Abstract
Objectives:
Frequent consumption of sugars-containing carbonated beverages has been associated with caries, but the consequences on the dental biofilm remain unclear. The aim was to evaluate the effect of commercial carbonated beverages and their sugar-free version on enamel and dentine demineralization and on the cariogenic properties of Streptococcus mutans biofilms.
Materials and Methods:
Biofilms of S. mutans UA159 were grown on enamel and dentin slabs and exposed 3 times/day for 5 min, to a commercial cola or orange-flavored carbonated beverage or to their sugar-free version. Biofilms/slabs were recovered to assess biomass, viable microorganisms, protein content and polysaccharides. Demineralization was estimated by the variation of Knoop surface microhardness.
Results:
Exposures to the biofilm with sugars-containing carbonated beverages resulted in similar biomass, viable microorganisms, proteins, and polysaccharides than sucrose (P < 0.05). The sugar-free cola and orange-flavored drink showed lower effect on the biofilm, as compared with sucrose or their sugared version (P < 0.05). All of the products tested, included the sugar-free, showed higher demineralization than the negative control (P < 0.05).
Conclusions:
Sugars-containing carbonated beverages enhance cariogenic activity of S. mutans biofilms, comparable with sucrose. Sugar-free carbonated beverages also have a high demineralizing potential, without affecting biofilm properties.
Keywords: Carbonated beverages, cariogenicity, dental caries, oral biofilm, soft drinks, Streptococcus mutans, sucrose
INTRODUCTION
According to the most recent conceptualization of its pathogenesis, dental caries may be considered as an ecological sugars-dependent dysbiosis caused by pathobionts.[1] The frequent presence of dietary sugars causes an imbalance in the ecological equilibrium of the resident microorganisms of the dental biofilm leading to a pH drop and the subsequent demineralization of the hard dental tissues.[2] In this scenario, sugars must be considered as the main causative factor of caries. Indeed, the essential role of sugars in caries etiology has been recently emphasized, stating that “sugars is the only crucial factor that determines the caries process in practice.”[3] This research focuses on sugars-containing carbonated beverages and their cariogenic potential, including their “sugar-free” versions. These products are also thought to be important contributors for chronic diseases, such as overweight and obesity,[4] diabetes,[5] and cardiovascular diseases.[6] In a time of spiking rates of obesity and diabetes worldwide, at an alarming level, sugars consumption is a matter of high concern for many disciplines, not only dentistry.
Carbon dioxide-containing beverages, named as soft drinks, carbonated beverages, among many others, are one of the chief contributors to the increased sugars daily intake.[7] A high and increasing consumption of carbonated beverages has been reported worldwide. Between 1970 and 1996, per capita consumption of soft drinks increased 23% in the USA,[8] which represents an increase of 13.2%–15.8% in the total amount of calories.[9] It has been reported that soft drink consumption per person/year is 162 L.[10] Since soft drinks typically contain a high concentration of sucrose, glucose or fructose, all of them biofilm-fermentable carbohydrates, analyzing these beverages in terms of their cariogenic potential becomes relevant.
Although there is an irrefutable association between carbonated drink consumption and a high caries experience, reported in several clinical and epidemiological studies,[11,12,13,14] there is a lack of clarity on the effect of soft drink consumption on the dental biofilm. A study reported some biochemical characteristics of human whole saliva and dental plaque resulting from the daily consumption of commercial sugary beverages.[15] The results indicated that moderate consumption of a cola drink failed to produce distinct changes related with caries. Adding to the confusion, a milestone study from the 50s in hamsters showed that soda had the lowest decalcification potential, when compared with the rest of the foodstuffs under study.[16]
As a way to palliate the deleterious effect of sucrose in the diet and in particular in soft drinks, many commercial products have replaced sucrose for natural or artificial sweeteners. Thus, carbonated beverages without sugars or “sugar-free” are widely considered caries safe. Although some sweeteners, including some of those contained in sugar-free soft drinks could be non- or anticariogenic by themselves,[17] in practice, these products are usually combined with other fermentable polysaccharides, such as maltodextrins or starches. It has been reported that commercial sweeteners would be less cariogenic than sucrose,[18] but still retaining some considerable demineralization potential.
Given the scarce information available on the effect of carbonated beverages with or without sucrose on demineralization and on the dental biofilm, the purpose of this study was to determine the cariogenic potential of two of the most highly consumed commercial carbonated beverages in the world. Likewise, we aimed to compare, side-by-side, the cariogenic potential of the sugars-containing with the sugar-free commercial versions of both products, in a relevant experimental caries model with Streptococcus mutans biofilms.
MATERIALS AND METHODS
Experimental design
A previously validated[19] and modified[20] experimental caries model with S. mutans biofilms was used for these experiments. Bovine enamel and dentin slabs served as substrates for S. mutans biofilm formation. To estimate demineralization, initial Knoop surface microhardness (SHi) was assessed on each enamel and dentin slab to compare with the values by the end of the experimental phase. Slabs were randomly arranged in seven treatment groups, as described below. Biofilms were exposed to the different treatments for 5 min, 3 times/day. Culture medium was changed twice per day. At the end of the experimental phase of 4 days for enamel and three for dentin, biofilms were separated from the slabs to assess the following dependent variables: Biofilm biomass, viable bacterial, extra- and intra-polysaccharide production and the total amount of soluble proteins within the biofilms. Slabs were retrieved to evaluate demineralization occurred throughout the experiment. Biofilm acidogenicity was estimated by pH variations in the culture medium, before each medium replacement. The entire experiment was repeated twice with each condition in triplicate (n = 6).
Enamel and dentin slab preparation
Bovine incisors were obtained and disinfected with 5% NaOCl and then stored in 0.9% NaCl, for no longer than 30 days until being used. Slabs (4 mm × 7 mm × 1 mm) of enamel, from the middle part of the crown, and of dentin, from the root portion, were prepared with a cutting machine (LECO VC50 Diamond Saw, Michigan, USA) and polished thoroughly polished. SHi was obtained by a row of three indentations on the slabs, 100 μm apart from each other, with a Knoop microindenter with a microhardness tester (402 MVD, Wolpert Wilson Instruments, Norwood, USA) at 50 g for 5 s in enamel and 10 g for 5 s in dentin. To start the experiments in similar conditions and avoid variability, only enamel slabs with SHi of 364.19 ± 36.4 kg/mm2 (n = 42) and dentin slabs with SHi 58.51 ± 5.8 kg/mm2 (n = 42) were included. Dentin slabs were sterilized with ethylene oxide[21] and enamel slabs in autoclave at 121°C for 15 min.
Streptococcus mutans biofilm formation
Two healthy young volunteers donated saliva in the morning of the 1st day of the experiments. Pooled saliva was sterilized through ultrafiltration with filters of 0.22 μm and treated for 30 min with a protease inhibitor cocktail. To enable S. mutans adhesion to the dental tissue for biofilm formation, slabs were covered with the sterile saliva, thus creating an acquired pellicle-like structure.[22] Once treated with the saliva, slabs were placed in individual wells of a 24-well culture plate (Corning Costar, Lowell MA, USA) by a specially designed hanger made of orthodontic wire. Frozen samples of S. mutans UA159 were reactivated in brain heart infusion (BHI) agar with 1% glucose (Merck, Darmstadt, Germany) at 37°C and 10% CO2 for 18 h. Culture optical density was adjusted at 0.8–1.0 (600 nm) and an aliquot of 100 μL was transferred to 50 mL of 1% sucrose-supplemented BHI broth, homogenized and 2 mL of the inoculated medium transferred to each well of the 24-well plate. Slabs were placed in the wells to form the sucrose-induced biofilm at 37°C and 10% CO2 for 8 h.[23] After the initial biofilm formation, samples were transferred to wells containing 0.1 mM glucose to allow biofilm maturation under a physiological basal glucose concentration[19] for 16 h to complete 24 h of growth and maturation. Enamel and dentin slabs were exposed to the carbonated beverages and to the controls under study.
Treatments
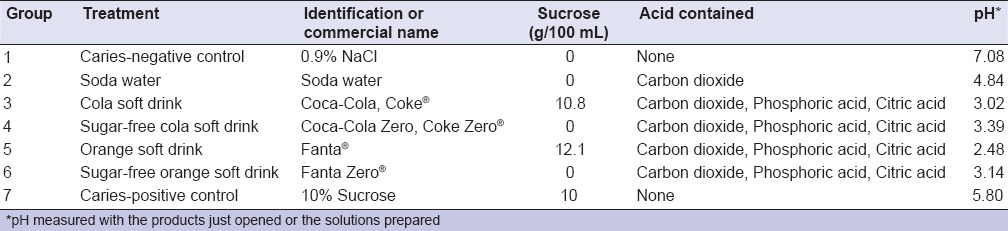
Each slab and the biofilm associated was randomly allocated to one of the following treatments: 0.9% NaCl (negative caries control), soda water (negative caries control), cola soft drink (Coca-Cola®, The Coca-Cola Company, Atlanta, USA), a sugar-free cola soft drink (Coca-Cola Zero®), orange soft drink (Fanta®), a sugar-free orange soft drink (Fanta Zero®) and 10% sucrose (caries-positive control). The choice of using Coca-Cola® and Fanta® was made because both are among the top 10 most sold soft drinks in the world, present in every country and represent 31% of the market in the USA.[10] Products tested here were those available in the Chilean market. To simulate a moderate ingestion pattern of soft drinks, treatments were applied 3 times/day (9:00 AM, 1:00 PM and 5:00 PM) for 5 min on each occasion by immersion in a well of a new 24-well plate containing 2 mL of the treatment, washed with 0.9% NaCl 3 times and relocated in the BHI medium supplemented with 0.1 mM glucose. To simulate pH-cycling conditions, like in the mouth, culture medium was changed twice per day, before the first and after the last treatment. Treatment cycles were repeated until completion of the experiment.
Biofilm acidogenicity
The pH-cycling model led to a lower pH in the spent medium by the end of the day, after the series of three beverage exposures, and a higher pH values after overnight culture in glucose-containing medium. These variations were registered to estimate acidogenicity from the S. mutans biofilms in response to the treatments. Thus, pH was measured twice daily in the triplicate wells using a microelectrode (HI 1083B, Hanna Instruments, Rumania) coupled to a portable pH meter (HI 9126-02, Hanna Instruments).
Slab demineralization
Loss of surface hardness (SH) has been extensively used as a reliable methodology to evaluate demineralization.[24] Given that dentin is more prone to acid dissolution, the length of the experimental phase for dentin was shortened 24 h. Slabs/biofilms were washed 3 times with 0.9% NaCl and vortexed (Maxi Mix II tipo 37600 Mixer, Thermolyne, Iowa, USA) for 30 s to separate biofilms from the dental substrate. The resulting biofilm suspension was kept for further biofilm analysis. Final SH (SHf) of the slabs was measured, in the same way as the SHi, to estimate demineralization produced throughout the experimental period. A new set of three indentations was used to calculate the variation of SH occurred during the experiment. Mean values from the SHi and SHf were used to obtain the percentage of SH loss (%SHL) calculated as: (mean SHi− mean SHf) × 100/SHi.
Characterization of the Streptococcus mutans biofilm exposed to the carbonated beverages
Biofilm suspensions separated from the slabs were analyzed for biomass, viable bacteria, soluble proteins and extra and intracellular polysaccharide (IPS) production, as briefly described.
Biomass
Sample dry weights were used to assess biomass.[22] 200 μL from the biofilm suspension was transferred to preweighted tubes and incubated in 100% ethanol at −20°C for 15 min. The resulting suspension was centrifuged for 10 min at 10,000 ×g and 4°C (Heraeus Megafuge 16R, Thermo Fisher Scientific, Waltham, MA, USA). The pellet was washed with 500 μL of 75% ethanol and centrifuged again. Biofilms were desiccated to obtain the dry weight in an incubator at 37°C for 24 h (MCO-19M, Panasonic, Osaka, Japan). To obtain biomass values, final weight was subtracted from the initial weight of each tube and expressed as mg per mL of biofilm suspension.
Viable bacteria
Serial dilutions in 0.9% NaCl (v/v) of the biofilm suspension were prepared and three drops (20 μL each) were seeded onto BHI agar culture medium, in duplicate.[19] Plates were incubated anaerobically for 24 h at 37°C (MCO-19M, Panasonic) and colonies counted from the dilution that allowed better visualization of isolated colonies. Counting was made under a magnification lens (×4), in the dilution that had at least 20 separated colonies. The number of individual colonies from each plate was corrected by the dilution factor and expressed as CFU/mg of biofilm dry weight.[25]
Biofilm soluble proteins
An aliquot of 50 μL of the biofilm suspension was treated with 2M NaOH and incubated at 100°C for 15 min. Final suspension was centrifuged at 10,000 ×g for 10 min at 4°C and the supernatant was used to determine total protein concentration through the Bradford method.[26] Protein concentration in each biofilm sample was assessed by a microplate reader at 595 nm (Biotek, ELx800, Winooski, Vermont, USA) and the results expressed as μg/mg of biomass.
Intra- and extra-polysaccharides
The procedures to assess polysaccharide concentration in the biofilms were taken from previous reports.[27] In brief, three polysaccharide fractions were analyzed: soluble extracellular polysaccharides (SEPS), insoluble extracellular polysaccharides (IEPS), and IPS. A 200 μL aliquot from the biofilm suspension was centrifuged at 10,000 ×g for 5 min at 4°C (Heraeus Megafuge 16R, Thermo Fisher Scientific) to determine SEPS from the supernatant.[25] The remaining pellet was treated with 200 μL 1 M NaOH, homogenized and centrifuged to acquire IEPS from the supernatant. The pellet from the previous steps containing the IPS was incubated with 200 μL 1M NaOH for 15 min at 100°C and centrifuged (10,000 ×g for 5 min at 4°C). The three supernatant fractions from each extraction step were separately treated with 3 volumes of cold 100% ethanol and incubated for 30 min at −20°C. Samples were immediately centrifuged and the resulting pellet was washed with cold 70% ethanol and centrifuged again (10,000 ×g for 5 min at 4°C). The resulting pellet of each fraction was resuspended in 1M NaOH and total carbohydrate concentration was estimated by the sulfuric phenol method,[28] using a microplate reader (ELx800, Biotek) and the results were standardized by dry weight of biofilm and expressed as % polysaccharide/mg of biomass.
Statistical analysis
Normal distribution of the data was verified and values obtained from the different treatment groups were compared by one-way analysis of variance followed by the post hoc Tukey test, using the Statistical Package for Social Sciences 15.0 software for Windows (IBM Corporation, NY, USA). Differences were considered significant at a 95% confidence level.
RESULTS
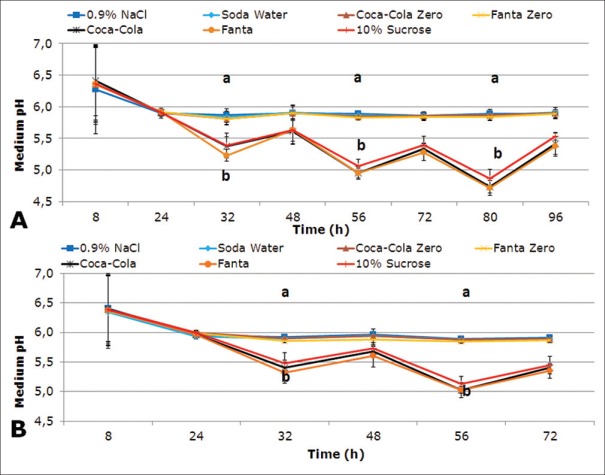
Biofilm acidogenicity induced by the carbonated beverages and measured by medium pH after each medium change is illustrated in Figure 1A and B, for enamel and dentin, respectively. At 32 h, pH dropped notoriously in the biofilms treated with the sugary carbonated beverages Coca-Cola® and Fanta® (P < 0.0001), in enamel and dentin, similar to the caries-positive control. From this time-point onward, pH kept decreasing throughout the experiment. Acidogenicity induced by Soda water, Fanta Zero®, and Coca-Cola Zero® remained similar to 0.9% NaCl, above 5.5.
Figure 1.
Acidogenicity from Streptococcus mutans biofilms formed on enamel (A) and dentin (B), exposed to tested carbonated beverages. Plot shows pH of the culture medium. Each point in the plot represents mean pH of 2 independent experiments in triplicate wells (n = 6). Error bars represent standard deviation. Different letters represent statistically significant differences among treatments (P < 0.05)
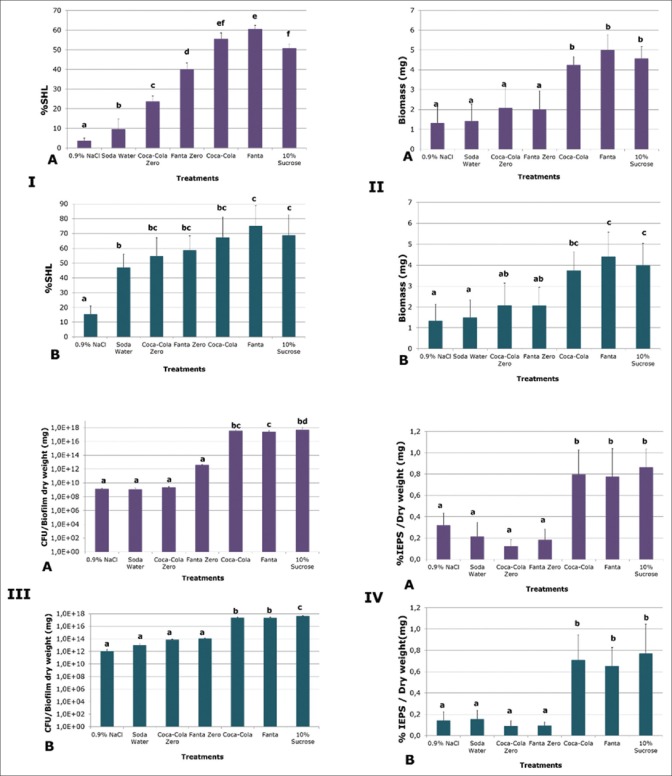
Demineralization induced by all carbonated beverages and tested through % SHL was higher than the negative control (P < 0.0001) [Figure 2-IA and B]. Fanta led to the highest demineralization among the treatments, higher than sucrose (P < 0.0001), but similar to Coca-Cola® [Figure 2-IA]. Sugar-free version of the beverages showed lower values than their sugars-containing counterparts (P < 0.05), but higher than soda water [Figure 2-IA]. Interestingly, soda water induced approximately 5% more demineralization than 0.9% NaCl on enamel (P = 0.025) and 30% on dentin (P = 0.001). Dentin showed high levels of demineralization with all the treatments assayed [Figure 2-IB], regardless of the sugars content of the soft drinks.
Figure 2.
Effect of the different beverages tested on the outcomes of the study: Demineralization on enamel (A) and dentin (B). Bars represent mean percentage of surface hardness loss of the slabs. (II) Biomass induced by each experimental condition. Enamel (A) and dentin (B). Bars represent mean biomass (mg) obtained after exposure to each experimental condition. (III) Viable Streptococcus mutans cells in the biofilms formed on enamel (A) and on dentin (B). Bacterial cells retrieved from each biofilm exposed to test solutions were seeded on plates, counted and expressed as CFU/biofilm dry weight (mg). (IV) Insoluble extracellular polysaccharides produced by the biofilms in response to carbonated beverages. Enamel (A) and dentin (B) biofilms by the different conditions were measured and expressed as mg/mg of biofilm. Bars indicate mean values for each treatment. Error bars indicate standard deviation (n = 6). Different letters represent significant differences among treatments (P < 0.05)
Similar to 10% sucrose, biofilms exposed to Fanta® and Coca-Cola® with sugars showed more biomass formation (P < 0.0001) than the other treatments [Figure 2-IIA and B]. In this regard, no differences were detected among sugar-free commercial carbonated beverages, soda water, and 0.9% NaCl in either tissue.
The amount of viable bacteria within the biofilms after the experimental phase was assessed and compared among the different treatments. Exposure to the carbonated beverages Fanta® and Coca-Cola® induced the formation of biofilms with similar quantities of viable S. mutans cells to 10% sucrose and higher (P < 0.0001) than the other treatments. The sugar-free beverages were unable to induce bacterial proliferation, at least, at the level showed by the other commercial drinks. The same trend was observed in enamel [Figure 2-IIIA] and in dentin biofilms [Figure 2-IIIB].
Extracellular polysaccharide production by the biofilm in response to the carbonated beverages revealed that sugary drinks induced higher IEPS [Figure 2-IVA and B], SEPS, and total proteins (data not shown) than the sugar-free version, in a comparable level to that induced by sucrose. The same trend was verifiable in biofilms formed on enamel and dentin. In the case of the IPS, there was not a difference among any of the treatments in either dental tissue (data not shown), suggesting a lack of regulatory activity on this fraction induced by the nature of the beverage.
DISCUSSION
One of the most important sources of sucrose in the population is the consumption of carbonated beverages.[4] In this context, the role of sugary foods, highly consumed by the population, is key to first understand the process and more importantly and to control its very high prevalence. A dose-dependent association between soft drink consumption and caries has been recently proposed.[12] How these products modify the dynamic of the dental biofilm is less clear. This research, therefore, aimed to shed light on the mechanisms involved in the cariogenicity of some of the most consumed sugary drinks, included their sugar-free version. Our findings clearly showed that commercial carbonated beverages containing sucrose and presented to the biofilm at least 3 times/day were more cariogenic than those exposed to the sugar-free version. The choice of three daily exposures to the biofilm of soft drinks was based on a study that showed that daily between-meal consumption of at least 3 times associated with a 179% increase in the probability of having high caries experience.[29] Furthermore, a prospective 4-year study indicated that there may be a dose-response relationship between frequency of soft drink intake and increasing caries rates in adults, with 3 or more exposures representing a 33% higher decayed, missing, or filled teeth as compared to those that reported no consumption.[12]
Sucrose concentration in the commercial carbonated beverages Coca-Cola® and Fanta® is 10.8% and 12.1%, respectively. Given that both products contain a similar sucrose content to the caries-positive control (10%), it was expected that both acted like sucrose control, in terms of cariogenicity. How can it be explained, then, that a commercial product that is labeled as containing no sugar added is capable to exhibit an important demineralizing potential? In fact, both Zero versions (sugar-free) of the commercial carbonated beverages showed significantly higher demineralization than the 0.9% NaCl negative control [Figure 2-I]. The reason may arise from the acidity of the sugar-free soft drinks. Besides carbon dioxide, both products contain phosphoric and citric acids, according to the manufacturer [Table 1]. We measured the pH of the drinks before exposure to the biofilms and all of them had a pH below 3.5. Interestingly, the effect of the sugar-free carbonated beverages was only on the demineralization, without affecting the biomass, the number of bacteria, or the polysaccharide production. The latter strongly suggests a rather erosive potential of these acidic beverages. Once in contact with the dental biofilm, acid-containing soft drinks entail two different risks; first, sugars may lead to caries by acid production by the acidogenic microbiota with subsequent demineralization. On the other hand, acid content and low pH in the drink may cause erosion on the hard dental tissues. Erosion caused by acidic beverages has been widely discussed[30,31,32] and it is still a growing field of study. Moreover, erosion derived from frequent acidic soft drink consumption has been associated with dentin hypersensitivity.[33] Thus, sugar-free carbonated beverages must not be considered cariogenic, in the sense that they mediate the biological process of caries, but dangerous, as they can induce erosion and produce dentin hypersensitivity.
Table 1.
Test groups and their characteristics. Type of product and commercial name, sucrose and acid content and pH of the solution.
Sucrose promotes biofilm growth and proliferation, mainly through polysaccharide production.[34] Given the sucrose content of the carbonated beverages assayed, it was reasonable to retrieve biofilms with similar values of total biomass, viable bacteria and polysaccharide formation, than those elicited with sucrose control. As expected, a carbonated sugar-free soft drink may exert its deleterious activity chemically, without interfering with the properties of the biofilm, like we reported here. In an in vivo situation, the effect of the sugar-free carbonated beverage may include alternative mechanisms, nonetheless. When the actual clinical biofilm is exposed to acidic soft drinks without sugars, many species may find hostile environmental conditions due to the low pH, as shown here. Commensal microbiota may be impaired to outcompete aciduric microbiota, like S. mutans and others. These aciduric and acidophilic microorganism are endowed with a powerful regulatory machinery to withstand stressful and demanding conditions.[35]
One interesting finding was that Fanta® induced higher demineralization than sucrose on enamel [Figure 2-IA]. We believe that the explanation may be 2-fold.First, Fanta® contains more sucrose (12.1%) than the caries-positive control (10%), hence, an increased production of acids. On the other hand, and according to the manufacturer, Fanta® is made with modified starches as thickeners, unlike Coca-Cola®. Those components may create a more viscous solution that is metabolized by the biofilm. It has been reported that starches enhance cariogenicity of sucrose, when both are combined and exposed to the biofilm.[27] When starch derivatives, such as maltodextrins, are added to products containing noncariogenic sweeteners, the biofilm is yet capable to ferment and induce acid production.[18]
It is generally accepted that sparkling soda or soda water is harmless in terms of dental health and many dentists actually prescribe it as an alternative to sugars-containing carbonated beverages. We decided to use soda water as a control, so the sugar-free carbonated beverages could be compared with a noncariogenic carbonated control and to estimate the demineralizing potential of carbon dioxide. Although demineralization induced by soda water in enamel was slightly higher than the negative control and lower than the Zero drinks [Figure 2-IA], in dentin, the demineralization was much higher than 0.9% NaCl and similar to the sugar-free soft drinks [Figure 2-IB]. This result remarks the lower demineralization threshold of dentin (pH 6.5) with regard to enamel (pH 5.5).[36] Based on the fact that the world is aging at a very high rate, root caries in this more susceptible population must be a concern. Gingival recession is common in older adults and is a risk factor for root caries.[37] Hence, dietary counseling to older adults must take into account the higher susceptibility to root caries of this population and the hazard imposed by the consumption of carbonated soft drinks, including sugar-free versions and the erosive potential of soda water on root surfaces.
The high protein and polysaccharide [Figure 2-IV] production by the biofilms exposed to the carbonated beverages with sucrose is no surprise. Sucrose induces a mature biofilm by production of polysaccharides in a dose-dependent manner.[25] The fact that the IPS did not show variation across the experimental groups suggests that these molecules are used only when bacterial cells are under severe starvation, as a reservoir for carbohydrates.[38]
We acknowledge the limitations of this in vitro approach. Alternative methodologies to assess demineralization may be used for further experiments, though microhardness has been extensively used to estimate demineralization in caries. A single-species biofilm, the absence of salivary proteins and salivary remineralizing ions, restrict drawing final conclusions. Yet, this methodological approach to test carbonated beverages overcomes obvious ethical limitations of an in vivo study and allows a better control of the variables, so commercial products can be compared in a side-by-side fashion. Further studies can contribute to expand these findings with alternative methodological strategies.
These results highlight the importance of controlling sugars consumption, especially due to the availability of sugars-containing soft drinks, their low prices, and their increasingly bigger volumes. For example, just one sugary drink contributes 295 kcal/day, which represent about 35% of the sugars needed per day.[39] Consumption of carbonated beverages has not decreased. A highly frequent consumption of these beverages create an ecological imbalance within the dental biofilm with loss of ecological balance leading to caries.[40] On the other hand, it has been claimed that reducing consumption of free sugars below a threshold of 15–20 kg/person/year or 40–55 g/day or 10% of total energy intake) seems to result in a lower risk of caries.[41] Unfortunately, most processed foods contain important amounts of added sugars and achieving this goal is not an easy task. Data between 1988 and 1994 from the “Third National Health and Nutrition Examination Survey” showed that carbonated beverages may have an cumulative effect after years of consumption.[14]
The multifactorial nature of the disease actually is under scrutiny, as the other involved factors, i.e., saliva, fluoride, tooth structure, socioeconomic status, among others, are not necessary factors for caries causation, but modifiers of the relationship of sugars and the dental biofilm. Hence, preventive measures should mainly consider the causative factors and secondarily the modifiers. In general, most of the energy is used to tackle the modifiers rather than the causative ones. Caries prevention must be oriented to control sugars consumption. Emphasizing on fluoride exposure or toothbrushing as the sole mechanisms to prevent caries will continue to fail, as the burden of disease clearly indicates.[42] The dental profession, therefore, has an opportunity to integrate the multidisciplinary efforts to control diseases directly linked with sugars consumption, such as obesity and diabetes. Furthermore, other deleterious implications of sugar consumption are being considered. For example, sugars consumption has been associated with reduced white blood cell phagocytosis and inflammatory cytokine markers.[43]
CONCLUSION
Commercial sugary carbonated beverages seem to be as cariogenic as sucrose. The sugar-free versions may be less cariogenic than their sucrose-containing counterpart, albeit preserving a dangerous cariogenic and erosive potential. The cariogenic effect of these beverages on the biofilm appears to derive from both, the stimulating activity of sucrose on biofilm proliferation and also from the acidity of this type of soft drink, which increases demineralization of the hard tissues, particularly root dentin. This harmful activity may be enhanced by the common consumption pattern of high quantities and high frequency of the carbonated drinks. Preventive caries measures should take into account this risk and include recommendations on avoiding consumption, especially those containing sucrose.
Financial support and sponsorship
This study was supported by a Chilean Government Grant FONDECYT 1140623 to RAG.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Simón-Soro A, Mira A. Solving the etiology of dental caries. Trends Microbiol. 2015;23:76–82. doi: 10.1016/j.tim.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 2.Fejerskov O. Changing paradigms in concepts on dental caries: Consequences for oral health care. Caries Res. 2004;38:182–91. doi: 10.1159/000077753. [DOI] [PubMed] [Google Scholar]
- 3.Sheiham A, James WP. Diet and dental caries: The pivotal role of free sugars reemphasized. J Dent Res. 2015;94:1341–7. doi: 10.1177/0022034515590377. [DOI] [PubMed] [Google Scholar]
- 4.Vartanian LR, Schwartz MB, Brownell KD. Effects of soft drink consumption on nutrition and health: A systematic review and meta-analysis. Am J Public Health. 2007;97:667–75. doi: 10.2105/AJPH.2005.083782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malik VS, Popkin BM, Bray GA, Després JP, Willett WC, Hu FB, et al. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: A meta-analysis. Diabetes Care. 2010;33:2477–83. doi: 10.2337/dc10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malik VS, Hu FB. Sugar-sweetened beverages and health: Where does the evidence stand? Am J Clin Nutr. 2011;94:1161–2. doi: 10.3945/ajcn.111.025676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tahmassebi JF, Duggal MS, Malik-Kotru G, Curzon ME. Soft drinks and dental health: A review of the current literature. J Dent. 2006;34:2–11. doi: 10.1016/j.jdent.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Johnson RK, Frary C. Choose beverages and foods to moderate your intake of sugars: The 2000 dietary guidelines for Americans – What's all the fuss about? J Nutr. 2001;131:2766S–71S. doi: 10.1093/jn/131.10.2766S. [DOI] [PubMed] [Google Scholar]
- 9.Krebs-Smith SM. Choose beverages and foods to moderate your intake of sugars: Measurement requires quantification. J Nutr. 2001;131:527S–35S. doi: 10.1093/jn/131.2.527S. [DOI] [PubMed] [Google Scholar]
- 10.Beverage-Digest. U.S. Beverage Business Results for 2014 March, 26. 2015. [Last accessed on 2016 Feb 03]. p. 66. Available from: http://www.beverage-digest.com/pdf/top-10_2015.pdf .
- 11.Al-Haboubi M, Klass C, Jones K, Bernabé E, Gallagher JE. Inequalities in the use of dental services among adults in inner South East London. Eur J Oral Sci. 2013;121:176–81. doi: 10.1111/eos.12043. [DOI] [PubMed] [Google Scholar]
- 12.Bernabé E, Vehkalahti MM, Sheiham A, Aromaa A, Suominen AL. Sugar-sweetened beverages and dental caries in adults: A 4-year prospective study. J Dent. 2014;42:952–8. doi: 10.1016/j.jdent.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Han DH, Kim DH, Kim MJ, Kim JB, Jung-Choi K, Bae KH, et al. Regular dental checkup and snack-soda drink consumption of preschool children are associated with early childhood caries in Korean caregiver/preschool children dyads. Community Dent Oral Epidemiol. 2014;42:70–8. doi: 10.1111/cdoe.12065. [DOI] [PubMed] [Google Scholar]
- 14.Heller KE, Burt BA, Eklund SA. Sugared soda consumption and dental caries in the United States. J Dent Res. 2001;80:1949–53. doi: 10.1177/00220345010800101701. [DOI] [PubMed] [Google Scholar]
- 15.Tenovuo J. Salivary parameters of relevance for assessing caries activity in individuals and populations. Community Dent Oral Epidemiol. 1997;25:82–6. doi: 10.1111/j.1600-0528.1997.tb00903.x. [DOI] [PubMed] [Google Scholar]
- 16.Bibby BG, Goldberg HJ, Chen E. Evaluation of caries-producing potentialities of various foodstuffs. J Am Dent Assoc. 1951;42:491–509. doi: 10.14219/jada.archive.1951.0088. [DOI] [PubMed] [Google Scholar]
- 17.Matsukubo T, Takazoe I. Sucrose substitutes and their role in caries prevention. Int Dent J. 2006;56:119–30. doi: 10.1111/j.1875-595x.2006.tb00083.x. [DOI] [PubMed] [Google Scholar]
- 18.Giacaman RA, Campos P, Muñoz-Sandoval C, Castro RJ. Cariogenic potential of commercial sweeteners in an experimental biofilm caries model on enamel. Arch Oral Biol. 2013;58:1116–22. doi: 10.1016/j.archoralbio.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Ccahuana-Vásquez RA, Cury JA. S. mutans biofilm model to evaluate antimicrobial substances and enamel demineralization. Braz Oral Res. 2010;24:135–41. doi: 10.1590/s1806-83242010000200002. [DOI] [PubMed] [Google Scholar]
- 20.Giacaman RA, Jobet-Vila P, Muñoz-Sandoval C. Fatty acid effect on sucrose-induced enamel demineralization and cariogenicity of an experimental biofilm-caries model. Odontology. 2015;103:169–76. doi: 10.1007/s10266-014-0154-5. [DOI] [PubMed] [Google Scholar]
- 21.Thomas RZ, Ruben JL, ten Bosch JJ, Huysmans MC. Effect of ethylene oxide sterilization on enamel and dentin demineralization in vitro. J Dent. 2007;35:547–51. doi: 10.1016/j.jdent.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Koo H, Hayacibara MF, Schobel BD, Cury JA, Rosalen PL, Park YK, et al. Inhibition of Streptococcus mutans biofilm accumulation and polysaccharide production by apigenin and tt-farnesol. J Antimicrob Chemother. 2003;52:782–9. doi: 10.1093/jac/dkg449. [DOI] [PubMed] [Google Scholar]
- 23.Nascimento MM, Gordan VV, Garvan CW, Browngardt CM, Burne RA. Correlations of oral bacterial arginine and urea catabolism with caries experience. Oral Microbiol Immunol. 2009;24:89–95. doi: 10.1111/j.1399-302X.2008.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zero DT. In situ caries models. Adv Dent Res. 1995;9:214–30. doi: 10.1177/08959374950090030501. [DOI] [PubMed] [Google Scholar]
- 25.Aires CP, Del Bel Cury AA, Tenuta LM, Klein MI, Koo H, Duarte S, et al. Effect of starch and sucrose on dental biofilm formation and on root dentine demineralization. Caries Res. 2008;42:380–6. doi: 10.1159/000154783. [DOI] [PubMed] [Google Scholar]
- 26.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 27.Duarte S, Klein MI, Aires CP, Cury JA, Bowen WH, Koo H, et al. Influences of starch and sucrose on Streptococcus mutans biofilms. Oral Microbiol Immunol. 2008;23:206–12. doi: 10.1111/j.1399-302X.2007.00412.x. [DOI] [PubMed] [Google Scholar]
- 28.Dubois M, Gilles K, Hamilton JK, Rebers PA, Smith F. A colorimetric method for the determination of sugars. Nature. 1951;168:167. doi: 10.1038/168167a0. [DOI] [PubMed] [Google Scholar]
- 29.Ismail AI, Burt BA, Eklund SA. The cariogenicity of soft drinks in the United States. J Am Dent Assoc. 1984;109:241–5. doi: 10.14219/jada.archive.1984.0346. [DOI] [PubMed] [Google Scholar]
- 30.Ehlen LA, Marshall TA, Qian F, Wefel JS, Warren JJ. Acidic beverages increase the risk of in vitro tooth erosion. Nutr Res. 2008;28:299–303. doi: 10.1016/j.nutres.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fujii M, Kitasako Y, Sadr A, Tagami J. Roughness and pH changes of enamel surface induced by soft drinks in vitro-applications of stylus profilometry, focus variation 3D scanning microscopy and micro pH sensor. Dent Mater J. 2011;30:404–10. doi: 10.4012/dmj.2010-204. [DOI] [PubMed] [Google Scholar]
- 32.Owens BM, Kitchens M. The erosive potential of soft drinks on enamel surface substrate: An in vitro scanning electron microscopy investigation. J Contemp Dent Pract. 2007;8:11–20. [PubMed] [Google Scholar]
- 33.Mafla AC, Lopez-Moncayo LF. Dentine sensitivity risk factors: A case-control study. Eur J Dent. 2016;10:1–6. doi: 10.4103/1305-7456.175678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koo H, Xiao J, Klein MI. Extracellular polysaccharides matrix – An often forgotten virulence factor in oral biofilm research. Int J Oral Sci. 2009;1:229–34. doi: 10.4248/IJOS.09086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith EG, Spatafora GA. Gene regulation in S. mutans: Complex control in a complex environment. J Dent Res. 2012;91:133–41. doi: 10.1177/0022034511415415. [DOI] [PubMed] [Google Scholar]
- 36.Melberg JR. Demineralization and remineralization of root surface caries. Gerodontology. 1986;5:25–31. doi: 10.1111/j.1741-2358.1986.tb00380.x. [DOI] [PubMed] [Google Scholar]
- 37.Gregory D, Hyde S. Root caries in older adults. J Calif Dent Assoc. 2015;43:439–45. [PubMed] [Google Scholar]
- 38.Busuioc M, Mackiewicz K, Buttaro BA, Piggot PJ. Role of intracellular polysaccharide in persistence of Streptococcus mutans. J Bacteriol. 2009;191:7315–22. doi: 10.1128/JB.00425-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ervin RB, Ogden CL. Consumption of added sugars among U.S. adults 2005-2010. NCHS Data Brief. 2013;122:1–8. [PubMed] [Google Scholar]
- 40.Díaz-Garrido N, Lozano C, Giacaman RA. Frequency of sucrose exposure on the cariogenicity of a biofilm-caries model. Eur J Dent. 2016;10:345–50. doi: 10.4103/1305-7456.184163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheiham A, James WP. A reappraisal of the quantitative relationship between sugar intake and dental caries: The need for new criteria for developing goals for sugar intake. BMC Public Health. 2014;14:863. doi: 10.1186/1471-2458-14-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJ, Marcenes W, et al. Global burden of untreated caries: A systematic review and metaregression. J Dent Res. 2015;94:650–8. doi: 10.1177/0022034515573272. [DOI] [PubMed] [Google Scholar]
- 43.Myles IA. Fast food fever: Reviewing the impacts of the Western diet on immunity. Nutr J. 2014;13:61. doi: 10.1186/1475-2891-13-61. [DOI] [PMC free article] [PubMed] [Google Scholar]