Abstract
Background
Vector control is a crucial element of anti-malaria campaigns and works best when there is a thorough knowledge of the biology and behaviour of the Anopheles vector species responsible for transmitting malaria within a given locale. With the push to eradicate malaria stronger than ever, there is a growing need to develop and deploy control strategies that exploit the behavioural attributes of local vector species. This is especially true in regions where the vectors are exophagic (i.e., prefer to bite outdoors), exophilic (i.e., prefer to remain outdoors), and zoophagic (i.e., as likely to feed on non-humans as humans). One promising strategy targeting vectors with these behavioural traits is the administration of avermectin-based endectocides, such as ivermectin, to humans and livestock. When ingested in a blood meal, ivermectin has been shown to reduce mosquito survivorship and fecundity in a number of Anopheles species. In this study, the relative toxicity of ivermectin was compared between two zoophagic, exophilic malaria vectors—Anopheles albimanus and Anopheles stephensi.
Results
Toxicity of ivermectin was assessed using membrane feedings, intrathoracic injections, and mosquito feedings on treated mice. When ingested in a blood meal, ivermectin was much less toxic to An. albimanus (4-day oral LC50 = 1468 ng/ml) than to An. stephensi (4-day oral LC50 = 7 ng/ml). However when injected into the haemocoel of An. albimanus, ivermectin was much more toxic (3-day parenteral LC50 = 188 ng/ml). Because the molecular targets of ivermectin (i.e., glutamate-gated chloride channels) reside outside the midgut in nerves and muscles, this suggests that ingested ivermectin was not readily absorbed across the midgut of An. albimanus. In contrast, ivermectin was considerably more toxic to An. stephensi when ingested (4-day oral LC50 = 7 ng/ml) than when injected (3-day parenteral LC50 = 49 ng/ml). This suggests that metabolic by-products from the digestion of ivermectin may play a role in the oral toxicity of ivermectin to An. stephensi. Blood meal digestion and subsequent oviposition rates were significantly hindered in both species by ingested ivermectin but only at concentrations at or above their respective oral LC50 concentrations. To test mosquitocidal activity of ivermectin in a live host system, two groups of three mice each received subcutaneous injections of either ivermectin (600 µg/kg BW) or saline (control). One day after injection, the ivermectin-treated mice (n = 3) exhibited significant mosquitocidal activity against both An. stephensi (85% mortality vs 0% in control-fed) and, to a lesser degree, An. albimanus (44% mortality vs 11% in control-fed). At 3 days, the mosquitocidal activity of ivermectin-treated mice waned and was effective only against An. stephensi (31% mortality vs 3% in control-fed).
Conclusions
Ivermectin was not uniformly toxic to both Anopheles species. Previous studies indicate that ivermectin is a good choice of endectocide to use against malaria vectors in southeast Asia and Africa. However, these data suggest that ivermectin may not be the optimal endectocide to use in Central America or the Caribbean where An. albimanus is a major malaria vector species. If endectocides are to be used to help eradicate malaria, then additional efficacy data will be needed to define the activity of specific endectocides against the major malaria vector species of the world.
Keywords: Anopheles albimanus, Anopheles stephensi, Endectocide, Ivermectin, Vector control
Background
Malaria remains a major public health problem throughout the tropics [1] and is transmitted by Anopheles mosquitoes. Vector control is an essential element of malaria control programmes. Throughout sub-Saharan Africa, indoor residual spraying (IRS) and insecticide treated bed-nets (ITN) have been successful vector control tactics because the primary vector species, Anopheles gambiae feed almost exclusively on people sleeping in their houses at night and rest inside the house after blood-feeding [2–4]. Other important malaria vector species in Africa (e.g., Anopheles arabiensis), Asia (e.g., Anopheles stephensi) and the neotropics (e.g., Anopheles albimanus) are exophagic (i.e., prefer to bite outdoors), exophilic (i.e., prefer to rest outdoors), and/or zoophagic (i.e., as likely to feed on non-humans as humans) [5]. Effective control of Anopheles species with these behavioural characteristics requires alternative tactics. The primary non-human blood sources for zoophagic malaria vectors are peridomestic livestock (cattle, goats). Livestock represent mosquito blood sources that humans can manage and control. It is logical that any comprehensive strategy against zoophagic malaria vectors should include some sort of livestock management component. One tactic to control zoophagic malaria vectors is to treat livestock with ivermectin.
Ivermectin is a lipophilic drug that belongs to the avermectin class of macrocyclic lactone compounds. Ivermectin acts as an endectocide (i.e., kills both endoparasites and ectoparasites). Ivermectin binds to and activates the glutamate-gated chloride channels of nerve and muscle cells of a wide variety of nematode and arthropod species, causing uncontrolled influx of chloride ions into the cells and leading to paralysis and death of the organism [6]. Ivermectin has a high safety profile in humans and livestock [7] and is one of the most commonly-used anti-helminthic drug in the livestock industry to control intestinal nematodes.
The large-scale administration of oral ivermectin to people has become a cornerstone in the global eradication campaign against human onchocerciasis and lymphatic filariasis [8, 9]. The recommended dose of ivermectin used to treat humans for filarial infection [i.e., 150 mg/kg body weight (BW)] yields peak plasma concentrations of ca. 40–45 ng/ml [10]. At these plasma concentrations, ivermectin can significantly reduce the survival of Anopheles mosquitoes that ingest treated blood. Anopheles species shown to be susceptible to ivermectin at these concentrations include major vectors from Africa (An. gambiae [11–13], An. arabiensis [14]), Southeast Asia (Anopheles campestris, Anopheles dirus, Anopheles minimus, Anopheles swandwongporni [15]) and Latin America (Anopheles aquasalis [16], Anopheles darlingi [17]). Pilot field trials in western Africa indicate that the mass drug administration of ivermectin against onchocerciasis and lymphatic filariasis can simultaneously reduce survival of An. gambiae and local malaria transmission [18, 19].
Similarly, ivermectin treatment of livestock could theoretically reduce vector abundance and lower malaria transmission [20, 21]. At the same time, treatment of herds may also reduce tick and intestinal worm burdens, leading to increases in livestock weight gain, milk production, and ultimately increases in the health and economic prosperity of livestock owners. However, it is currently unknown if all species of Anopheles vectors are equally susceptible to ivermectin. This study compared the relative toxicity of ivermectin to two known malaria vectors: Anopheles (Nyssorhynchus) albimanus, a major vector along the coastal regions of Mexico, Central America, the Caribbean, and northern South America [22], and Anopheles (Cellia) stephensi, an important malaria vector in southern and western Asia [23]. Both species are highly zoophagic, exophilic and exophagic, making them appropriate species to target with a strategy involving ivermectin treatment of livestock.
Methods
Mosquitoes
Laboratory colonies of An. albimanus STECL strain and An. stephensi STE2 strain were obtained as eggs through BEI Resources (Manassas, VA USA). Mosquitoes were reared in the University of North Dakota insectary at a photoperiod of 12-h light:12-h dark and a temperature of 26 °C. Eggs were hatched in trays of dechlorinated water. Larvae were fed fish food (Tetra Pond Sticks, Tetra, Blacksburg, VA USA) ground to a fine powder in an electric coffee grinder. Pupae were placed into ca. 28 l wire mesh cages to emerge. Adults were given access to water and a sugar source. Female mosquitoes 3–7 days old for use in toxicity studies were aspirated from rearing cages into smaller (ca. 0.5 l), cylindrical cardboard containers with mesh tops at a density of 15–40 mosquitoes per cage.
Membrane feeding
For each membrane feeding trial, age-matched cohorts of An. albimanus and An. stephensi were blood fed simultaneously on identical ivermectin preparations. For each feeding trial, stock solutions were prepared fresh by diluting powdered technical grade ivermectin (Sigma-Aldrich, St. Louis MO, USA) into dimethyl sulfoxide (DMSO) at a concentration of 2 mg/ml. The stock solution was diluted to starting concentrations in phosphate-buffered saline (PBS). From that, serial dilutions were made in 1.5 ml polypropylene microfuge tubes containing whole bovine blood with sodium heparin (10 U/ml, Pel-Freez Biologicals, Rodgers AR, USA) for a total volume of 1 ml. A control group (i.e., 10 µl PBS added to 990 µl blood) was included with each trial. Prior to feeding, the tubes were inverted a minimum of three times to mix the solutions and kept in warm water. Natural sausage casing (Dewied International, San Antonio TX, USA) was rinsed thoroughly to remove salt preservative and cut to fit across the bottom of glass membrane feeders. Feeders were connected to one another with rubber tubing and heated water (ca. 37 °C) was circulated to warm the feeders. Membrane feeders were placed one per cage, and the blood-ivermectin mixtures were pipetted into the feeders. Mosquitoes were allowed to feed for ca. 30 min. Unfed mosquitoes were removed. Engorged mosquitoes were maintained in the insectary and provided with cotton soaked in a 10% sucrose solution. Cages were checked every day. Dead mosquitoes were counted and removed. For most trials, the number of surviving mosquitoes was counted at 4 days. In one trial, survival counts were extended to 5 days to determine if mortality had stabilized. By day 5, the rate of mortality stabilized and became asymptotic. Thus, 4 days was selected as an appropriate time on which to base LC50 determinations for oral toxicity. After preliminary trials to determine an appropriate range of concentrations, feedings were replicated five times using 4–5 ivermectin concentrations, plus a control, for each feeding trial.
Intrathoracic injection
Mosquitoes were immobilized by chilling (− 20 °C for ca. 20 s), then quickly transferred to filter paper onto a chill table (BioQuip, Rancho Dominguez, CA, USA). To perform injections, a semi-automated micro-injector (TriTech Research, Los Angeles CA, USA) was fitted with fine-tipped glass needles made with a micropipette puller (World Precision, Sarasota FL, USA). A stock solution was prepared as described above and from that, serial dilutions were made in 1.5 ml polypropylene microfuge tubes containing an insect salt solution [24]. Mosquitoes were injected with 0.4 µl of solution into the thorax, near the base of the wing. Mosquitoes were then placed into appropriately labeled recovery containers. Injected mosquitoes were provided 10% sucrose solution and checked daily for 3 days. Dead mosquitoes were counted and removed over the course of 3 days, at which time the surviving mosquitoes were counted. In one trial, survival counts were extended to 4 days to determine if mortality had stabilized. By day 4, the rate of mortality stabilized and became asymptotic. Thus, 3 days was selected as an appropriate time on which to base LC50 determinations for parenteral toxicity. After preliminary trials to determine an appropriate range of concentrations, injections were replicated three times for An. stephensi and four times for An. albimanus, using 4–5 ivermectin concentrations plus a control at each injection trial.
Fecundity
Mosquitoes were fed with membrane feeders on blood containing various doses of ivermectin, as described previously. Anopheles stephensi were given a single experimental blood meal and monitored for egg production. However during colony rearing of An. albimanus, it was noted that this species required more than one blood feeding in order to produce sufficient eggs to maintain the colony. To accurately assess the effect of ivermectin on An. albimanus fecundity, it was necessary that control-fed females undergo full gonotrophic development. Therefore, An. albimanus were first given a normal blood meal (i.e., cow blood with no drugs). Unfed mosquitoes were removed and engorged An. albimanus were held for 2 days without oviposition sites. The mosquitoes were then offered a second, experimental blood meal. Unfed mosquitoes were removed. One day after experimental blood meals, fully engorged mosquitoes were immobilized by chilling and each mosquito was placed into an individual 30 ml glass vial with screened top and single strip of filter paper on which to rest. Raisins were placed on top of each vial as a sucrose source. After mosquitoes fully recovered and could fly, 2 ml of aged tap water was introduced into the vial for mosquitoes to lay their eggs. Vials were maintained at 26 °C for 5 days, after which vials were filled with ethanol to kill the parent mosquito and the eggs and hatchlings. Eggs and hatchling larvae were counted the same day. Midguts and ovaries from the parent mosquitoes were dissected to quantify blood digestion and ovarian development, based on the Sella scale [25]. For simplicity, Sella stages were combined into two groups: early stages (Sella stages II, III, IV) indicating that minimal vitellogenesis had occurred, and late stages (Sella stages V, VI, VII) indicating that complete or nearly complete vitellogenesis had occurred.
Feeding on treated mice
Ivermectin stock solution (2000 µg/ml DMSO) was diluted in saline to a working concentration of 80 µg/ml. Three outbred, white mice were weighed and subcutaneously injected with an appropriate volume of ivermectin solution to achieve a dose of 600 µg/kg BW. Three control mice were injected subcutaneously with equivalent volumes of saline. The next day (= 24 h after treatment), each mouse was anesthetized (pentobarbital 60 µg/gm BW, ip) and individually placed onto a screened-top container containing 20–30 An. albimanus mosquitoes. Mosquitoes were allowed to feed for ca. 20 min, after which anesthetized mice were immediately transferred to screen-top containers containing 20–30 An. stephensi mosquitoes. Mosquitoes were allowed ca. 30 min to feed. Afterwards, mice were returned to the vivarium. Unfed mosquitoes were removed from the cages. Engorged mosquitoes were provided with moistened cotton and a sucrose source (chopped prune), and maintained at 26 °C. Four days after blood feeding, surviving mosquitoes from each mouse were subdivided into aliquots of 3–6 mosquitoes each and gently introduced into new containers containing a cup of water for oviposition. Three days after mice had received injections, a fresh batch of An. albimanus and An. stephensi mosquitoes were fed on the mice and the process described above was repeated. Dead mosquitoes were removed and counted daily for 6 days, after which the number of mosquitoes left alive and total number of eggs laid were counted.
Data analysis
Mosquito mortalities observed within experimental groups were adjusted for any mortality that occurred within corresponding control groups using Abbott’s formula [26]. Only experimental trials having control mortalities less than 20% were used for further data analyses. Log-probit analyses were conducted on the corrected percent moralities to estimate LC50 values (Minitab Inc., State College PA, USA). Mosquito survivorship was analysed with a Kaplan–Meier survival analysis and Log-rank Mantel-Cox test (GraphPad Software, La Jolla, CA, USA). Mosquito fecundities (i.e., average number of eggs laid per female) were compared among treatments using one-way analysis of variance (ANOVA) on log10 transformed egg counts. If an ANOVA showed a significant effect due to treatment, the Tukey Honest Significant Differences (Tukey HSD) post hoc test was used to separate statistical differences between groups. Rates of mosquito oviposition and egg hatch, and blood digestion (based on early Sella scores) in treatment groups were compared with their corresponding controls with Chi square analyses (Statistix, Tallahassee FL, USA). A 0.05 level of significance was used throughout.
Results
Membrane feeding and intrathoracic inoculations
A total of 573 An. stephensi and 582 An. albimanus were used to determine the relative oral toxicities of ingested ivermectin. A total of 606 An. stephensi and 293 An. albimanus were used to determine the relative parenteral toxicities of inoculated ivermectin. When ingested, ivermectin was much more toxic to An. stephensi (oral 4-day LC50 = 7 ng/ml) than to co-fed An. albimanus (oral 4-day LC50 = 1468 ng/ml; Table 1). Similarly, intrathoracially-inoculated ivermectin was more toxic to An. stephensi (parenteral 3-day LC50 = 49 ng/ml) than to An. albimanus (parenteral 3-day LC50 = 188 ng/ml; Table 1). Lethality of ingested ivermectin did not occur immediately. Instead, mosquito mortality progressed over a period of several days after ingestion of treated blood and treated mosquitoes of both species experienced significant reduction in survivorship over a 4-day period (Fig. 1). Significant reductions of mosquito survival occurred with An. stephensi at all doses tested (Fig. 1a) whereas with An. albimanus, significant reductions occurred only at the highest doses tested (Fig. 1b). Mosquito mortality occurred more rapidly when ivermectin was inoculated versus when the drug was ingested (Fig. 2).
Table 1.
Comparative toxicities of ivermectin to Anopheles mosquitoes when ingested (oral LC50) or injected (parenteral LC50)
| Species | Oral LC50 (95% CL) | Parenteral 3-day LC50 (95% CL) | Citation |
|---|---|---|---|
| An. arabiensis | 7.9 (6.2, 9.9) at 9 days post-feed | – | [14] |
| An. stephensi | 7.0 (5.2, 8.6) at 4 days post-feed | 48.8 (42.6, 56.9) | present study |
| An. minimus | 16.3 (11.6, 19.4) at 7 days post-feed | – | [15] |
| An. gambiae s.l. | 19.8 (± 2.8) at 9 days post-feed | – | [11] |
| An. gambiae s.s. | 22.4 (18.0, 26.9) at 5 days post-feed | – | [12] |
| An. campestris | 26.4 (21.9, 30.5) at 7 days post-feed | – | [15] |
| An. sawadwongporni | 26.9 (24.8, 28.8) at 7 days post-feed | – | [15] |
| An. dirus | 55.6 (52.3, 59.1) at 7 days post-feed | – | [15] |
| An. aquasalis | 47.0 (44.7, 49.4) at 5 days post-feed | – | [16] |
| An. albimanus | 1468.0 (1153.5, 1965.5) at 4 days post-feed | 187.9 (136.2, 239.0) | present study |
| An. darlingi | 43.2 (37.5, 48.6) at 7 days post-feed | – | [17] |
LC50 values are expressed as ng/ml. Italic text indicates data resulting from the present study
Fig. 1.
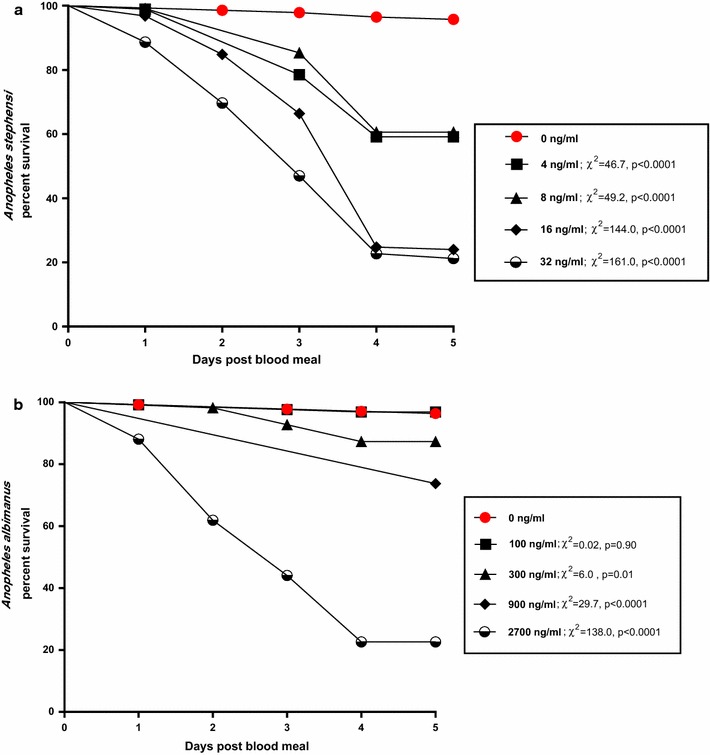
Daily proportion of surviving mosquitoes after ingesting ivermectin at various concentrations. a Anopheles stephensi, b Anopheles albimanus. Survival curves for ivermectin-exposed mosquitoes were compared statistically to the survival curves of control mosquitoes using Log-rank Mantel-Cox tests. Test values and p values at each ivermectin concentration are shown
Fig. 2.
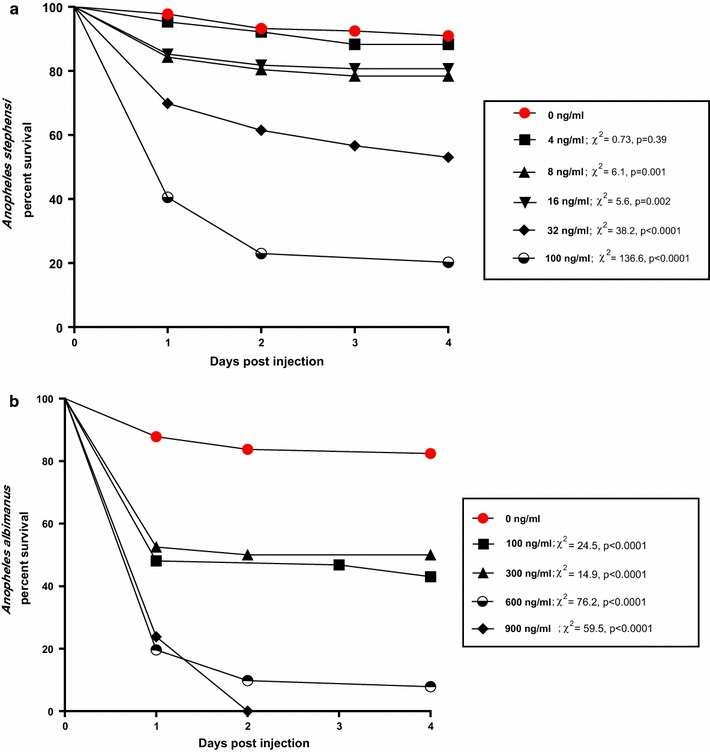
Daily proportion of surviving mosquitoes after intrathoracic inoculation of ivermectin at various concentrations. a Anopheles stephensi, b Anopheles albimanus. Survival curves for ivermectin-exposed mosquitoes were compared statistically to the survival curves of control mosquitoes using Log-rank Mantel-Cox tests. Test values and p values at each ivermectin concentration are shown
Fecundity
The oviposition rate for control-fed An. stephensi after ingesting one blood meal (82%, n = 38) was similar to that of control-fed An. albimanus after ingesting two blood meals (84%, n = 62) (Table 2). Significant reductions in oviposition rates occurred in An. stephensi (33%, n = 6, cχ2 = 4.1, p = 0.04) and An. albimanus (61%, n = 51, cχ2 = 6.5, p = 0.01) that ingested ivermectin at 32 and 1300 ng/ml respectively (Chi square tests, cχ2 ≥ 4.1, p < 0.05) but not for mosquitoes that ingested lower concentrations of ivermectin (Table 2, Chi square tests, p > 0.68).
Table 2.
Reproductive capacity of Anopheles mosquitoes ingesting various concentrations of ivermectin via membrane feeder
| Mosquito species | Conc (ng/ml) | Total no. surviving females | Oviposition rate of survivors (%) | Geometric mean no. of eggs laid per ovipositing female (95% CI) | Overall hatch rate (%) | Theoretical number of larvae produced per 1000 surviving females |
|---|---|---|---|---|---|---|
| An. stephensi | 0 | 38 | 82A | 50.1 (34.9, 71.9)A | 73.3A | 30,113 |
| 4 | 14 | 71A | 41.6 (22.0, 78.5)A | 70.1A | 20,705 | |
| 8 | 12 | 83A | 29.8 (13.1, 46.7)A | 56.1B | 13,876 | |
| 16 | 12 | 83A | 24.7 (15.8, 56.2)A | 76.0A | 15,581 | |
| 32 | 6 | 33B | 10.4 (2.5, 43.0)A | 52.4A | 1798 | |
| An. albimanus | 0 | 62 | 84A | 50.0 (41.0, 60.0)A | 47.9A | 20,118 |
| 300 | 53 | 87A | 49.2 (41.2, 60.6)A | 46.2A | 19,776 | |
| 1300 | 51 | 61B | 39.2 (30.9, 50.0)A | 18.4B | 4400 |
Anopheles stephensi mosquitoes were blood fed one time on either treated or untreated blood. Anopheles albimanus mosquitoes were blood fed twice; once on untreated blood and again, 2 days later, on either treated or untreated blood. Engorged mosquitoes were held individually in oviposition vials. Superscripts indicate statistically significant differences for parameters within a species
There were no differences in the average number of eggs laid per ovipositing female between control-fed and ivermectin-fed mosquitoes at any of the dosages tested for both An. stephensi (F = 2.05 df = 4, 58; p = 0.10) and An. albimanus (F = 1.48, df = 2, 126; p = 0.23) (Table 2). However, the egg hatching rates for An. stephensi that ingested 8 ng/ml ivermectin (56%, n = 12, cχ2 = 43.1, p < 0.0001) and An. albimanus that ingested 1300 ng/ml ivermectin (18%, n = 51, cχ2 = 389.1, p < 0.0001) were significantly less than egg hatch rates of the corresponding control-fed groups (73 and 48%, respectively) (Table 2). The egg hatch rate for An. stephensi that ingested 32 ng/ml ivermectin (52%) was lower than that of control-fed mosquitoes (73%), but the difference was not quite statistically significant (cχ2 = 3.62, p = 0.057). This was likely due to the excessive adult mortality that occurred at this dose and the small number of surviving females (n = 6) left to oviposit (Table 2). By multiplying oviposition rates, fecundity and hatch rates for each treatment group (Table 2, last column), notable reductions in F1 larva production occurred only at doses that exceeded the oral LC50 values for each species (see Table 1). Five days after blood feeding, the majority (≥ 90%) of control-fed mosquitoes in both species had either laid their eggs, or were fully gravid in late Sella stages. However with increasing dosages of ivermectin, the proportion of mosquitoes that laid eggs by day 5 diminished (Fig. 3). With exception of An. albimanus fed at 100 ng/ml concentration, significantly greater proportions of ivermectin-fed mosquitoes retained undigested blood in their guts and underdeveloped ovaries (early Sella stages), compared to midguts and ovaries observed in control-fed mosquitoes (Fisher’s exact test, p values ≤ 0.007, Fig. 3).
Fig. 3.
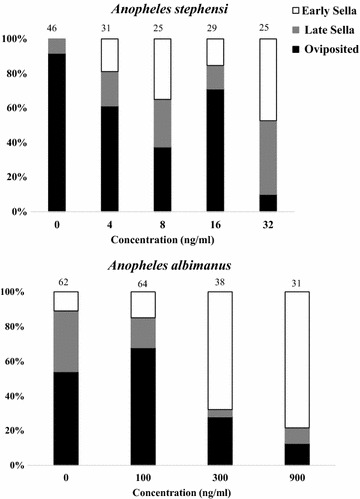
Proportions of Anopheles stephensi and Anopheles albimanus that completed vitellogensis and successfully oviposited (= Oviposited), underwent extensive vitellogenesis but did not oviposit (= late Sella), or underwent minimal vitellogenesis and minimal blood meal digestion (= early Sella). Mosquitoes were dissected 5 days after ingesting various concentrations of ivermectin via membrane feeding. Numbers above histograms indicate numbers of mosquitoes examined
Feeding on treated mice
One day after mice received injections, there were significant reductions in the 6-day survival rate of An. stephensi (15%) and An. albimanus (56%) fed on ivermectin-injected mice compared to mosquitoes fed on saline-injected control mice (100 and 89%, respectively, Chi square tests with Yate’s correction, cχ2 > 11, p < 0.001; Table 3). Average fecundity in An. stephensi fed on ivermectin-treated mice (0.9 ± 1.6 eggs/female) was significantly less than mosquitoes fed on control mice (46.1 ± 25.9 eggs/female) (t test, T = 8.2, df = 13, p < 0.0001). No significant reduction in fecundity was observed for An. albimanus fed on ivermectin-treated mice. Three days after mice were treated with ivermectin, there remained a significant, albeit smaller, reduction in the 6-day survival of An. stephensi fed on ivermectin-treated mice (62%) versus that of An. stephensi fed on control mice (96%) (Chi square tests with Yate’s correction, value = 16, p < 0.001; Table 3). No significant difference was observed in the 6-day survival of An. albimanus fed on mice 3 days after treatment. By 3 days after mouse injections, there were no significant differences in mosquito fecundity for either species fed on ivermectin-treated versus control mice (Table 3).
Table 3.
Survival and fecundity of Anopheles mosquitoes at 6 days after feeding on ivermectin treated versus untreated mice
| Species | Treatment | 1 day after mouse injections | 3 days after mouse injections | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Percent surviving (n) | Eggs per female (± SD) | Percent surviving (n) | Eggs per female (± SD) | ||||||
| An. stephensi | Ivermectin | 15% (52) | cχ2 = 76.5 p < 0.001 |
0.9 ± 1.6 | T = 8.2 df = 13 p < 0.001 |
62% (66) | cχ2 = 15.7 p < 0.001 |
30.8 ± 8.7 | T = 1.0 df = 4 p = 0.373 |
| Control | 100% (56) | 46.1 ± 25.9 | 96% (48) | 44.7 ± 18.8 | |||||
| An. albimanus | Ivermectin | 56% (56) | cχ2 = 11.3 p < 0.001 |
4.7 ± 8.1 | T = 1.5 df = 17 p = 0.143 |
95% (57) | cχ2 = 0.9 p = 0.332 |
25.2 ± 7.2 | T = 0.2 df = 4 p = 0.864 |
| Control | 89% (52) | 12.8 ± 13.2 | 85% (48) | 24.8 ± 10.0 | |||||
Mice received subcutaneous injections of either ivermectin (600 mg/kg BW) or saline (control group). Three mice were used for each treatment. Cohorts of age-matched mosquitoes (n = sample size) were blood-fed 1 day after injections and 3 days after injections
Discussion
Ivermectin ingested in a blood meal has been shown to be extremely potent against at least eight different anopheline species that have been tested to-date [11–17]. This study found ingested ivermectin to be similarly potent against An. stephensi (Table 1). However, An. albimanus is the most ivermectin-tolerant Anopheles species evaluated to-date, with an oral LC50 over 25-fold greater than other species tested. The relative insensitivity of An. albimanus to ingested ivermectin was evident regardless of whether ivermectin was administered in cow blood via membrane feeders or via mosquito feedings on treated mice (Table 3).
To investigate the mechanism(s) of this insensitivity, serial dilutions of ivermectin were injected directly into the haemocoels of An. albimanus and An. stephensi and the resulting parenteral LC50 values were compared with the oral LC50 values derived from membrane feeding trials (Table 1). The rationale for injecting ivermectin is that the target molecules for ivermectin (i.e., glutamate-gated chloride channels) reside within nervous and muscle tissue outside of the midgut [27]. Injection bypasses the midgut. When injected directly into the haemocoel of An. albimanus, ivermectin not only worked more rapidly (Fig. 2B) but was also tenfold more toxic than when it was ingested in a blood meal (Table 1). This indicates that ingested ivermectin was poorly absorbed across the gut of An. albimanus. Even so, injected ivermectin was still significantly less toxic to An. albimanus (LC50 = 188 ng/ml) than to An. stephensi (LC50 = 49 ng/ml), suggesting that the binding affinity of ivermectin and/or its stimulatory effect on the glutamate-gated chloride channels of An. albimanus was less than that of the glutamate-gated chloride channels of An. stephensi. The relative insensitivity of An. albimanus to ingested ivermectin may be attributable to (1) poor absorption of ivermectin across the gut of An. albimanus, (2) potential structural differences between the glutamate-gated chloride channels among anopheline species, or (3) more efficient detoxification processes occurring in An. albimanus compared to other species of Anopheles tested.
Curiously, ivermectin was significantly more toxic to An. stephensi when ingested (LC50 = 7 ng/ml) than when injected (LC50 = 49 ng/ml). This suggests that ivermectin metabolites resulting from mosquito digestion of the blood meal may have a contributory role in the overall oral toxicity of ivermectin to An. stephensi.
Ingestion of ivermectin also reduced the reproductive potential of An. albimanus and An. stephensi by inhibiting blood meal digestion, vitellogenesis, oviposition rate, fecundity, and egg hatch (Fig. 3, Tables 2 and 3). However, statistically significant reductions in these reproductive parameters occurred only when mosquitoes ingested ivermectin at or above the respective LC50 concentrations for each species. This is similar to that reported for ivermectin in An. arabiensis [14] but stands in contrast to results reported for An. aquasalis [16], where significant reductions in egg production and hatching were observed after mosquitoes had ingested ivermectin at a substantially lower potency, i.e., a concentration equivalent to the LC5 for that species (= 18 ng/ml). Defining the effects of ingested ivermectin on mosquito reproduction is important because significant net reductions in mosquito reproduction could still act to reduce mosquito populations, even at sub-lethal concentrations (see Table 2).
According to the recommendations of most commercial manufacturers of endectocide livestock products, a subcutaneous dose of ivermectin at 200 mg/kg BW is standard for control of intestinal nematodes. At that dose and mode of delivery, the peak plasma concentrations of ivermectin in cattle ranges between 30 and 46 ng/ml [28–30]. Field studies have shown that this amount of ivermectin (200 mg/kg BW) in the blood of treated cattle is sufficient to cause significant mortality and reproductive losses in several species of Anopheles mosquitoes, including An. arabiensis [31], Anopheles culicifacies and An. stephensi [32]. This study suggests that plasma levels of ivermectin in this range would not produce any mortality or loss of reproduction in An. albimanus that feed on ivermectin-treated cattle.
To successfully implement endectocides for vector control, it is essential to fill the gaps in knowledge about how different mosquito species react to ivermectin. This study found wide differences in oral and parenteral toxicities of ivermectin to the Asian vector, An. stephensi, and the Latin American vector An. albimanus. These studies utilized long established laboratory strains of mosquitoes. An important next step will be to define comparative toxicities of ivermectin and other endectocides against field strains of Anopheles mosquitoes.
Conclusions
Ivermectin has been shown to be a promising candidate for Anopheles vector control in many parts of the world. However, there are several important species of Anopheles that have yet to be tested for their susceptibility to ivermectin. This study found that An. stephensi was highly susceptible to ivermectin. Conversely, An. albimanus was nearly impervious to the effects of ivermectin when compared to other Anopheles species tested to date. Significant mortality and sterility of An. albimanus were achieved only at ivermectin concentrations so high that they would not be feasible or desirable to use in livestock. Fortunately, there are a number of other related avermectin-based endectocides currently registered for use in livestock in many countries. Further efficacy testing of alternative endectocides should be conducted against Central and South American malaria vectors if endectocides are to be successfully implemented in the Americas for zoophagic vector control.
Authors’ contributions
SMD and JAV conceived and designed the experiments; SMD, KJM and JAV performed the experiments; SMD and JAV analysed the data; SMD and JAV wrote or revised the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The mosquitoes used in this study (Anopheles albimanus, Strain STECLA, Eggs, MRA-126 contributed by Mark Q. Benedict, and Anopheles stephensi, Strain STE2, Eggs, MRA-128 contributed by Mark Q. Benedict) were obtained through the BEI Resources, NIAID, NIH, USA.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The data analysed during this study are available on request from the corresponding author.
Ethics approval and consent to participate
The use of vertebrate animals was approved by the University of North Dakota Institutional Animal Care and Use Committee (1508-1C).
Funding
This study was supported by Grant R21AI119771 from the National Institute of Allergy and Infectious Diseases, NIH, USA. KJM was supported on an assistantship from the National Science Foundation and the North Dakota EPSCoR program, #IIA-1355466.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- ANOVA
analysis of variance
- BW
body weight
- DMSO
dimethyl sulfoxide
- IP
intraperitoneal
- IRS
indoor residual spraying
- ITN
insecticide-treated bednet
- PBS
phosphate-buffered saline
References
- 1.World Health Organization . World malaria report 2017. Geneva: World Health Organization; 2017. pp. 1–196. [Google Scholar]
- 2.Molineaux L, Gramiccia G. The Garki project. Geneva: World Health Organization; 1980. p. 311. [Google Scholar]
- 3.Graves PM, Brabin BJ, Charlwood JD, Burkot TR, Cattani JA, Ginny M, et al. Reduction in incidence and prevalence of Plasmodium falciparum in under-5-year-old children by permethrin impregnation of mosquito nets. Bull World Health Organ. 1987;65:869–877. [PMC free article] [PubMed] [Google Scholar]
- 4.Lindblade KA, Mwandama D, Mzilahowa T, Steinhardt L, Gimnig J, Shah M, et al. A cohort study of the effectiveness of insecticide-treated bed nets to prevent malaria in an area of moderate pyrethroid resistance, Malawi. Malar J. 2015;14:31. doi: 10.1186/s12936-015-0554-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruce-Chwatt LJ, Garrett-Jones C, Weitz B. Ten years’ study (1955–64) of host selection by anopheline mosquitos. Bull World Health Organ. 1966;35:405–439. [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell WC, Fisher MH, Stapley EO, Albers-Schönberg G, Jacob TA. Ivermectin: a potent new antiparasitic agent. Science. 1983;221:823–828. doi: 10.1126/science.6308762. [DOI] [PubMed] [Google Scholar]
- 7.Crump A, Omura S. Ivermectin, “Wonder drug” from Japan: the human use perspective. PJA Series B. 2011;87:13–28. doi: 10.2183/pjab.87.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crump A, Morel CM, Omura S. The onchocerciasis chronicle: from the beginning to the end? Trends Parasitol. 2012;28:280–288. doi: 10.1016/j.pt.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Rebollo MP, Bockarie MJ. Toward the elimination of lymphatic filariasis by 2020: treatment update and impact assessment for the endgame. Expert Rev Anti Infect Ther. 2013;11:723–731. doi: 10.1586/14787210.2013.811841. [DOI] [PubMed] [Google Scholar]
- 10.Canga AG, Prieto AMS, Liebana MJD, Martinez NG, Vega MS, Vieitez JJG. The pharmacokinetics and interactions of ivermectin in humans—a mini-review. AAPS J. 2008;10:42–46. doi: 10.1208/s12248-007-9000-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fritz ML, Siegert PY, Walker ED, Bayoh MN, Vulule JR, Miller JR. Toxicity of bloodmeals from ivermectin-treated cattle to Anopheles gambiae s.1. Ann Trop Med Parasitol. 2009;103:539–547. doi: 10.1179/000349809X12459740922138. [DOI] [PubMed] [Google Scholar]
- 12.Kobylinski KC, Deus KM, Butters MP, Hongyu T, Gray M, Marques da Silva I, et al. The effect of oral anthelmintics on the survivorship and re-feeding frequency of anthropophilic mosquito disease vectors. Acta Trop. 2010;116:119–126. doi: 10.1016/j.actatropica.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butters MP, Kobylinski KC, Deus KM, Marques da Silva I, Gray M, Sylla M, et al. Comparative evaluation of systemic drugs for their effects against Anopheles gambiae. Acta Trop. 2012;121:34–43. doi: 10.1016/j.actatropica.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fritz ML, Walker ED, Miller JR. Lethal and sublethal effects of avermectin/milbemycin parasiticides on the African malaria vector, Anopheles arabiensis. J Med Entomol. 2012;49:326–331. doi: 10.1603/ME11098. [DOI] [PubMed] [Google Scholar]
- 15.Kobylinski KC, Ubalee R, Ponlawat A, Nitatsukprasert C, Phasomkulsolsil S, Wattanakul T, et al. Ivermectin susceptibility and sporontocidal effect in Greater Mekong Subregion Anopheles. Malar J. 2017;16:280. doi: 10.1186/s12936-017-1923-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sampaio VS, Beltrán TP, Kobylinski KC, Melo GC, Lima JBP, Silva SGM, et al. Filling gaps on ivermectin knowledge: effects on the survival and reproduction of Anopheles aquasalis, a Latin American malaria vector. Malar J. 2016;15:491. doi: 10.1186/s12936-016-1540-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobylinski KC, Escobedo-Vargas KS, López-Sifuentes VM, Durand S, Smith ES, Baldeviano GC, et al. Ivermectin susceptibility, sporontocidal effect, and inhibition of time to re-feed in the Amazonian malaria vector Anopheles darlingi. Malar J. 2017;16:479. doi: 10.1186/s12936-017-2121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sylla M, Kobylinski KC, Gray M, Chapman PL, Sarr MD, Rasgon JL, et al. Mass drug administration of ivermectin in south-eastern Senegal reduces the survivorship of wild-caught, blood fed malaria vectors. Malar J. 2010;9:365. doi: 10.1186/1475-2875-9-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alout H, Krajacich BJ, Meyers JI, Grubaugh ND, Brackney DE, Kobylinski KC, et al. Evaluation of ivermectin mass drug administration for malaria transmission control across different West African environments. Malar J. 2014;13:417. doi: 10.1186/1475-2875-13-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saul A. Zooprophylaxis or zoopotentiation: the outcome of introducing animals on vector transmission is highly dependent on the mosquito mortality while searching. Malar J. 2003;2:32. doi: 10.1186/1475-2875-2-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franco AO, Gomes MGM, Rowland M, Coleman PG, Davies CR. Controlling malaria using livestock-based interventions: a one-health approach. PLoS ONE. 2014;9:e101699. doi: 10.1371/journal.pone.0101699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zimmerman RH. Ecology of malaria vectors in the Americas and future direction. Mem Inst Oswaldo Cruz. 1992;87(3):371–383. doi: 10.1590/S0074-02761992000700064. [DOI] [PubMed] [Google Scholar]
- 23.Sinka ME, Bangs MJ, Manguin S, Rubio-Palis Y, Chareonviriyaphap T, Coetzee M, et al. A global map of dominant malaria vectors. Parasit Vectors. 2012;5:69. doi: 10.1186/1756-3305-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zilberstein Y, Ayali A. The role of the frontal ganglion in locust feeding and moulting related behaviours. J Exp Biol. 2002;205:2833–2841. doi: 10.1242/jeb.205.18.2833. [DOI] [PubMed] [Google Scholar]
- 25.Detinova TS, Beklemishev WN, Bertram DS. Age-grouping methods in Diptera of medical importance. World Heal Organ Monogr Ser. 1962;47:1–213. [PubMed] [Google Scholar]
- 26.Abbott WS. A method of computing the effectiveness of an insecticide. J Econ Entomol. 1925;18:265–267. doi: 10.1093/jee/18.2.265a. [DOI] [Google Scholar]
- 27.Meyers JI, Gray M, Kuklinski W, Johnson LB, Snow CD, Black WC, 4th, et al. Characterization of the target of ivermectin, the glutamate-gated chloride channel, from Anopheles gambiae. J Exp Biol. 2015;218:1478–1486. doi: 10.1242/jeb.118570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toutain PL, Upson DW, Terhune TN, McKenzie ME. Comparative pharmacokinetics of doramectin and ivermectin in cattle. Vet Parasitol. 1997;72:3–8. doi: 10.1016/S0304-4017(97)00070-8. [DOI] [PubMed] [Google Scholar]
- 29.Lanusse C, Lifschitz A, Virkel G, Alvarez L, Sanchez S, Sutra JF, et al. Comparative plasma disposition kinetics of ivermectin, moxidectin and doramectin in cattle. J Vet Pharmacol Ther. 1997;20:91–99. doi: 10.1046/j.1365-2885.1997.00825.x. [DOI] [PubMed] [Google Scholar]
- 30.Ndong TB, Kane Y, Ba MA, Sane I, Sutra JF, Alvinerie M. Pharmacokinetics of ivermectin in zebu Gobra (Bos indicus) Vet Parasitol. 2005;128:169–173. doi: 10.1016/j.vetpar.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 31.Lyimo IN, Kessy ST, Mbina KF, Daraja AA, Mnyone LL. Ivermectin-treated cattle reduces blood digestion, egg production and survival of a free-living population of Anopheles arabiensis under semi-field condition in south-eastern Tanzania. Malar J. 2017;16:239. doi: 10.1186/s12936-017-1885-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naz S, Maqbool A, Ahmad M, Anjum AA. Efficacy of ivermectin for control of zoophilic malaria vectors in Pakistan. Pak J Zool. 2013;45:1585–1591. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data analysed during this study are available on request from the corresponding author.


