Abstract
Objectives:
The aim of this study is to assess the antibacterial effectiveness of probiotic-experimental-based mouthwash (MW) against Streptococcus mutans in vitro.
Materials and Methods:
Antimicrobial screening of two active additives (probiotic-zamzam) was tested against S. mutans using disc diffusion method. A total of three MWs; (1) an experimental MW base formula, (2) an experimental MW base formula with the two active additives, and (3) commercial MW (hexitol), were evaluated against S. mutans by well diffusion method after 24 h and 72 h storage period. The survival profile of probiotic strain in the experimental MW was determined using colony counting method as well as the pH changes at three intervals. Statistical analysis was performed using one-way ANOVA and t-test to compare the inhibition zone diameter.
Results:
For active additives, probiotic strain exhibited higher mean inhibition zones values than zamzam water against S. mutans. Regarding the inhibition zones for the three tested MWs, the experimental MW showed significant increase in the inhibition zone after 72 h, while there was insignificant change with commercial MW. For probiotic count in MW, there was insignificant change in bacterial count after 24 h, and significant decrease after 15 days, followed by insignificant change after 30 days. For the pH values of the experimental MW, a statistically insignificant change was found after 24 h, significant decrease after 15 days and insignificant change after 30 days.
Conclusions:
The probiotic-zamzam experimental MW was effective in reducing S. mutans. Zamzam water could be considered as prebiotic ingredient. Therefore, the probiotic-zamzam MW has a potential therapeutic value.
Keywords: Lactobacillus rhamnosus, mouthwash, prebiotics, probiotics, Streptococcus mutans, zamzam
INTRODUCTION
Dental caries is one of the most common chronic pathological infectious diseases worldwide. Caries prevention has been evolving over a period in reducing the risk in highly prone individuals. Dietary counseling and proper oral hygiene measures are essential for its control.[1,2] Prevention of bacterial growth and colonization prevents destruction of tooth structure. Dental biofilms are however not easily controlled by mechanical means; as well its success is limited in part because it is regarded as time-consuming and technically difficult by most individuals. Thus, it seems reasonable to control caries by agents that prevent the formation or/and disrupt biofilms, inhibit acid formation, or stimulate base formation by dental biofilms.[3]
Mouthwashes (MWs) have been particularly well accepted by individuals due to their ease of use. It is an effective method for delivery of antimicrobial agents thus preventing bacterial adhesion, colonization, and metabolism. However, the emergence of bacterial resistance to such agents has become a common phenomenon, which is represents a major problem.[1,4] This has encouraged the development of alternative strategies to tackle drug-resistance problems. Among them are probiotics that have introduced as novel antimicrobial agent.[5,6]
Probiotics is the appellation on living microorganisms that have a positive impact on health which, through different means, compete with pathogenic bacteria. It can be considered as a viable alternative for oral health care that beneficially influence the health of the host.[6,7] It is now clear that dairy products such as milk, milk drink, and yoghurt contain certain probiotics which can suppress caries progression and some which can exert “active” caries preventive effects.[8] Lactobacillus rhamnosus is one of the most extensively studied probiotics in oral biology since it does not readily ferment sucrose and is safer for teeth than lactic acid-producing bacteria. Controlled studies have shown the effectiveness of L. rhamnosus in reducing caries.[9,10] Streptococcus mutans is considered one of the most important cariogenic species of the human oral microbial flora. There are ample of evidences from both cross-sectional and longitudinal studies showing the strong association between S. mutans and dental caries. All the available evidence indicates that any preventive strategy should have S. mutans as its principal target.[11,12,13]
MWs are available in different compositions and many claims asserted to have antimicrobial properties. Constituents of MWs include water as chief constituent. The results of the water samples tested by the European laboratories showed that zamzam water has a special physique that makes it advantageous water as there is not any biological growth in and vegetation which is usually take place in ordinary water. Furthermore, in zamzam water no bacteria can form in contrast to ordinary water in which the change in taste, color and smell could be attributed to algae growth.[14] In addition, the quantity of calcium and magnesium salts in zamzam water was slightly higher. The chemical analysis of zamzam water contains some inorganic elements such as calcium (Ca), sodium (Na), potassium (K), fluorine (Fl), magnesium (Mg), chloride (Cl), bicarbonate (HCO3), nitrate (3 – NO), sulfate (SO4), and totally dissolved salts.[15] Moreover, zamzam water effectively increases tooth resistance against acid dissolution due to its fluoride content; therefore, it is useful to harden enamel surface against dental caries challenge.[15,16,17] Based on these considerations, this in vitro study was conducted to assess the effect of inoculating probiotic strain into MW as an attempt to develop a novel MW with anticaries properties.
MATERIALS AND METHODS
Preparation of probiotic experimental mouthwash
Preparation of probiotic strain
L. rhamnosus B-445 was selected as an example of probiotic species and provided in lyophilized form by the Northern Regional Research Laboratory, Illinois, USA.
Culture and enumeration of live probiotic bacterial cells
For De Man, Rogosa, and Sharpe medium broth (MRS; Fluka and catalogue no. 69966 MRS broth, Sigma-Aldrich) was used to grow L. rhamnosus and was incubated anaerobically (Gas Generating Kit Anaerobic System, Oxoid, UK) at 37°C for 48 h. After incubation, the cultured bacteria were centrifuged at 5000 rpm for 20 min to obtain pure cells (pellet). Then, the number of the live bacteria cells in 1 g of the obtained pellet was enumerated by colony counting method. Serial dilutions of 1 g of the previously obtained pellet was prepared in 9 ml sterile saline and 1 ml from each dilution 10−7 and 10−8 was placed to Petri plate (triplicate plates for each dilution), then MRS agar medium was poured into the previous prepared Petri plates. The pour plates were incubated at 37°C for 48 h. After incubation, the most countable plate was counted according to Shan et al., 2015, using the following formula:
Live cells (colony-forming units [CFUs]/g) = number of colonies in the agar plate x dilution factor /volume of culture plate.[18] After calculation, it was found that each 1 g of the previously prepared pellet contained 15 × 108 cells.
Incorporation of the active ingredients into the experimental mouthwash base formula
Ten milliliters of experimental MW contained zamzam water (29% w/v) (National Water Company, Masjid al-Haram in Mecca, Saudi Arabia) in distilled water aqueous base, propylene glycol, and menthol was inoculated with 1 g pellet of 15 × 108 L. rhamnosus.
Isolation and culture of Streptococcus mutans
Collection of plaque sample
The dental plaque sample was obtained by swabbing all surfaces of the teeth of high caries index patient, using sterile cotton-tipped swab (Q-tips, Dermacea, Sherwood Medical, and St Louis, USA). The swab was placed in a 5 mL sterile container containing 2 mL phosphate-buffered saline (PBS) and stored at 4°C until plated. The swab in PBS was vortexed (Thermolyne Maxi Mix II, Iowa, and USA) for 5 min to dislodge bacteria.[19]
Identification of Streptococcus mutans
Serial dilutions of the previously PBS were prepared in three 9 ml sterile saline test tube to form dilution at 10−1, 10−2, and 10−3 using an automatic micropipette. The selective media Mitis salivarius-bacitracin agar (MSB; Fluka and catalogue no. 01337, Sigma-Aldrich) was used to isolate and grow S. mutans. A volume of 50 μl of diluted PBS was aseptically transferred from the previous dilutions (10−2 and 10−3) to Petri plates then MSB agar was poured into them. After the pour plates were hardened, they were inverted and incubated for 18–24 h at 37°C in an incubator [Figure 1].[20]
Figure 1.
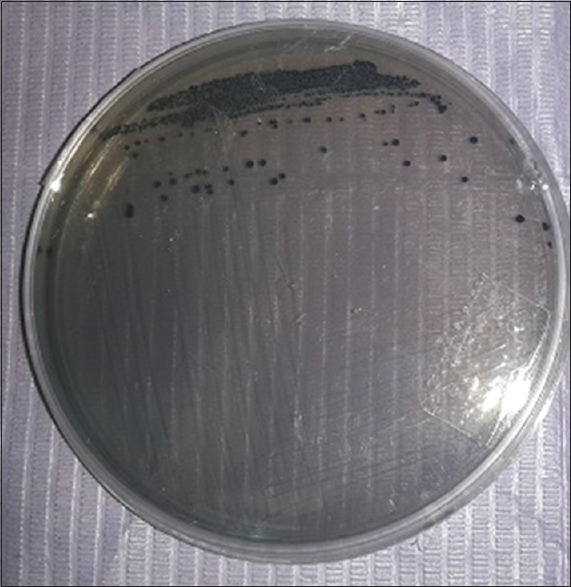
Identification of Streptococcus mutans on Mitis salivarius-bacitracin selective medium
Isolation of Streptococcus mutans
A single colony of S. mutans was isolated and placed in a test tube containing 10 ml of Tryptone Soya broth (TSB; Difco, Detroit, MI USA). Then, it was incubated at 37°C for 24 h to grow.[21] The culture was diluted into fresh media until a concentration –1× 106 CFU/ml and this solution was the working microbial solution.
Testing procedure
Antimicrobial screening of the two active additives using disc diffusion method
Tryptone soya agar (TSA; Difco, Detroit, MI USA) was poured into sterile Petri dishes (15 ml each) and 20 μl of S. mutans in the previously prepared working microbial solution were dispersed on the surface of each agar plate. One gram of the formerly obtained pellet (L. rhamnosus) was dissolved in 4 ml saline. About 6 mm diameter of sterile filter paper discs (Whatman No. 1 filter paper) were impregnated with 50 μl of each ingredient (zamzam water and previously dissolved pellet), then two sterile filter paper discs for each ingredient were placed on surface agar plate which inoculated by S. mutans (triplicate plates) and incubated at 37°c for 24 h. At the end of incubation period, the antimicrobial activity of the two ingredients was evaluated by determining the diameter (mm) of inhibition zones around each disc of active additives [Figure 2].[13]
Figure 2.
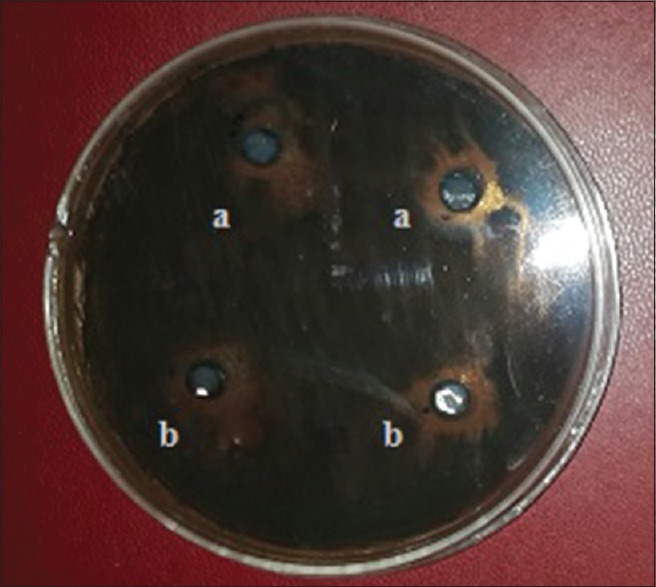
The antimicrobial effect of each ingredient of the experimental mouthwash against Streptococcus mutans; (a): zamzam water and (b): probiotic strain
Antimicrobial effectiveness of the tested mouthwashes using well diffusion method
Three main groups of MWs were evaluated immediately after preparation and after 72 h storage at room temperature; Group 1: Experimental MW base formula (negative control), Group 2: Experimental MW base formula with active additives (zamzam-probiotic), and Group 3: hexitol MW (positive control); 100 ml of chlorhexidine HCL 125 mg, ADCO, Cairo, Egypt. The antimicrobial activity of each MW of the three main groups was determined by modified agar well diffusion method.[22] Twenty microliters of diluted S. mutans was spread on the surface of Tryptone Soya agar plate and a sterile 5-mm cork borer was used to three wells at equidistance in the plate. Fifty microliters of each MW will be completely filled each well on agar plate. The plate will be incubated at 37°C for 24 h. Again, the experiment was repeated after 72 h MWs storage at room temperature and the inhibition zones diameter for each MW was evaluated as mentioned above.
Determination the survival profile of probiotic strain in the experimental mouthwash
Serial dilutions of 1 ml of an experimental MW were made in a physiological saline solution till dilution at 108. About 1 ml saline from each dilution was seeded on triplicate plates of MRS agar medium at 37°C for 24 h and the viability of the probiotic strain (L. rhamnosus cells) in the freshly prepared experimental MW was determined.[23] The method was repeated after 24 h, 72 h, and 30 days from the storage of the experimental MW at room temperature.
Acidity test
A pH meter (Mettler-Toledo, USA) was used to determine the pH changes in the experimental MW at three intervals during storage periods; immediately, after 1 month, and after 3 months. To measure the pH of the experimental MW, 5 ml of mouthrinse was added to 5 ml of tap water in a beaker, and then stirred with a glass stirrer. Finally, pH-sensitive electrode was dipped into the beaker then the digital reading was allowed to stabilize for a few seconds and the pH reading was recorded.[24]
Statistical analysis
Microsta7 for Windows statistical package was used for statistical analysis of this study. Student paired “t”-test was used to compare between immediate and after 72 h parameters in the same group. Independent student “t”-test was used to compare mean values of tested groups in each time. One-way ANOVA was used to compare values throughout the study period, followed by calculating the least significant difference for paired comparisons.
RESULTS
The inhibition zones produced by the MWs against tested microorganism are presented in Figure 3. The mean values and standard deviations of the inhibition zones produced by the two active additives against S. mutans are shown in Table 1 and Figure 4. The inhibition zone diameter in probiotic strains group was statistically significantly higher than that of base formula MW group.
Figure 3.
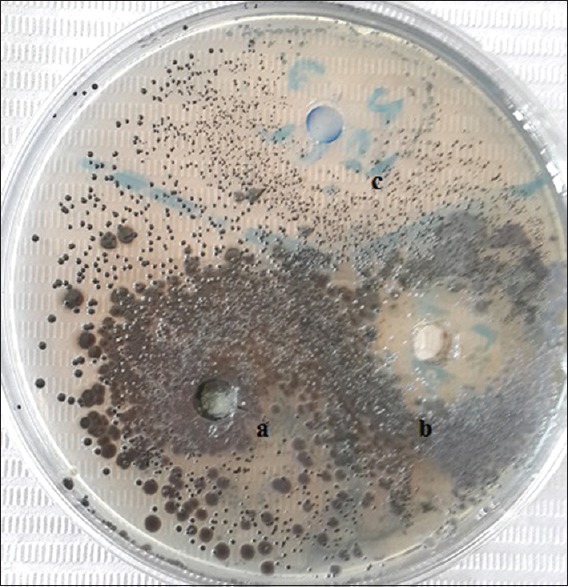
The antimicrobial effect of (a): an experimental mouthwash base formula, (b): an experimental mouthwash, (c): a commercial mouthwash after 72 h mouthwashes storage at room temperature
Table 1.
Comparing inhibition zones between active additives
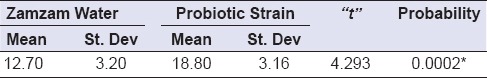
Figure 4.
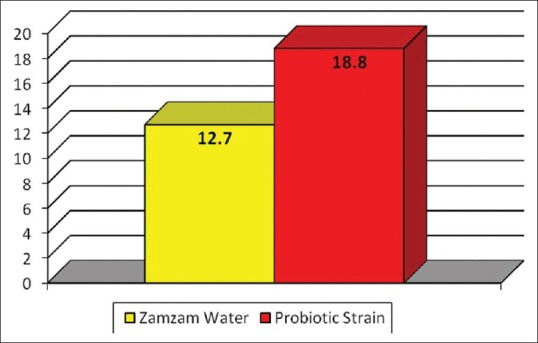
Mean values of inhibition zone diameter in both groups
Comparison between change in inhibition zone diameter in base MW group after 24 h and 72 h revealed that no changes were detected. However, in the experimental MW group, there was statistically significant increase in the inhibition zone after 72 h in comparison to 24 h (P = 0.00002). In hexitol MW, there was statistically insignificant change in the inhibition zone diameter between 24 h and 72 h (P = 0.138). When comparing experimental MW with hexitol MW, there was statistically significant difference between both groups after both 24 h and 72 h intervals in the inhibition zone diameter with (P = 0.0009 and P = 0.043), respectively [Figure 5]. The total change in inhibition zone in experimental MW was statistically significantly higher than that in hexitol MW with (means = 9.2 ± 3.91 and 1.5 ± 4.09), respectively.
Figure 5.
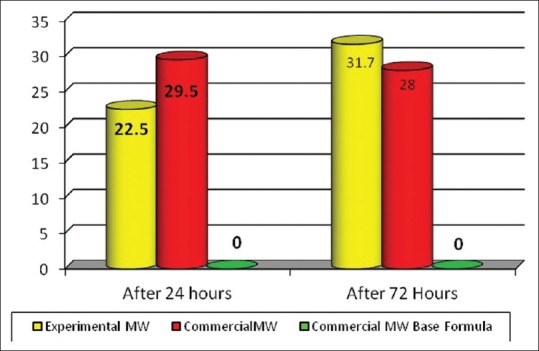
Mean values of inhibition zone diameter between tested mouthwashes at each incubation period (after 24 h and 72 h)
One-way ANOVA comparing L. rhamnosus bacterial counts (Log10 CFU/g) in the experimental MW at different time periods of storage showed that there was statistically insignificant change in bacterial count after 24 h, followed by statistically significant decrease after 15 days, followed by statistically insignificant change after 30 days [Table 2 and Figure 6]. Moreover, the results of the mean difference of pH values of the experimental MW in different storage intervals revealed a statistically insignificant change in pH value after 24 h, followed by statistically significant decrease after 15 days, with insignificant change after 30 days [Table 3 and Figure 7].
Table 2.
One-way ANOVA comparing Lactobacillus rhamnosus bacterial counts (Log10 CFU/g) in the experimental mouthwash at different time periods of storage
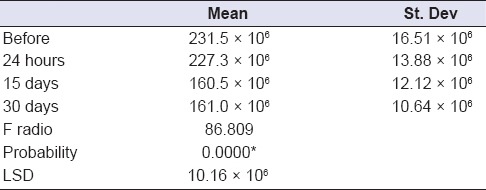
Figure 6.
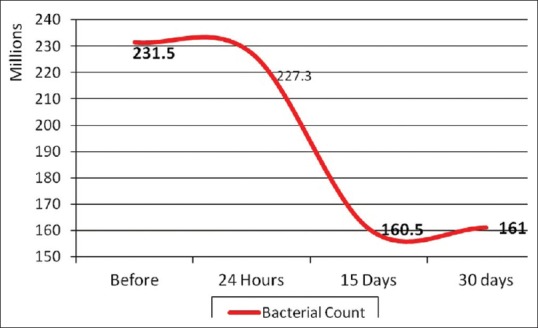
Effect of time on mean values of Lactobacillus rhamnosus bacterial counts (Log10 CFU/g) in the experimental mouthwash at different time periods of storage
Table 3.
One-way ANOVA comparing mean difference of pH values of the experimental mouthwash in different storage times
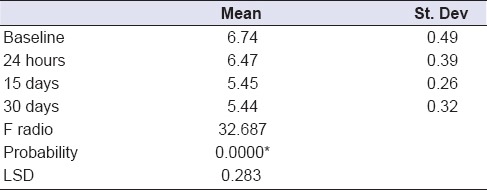
Figure 7.
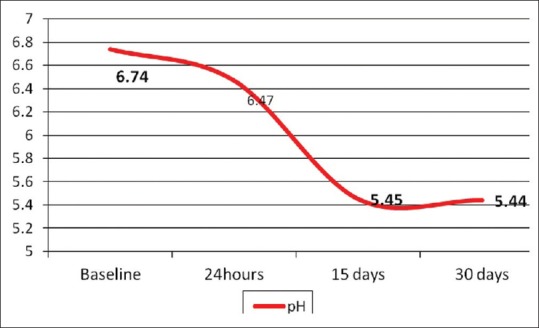
Effect of time on mean values of pH values of experimental mouthwash throughout the storage times
DISCUSSION
Probiotic MWs have been developed to provide a natural defense against harmful oral bacteria only.[1] The active ingredients used in this study may possess many medical properties.[10,15] However, data concerning the substantively of these ingredients are spare. This study was conducted to assess the combined effect of probiotic and zamzam water in the inhibition of S. mutans in comparison to hexitol MW containing chlorhexidine HCL. Chlorhexidine formulations are considered as the gold standard antiplaque and antigingivitis MW as result of its broad-spectrum antimicrobial activity.[25] To evaluate the antimicrobial activity, disc diffusion test was used. The agar method is considered a reliable and standardized evaluation method for a large number of bacterial strains. The diameter of inhibition zone is considered to be directly proportional to the antimicrobial activity of the tested substance.[22] In the present study, S. mutans was selected due to its direct correlation with dental caries.[1]
Probiotic bacteria are living microorganisms which when administrated in adequate amounts beneficially influence the host health. The first probiotic species defined as having probiotic properties belong to the genera of Lactobacillus and Bifidobacterium by Hull et al.[26] and Holcomb et al.[27] They are nowadays added to a variety of commercial dairy products such as cheese, yogurt, and milk. The exact mechanism by which probiotics exert their effects are exactly unknown, but they may act by different mechanisms including production of antimicrobial substances, competing for nutrients or binding site, degradation of toxins, and regulation of immune response.[28] Several factors influencing the function of probiotics such as strain characteristics, stability, fermentation technology, viability and nonviability, microencapsulation and target prebiotics.[29] The term “prebiotic” was introduced by Gibson and Roberfroid.[30] Prebiotics of proven efficacy are able to modulate the microbiota by stimulating indigenous beneficial flora while inhibiting the growth and activity of pathogenic bacteria. Most of the prebiotics used as food adjuncts are derived from plants.
Probiotic therapy can be considered as a viable alternative for oral health care.[5] Probiotics can be delivered by various vehicles such as lozenges, MW, gelatin, powder, straw, or tablets. Probiotic MWs containing living microbes are nowadays recommended. They are not harmful to the oral cavity, no susceptibility for antibiotic resistance, and there are no proven toxicities. L. rhamnosus was chosen in this study as a probiotic bacterium because this bacterium has been extensively studied probiotic for its health benefits in humans since it does not readily ferment sucrose and is safer for teeth. Simark-Mattsson et al.[31] found that the species with maximum interference capacity against S. mutans included Lactobacillus paracasei, Lactobacillus plantarum, and L. rhamnosus.
The results of the inhibition zones produced by the two active additives provide evidence that both probiotic and zamzam water have an inhibitory effect on S. mutans but with statistically significantly higher values with probiotic strain group than that of zamzam water group. The effect of zamzam water could be attributed to the presence of high concentrations of bicarbonates rendering it alkaline (pH ranges from 7.9 to 8.2). Zamzam water is naturally hard carbonated water and sterile that has no germ in it. The inhibitory effect could also be referred to the presence of high concentrations of bicarbonates, potassium, and calcium and/or the low concentrations of some inorganic minerals such as selenium, arsenic, and lithium.[32] However, this activity of probiotic strain in our study is either due to competition for nutrients in agar medium between the pathogen and the probiotic strain, or production of antimicrobial substances that can inhibit the growth of the pathogen. The latter results are in agreement with Saha et al.[33] and Stamatova and Meurman.[34] who demonstrated that the beneficial role of probiotics is mainly based on their antagonistic effect on pathogens.
In probiotic-zamzam experimental MW, there was a sustained increase in inhibition zone with statistically significance increase after 72 h as exhibited by agar disc diffusion method. Various studies have been conducted to evaluate the effect of probiotics on caries causing bacteria. A study by Ahola et al.[35] aimed at showing the benefit of incorporating L. rhamnosus into cheese showed that a highest reduction in level of S. mutans was detected. Caglar et al.[7] investigated the effect of probiotic Lactobacilli delivered through medical device containing probiotic lozenge and revealed a reduction in the levels of salivary S. mutans and Lactobacilli. Zahradnik et al.[36] studied the safety and effectiveness of a probiotic MW and found that it was safe for daily use as an aid in maintaining the health of dental and periodontal tissues. Näse et al.[10] evaluated the effect of long-term consumption of a L. rhamnosus in milk on dental caries and concluded that probiotic bacterium had beneficial effect on dental health. Tahmourespour and Kermanshahi[12] investigated the ability of Lactobacillus probiotic strains on the adhesion of streptococcal strains on the surfaces and concluded that adhesion reduction is due to bacterial interactions and colonization of adhesion sites, thus decreasing the cariogenic potential of oral Streptococci. Keller and Twetman[37] evaluated the acid production in dental plaque after exposure to probiotic bacterium and revealed that no evidence of an increase in plaque acidity after exposure to probiotic.
When probiotic-zamzam MW was compared with experimental base MW and hexitol MW, probiotic and zamzam MW gave the greatest increase in inhibition in zone mean value. It was probably due to the synergistic effect between active ingredients of the experimental MW. However, in hexitol group, the inhibition zone could be attributed to the lethal effects of chlorhexidine as result of its broad-spectrum antibacterial activity through membrane disruption causing a concentration-dependent growth inhibition and cell death. In addition, it strongly inhibits plaque growth as result of its cationic nature which helps it to bind to the tooth structure thus reducing pellicle formation and increasing substantively through controlled release of the agent.[11,25] Moreover, the increase in inhibition zone in probiotic-zamzam group was significant after 72 h, while in chlorhexidine group, the nonsignificant change could be attributed to bacterial drug resistance toward chlorhexidine. Milward and Wilson[38] evaluated the effect of chlorhexidine on Streptococcus sanguinis biofilm and revealed that 72 h biofilms tend to be more resistant to chlorhexidine than 24 h plaque biofilm. Further presumption could be due to the bacteriocin-like inhibitory substance (BLIS) which secreted by the probiotic would diffuse through the agar and produce inhibition well away from the location of the bacteria that secreted them.
There are no previous studies on the behavior of probiotic during storage in zamzam water. The viability of L. rhamnosus in zamzam water up to 30 days indicates that zamzam water could be considered as a prebiotic as it selectively stimulates the growth of the probiotic microbiota while it inhibits the growth and activity of pathogenic bacteria. Hence, the probiotic and zamzam combination could be referred as “synbiotic” because it alludes to synergism in which the prebiotic compound selectively favors the probiotic compound.
Regarding pH values of the experimental MW in different storage intervals, the neutral or alkalinity of oral hygiene measures could attribute to the adherence of mucoproteins which present in the mouth to the surface of the teeth in the form of a slimy film and are difficult to remove. While other oral measures have a pH value 6.0 or less aiding the mucoproteins lose this adhesive quality and remove from the surface of the teeth easily by rinsing.[39] These findings support our results as the pH value of the experimental MW that obtained after 30 days by pH meter was 5.4 [Figure 7]. Moreover, the decrease in pH could be due to hydrogen peroxide or (BLIS) which have a wide inhibitory effect on pathogens as discussed earlier.[34,40]
The data showed that, probiotic–zamzam- based MW formulation exhibited antimicrobial effectiveness against tested microorganism as result of both the inhibitory effect of probiotics and the anticarcinogenic effect of the fluoride content of zamzam water. Furthermore, the combination of both antimicrobials in experimental MW may potentiate their individual effects and allow the MW to interfere with several aspects of oral biochemistry, helping to promote and maintain an adequate oral health status. However, the results obtained in this study can serve a guide for further clinical investigations. In view of the limitations of in vitro studies, in vivo studies are needed to support the efficacy of probiotics. In addition, long-term, controlled trials are also essential, where the combined effect of saliva, oral environment, and the effect of brushing and dentifrices are objectively assessed. Therefore, the results of this study can aid in the evaluation of tested material for clinical use; with the recommendation that clinical evidence must be provided in the future.
CONCLUSIONS
The probiotic-zamzam MW tested was effective in reducing S. mutans. Zamzam water could be considered as a prebiotic ingredient as it selectively stimulating the growth and/or activity of bacteria. Therefore, the probiotic-zamzam MW has a potential therapeutic value and further long-term clinical study is recommended to determine its efficacy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Jothika M, Vanajassun PP, Someshwar B. Effectiveness of probiotic, chlorhexidine and fluoride mouthwash against Streptococcus mutans-randomized, single-blind, in vivo study. J Int Soc Prev Community Dent. 2015;5:S44–8. doi: 10.4103/2231-0762.156153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Culp DJ, Robinson B, Parkkila S, Pan PW, Cash MN, Truong HN, et al. Oral colonization by Streptococcus mutans and caries development is reduced upon deletion of carbonic anhydrase VI expression in saliva. Biochim Biophys Acta. 2011;1812:1567–76. doi: 10.1016/j.bbadis.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scheie AA, Petersen FC. Antimicrobials in caries control. In: Ole F, Edwina AK, Bente N, Vibeke B, editors. Dental Caries: The Disease and Its Clinical Management. 2nd ed. Oxford, UK: Blackwell Munksgaard Publishing Ltd; 2008. pp. 266–76. [Google Scholar]
- 4.Sharma U, Jain RL, Pathak A. A clinical assessment of the effectiveness of mouthwashes in comparison to toothbrushing in children. J Indian Soc Pedod Prev Dent. 2004;22:38–44. [PubMed] [Google Scholar]
- 5.Jain P, Sharma P. Probiotics and their efficacy in improving oral health: A review. J Appl Pharm Sci. 2012;2:151–63. [Google Scholar]
- 6.Harini PM, Anegundi RT. Efficacy of a probiotic and chlorhexidine mouth rinses: A short-term clinical study. J Indian Soc Pedod Prev Dent. 2010;28:179–82. doi: 10.4103/0970-4388.73799. [DOI] [PubMed] [Google Scholar]
- 7.Caglar E, Kuscu OO, Cildir SK, Kuvvetli SS, Sandalli N. A probiotic lozenge administered medical device and its effect on salivary mutans streptococci and lactobacilli. Int J Paediatr Dent. 2008;18:35–9. doi: 10.1111/j.1365-263X.2007.00866.x. [DOI] [PubMed] [Google Scholar]
- 8.Cildir SK, Germec D, Sandalli N, Ozdemir FI, Arun T, Twetman S, et al. Reduction of salivary mutans streptococci in orthodontic patients during daily consumption of yoghurt containing probiotic bacteria. Eur J Orthod. 2009;31:407–11. doi: 10.1093/ejo/cjn108. [DOI] [PubMed] [Google Scholar]
- 9.Marchand J, Vandenplas Y. Micro-or-ganisms administered in the benefit of the host: Myths and facts. Eur J Gastroenterol Hepatol. 2000;12:1077–88. doi: 10.1097/00042737-200012100-00003. [DOI] [PubMed] [Google Scholar]
- 10.Näse L, Hatakka K, Savilahti E, Saxelin M, Pönkä A, Poussa T, et al. Effect of long-term consumption of a probiotic bacterium, Lactobacillus rhamnosus GG, in milk on dental caries and caries risk in children. Caries Res. 2001;35:412–20. doi: 10.1159/000047484. [DOI] [PubMed] [Google Scholar]
- 11.Järvinen H, Tenovuo J, Huovinen P. In vitro susceptibility of Streptococcus mutans to chlorhexidine and six other antimicrobial agents. Antimicrob Agents Chemother. 1993;37:1158–9. doi: 10.1128/aac.37.5.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tahmourespour A, Kermanshahi RK. The effect of a probiotic strain (Lactobacillus acidophilus) on the plaque formation of oral streptococci. Bosn J Basic Med Sci. 2011;11:37–40. doi: 10.17305/bjbms.2011.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parkar SM, Thakkar P, Shah K. Antimicrobial activity of four commercially available mouthwashes against Streptococcus mutans: An in vitro study. Univ Res J Dent. 2013;3:108–12. [Google Scholar]
- 14.Mashat BH. The microbiological quality of sabil free drinking water in Makkah Al-Mukarramah. JKAU Meteoral Environ Arid Land Agric Sci. 2010;21:87–100. [Google Scholar]
- 15.Nauman K, Asif A, Sumera K, Anwaar A, Muhammad I. Mineral composition and health functionality of zamzam water: A review. Int J Food Properties. 2014;17:661–77. [Google Scholar]
- 16.Alfadul SM, Khan MA. Water quality of bottled water in the Kingdom of Saudi Arabia: A comparative study with Riyadh municipal and Zamzam water. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2011;46:1519–28. doi: 10.1080/10934529.2011.609109. [DOI] [PubMed] [Google Scholar]
- 17.Vani NV, Idris AM, Abuhaya AH, Jafer M, Almutari DA. Assessment of calcium, magnesium, and fluoride in bottled and natural drinking water from Jazan Province of Saudi Arabia and a brief review on their role in tooth remineralization. J Int Oral Health. 2016;8:1012–5. [Google Scholar]
- 18.Shan J, Xiaojing H, Chengfei Z, Zhiyu C, Ting Z. Morphological and proteomic analyses of the biofilms generated by Streptococcus mutans isolated from caries-active and caries-free adults. Journal of Dental Sciences. 2015;10:206–15. [Google Scholar]
- 19.Elgamily H, Moussa A, Elboraey A, El-Sayed H, Al-Moghazy M, Abdalla A, et al. Microbiological assessment of Moringa oleifera extracts and its incorporation in novel dental remedies against some oral pathogens. Open Access Maced J Med Sci. 2016;4:585–90. doi: 10.3889/oamjms.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sánchez-García S, Gutiérrez-Venegas G, Juárez-Cedillo T, Reyes-Morales H, Solórzano-Santos F, García-Peña C, et al. A simplified caries risk test in stimulated saliva from elderly patients. Gerodontology. 2008;25:26–33. doi: 10.1111/j.1741-2358.2007.00184.x. [DOI] [PubMed] [Google Scholar]
- 21.Munir A, Umar J, Hameed A, Ahmed S. The effect of commercially available local brand of toothpastes against oral bacteria. Pak Oral Dent J. 2005;25:35–40. [Google Scholar]
- 22.Esteban-Tejeda L, Prado C, Cabal B, Sanz J, Torrecillas R, Moya J. Antibacterial and antifungal activity of ZnO containing glasses. PLoS One. 2015;10:e0132709. doi: 10.1371/journal.pone.0132709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zyzelewicz D, Nebesny E, Moty I, Libudsisz Z. Effect of milk chocolate supplementation with lyophilized Lactobacillus cells on its attributes. Czech J Food Sci. 2010;28:392–406. [Google Scholar]
- 24.Soham B, Srilatha KT, Seema D. Effects of fluoridated toothpaste and mouth rinse on salivary pH in children – An in vivo study. J Oral Hyg Health. 2015;3:192. [Google Scholar]
- 25.Lang NP, Hotz P, Graf H, Geering AH, Saxer UP, Sturzenberger OP, et al. Effects of supervised chlorhexidine mouthrinses in children. A longitudinal clinical trial. J Periodontal Res. 1982;17:101–11. doi: 10.1111/j.1600-0765.1982.tb01135.x. [DOI] [PubMed] [Google Scholar]
- 26.Hull RR, Roberts AV, Mayes JJ. Survival of lactobacillus acidophilus in yogurt. Aust J Dairy Technol. 1984;39:164–6. [Google Scholar]
- 27.Holcomb JE, Frank JF, McGregor JU. Viability of lacto-bacillus acidophilus and Bifidobacterium bifidum in soft- serve frozen yogurt. Cult Dairy Prod J. 1991;26:4–5. [Google Scholar]
- 28.Mercenier A, Pavan S, Pot B. Probiotics as biotherapeutic agents: Present knowledge and future prospects. Curr Pharm Des. 2003;9:175–91. doi: 10.2174/1381612033392224. [DOI] [PubMed] [Google Scholar]
- 29.Bandyopadhyay B, Mandal N. Probiotics, prebiotics and synbiotics – In health improvement by modulating gut microbiota: The concept revisited. Int J Curr Microbiol Appl Sci. 2014;3:410–20. [Google Scholar]
- 30.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J Nutr. 1995;125:1401–12. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 31.Simark-Mattsson C, Emilson CG, Håkansson EG, Jacobsson C, Roos K, Holm S, et al. Lactobacillus-mediated interference of mutans streptococci in caries-free vs.caries-active subjects. Eur J Oral Sci. 2007;115:308–14. doi: 10.1111/j.1600-0722.2007.00458.x. [DOI] [PubMed] [Google Scholar]
- 32.Al-Zuhair A, Al-Ghamdi H, Noorwali M. The Institute of the Custodian of the Two Holy Mosques for Al Hajj Research Centre. Makkah: Om Al Qura University; 2005. Analytical Report of Zamzam Water during the Ramadan and Hajj Seasons 1425H. [Google Scholar]
- 33.Saha S, Tomaro-Duchesneau C, Malhotra M, Tabrizian M, Prakash S. Suppression of Streptococcus mutans and Candida albicans by probiotics: An in vitro study. Dentistry. 2012;2:141. [Google Scholar]
- 34.Stamatova I, Meurman JH. Probiotics: Health benefits in the mouth. Am J Dent. 2009;22:329–38. [PubMed] [Google Scholar]
- 35.Ahola AJ, Yli-Knuuttila H, Suomalainen T, Poussa T, Ahlström A, Meurman JH, et al. Short-term consumption of probiotic-containing cheese and its effect on dental caries risk factors. Arch Oral Biol. 2002;47:799–804. doi: 10.1016/s0003-9969(02)00112-7. [DOI] [PubMed] [Google Scholar]
- 36.Zahradnik RT, Magnusson I, Walker C, McDonell E, Hillman CH, Hillman JD, et al. Preliminary assessment of safety and effectiveness in humans of proBiora3, a probiotic mouthwash. J Appl Microbiol. 2009;107:682–90. doi: 10.1111/j.1365-2672.2009.04243.x. [DOI] [PubMed] [Google Scholar]
- 37.Keller MK, Twetman S. Acid production in dental plaque after exposure to probiotic bacteria. BMC Oral Health. 2012;12:44. doi: 10.1186/1472-6831-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milward TA, Wilson M. The effect of chlorhexidine on Streptococcus sangius biofilms. Microbios. 1989;58:155–64. [PubMed] [Google Scholar]
- 39.Chand S, Gulati P, Dhingra S, Swatika Estimating the pH of commercially available dentifrices and evaluating its effect on salivary pH after brushing. J Oral Health Community Dent. 2013;7:12–6. [Google Scholar]
- 40.Collado MC, Meriluoto J, Salminen S. Measurement of aggregation properties between probiotics and pathogens: In vitro evaluation of different methods. J Microbiol Methods. 2007;71:71–4. doi: 10.1016/j.mimet.2007.07.005. [DOI] [PubMed] [Google Scholar]


