Abstract
Background:
Thyroid cancer has been growing rapidly during the last decades. Radioiodine-131 (I-131) as an appropriate therapy modality is currently using in the treatment of cancer and hyperthyroidism diseases. This radiotracer is considered as a cause of oxidative DNA damage in nontarget cells and tissues. The aim of this study was to investigate the effects of curcumin and trehalose on the level of DNA double-strand breaks (DSBs) caused by I-131 in human lymphocytes.
Materials and Methods:
First, 6-mL blood samples were taken from each of the five volunteers. After 1 h of preincubation with the antioxidants, a total of 20 μCi I-131/2 mL (blood + NaCl) was added to each sample, and then, the samples were reincubated for 1 h. Lymphocytes were separated and the mean DSB levels were measured for each sample through γ-H2AX assay to evaluate the effects of antioxidants.
Results:
After 1-h incubation with I-131, the DSBs increased by 102.9% compared to the control group (0.343 vs. 0.169 DSB/cell; P = 0.00). Furthermore, compared to the control + I-131 group, curcumin and trehalose reduced the DSBs by 42% and 38%, respectively. There was a significant decrement (P = 0.00) in the levels of DSBs of the curcumin + I-131 and trehalose + I-131 subgroups compared to the control + I-131 subgroup. Furthermore, there was no significant relationship between the radioprotective effect of curcumin and trehalose (P = 0.95).
Conclusion:
The use of curcumin and trehalose as antioxidant can reduce the numbers of DSBs caused by I-131. Meanwhile, the radioprotective effect of curcumin was more than trehalose.
Keywords: Antioxidants, DNA Breaks, curcumin, nuclear medicine, trehalose
Introduction
Thyroid is an endocrine gland which plays a key role in the regulation of body metabolism.[1] Disorders of the gland can consist of hypothyroidism, Graves' disease, thyroiditis, euthyroid Graves' ophthalmopathy, multinodular Goiter, benign adenocarcinoma, and thyroid carcinoma.[2]
Thyroid cancer as a well-known disease has been growing rapidly during the last decades in which such a shifting trend is up to ten times, depending on the geographical area.[3,4] Standard treatment of thyroid cancer includes surgical resection and radioiodine-131 (I-131) therapy.[5] One of the primary objectives of treatment with I-131 after surgery is ablating residual tumoral cells of thyroid.[6] It is notable that thyroid gland is the serious organ for iodine and is taken up by its follicular cells. The metabolic activity of the cells is the main factor in retention of iodine in the cells.[7] I-131 with the effective half-life of 7.6 days in the living body emits beta (β) and gamma (γ) radiations; as its beta component (with energy 606 Kev) is used for the treatment.[7] In thyroid tumoral cells, radiation leads to irreparable injury to critical targets within the cell, such as DNA, RNA, lipids, and proteins, leading to cell death.[5]
The use of radioactive iodine has several adverse effects such as gastrointestinal symptoms, sialadenitis, xerostomia, neoplasia, and temporary bone-marrow suppression.[8,9,10] Furthermore, it may induce chromosomal instability and genetic damage in normal cells that may cause in secondary cancers.[11,12,13] The cellular damage resulting from I-131 can be through direct ionization or creation of reactive oxygen spaces.[14] One of the vital aims during the interaction between ionization radiations and the cells is the cell DNA in which can cause a range of damages such as single-strand break and double-strand break (DSB). Due to a higher risk of repair, DSBs are more important than others which is the initiator of malignity and cause of death.[15] According to the results of nuclear medicine studies, level of the DSBs increases after the administration of I-131 even after 144 h.[16,17] Therefore, this treatment modality is not completely safe. The DSBs can be counted and identified by the γ-H2AX assay, as a sensitive and precise method. In this method, the DSBs can be evaluated by isolation of lymphocytes and application of monoclonal antibodies which have been attached to the phosphorylated histones by radiation.[18]
Application of herbal radioprotective materials and antioxidants could reduce the genetically damages associated with ionizing radiation and subsequently, decreasing the induction of secondary cancers. Curcumin pigment present in the turmeric plant is using as a spice in food. Such a food substance, rather than having an anti-inflammatory purpose, is considered as a powerful antioxidant and its ability on preservation of vital structures against the oxidative effects has been recognized by several studies.[19,20,21] Furthermore, Trehalose, a nonreducing disaccharide of glucose, has antioxidative effects and hence, it is frequently using during the freezing process. Based on the studies, trehalose can reduce the freeze-induced oxidative stress and improve the antioxidation activity.[22,23] Furthermore, trehalose as an antioxidant agent can increase the vitamin E concentration during the sperm freezing process.[24]
To the best of our knowledge, the effect of trehalose on the extent of damages induced by the ionizing radiation has not been studied. Furthermore, the use of γ-H2AX method in evaluation of the radioprotective effect of curcumin has been not reported. Therefore, this study aimed to investigate the radioprotective effect of curcumin and trehalose against damage caused by I-131 using γ-H2AX method.
Materials and Methods
Study design and sampling
This study was approved by the Ethics Committee of Kashan University of Medical Sciences, Iran (code number: IR. KAUMS. REC.1395.81) and conducted after obtaining written consent from volunteers. Five volunteers participated in the study including four men and one woman (mean age ± standard deviation [SD] of 35.8 ± 6.7). The inclusion criteria were being healthy, nonsmoking, without a history of lymphoma and leukemia diseases, no exposure to nuclear medicine, radiotherapy or chemotherapy at least 6 months before the sampling.
Six-milliliter blood was taken from the antecubital vein each volunteer and was equally divided into 6 vials containing ethylenediaminetetraacetic acid. Based on the incomplete factorial method, the cases were divided into two major groups including without radiation and irradiated with I-131. Each group was classified into three subgroups: control, curcumin, and trehalose. In the other words, the six groups are control, curcumin, trehalose, control + I-131, curcumin + I-131, and trehalose + I-131.
Antioxidants
The concentrations of curcumin and trehalose were calculated according to Shafaghati et al.[21] and it was assumed that each person totally has 6 L blood. Consequently, the concentrations of 50 μg curcumin per mL of blood and 5.738 mg trehalose per mL of blood, according to the GRAS notification for Hayashibara trehalose submitted by Hayashibara International, Inc., were calculated and added to tube containing blood and I-131.
Treatment and irradiation
The subgroups without I-131 after adding the curcumin and trehalose were incubated for 2 h at 37°C. The samples of irradiated group were also incubated for 1 h with antioxidants at 37°C. Then, 20 μCi I-131 (provided by Nuclear Medicine Center of Kashan Shahid Beheshti Hospital, Iran) with normal saline (Nacl), in a total volume of 1 mL, were added to the samples and the final volume of each vial was 2 ml. These samples were again incubated at 37°C for 1 h. After incubation, the irradiated group was centrifuged at 2000 g for 15 min. The supernatant containing I-131 was removed and discarded. The deposited layer was washed twice in phosphate-buffered saline (PBS), and the samples were prepared for the H2AX process.
γ H2AX process
The present H2AX method was based on details described in previous studies.[16,17] Blood samples were diluted with PBS (1:1). The white blood cells were separated by centrifugation at 1700 g for 15 min (4°C). The cells were washed twice with PBS (each for 5 min) and fixed for 15 min with 4% paraformaldehyde in PBS. After washing, approximately 20 μl of solution were duplicated on slide followed by permeabilization in cold acetone for 10 min. The cells were washed 3 times with PBS and blocked in PBS, 5% bovine serum albumin (BSA) (Sigma Co.) and 0.2% triton x-100 at room temperature. The cells were stained overnight using a specific γ-H2AX antibody (Millipore, Germany, clone JBW301) (dilution 1:500 in PBS containing 1% BSA and 0.05% x-triton) at 4°C. The slides were washed 3 times (each for 10 min) and incubated with secondary antibody (Alexa Fluor 488) (dilution 1:500 in PBS containing 1% BSA and 0.05% x-triton) for 1 h at room temperature in the darkness. Then, the cells were washed 3 times (each for 15 min) and mounted with propidium iodide (dilution 1:50) (Invitrogen). Finally, in each sample, the numbers of γ-H2AX foci (DSBs) were visually counted over 100 lymphocyte cells using fluorescence microscope (Magnum _ T, Ceti Co., Medline UK), with ×1000, by two blind observers. Granulocytes and monocytes were omitted by morphological criteria, and the average number of DSB/cell was calculated.
Statistical analysis
All results were expressed as mean values ± SD. The Kolmogorov–Smirnov test was used to evaluate the normal distribution of continuous data. In addition, increasing the level of DSBs was calculated using the Formula 1:
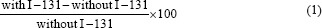
The decreased level of DSBs was also calculated using Formula 2:
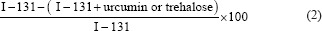
Statistical differences in the mean values of groups were tested with the independent t-test and ANOVA. Furthermore, to evaluate multivariate effects, a factorial design based on generalized linear model (GLM) was used. Finally, P < 0.05 was considered statistically significant.
Results
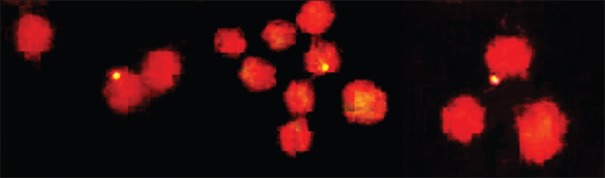
Figure 1 shows the γ-H2AX foci in human lymphocytes in the without I-131 and with I-131 groups.
Figure 1.
Exemplary microscope image of γ-H2AX foci, obtained from human lymphocyts. The thyrocytes were stained with propidium iodide, observed in fluorescent microscopy at × 1000. The light dots are DNA double-strand breaks
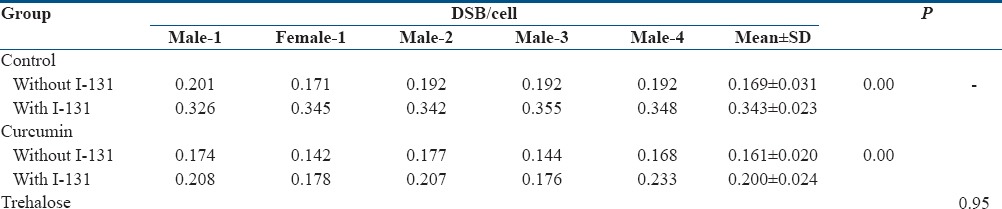
As shown in Figure 2 and Table 1, the number of DSBs per cell in the control (without I-131 and antioxidant), curcumin, and trehalose subgroups was 0.169 ± 0.031, 0.161 ± 0.020, and 0.160 ± 0.023, respectively. There was no significant difference between the samples of nonirradiated subgroups (P = 0.65). The number of DSBs per cell in the irradiated group without antioxidant was 0.343 ± 0.023. After incubation of I-131 in trehalose and curcumin samples, the number of DSBs per cell was 0.200 ± 0.024 and 0.210 ± 0.028, respectively. There was a significant decrement (P = 0.00) in the levels of DSBs of the curcumin + I-131 and trehalose + I-131 subgroups compared to the control + I-131 subgroup. Furthermore, no significant relationship was found between the radioprotective effect of curcumin and trehalose (P = 0.95).
Figure 2.
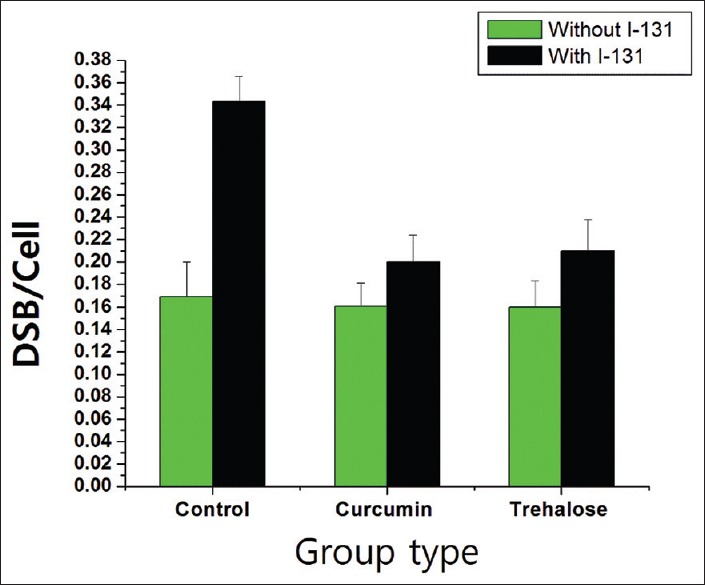
The graph presents double strand break/cell (mean ± standard deviation) in lymphocytes DNA with and without I-131
Table 1.
Double strand breaks values of each volunteer in both groups (with and without radioiodine-131)
Also, for the multivariate analysis of the effects of curcumin and trehalose, with and without using I-131, a GLM was employed. The results of GLM indicated the effect of different groups on the level of DSBs in the presence or absence of I-131. In addition, the interactive effect of antioxidants on the level of DSBs was evaluated, based on the presence or absence of I-131 (P = 0.00).
Discussion
In the current study, the radioprotective effects of curcumin and trehalose on genetic damages caused by I-131 in human lymphocytes were evaluated. To assess the genetic damages in the current study, the γ-H2AX method was used. DSB formation and phosphorylation of histone H2AX were among the first reactions of the cell to radiation. The phosphorylated histones can be identified by the γ-H2AX method using the specific antibody. According to the conducted studies, there was a linear relationship between the received radiation dose and the number of formed γ-foci; hence, it can be considered as a specific and accurate method to measure the DSBs.[16,17,25,26]
Iodine-131 is extensively applied for the treatment of thyroid-related diseases. The use of radioiodine treatment with high dose is associated with dose-limited side effects. In the current study, 20 μCi I-131/2 mL (blood + NaCl) was used. Based on the study of Eberlein et al.,[27] this amount of radioiodine leads to 40 mGy absorbed dose in blood and according to, the current study was setup. The dose of 40 mGy is almost similar to the mean absorbed dose to blood in the patients within the first 2 h of I-131 therapy; as the highest number of DSBs was observed during this time.[16] In a study by Lassmann et al.,[16] the number of γ-H2AX foci at different times of receiving Iodine-131 in the patients undergoing I-131 therapy was evaluated. They reported that the number of DSBs in the lymphocytes increased and reached its maximum within the first 2 h of I-131 therapy (median: 0.227 γ-H2AX foci/cell). In our study, after irradiation of the blood samples with a 40 mGy dose of Iodine-131, the number of DSBs reached 0.343 ± 0.023 γ-H2AX foci/cell. The difference between findings of these studies may arise from the types of study (in vivo vs. in vitro) and also different levels of repair in the patients, compared with the blood samples of the current study.
Compared to the control group, the level of DSB in the control + I-131 group increased to 102.9% (0.343 vs. 0.169 DSB/cell; P = 0.00) after 1-h incubation with I-131 (without antioxidant). Hosseinimehr et al. evaluated the effect of three dose values of I-131 on a human blood sample. They reported that 10 and 100 microcurie doses of I-131 increased the number of micronuclei 8.5 times more than the control group.[13] In another study, Eberlein et al. evaluated the DSBs caused by I-131 and Lu-137 and showed a linear relationship between the absorbed radiation dose and the number of DSBs.[27] Our results were consistent with the results of these studies; as the ionizing ray of I-131 leads to increasing the genetic damage.
The antioxidant effect of curcumin on damages of I-131 by the γ-H2AX method was evaluated; as the findings indicated that curcumin can reduce the rate of DSBs by 42% (0.200 vs. 0.343 DSB/cell). In a study, Shafaghati et al. evaluated the effect of curcumin on oxidative stress caused by I-131 at different doses by micronuclei assay in vitro. They showed that 50 μg/mL curcumin reduced the number of micronuclei by 52%.[21] To evaluate the radioprotective effect of curcumin as well as the effectiveness of the γ-H2AX method, the curcumin concentration used in the current study was similar to study of Shafaghati et al. (50 μg/mL). The difference between the two studies could be due to differences in the sensitivity and precision of the used method as well as the dose values of the radionuclide (100 μCi vs. 20 μCi).
Furthermore, Trehalose is a sugar with sweetness of 45% more than sucrose used as an antioxidant agent in freezing procedures. Trehalose protects the cell against oxidative stress through removing free radicals and increasing intracellular antioxidant activities.[23,28,29] In the current study, the use of trehalose, as a radioprotector on ionizing ray of I-131, showed a reduction of 38% in DSBs (0.210 vs. 0.343 DSB/cell). In addition, it was shown that the radioprotective effect of curcumin is more than trehalose (42% vs. 38%). It is noteworthy that curcumin and trehalose can protect the vital organelle of DNA by removing free radicals. Furthermore, the protective effects of curcumin can be related to the enhancement of enzymatic and nonenzymatic antioxidants such as glutathione in the cells treated with curcumin.[30,31] In addition, anti-cancer and anti-inflammatory properties of curcumin have been proven in various studies.[32,33,34] In a study, Agrawal and Mishrareferred to the effects of curcumin on cell signaling pathways, including apoptosis (activation of caspases and downregulation of antiapoptotic gene products), proliferation (human epidermal growth factor receptor 2, epidermal growth factor receptor, and activator protein-1), angiogenesis (vascular endothelial growth factor), and inflammation (nuclear factor-κB, tumor necrosis factor-alpha, interleukin 6 [IL-6], IL-1, cyclooxygenase-2, and 5-lipoxygenase).[35]
However, the following particular points should be considered: in the current study, the effect of such agents was only investigated on lymphocytes, but their effectiveness on other cells and organs is unclear. On the other hand, the effect of these agents should be investigated at different incubation time and doses, as well as in animal studies and clinical trials. In addition, it is necessary to consider the gastrointestinal absorption of the agents in oral consumptions in vivo. Furthermore, due to the high cost of antibodies as well as issues regarding to radiation protection, it was not possible to the evaluation of the combined effects of these two radioprotectors at the moment, but this work is suggested as a future study.
Conclusion
It has been proven that genetic damage increases in patients after I-131 therapy. In the current study, the level of DSB in the control + I-131 group in comparison with the control group increased to 102.9%. Furthermore, the results indicated that the number of DSBs per cell decreases by 42% and 38% in subgroups related to curcumin and trehalose radioprotectors, respectively. As it is understood the radioprotective effect of curcumin against genetic damage caused by I-131 is more than trehalose.
Financial support and sponsorship
Kashan University of Medical Sciences (Kashan, Iran) has financially supported the work, and this is stated in the acknowledgment section of the article.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
This study is part of an M. Sc. thesis supported by Deputy of Research, Kashan University of Medical Science (grant No. 94139).
References
- 1.Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev. 2014;94:355–82. doi: 10.1152/physrev.00030.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farhood B, Bahreyni Toossi MT, Vosoughi H, Khademi S, Knaup C. Measurement of thyroid dose by TLD arising from radiotherapy of breast cancer patients from supraclavicular field. J Biomed Phys Eng. 2016;6:147–56. [PMC free article] [PubMed] [Google Scholar]
- 3.Kilfoy BA, Zheng T, Holford TR, Han X, Ward MH, Sjodin A, et al. International patterns and trends in thyroid cancer incidence, 1973-2002. Cancer Causes Control. 2009;20:525–31. doi: 10.1007/s10552-008-9260-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide increasing incidence of thyroid cancer: Update on epidemiology and risk factors. J Cancer Epidemiol 2013. 2013:965212. doi: 10.1155/2013/965212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klubo-Gwiezdzinska J, Costello J, Jr, Jensen K, Patel A, Tkavc R, Van Nostrand D, et al. Amifostine does not protect thyroid cancer cells in DNA damagingin vitro models. Endocr Connect. 2017;6:469–78. doi: 10.1530/EC-17-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fard-Esfahani A, Emami-Ardekani A, Fallahi B, Fard-Esfahani P, Beiki D, Hassanzadeh-Rad A, et al. Adverse effects of radioactive iodine-131 treatment for differentiated thyroid carcinoma. Nucl Med Commun. 2014;35:808–17. doi: 10.1097/MNM.0000000000000132. [DOI] [PubMed] [Google Scholar]
- 7.Wyszomirska A. Iodine-131 for therapy of thyroid diseases. Physical and biological basis. Nucl Med Rev Cent East Eur. 2012;15:120–3. [PubMed] [Google Scholar]
- 8.Bushnell DL, Boles MA, Kaufman GE, Wadas MA, Barnes WE. Complications, sequela and dosimetry of iodine-131 therapy for thyroid carcinoma. J Nucl Med. 1992;33:2214–21. [PubMed] [Google Scholar]
- 9.Noaparast Z, Hosseinimehr SJ. Radioprotective agents for the prevention of side effects induced by radioiodine-131 therapy. Future Oncol. 2013;9:1145–59. doi: 10.2217/fon.13.79. [DOI] [PubMed] [Google Scholar]
- 10.Safaei M, Jafarpour SM, Mohseni M, Salimian M, Akbari H, Karami F, et al. Vitamins E and C prevent DNA double-Strand breaks in peripheral lymphocytes exposed to radiations from iodine-131. Indian J Nucl Med. 2018;33:20–4. doi: 10.4103/ijnm.IJNM_89_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baugnet-Mahieu L, Lemaire M, Léonard ED, Léonard A, Gerber GB. Chromosome aberrations after treatment with radioactive iodine for thyroid cancer. Radiat Res. 1994;140:429–31. [PubMed] [Google Scholar]
- 12.Watanabe N, Kanegane H, Kinuya S, Shuke N, Yokoyama K, Kato H, et al. The radiotoxicity of 131I therapy of thyroid cancer: Assessment by micronucleus assay of B lymphocytes. J Nucl Med. 2004;45:608–11. [PubMed] [Google Scholar]
- 13.Hosseinimehr SJ, Shafaghati N, Hedayati M. Genotoxicity induced by iodine-131 in human cultured lymphocytes. Interdiscip Toxicol. 2013;6:74–6. doi: 10.2478/intox-2013-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosseinimehr SJ. Flavonoids and genomic instability induced by ionizing radiation. Drug Discov Today. 2010;15:907–18. doi: 10.1016/j.drudis.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. Philadelphia: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 16.Lassmann M, Hänscheid H, Gassen D, Biko J, Meineke V, Reiners C, et al. in vivo formation of gamma-H2AX and 53BP1 DNA repair foci in blood cells after radioiodine therapy of differentiated thyroid cancer. J Nucl Med. 2010;51:1318–25. doi: 10.2967/jnumed.109.071357. [DOI] [PubMed] [Google Scholar]
- 17.Doai M, Watanabe N, Takahashi T, Taniguchi M, Tonami H, Iwabuchi K, et al. Sensitive immunodetection of radiotoxicity after iodine-131 therapy for thyroid cancer using γ-H2AX foci of DNA damage in lymphocytes. Ann Nucl Med. 2013;27:233–8. doi: 10.1007/s12149-012-0678-0. [DOI] [PubMed] [Google Scholar]
- 18.Valdiglesias V, Giunta S, Fenech M, Neri M, Bonassi S. ΓH2AX as a marker of DNA double strand breaks and genomic instability in human population studies. Mutat Res. 2013;753:24–40. doi: 10.1016/j.mrrev.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Cao J, Jia L, Zhou HM, Liu Y, Zhong LF. Mitochondrial and nuclear DNA damage induced by curcumin in human hepatoma G2 cells. Toxicol Sci. 2006;91:476–83. doi: 10.1093/toxsci/kfj153. [DOI] [PubMed] [Google Scholar]
- 20.Hosseinimehr SJ, Hosseini SA. Radiosensitive effect of curcumin on thyroid cancer cell death induced by radioiodine-131. Interdiscip Toxicol. 2014;7:85–8. doi: 10.2478/intox-2014-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shafaghati N, Hedayati M, Hosseinimehr SJ. Protective effects of curcumin against genotoxicity induced by 131-iodine in human cultured lymphocyte cells. Pharmacogn Mag. 2014;10:106–10. doi: 10.4103/0973-1296.131020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aisen E, Quintana M, Medina V, Morello H, Venturino A. Ultramicroscopic and biochemical changes in ram spermatozoa cryopreserved with trehalose-based hypertonic extenders. Cryobiology. 2005;50:239–49. doi: 10.1016/j.cryobiol.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Liu Q, Wang X, Wang W, Zhang X, Xu S, Ma D, et al. Effect of the addition of six antioxidants on sperm motility, membrane integrity and mitochondrial function in red seabream (Pagrus major) sperm cryopreservation. Fish Physiol Biochem. 2015;41:413–22. doi: 10.1007/s10695-014-9993-9. [DOI] [PubMed] [Google Scholar]
- 24.Bucak MN, Ateşşahin A, Varişli O, Yüce A, Tekin N, Akçay A, et al. The influence of trehalose, taurine, cysteamine and hyaluronan on ram semen microscopic and oxidative stress parameters after freeze-thawing process. Theriogenology. 2007;67:1060–7. doi: 10.1016/j.theriogenology.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Rothkamm K, Löbrich M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proc Natl Acad Sci U S A. 2003;100:5057–62. doi: 10.1073/pnas.0830918100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rogakou EP, Boon C, Redon C, Bonner WM. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol. 1999;146:905–16. doi: 10.1083/jcb.146.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eberlein U, Peper M, Fernández M, Lassmann M, Scherthan H. Calibration of the γ-H2AX DNA double strand break focus assay for internal radiation exposure of blood lymphocytes. PLoS One. 2015;10:e0123174. doi: 10.1371/journal.pone.0123174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonnema SJ, Hegedüs L. Radioiodine therapy in benign thyroid diseases: Effects, side effects, and factors affecting therapeutic outcome. Endocr Rev. 2012;33:920–80. doi: 10.1210/er.2012-1030. [DOI] [PubMed] [Google Scholar]
- 29.Hu JH, Zan LS, Zhao XL, Li QW, Jiang ZL, Li YK, et al. Effects of trehalose supplementation on semen quality and oxidative stress variables in frozen-thawed bovine semen. J Anim Sci. 2010;88:1657–62. doi: 10.2527/jas.2009-2335. [DOI] [PubMed] [Google Scholar]
- 30.Srinivasan M, Rajendra Prasad N, Menon VP. Protective effect of curcumin on gamma-radiation induced DNA damage and lipid peroxidation in cultured human lymphocytes. Mutat Res. 2006;611:96–103. doi: 10.1016/j.mrgentox.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Cho YJ, Yi CO, Jeon BT, Jeong YY, Kang GM, Lee JE, et al. Curcumin attenuates radiation-induced inflammation and fibrosis in rat lungs. Korean J Physiol Pharmacol. 2013;17:267–74. doi: 10.4196/kjpp.2013.17.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelkel M, Jacob C, Dicato M, Diederich M. Potential of the dietary antioxidants resveratrol and curcumin in prevention and treatment of hematologic malignancies. Molecules. 2010;15:7035–74. doi: 10.3390/molecules15107035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kunnumakkara AB, Diagaradjane P, Guha S, Deorukhkar A, Shentu S, Aggarwal BB, et al. Curcumin sensitizes human colorectal cancer xenografts in nude mice to gamma-radiation by targeting nuclear factor-kappaB-regulated gene products. Clin Cancer Res. 2008;14:2128–36. doi: 10.1158/1078-0432.CCR-07-4722. [DOI] [PubMed] [Google Scholar]
- 34.Taylor RA, Leonard MC. Curcumin for inflammatory bowel disease: A review of human studies. Altern Med Rev. 2011;16:152–6. [PubMed] [Google Scholar]
- 35.Agrawal DK, Mishra PK. Curcumin and its analogues: Potential anticancer agents. Med Res Rev. 2010;30:818–60. doi: 10.1002/med.20188. [DOI] [PubMed] [Google Scholar]