cancergenome.nih.gov/.
Funding Statement
This work was supported by the Maynard Chair in Breast Cancer Epigenomics at the University of Arizona Cancer Center and the Cancer Center Support Grant (P30 CA023074).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 3 approved]
References
- 1. Vrba L, Garbe JC, Stampfer MR, et al. : A lincRNA connected to cell mortality and epigenetically-silenced in most common human cancers. Epigenetics. 2015;10(11):1074–83. 10.1080/15592294.2015.1106673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. NCI, NHGRI, NIH: The Cancer Genome Atlas. NIH, National Cancer Institute, National Human Genome Research Institute,2016. Reference Source [Google Scholar]
- 3. Abba MC, Gong T, Lu Y, et al. : A Molecular Portrait of High-Grade Ductal Carcinoma In Situ. Cancer Res. 2015;75(18):3980–90. 10.1158/0008-5472.CAN-15-0506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fleischer T, Frigessi A, Johnson KC, et al. : Genome-wide DNA methylation profiles in progression to in situ and invasive carcinoma of the breast with impact on gene transcription and prognosis. Genome Biol. 2014;15(8):435. 10.1186/PREACCEPT-2333349012841587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johnson KC, Koestler DC, Fleischer T, et al. : DNA methylation in ductal carcinoma in situ related with future development of invasive breast cancer. Clin Epigenetics. 2015;7:75. 10.1186/s13148-015-0094-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Luo Y, Wong CJ, Kaz AM, et al. : Differences in DNA methylation signatures reveal multiple pathways of progression from adenoma to colorectal cancer. Gastroenterology. 2014;147(2):418–29.e8. 10.1053/j.gastro.2014.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Qu X, Sandmann T, Frierson H, Jr, et al. : Integrated genomic analysis of colorectal cancer progression reveals activation of EGFR through demethylation of the EREG promoter. Oncogene. 2016;35(50):6403–6415. 10.1038/onc.2016.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reyngold M, Turcan S, Giri D, et al. : Remodeling of the methylation landscape in breast cancer metastasis. PLoS One. 2014;9(8):e103896. 10.1371/journal.pone.0103896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vrba L, Futscher BW: Epigenetic Silencing of MORT Is an Early Event in Cancer and Is Associated with Luminal, Receptor Positive Breast Tumor Subtypes. J Breast Cancer. 2017;20(2):198–202. 10.4048/jbc.2017.20.2.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Team RC: R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing,2017. Reference Source [Google Scholar]
- 11. Zhao LP, Li RH, Han DM, et al. : Independent prognostic Factor of low-expressed LncRNA ZNF667-AS1 for cervical cancer and inhibitory function on the proliferation of cervical cancer. Eur Rev Med Pharmacol Sci. 2017;21(23):5353–60. 10.26355/eurrev_201712_13920 [DOI] [PubMed] [Google Scholar]