Abstract
Background
Although the study of calcium (Ca2+) is classically associated with excitable cells such as myocytes or neurons, the ubiquity of this essential element in all cellular processes has led to interest in other cell types. The importance of Ca2+ to apoptosis, cell signaling, and immune activation is of special import in cancer.
Main
Here we review the current understanding of Ca2+ in each of these processes vital to the initiation, spread, and drug resistance of malignancies. We describe the involvement of Ca2+, and Ca2+ related proteins in cell cycle checkpoints and Ca2+ dependent apoptosis and discuss their roles in cellular immortalization. The role of Ca2+ in inter-cellular communication is also discussed in relevance to tumor-stromal communication, angiogenesis, and tumor microinvasion. The role that Ca2+ plays in immune surveillance and evasion is also addressed. Finally, we discuss the possibility of targeting Ca2+ singling to address the most pressing topics of cancer treatment: metastatic disease and drug resistance.
Conclusion
This review discusses the current understanding of Ca2+ in cancer. By addressing Ca2+ facilitated angiogenesis, immune evasion, metastasis, and drug resistance, we anticipate future avenues for development of Ca2+ as a nexus of therapy.
Keywords: Calcium signaling, Cancer, Immortalization, Tumor-stromal interaction, Metastasis, Drug resistance
Background
Investigational interest in calcium (Ca2+) began over 100 years ago with the discovery of the requirement for Ca2+ in the contraction of rat cardiac muscle [1]. Due to this initial discovery, Ca2+ was thoroughly characterized in ventricular action potential and other muscle cell types before the same basic principles were applied to other excitatory cells types, such as neuronal cells [2]. The importance of active zone localized Ca2+ channels to neurotransmitter release further reinforced the importance of Ca2+ in proper cell function. Today, Ca2+ is known to be an essential element vital to the health and function of every cell type. Amplification in the magnitude and duration of Ca2+ changes in the cytosol could mean the difference between cellular migration and cell death [3, 4]. Similarly, increases in mitochondrial Ca2+ can signal either increased ATP synthesis or trigger cell death [5]. This fine control of cytosolic and organelle Ca2+ levels relies on an intricate symphony between a wide variety of Ca2+ channels pumps and exchangers [2]. In this review, we provide an overview of how disruptions in Ca2+ regulation affect cancer progression, from its involvement in the immortalization of tumor cells, to its role in tumor-stromal interactions and epithelial–mesenchymal transition, and finally to current research on Ca2+ in drug resistance.
Role of intracellular Ca2+ in cell cycle and death
Given the greater than ten-fold gradient between cytosolic (~ 100 nM) and extracellular (> 1 mM) Ca2+ levels, opening of intramembrane Ca2+ channels leads to an immediate influx of Ca2+ [1]. Upon reaching the cytoplasm, Ca2+ often forms complexes with calmodulin to regulate a variety of kinases and cyclins, which regulate cell proliferation and apoptosis [6, 7]. Ca2+ regulates global cellular processes in such a way that any disturbances to Ca2+ homeostasis via alterations in expression or folding of Ca2+ channels and Ca2+ binding proteins can disrupt the cell-cycle [8]. As a result, dysregulation of intracellular Ca2+ levels can affect the ability of cells to regulate progression through the cell-cycle and lead to unchecked proliferation and tumorigenesis [9], two of the ten hallmarks of cancer (Fig. 1).
Fig. 1.
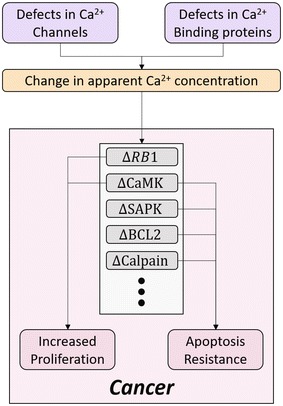
Ca2+ and associated protein involvement in cellular proliferation. The affect of Ca2+ concentration on key cellular proteins are diagrammed
In the normal cell, progression from G1 to S-phase is accomplished via phosphorylation and subsequent inactivation of the tumor suppressor, RetinoBlastoma protein 1 (RB1), as illustrated in Fig. 2 [10]. Endogenous RB1 inactivation or deletion removes this check on the cell cycle and allows affected cells to undergo unchecked DNA synthesis, leading to an accumulation of potentially oncogenic DNA damage. Normally, cytosolic Ca2+ levels modulate the activity of guanosine exchange factor (GEF), a Ras stimulator, and GTPase activating protein (GAP), a Ras inhibitor. When activated, Ras stimulates the proliferative mitogen-activated protein kinase (MAPK) pathway, which results in upregulation of cyclin D1 in the cytoplasm, with ultimate phosphorylation of RB1 and release of the E2F transcription factor which initiates the cells transition into S-phase (Fig. 2). This connection between calcium and RB1 indicates that increased cytosolic Ca2+ levels can lead to constitutive activation of the MAPK pathway, causing removal of the G1-S transition check point. Ca2+ is also involved in signaling entry into G1, as well as the transition from G2 to M, although the mechanisms of its involvement at these check points are not well understood [11].
Fig. 2.
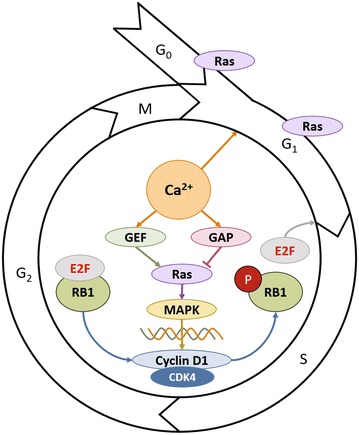
Schematic of cell cycle, and the influence of calcium in G1/S transitioning via the MAPK pathway. Note that Ras, a protein under control of cytosolic calcium levels, also regulates the G0/G1 transition, and is important throughout G1 phase
Other cell cycle related families, like the Ca2+/calmodulin-dependent protein kinases (CaMKs) are also known to facilitate proliferation and avoid death by promoting passage through the cell cycle and resisting apoptotic mechanisms [12]. CaMK levels have been shown to vary in lymphoma, ovarian cancer, and hepatocellular carcinoma, among others [13–15].
Changes in Ca2+ conduction and levels can lead to apoptosis evasion and immortalization
In normal tissue, large, sustained changes to cytosolic Ca2+ can initiate cell death. Ca2+ flux from the endoplasmic reticulum (ER) to the mitochondria can also result in increased mitochondrial sensitivity to apoptotic stimuli. Chronic Ca2+ depletion is also known to cause ER stress and activation of stress activated protein kinases (SAPKs), which leads to apoptosis [11]. Finally, high cytosolic levels of Ca2+ can lead to cell death by activating calpain, a cysteine protease that specifically lyses BCL2, an anti-apoptotic regulatory protein [16, 17]. Alterations in Ca2+ levels can help cancer cells evade the first of these pathways by interrupting the transfer of Ca2+ from the ER to the mitochondria. Specifically, Ca2+-permeable Inositol 1,4,5-triphosphate receptor (IP3R) channels that facilitate this pro-apoptotic flux of Ca2+ from the ER could be prevented form activating. This process is aided by the anti-apoptotic capabilities of BCL-2, which diminishes Ca2+ flux by binding IP3Rs or decreasing Ca2+ levels in the ER lumen [18, 19]. Certain cancer types are also known to regulate cytosolic Ca2+ to their advantage by bleeding off excess Ca2+ to create pro survival conditions. This is evident in breast cancer, where over-expression of plasma membrane calcium-ATPase 2 (PMCA2) allows for the release of Ca2+ in conditions of Ca2+ overload [20]. Potential therapeutics blocking BCL2 activation, promoting stability of the ER-mitochondrial linkage, or blocking the PMCA2 “emergency-release valve” could induce Ca2+-triggered apoptosis in tumor cells.
Tissue pressure, hypoxia and H+ can elicit Ca2+ changes
The cancer microenvironment consists of two interactive components: neoplastic cells and stroma [21]. The tumor stroma is a complex environment consisting of a non-cellular extracellular matrix (ECM), and fibroblasts, epithelial, endothelial, and immune cells [22]. This stroma is responsible for providing the nutrients, O2, and signaling molecules necessary to support tumor growth. In pancreatic adenocarcinoma, transient receptor potential cation channel 1 and 6 (TRPC1 and TRPC6) are activated by elevated pressure and hypoxia, respectively. This process also leads to Ca2+ entry and subsequent pro-angiogenic signaling cascade [23, 24]. In hepatocellular cancer cells, hypoxia also activates an ER Ca2+ sensor, stromal interaction molecule 1 (STIM1), which mediates activation of store-operated Ca2+ entry (SOCE) and leads to upregulation of hypoxia-inducible factor 1 (HIF-1) expression [25, 26]. HIF-1 then promotes release of growth factors (GFs) such as angiopoietin 2, placental GF, and stromal-derived factor 1 to promote angiogenesis [27]. In breast cancer, acid-sensing ion channel 1 (ASIC1) mediates Ca2+ influx. This pathway promotes tumor progression by forming reactive oxidative species and nuclear factor kB (NF-kB). Silencing ASIC1 has been shown to reduce tumor growth and metastasis in xenograft models [28]. Similarly, in pancreatic cancer cells, ASIC1 and ASIC3 mediate acidity-induced Ca2+ influx to promote epithelial–mesenchymal transition. Indeed, knockdown of ASIC1 and ASIC3 has been confirmed to suppress liver and lung metastasis in xenograft models.
Ca2+-dependent tumor-stromal signaling drives angiogenesis
Communication between tumor and stromal cells has been shown to maintain growth and expansion through Ca2+-dependent signaling [29]. Vascular endothelial growth factor (VEGF) released by tumor cells triggers signal transduction that facilitates Ca2+-activated proliferation in endothelial cells. Upon VEGF receptor 2 activation, phosphoinositide phospholipase C (PLCγ) is phosphorylated, which in turn hydrolyzes phospholipid phosphatidylinositol (4,5)-bisphosphate (PIP2), resulting in accumulation of diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). Accumulation of IP3 results in increase of intracellular Ca2+ and activation of the proliferative MAPK pathway [30, 31]. Proliferation in numerous subtypes of breast and gastrointestinal carcinomas, and glioblastomas is dependent upon this process [32–34]. Similarly, basic fibroblast growth factor (BFGF) activates the transient receptor potential cation channel subfamily V member 4 (TRPV4) in endothelial cells to facilitate Ca2+ influx, leading to endothelial cell proliferation, migration and angiogenesis [35, 36].
Ca2+-dependent signaling may promote or hinder tumor escape of immune surveillance
Ca2+ dependent signaling is critical in the functioning of tumor-associated macrophages (TAMs), which have the ability to both sustain tumor growth and exert anti-tumor effects under certain conditions [37]. TAMs induce tumor progression through chemokine ligand 18 (CCL18) production. In breast cancer, CCL18 binds to phosphatidylinositol transfer protein membrane-associated 3 (PITPNM3) at the plasma membrane and induces phosphorylation of PLCγ1 and protein kinase C zeta (PKCζ). This cascade increases levels of inositol 1,4,5-triphosphate 3-kinase isoform B (IP3KB), which are mediators in the Ca2+ signaling pathway. Indeed, the expression of CCL18 in blood or cancer stroma is associated with metastasis and reduced survival [38]. On the other hand, when T cell receptors (TCRs) on cytotoxic T lymphocytes binds to MHC-antigen receptors on a malignant cell, the resultant immune synapse triggers Ca2+ influx in the immune cell, leading to lytic granule release and tumor killing. TCR stimulation can also evoke Ca2+ release from the ER via signaling cascade involving Zeta-chain-associated protein kinase 70 (ZAP-70), lymphocyte-specific protein tyrosine kinase (Lck), linker of activation of T cells (LAT), PLC-γ, and IP3 [39, 40]. Similarly, Ca2+ entry through Orai1 channels is required for release of lytic granules and subsequent tumor cell destruction by natural killer cells [41]. Lastly, recent experiments with chimeric antigen receptor T (CAR T) cells, which have faster release-rates from dying tumor cells than T cell receptor (TCR) T cells do, have implied there is no difference in intensity of Ca2+ flux between the two cell types; therefore both trigger the release of tumor-killing particles at the same threshold level of Ca2+ [42]. Interactions between various components of stroma and tumor are shown in Fig. 3.
Fig. 3.
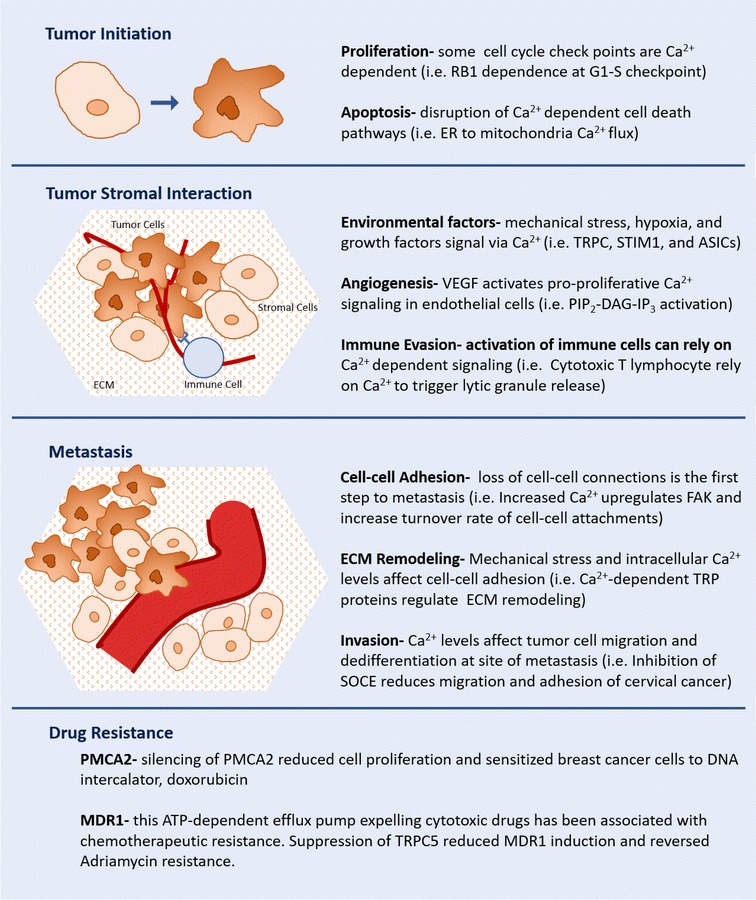
Ca2+ signaling in tumor progression. The involvement of Ca2+ in every step of tumor development, metastasis, and current knowledge on Ca2+ facilitated drug resistance
Developing areas in tumor stromal Ca2+-dependent signaling
Recent findings on transient receptor potential cation channel subfamily A member 1 (TRPA1) and secreted protein acidic and rich in cysteine (SPARC) point to areas in need of further exploration. In prostate cancer stromal cells, TRPA1 has been shown to act as a mechanosensor and have the ability to bind to Triclosan, an antibacterial agent [43]. This binding increases Ca2+ in stromal cells to trigger subsequent secretion of mitogenic factors, which lead to proliferation and/or migration of adjacent epithelial and endothelial cells to promote angiogenesis [21]. However, the specific stromal ligand activating this function has yet to be discovered. SPARC, a multifunctional, matricellular Ca2+ binding protein, overexpressed in glioblastoma and thyroid, esophageal, hepatocellular, and pancreatic carcinomas, has been clinically correlated with tumor progression [44–47]. SPARC contains an N-terminal low-affinity Ca2+-binding domain and a C-terminal high-affinity Ca2+-binding domain [48]. This protein plays a crucial role in cell rounding and focal adhesion disassembly during angiogenesis, tumor invasion, and metastasis [49]. While the prevalence of Ca2+ binding domains in this protein hint at a role in SPARC function, the exact pathway through which a Ca2+-SPARC complex elicits tumor advancement remains largely unknown [50]. The continued mystery surrounding the mechanism of Ca2+ associated TRPA1 and SPARC function identifies the needs to continued investigation into Ca2+-dependent signaling in the tumor stroma.
Impact of Ca2+ signaling on the epithelial–mesenchymal transition
The first step in metastasis is the loss of cell–cell connections. Focal adhesion kinase (FAK) is a ubiquitously expressed cytoplasmic tyrosine kinase that increases turnover of cell–cell contacts [51]. Overexpression of FAK is commonly associated with cancer, and seems to induce resistance to anoikis, death due to loss of attachment to a basement membrane. Increased intracellular Ca2+ upregulates FAK at focal adhesions through phosphorylation by the calmodulin-dependent protein kinase II (CaMKII) [52]. Thus, aberrant signaling resulting in elevated intracellular Ca2+ levels can lead to an increase in FAK and a higher turnover rate for cell–cell attachments [53]. Calcineurin, a protein regulated by Ca2+, recycles integrins in migrating cells and is another potential mediator of Ca2+-induced migration [54]. Except for this dysregulation of Ca2+, there are currently no other known differences between normal and malignant cells capable of migration [55].
Mechanical stress and intracellular Ca2+ levels affect cell–cell adhesion through TRP family proteins [56]. In addition to the above described role of TRP in cellular proliferation, TRP also plays a role in the epithelial–mesenchymal transition. High TRP levels are associated with the loss of cell adhesion, while TRP loss is associated with increased strength and number of focal adhesions [57]. Higher expression of TRP family member TRPV1 has been associated with increased migration in many different cancer cell lines [58, 59]. TRPV2 has also been shown to be an important regulator of matrix metalloproteases MMP2 and MMP9, which are required for the extensive ECM remodeling necessary for successful metastasis [60]. ECM remodeling enzymes are substantially upregulated or specifically induced in many cancers [61]. In addition, many ECM proteins themselves are controlled by calcium levels in the cell. From the glycoprotein fibrinogen which has multiple calcium binding sites critical for structure and function to fibrillin, which has several calcium binding epidermal growth factor domains to the thrombospondins which have multiple calcium binding repeats, calcium is a crucial player in normal physiology of the extracellular matrix. The overall effect of Ca2+ on ECM maintenance and remodeling remains an unanswered question, and an active area of research.
The epithelial–mesenchymal transition (EMT) is also associated with an increased capacity for invasion. This invasive capability has been connected to Ca2+ signaling in some cell types [62]. Davis et al. [63] have shown that when EMT is induced, there is an increase in cytosolic Ca2+ levels in human breast cancer cells. Chelating Ca2+ in this instance reduced epidermal growth factor levels and blocked the induction of EMT markers. Another important contributor to proliferative ability is the SOCE system, through which Ca2+ is pumped into the cytosol when ER Ca2+ is depleted. SOCE inhibitors have been shown to inhibit migration of cervical cancer and reduce the association of focal adhesion kinases at focal adhesion sites [62].
Extracellular Ca2+ levels have also demonstrated an effect on the re-differentiation of epithelial breast cancer lines. Re-differentiation after metastasis, is important in allowing cancer to survive in a novel niche after metastasis. Although physiological levels of Ca2+ inhibit proliferation and invasion, higher than normal extracellular levels increase estrogen receptor activity, which has been associated with more aggressive and invasive breast cancers [64]. High extracellular Ca2+ levels ultimately increase the risk of bony metastasis in both breast and prostate cancer [65].
Targeting Ca2+ as a treatment modality for metastatic disease
Tumor metastases cause the majority of cancer deaths. As such, development of preventative measures against and treatment of metastasis is an extremely active area of research. Metastatic transformation requires the loss of epithelial cell–cell connections and the transformation of primary tumor cells into a migratory mesenchymal cell. During this process, the cells must also degrade the ECM, cross basement membranes, and enter the circulatory system. As detailed above, Ca2+ signaling is involved in every step of this process [66–68]. Therapeutically, targeting Ca2+ signaling to prevent metastasis is challenging, as any inhibition is likely to impact normal cells as well. Coupling Ca2+ to a cancer specific target has been shown to reduce normal cell death in a prostate cancer study [69]. For example, a drug combining Thapsigargin, a sarcolemma and ER Ca2+-ATPase (SERCA) inhibitor, with the targeting peptide for a prostate-specific antigen was able to limit cell death to prostate cancer cells while sparing normal cells [70]. Despite such technological advances, Ca2+-dependent migration mechanisms between normal and cancerous cells are similar enough that another mode of targeting Ca2+ should be considered [71]. As we have learned from “undruggable” proteins like Ras and Myc, targeting downstream effectors of Ca2+-dependent signaling, such as proteins associated with cell–cell contacts and ECM degradation, may be a more practical approach [72].
Alterations in Ca2+ signaling in settings of drug resistance
In addition to being implicated in the described processes of tumor progression, Ca2+ might also play a significant role in facilitating drug resistance. In a recent study on breast cancer cell lines, increased mRNA levels of plasmalemmal Ca2+ efflux pump (PMCA2), which removes Ca2+ from the cell, was correlated with poor survival [73]. Silencing of PMCA2 reduced cell proliferation and sensitized these cells to doxorubicin. Elevated PMCA2 is commonly found in the mammary glands of lactating mice and may thus indicate high cellular metabolic activity, which is also frequently found in malignant cells. High levels of PMCA2 have also been confirmed in a variety of breast cancer cell lines. Another study confirmed the relationship between high PMCA2 expression and poor outcome, and demonstrated the ability of PMCA2 suppression to sensitize mammary epithelial cells to apoptosis [74].
P-glycoprotein or multidrug resistance protein 1 (MDR1), an ATP-dependent efflux pump that expels cytotoxic drugs, has also been associated with chemotherapeutic resistance in breast cancer [75]. Induction of this protein has been associated with upregulation of the Ca2+-permeable channel TRPC5 in adriamycin-resistant breast cancer cell lines. In both human and mice models, TRPC5 expression is often higher in tumor cells and concentrated to vesicles. Indeed, in the adriamycin-resistant breast cancer study, suppressing the activity of pro-oncotic TRPC5 reduced MDR1 induction and reversed adriamycin resistance both in vitro and in vivo [73]. TRPC5 suppression also appears to be essential to drug resistance in colorectal cancer, where suppression of TRPC5 expression reduced MDR1 induction, leading to 5-FU resistance via the canonical Wnt/β-catenin signal pathway.
A subtype of TRPC6 has also been implicated in another malignancy infamously recalcitrant to multiple chemotherapeutic regimens, hepatocellular carcinoma (HCC). A recent study has shown that a subtype of TRPC6, usually expressed at low levels in normal hepatocytes, mediates Ca2+ signaling and drug resistance in HCC. In this study, inhibition of Ca2+ signaling via TRPC6 inhibition resulted in restored sensitivity of HCC cells to various chemotherapeutic drugs and attenuation of the epithelial–mesenchymal transition [76]. These in vitro studies were further corroborated in xenograft models where TRPC6 inhibition enhanced doxorubicin efficacy. The same study also identified the STAT3 pathway as the mechanism of action for TRPC6/Ca2+ mediated drug sensitivity. Namely, reduction of intracellular Ca2+ via TRPC6 inhibition activates STAT3, which then stimulates re-differentiation of cells and restores drug sensitivity [77]. T-type Ca2+ channels have also been associated with drug resistance in ovarian and other high-morbidity gynecological malignancies. Experiments on mice models of ovarian cancer have shown mibefradil inhibition of T-type Ca2+ channels to sensitize the disease to carboplatin. Furthermore, both pharmaceutical and genetic inhibition of Ca2+ channels led to apoptotic growth suppression in the ovarian cancer cells [78].
Drug resistance, especially the development of multi-drug resistant disease, is of special concern in cancer therapy. The fact that Ca2+-mediated signaling can restore drug sensitivity in breast, colorectal, hepatocellular, and ovarian cancers suggests a possible role for Ca2+ channel blockers as an adjuvant therapy to standard-of-care chemotherapies.
Conclusions
From tumor initiation to metastasis and drug resistance, Ca2+ signaling is intrinsic to all aspects of cancer biology (Fig. 3). Ironically, the very ubiquity of Ca2+ signaling in cancer makes this essential element difficult to explore in detail and target for drug development. While multiple studies have shown the importance of Ca2+ signaling at every key disease turning point (immortalization, metastasis, and drug response), isolation of the specific effects remains elusive. This suggests development of therapies targeting Ca2+ should be constructed using experience from other “undruggable” targets like Ras and Myc. Instead of targeting Ca2+ itself, known Ca2+ associated proteins such as PMCA2, TRPC5, and MDR1 may serve as more discerning objectives.
Another emerging field of interest for Ca2+ signaling is immunotherapy. Recent publications have suggested that calcium signaling could be used to improve the efficiency of immunotherapy approaches by enhancing antigen presentation and in the adaptive immune response. In addition, the role of Ca2+ in killing by natural killer cells and cytotoxic T lymphocytes may also be exploited as high levels of intracellular Ca2+ are required for efficient cancer cell killing activity. Conversely, Ca2+ reduction has been showing to reduce growth of malignant cells themselves. Thus, it is necessary to identify the specific Ca2+ channels utilized in granule exocytosis so that immune system’s ability to kill malignant cells can be enhanced without simultaneously promoting tumor growth. Although immunotherapy is a promising field through which Ca2+ signaling could augment treatment efficacy, the ubiquity of Ca2+ in normal metabolism and cellular function makes greater understanding of specific mechanisms in Ca2+ signaling necessary before such dreams become attainable.
Authors’ contributions
MX, AS, MK, KSYJ, and HNB equally contributed to the writing of the manuscript. AS created Figs. 1 and 2. MMX compiled and edited the manuscript and created Fig. 3. All authors read and approved the final manuscript.
Acknowledgements
No applicable.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Not applicable.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ASIC1
acid-sensing ion channel 1
- BFGF
basic fibroblast growth factor
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- CaMK
Ca2+/calmodulin-dependent protein kinases
- CCL18
chemokine ligand 18
- DAG
diacylglycerol
- ECM
extracellular matrix
- EMT
epithelial–mesenchymal transition
- ER
endoplasmic reticulum
- FAK
focal adhesion kinase
- HIF-1
hypoxia-inducible factor 1
- IP3
inositol 1,4,5-trisphosphate
- IP3KB
inositol 1,4,5-triphosphate 3-kinase isoform B
- IP3R
1,4,5-triphosphate receptor
- LAT
linker of activation of T cells
- Lck
lymphocyte-specific protein tyrosine kinase
- MAPK
mitogen-activated protein kinase
- MDR1
p-glycoprotein or multidrug resistance protein 1
- NF-kB
nuclear factor kB
- PIP2
phospholipid phosphatidylinositol (4,5)-bisphosphate
- PITPNM3
phosphatidylinositol transfer protein membrane-associated 3
- PKCζ
protein kinase C zeta
- PLCγ
phosphoinositide phospholipase C
- PMCA2
plasma membrane calcium-ATPase 2, plasmalemmal Ca2+ efflux pump
- RB1
retinoblastoma protein 1
- SAPK
stress activated protein kinases
- SERCA
sarcolemma and ER Ca2+-ATPase
- SOCE
store-operated Ca2+ entry
- SPARC
secreted protein acidic and rich in cysteine
- STIM1
stromal interaction molecule 1
- TAMs
tumor-associated macrophages
- TCRs
T cell receptors
- TRPA1
transient receptor potential cation channel subfamily A member 1
- TRPC
transient receptor potential cation channel
- TRPV4
transient receptor potential cation channel subfamily V member 4
- VEGF
vascular endothelial growth factor
- ZAP-70
zeta-chain-associated protein kinase 70
Footnotes
MengMeng Xu, Andreas Seas, Musa Kiyani, Keven S. Y. Ji and Hannah N. Bell contributed equally to this work
Contributor Information
MengMeng Xu, Email: mengmeng.xu@duke.edu.
Andreas Seas, Email: andreas.seas@duke.edu.
Musa Kiyani, Email: musa.kiyani@duke.edu.
Keven S. Y. Ji, Email: seung.yong.ji@duke.edu
Hannah N. Bell, Email: hannah.bell@duke.edu
References
- 1.Carafoli E. Calcium signaling: a tale for all seasons. Proc Natl Acad Sci USA. 2002;99:1115–1122. doi: 10.1073/pnas.032427999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 3.Zhivotovsky B, Orrenius S. Calcium and cell death mechanisms: a perspective from the cell death community. Cell Calcium. 2011;50:211–221. doi: 10.1016/j.ceca.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Wei C, Wang X, Chen M, Ouyang K, Song LS, Cheng H. Calcium flickers steer cell migration. Nature. 2009;457:901–905. doi: 10.1038/nature07577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Benedetto G, Scalzotto E, Mongillo M, Pozzan T. Mitochondrial Ca(2)(+) uptake induces cyclic AMP generation in the matrix and modulates organelle ATP levels. Cell Metab. 2013;17:965–975. doi: 10.1016/j.cmet.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Humeau J, Bravo-San Pedro JM, Vitale I, Nunez L, Villalobos C, Kroemer G, et al. Calcium signaling and cell cycle: progression or death. Cell Calcium. 2017;70:3–15. doi: 10.1016/j.ceca.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Kahl CR, Means AR. Calcineurin regulates cyclin D1 accumulation in growth-stimulated fibroblasts. Mol Biol Cell. 2004;15:1833–1842. doi: 10.1091/mbc.E03-10-0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prevarskaya N, Ouadid-Ahidouch H, Skryma R, Shuba Y. Remodelling of Ca2+ transport in cancer: how it contributes to cancer hallmarks? Philos Trans R Soc Lond B Biol Sci. 2014;369:20130097. doi: 10.1098/rstb.2013.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Cook SJ, Lockyer PJ. Recent advances in Ca(2+)-dependent Ras regulation and cell proliferation. Cell Calcium. 2006;39:101–112. doi: 10.1016/j.ceca.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 11.Roderick HL, Cook SJ. Ca2+ signalling checkpoints in cancer: remodelling Ca2+ for cancer cell proliferation and survival. Nat Rev Cancer. 2008;8:361–375. doi: 10.1038/nrc2374. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez-Mora O, LaHair MM, Howe CJ, McCubrey JA, Franklin RA. Calcium/calmodulin-dependent protein kinases as potential targets in cancer therapy. Expert Opin Ther Targets. 2005;9:791–808. doi: 10.1517/14728222.9.4.791. [DOI] [PubMed] [Google Scholar]
- 13.Gu Y, Zhang J, Ma X, Kim BW, Wang H, Li J, et al. Stabilization of the c-Myc protein by CAMKIIgamma promotes T cell lymphoma. Cancer Cell. 2017;32(115–128):e117. doi: 10.1016/j.ccell.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kritsch D, Hoffmann F, Steinbach D, Jansen L, Mary Photini S, Gajda M, et al. Tribbles 2 mediates cisplatin sensitivity and DNA damage response in epithelial ovarian cancer. Int J Cancer. 2017;141:1600–1614. doi: 10.1002/ijc.30860. [DOI] [PubMed] [Google Scholar]
- 15.Marcelo KL, Means AR, York B. The Ca(2+)/calmodulin/CaMKK2 axis: nature’s metabolic CaMshaft. Trends Endocrinol Metab. 2016;27:706–718. doi: 10.1016/j.tem.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu TT, Peters AA, Tan PT, Roberts-Thomson SJ, Monteith GR. Consequences of activating the calcium-permeable ion channel TRPV1 in breast cancer cells with regulated TRPV1 expression. Cell Calcium. 2014;56:59–67. doi: 10.1016/j.ceca.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Akbulut Y, Gaunt HJ, Muraki K, Ludlow MJ, Amer MS, et al. (−)-Englerin A is a potent and selective activator of TRPC4 and TRPC5 calcium channels. Angew Chem Int Ed. 2017;54:3787–3791. doi: 10.1002/anie.201411511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foyouzi-Youssefi R, Arnaudeau S, Borner C, Kelley WL, Tschopp J, Lew DP, et al. Bcl-2 decreases the free Ca2+ concentration within the endoplasmic reticulum. Proc Natl Acad Sci USA. 2000;97:5723–5728. doi: 10.1073/pnas.97.11.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen R, Valencia I, Zhong F, McColl KS, Roderick HL, Bootman MD, et al. Bcl-2 functionally interacts with inositol 1,4,5-trisphosphate receptors to regulate calcium release from the ER in response to inositol 1,4,5-trisphosphate. J Cell Biol. 2004;166:193–203. doi: 10.1083/jcb.200309146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.VanHouten J, Sullivan C, Bazinet C, Ryoo T, Camp R, Rimm DL, et al. PMCA2 regulates apoptosis during mammary gland involution and predicts outcome in breast cancer. Proc Natl Acad Sci USA. 2010;107:11405–11410. doi: 10.1073/pnas.0911186107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Derouiche S, Mariot P, Warnier M, Vancauwenberghe E, Bidaux G, Gosset P, et al. Activation of TRPA1 channel by antibacterial agent triclosan induces VEGF secretion in human prostate cancer stromal cells. Cancer Prev Res (Phila) 2017;10:177–187. doi: 10.1158/1940-6207.CAPR-16-0257. [DOI] [PubMed] [Google Scholar]
- 22.Li H, Fan X, Houghton J. Tumor microenvironment: the role of the tumor stroma in cancer. J Cell Biochem. 2007;101:805–815. doi: 10.1002/jcb.21159. [DOI] [PubMed] [Google Scholar]
- 23.Dietrich A, Gudermann T. TRPC6: physiological function and pathophysiological relevance. Handb Exp Pharmacol. 2014;222:157–188. doi: 10.1007/978-3-642-54215-2_7. [DOI] [PubMed] [Google Scholar]
- 24.Nielsen N, Kondratska K, Ruck T, Hild B, Kovalenko I, Schimmelpfennig S, et al. TRPC6 channels modulate the response of pancreatic stellate cells to hypoxia. Pflugers Arch. 2017;469:1567–1577. doi: 10.1007/s00424-017-2057-0. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Guo B, Xie Q, Ye D, Zhang D, Zhu Y, et al. STIM1 mediates hypoxia-driven hepatocarcinogenesis via interaction with HIF-1. Cell Rep. 2015;12:388–395. doi: 10.1016/j.celrep.2015.06.033. [DOI] [PubMed] [Google Scholar]
- 26.Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Semenza GL. Cancer-stromal cell interactions mediated by hypoxia-inducible factors promote angiogenesis, lymphangiogenesis, and metastasis. Oncogene. 2013;32:4057–4063. doi: 10.1038/onc.2012.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta SC, Singh R, Asters M, Liu J, Zhang X, Pabbidi MR, et al. Regulation of breast tumorigenesis through acid sensors. Oncogene. 2016;35:4102–4111. doi: 10.1038/onc.2015.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mueller MM, Fusenig NE. Friends or foes—bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 30.Wang S, Li X, Parra M, Verdin E, Bassel-Duby R, Olson EN. Control of endothelial cell proliferation and migration by VEGF signaling to histone deacetylase 7. Proc Natl Acad Sci USA. 2008;105:7738–7743. doi: 10.1073/pnas.0802857105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keefe SM, Cohen MA, Brose MS. Targeting vascular endothelial growth factor receptor in thyroid cancer: the intracellular and extracellular implications. Clin Cancer Res. 2010;16:778–783. doi: 10.1158/1078-0432.CCR-08-2743. [DOI] [PubMed] [Google Scholar]
- 32.Brown LF, Berse B, Jackman RW, Tognazzi K, Manseau EJ, Senger DR, et al. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in adenocarcinomas of the gastrointestinal tract. Cancer Res. 1993;53:4727–4735. [PubMed] [Google Scholar]
- 33.Brown LF, Berse B, Jackman RW, Tognazzi K, Guidi AJ, Dvorak HF, et al. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in breast cancer. Hum Pathol. 1995;26:86–91. doi: 10.1016/0046-8177(95)90119-1. [DOI] [PubMed] [Google Scholar]
- 34.Plate KH, Breier G, Weich HA, Mennel HD, Risau W. Vascular endothelial growth factor and glioma angiogenesis: coordinate induction of VEGF receptors, distribution of VEGF protein and possible in vivo regulatory mechanisms. Int J Cancer. 1994;59:520–529. doi: 10.1002/ijc.2910590415. [DOI] [PubMed] [Google Scholar]
- 35.Antoniotti S, Fiorio Pla A, Pregnolato S, Mottola A, Lovisolo D, Munaron L. Control of endothelial cell proliferation by calcium influx and arachidonic acid metabolism: a pharmacological approach. J Cell Physiol. 2003;197:370–378. doi: 10.1002/jcp.10359. [DOI] [PubMed] [Google Scholar]
- 36.Liedtke W, Kim C. Functionality of the TRPV subfamily of TRP ion channels: add mechano-TRP and osmo-TRP to the lexicon! Cell Mol Life Sci. 2005;62:2985–3001. doi: 10.1007/s00018-005-5181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Costa da Silva M, Breckwoldt MO, Vinchi F, Correia MP, Stojanovic A, et al. Iron induces anti-tumor activity in tumor-associated macrophages. Front Immunol. 2017;8:1479. doi: 10.3389/fimmu.2017.01479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen J, Yao Y, Gong C, Yu F, Su S, Chen J, et al. CCL18 from tumor-associated macrophages promotes breast cancer metastasis via PITPNM3. Cancer Cell. 2011;19:541–555. doi: 10.1016/j.ccr.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hou X, Pedi L, Diver MM, Long SB. Crystal structure of the calcium release-activated calcium channel Orai. Science. 2012;338:1308–1313. doi: 10.1126/science.1228757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwarz EC, Qu B, Hoth M. Calcium, cancer and killing: the role of calcium in killing cancer cells by cytotoxic T lymphocytes and natural killer cells. Biochim Biophys Acta. 2013;1833:1603–1611. doi: 10.1016/j.bbamcr.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 41.Zhou X, Friedmann KS, Lyrmann H, Zhou Y, Schoppmeyer R, et al. A calcium optimum for cytotoxic T lymphocyte and natural killer cell cytotoxicity. J Physiol. 2018 doi: 10.1113/JP274964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davenport AJ, Cross RS, Watson KA, Liao Y, Shi W, et al. Chimeric antigen receptor T cells form nonclassical and potent immune synapses driving rapid cytotoxicity. Proc Natl Acad Sci. 2018;15:E2068–E2076. doi: 10.1073/pnas.1716266115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen J, Hackos DH. TRPA1 as a drug target–promise and challenges. Naunyn Schmiedebergs Arch Pharmacol. 2015;388:451–463. doi: 10.1007/s00210-015-1088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Infante JR, Matsubayashi H, Sato N, Tonascia J, Klein AP, Riall TA, et al. Peritumoral fibroblast SPARC expression and patient outcome with resectable pancreatic adenocarcinoma. J Clin Oncol. 2007;25:319–325. doi: 10.1200/JCO.2006.07.8824. [DOI] [PubMed] [Google Scholar]
- 45.Lau CP, Poon RT, Cheung ST, Yu WC, Fan ST. SPARC and Hevin expression correlate with tumour angiogenesis in hepatocellular carcinoma. J Pathol. 2006;210:459–468. doi: 10.1002/path.2068. [DOI] [PubMed] [Google Scholar]
- 46.Sage EH, Bornstein P. Extracellular proteins that modulate cell-matrix interactions. SPARC, tenascin, and thrombospondin. J Biol Chem. 1991;266:14831–14834. [PubMed] [Google Scholar]
- 47.Takano T, Hasegawa Y, Miyauchi A, Matsuzuka F, Yoshida H, Kuma K, et al. Quantitative analysis of osteonectin mRNA in thyroid carcinomas. Endocr J. 2002;49:511–516. doi: 10.1507/endocrj.49.511. [DOI] [PubMed] [Google Scholar]
- 48.Kaleagasioglu F, Berger MR. SIBLINGs and SPARC families: their emerging roles in pancreatic cancer. World J Gastroenterol. 2014;20:14747–14759. doi: 10.3748/wjg.v20.i40.14747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Framson PE, Sage EH. SPARC and tumor growth: where the seed meets the soil? J Cell Biochem. 2004;92:679–690. doi: 10.1002/jcb.20091. [DOI] [PubMed] [Google Scholar]
- 50.Pottgiesser J, Maurer P, Mayer U, Nischt R, Mann K, Timpl R, et al. Changes in calcium and collagen IV binding caused by mutations in the EF hand and other domains of extracellular matrix protein BM-40 (SPARC, osteonectin) J Mol Biol. 1994;238:563–574. doi: 10.1006/jmbi.1994.1315. [DOI] [PubMed] [Google Scholar]
- 51.Bouchard V, Demers MJ, Thibodeau S, Laquerre V, Fujita N, et al. Fak/Src signaling in human intestinal epithelial cell survival and anoikis: differentiation state-specific uncoupling with the PI3-K/Akt-1 and MEK/Erk pathways. J Cell Physiol. 2007;212:717–728. doi: 10.1002/jcp.21096. [DOI] [PubMed] [Google Scholar]
- 52.Giannone G, Ronde P, Gaire M, Beaudouin J, Haiech J, Ellenberg J, et al. Calcium rises locally trigger focal adhesion disassembly and enhance residency of focal adhesion kinase at focal adhesions. J Biol Chem. 2004;279:28715–28723. doi: 10.1074/jbc.M404054200. [DOI] [PubMed] [Google Scholar]
- 53.Tai YL, Chen LC, Shen TL. Emerging roles of focal adhesion kinase in cancer. Biomed Res Int. 2015;2015:690690. doi: 10.1155/2015/690690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lawson MA, Maxfield FR. Ca(2+)- and calcineurin-dependent recycling of an integrin to the front of migrating neutrophils. Nature. 1995;377:75–79. doi: 10.1038/377075a0. [DOI] [PubMed] [Google Scholar]
- 55.Prevarskaya N, Skryma R, Shuba Y. Calcium in tumour metastasis: new roles for known actors. Nat Rev Cancer. 2011;11:609–618. doi: 10.1038/nrc3105. [DOI] [PubMed] [Google Scholar]
- 56.Lee J, Ishihara A, Oxford G, Johnson B, Jacobson K. Regulation of cell movement is mediated by stretch-activated calcium channels. Nature. 1999;400:382–386. doi: 10.1038/22578. [DOI] [PubMed] [Google Scholar]
- 57.Su LT, Agapito MA, Li M, Simonson WT, Huttenlocher A, Habas R, et al. TRPM7 regulates cell adhesion by controlling the calcium-dependent protease calpain. J Biol Chem. 2006;281:11260–11270. doi: 10.1074/jbc.M512885200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Monet M, Lehen’kyi V, Gackiere F, Firlej V, Vandenberghe M, Roudbaraki M, et al. Role of cationic channel TRPV2 in promoting prostate cancer migration and progression to androgen resistance. Cancer Res. 2010;70:1225–1235. doi: 10.1158/0008-5472.CAN-09-2205. [DOI] [PubMed] [Google Scholar]
- 59.Waning J, Vriens J, Owsianik G, Stuwe L, Mally S, Fabian A, et al. A novel function of capsaicin-sensitive TRPV1 channels: involvement in cell migration. Cell Calcium. 2007;42:17–25. doi: 10.1016/j.ceca.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 60.Monet M, Gkika D, Lehen’kyi V, Pourtier A, Vanden Abeele F, Bidaux G, et al. Lysophospholipids stimulate prostate cancer cell migration via TRPV2 channel activation. Biochim Biophys Acta. 2009;1793:528–539. doi: 10.1016/j.bbamcr.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 61.Jiang M, Qiu J, Zhang L, Lu D, Long M, Chen L, et al. Changes in tension regulates proliferation and migration of fibroblasts by remodeling expression of ECM proteins. Exp Ther Med. 2016;12:1542–1550. doi: 10.3892/etm.2016.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.White C. The regulation of tumor cell invasion and metastasis by endoplasmic reticulum-to-mitochondrial Ca2+ transfer. Front Oncol. 2017;7:171. doi: 10.3389/fonc.2017.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Davis FM, Azimi I, Faville RA, Peters AA, Jalink K, Putney JW, Jr, et al. Induction of epithelial–mesenchymal transition (EMT) in breast cancer cells is calcium signal dependent. Oncogene. 2014;33:2307–2316. doi: 10.1038/onc.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Journe F, Dumon JC, Kheddoumi N, Fox J, Laios I, Leclercq G, et al. Extracellular calcium downregulates estrogen receptor alpha and increases its transcriptional activity through calcium-sensing receptor in breast cancer cells. Bone. 2004;35:479–488. doi: 10.1016/j.bone.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 65.Insua-Rodriguez J, Oskarsson T. The extracellular matrix in breast cancer. Adv Drug Deliv Rev. 2016;97:41–55. doi: 10.1016/j.addr.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 66.Chen YF, Chiu WT, Chen YT, Lin PY, Huang HJ, Chou CY, et al. Calcium store sensor stromal-interaction molecule 1-dependent signaling plays an important role in cervical cancer growth, migration, and angiogenesis. Proc Natl Acad Sci USA. 2011;108:15225–15230. doi: 10.1073/pnas.1103315108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Deliot N, Constantin B. Plasma membrane calcium channels in cancer: alterations and consequences for cell proliferation and migration. Biochim Biophys Acta. 2015;1848:2512–2522. doi: 10.1016/j.bbamem.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 68.Monteith GR, Prevarskaya N, Roberts-Thomson SJ. The calcium-cancer signalling nexus. Nat Rev Cancer. 2017;17:367–380. doi: 10.1038/nrc.2017.18. [DOI] [PubMed] [Google Scholar]
- 69.Cui C, Merritt R, Fu L, Pan Z. Targeting calcium signaling in cancer therapy. Acta Pharm Sin B. 2017;7:3–17. doi: 10.1016/j.apsb.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Denmeade SR, Isaacs JT. The SERCA pump as a therapeutic target: making a “smart bomb” for prostate cancer. Cancer Biol Ther. 2005;4:14–22. doi: 10.4161/cbt.4.1.1505. [DOI] [PubMed] [Google Scholar]
- 71.Chen YF, Chen YT, Chiu WT, Shen MR. Remodeling of calcium signaling in tumor progression. J Biomed Sci. 2013;20:23. doi: 10.1186/1423-0127-20-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen YT, Chen YF, Chiu WT, Wang YK, Chang HC, Shen MR. The ER Ca(2)(+) sensor STIM1 regulates actomyosin contractility of migratory cells. J Cell Sci. 2013;126:1260–1267. doi: 10.1242/jcs.121129. [DOI] [PubMed] [Google Scholar]
- 73.Peters AA, Milevskiy MJ, Lee WC, Curry MC, Smart CE, Saunus JM, et al. The calcium pump plasma membrane Ca2+-ATPase 2 (PMCA2) regulates breast cancer cell proliferation and sensitivity to doxorubicin. Sci Rep. 2016;6:25505. doi: 10.1038/srep25505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.VanHouten J, Sullivan C, Bazinet C, Ryoo T, Camp R, Rimm DL, et al. PMCA2 regulates apoptosis during mammary gland involution and predicts outcome in breast cancer. Proc Natl Acad Sci. 2010;107:11405–11410. doi: 10.1073/pnas.0911186107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13:714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 76.El Boustany C, Bidaux G, Enfissi A, Delcourt P, Prevarskaya N, Capiod T. Capacitative calcium entry and transient receptor potential canonical 6 expression control human hepatoma cell proliferation. Hepatology. 2008;47:2068–2077. doi: 10.1002/hep.22263. [DOI] [PubMed] [Google Scholar]
- 77.Courapied S, Sellier H, de Carné Trécesson S, Vigneron A, Bernard AC, Gamelin E, et al. The cdk5 kinase regulates the STAT3 transcription factor to prevent DNA damage upon topoisomerase I inhibition. J Biol Chem. 2010;285:26765–26778. doi: 10.1074/jbc.M109.092304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dziegielewska B, Casarez EV, Yang WZ, Gray LS, Dziegielewski J, Slack-Davis JK. T-type Ca2+ channel inhibition sensitizes ovarian cancer to carboplatin. Mol Cancer Ther. 2016;15:460–470. doi: 10.1158/1535-7163.MCT-15-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


