Abstract
Background
Stent underexpansion is a major risk factor for in-stent restenosis and acute in-stent thrombosis1Intravascular ultrasound (IVUS) is one of the standards for detection of stent underexpansion (de Feyter et al. 1999; Mintz et al., 2001). StentBoost (SB) enhancement allows an improved angiographic visualization of the stent (Koolen et al., 2005).
Aim of work
Comparison of stent expansion by IVUS and SB enhancement and detection of value of SB to guide dilatation post stent deployment.
Methodology
IVUS, SB enhancement and QCA were done in 30 patients admitted for elective stenting procedures .We compared measurements of mean ±standard deviations of (Max SD, Min SD, Mean SD, stent symmetry index) using IVUS, SB and QCA after stent deployment and after postdilatation whenever necessary to optimize stent deployment. The Stent symmetry index was calculated [(maximum stent diameter minus minimum stent diameter) divided by maximum stent diameter].
Results
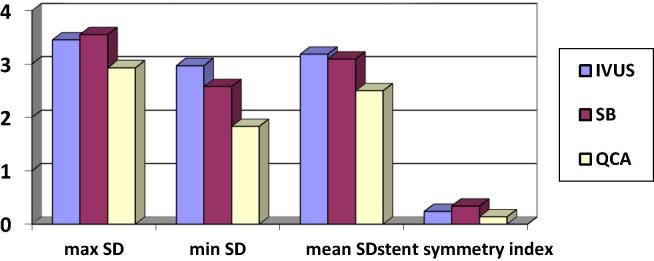
The Max SD was (3.45 ± 0.62 vs 3.55 ± 0.56 vs 2.97 ± 0.59) by IVUS vs SB vs QCA respectively. Max SD was significantly higher by IVUS vs QCA (p .009) and between SB vs QCA (p .001) while there was nonsignificant difference between IVUS vs SB (p .53). The Min SD was (2.77 ± 0.53 vs 2.58 ± 0.56 vs 1.88 ± 0.60) by IVUS vs SB vs QCA respectively. Min SD was significantly higher by IVUS vs QCA (p .001) and between SB vs QCA (p .001) while there was nonsignificant difference between IVUS vs SB (p .07). The stent symmetry index was (0.24 ±0.09 vs 0.34 ± 0.09 vs 0.14 ±0.27) by IVUS vs SB vs QCA respectively. It was significantly higher by IVUS vs QCA (p .001) and between SB vs QCA (p .001) while there was nonsignificant difference between IVUS vs SB (p .32). SB was positively correlated with IVUS measurements of Max SD (p < .0001 & r 0.74) and Min SD (p < .0001 & r 0.68). QCA was positively correlated with IVUS measurements of Max SD correlation (p < .0001 & r 0.69) and Min SD (p < .0001 & r 0.63). QCA was positively correlated with SB measurements of Max SD (p < .0001 & r 0.61) and Min SD (p .003 & r 0.49).
Conclusions
StentBoost enhancement has superior correlations for stent expansion measured by IVUS when compared with QCA. SB enhancement improved stent visualization and identification of stent underexpansion to guide stent postdilatation.
Keywords: Quantitative coronary angiography (QCA), Intravascular ultrasound (IVUS), Stent Boost (SB) enhancement, Maximal stent diameter (Max SD), Minimal stent diameter (Min SD)
1. Introduction
Despite advances in equipment and stent implantation techniques, both acute and long-term complications (stent thrombosis and in-stent restenosis) still occur.5 Stent underexpansion is a major risk factor for in-stent restenosis and acute in-stent thrombosis.1Traditional X-ray angiography presents certain problems which are potentially limiting to optimal stent visualize (see Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7).
Fig. 1.
Stent diameters assessed by IVUS, SB and QCA.
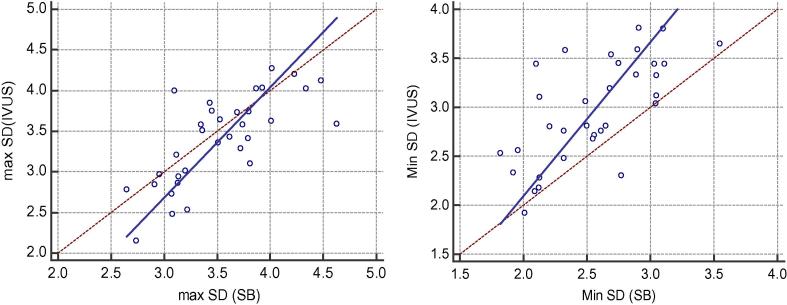
Fig. 2.
Correlations between IVUS & SB regarding Max SD and Min SD measurements.
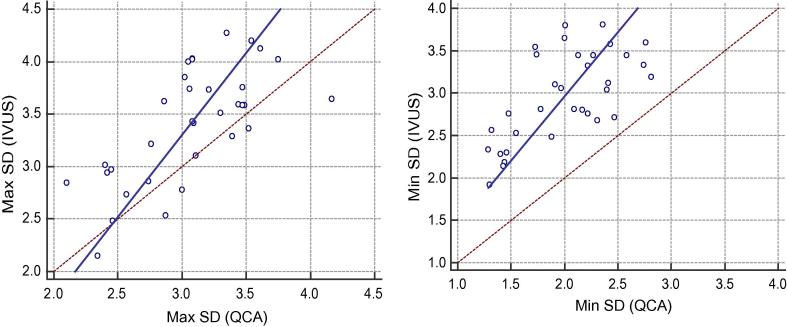
Fig. 3.
Correlations between Max SD and Min SD measurements by IVUS & QCA.
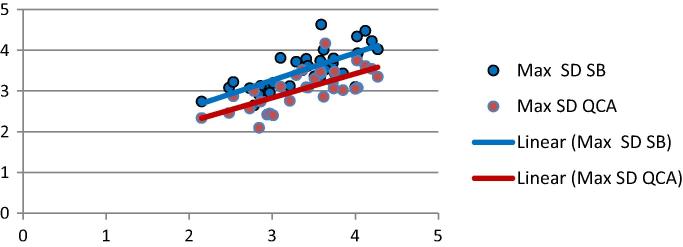
Fig. 4.
Correlation between Max SD measurements by IVUS, SB and QCA.
Fig. 5.
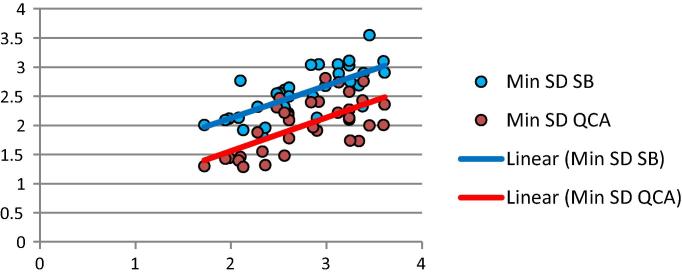
Correlation between Min SD measurements by IVUS, SB and QCA.
Fig. 6.
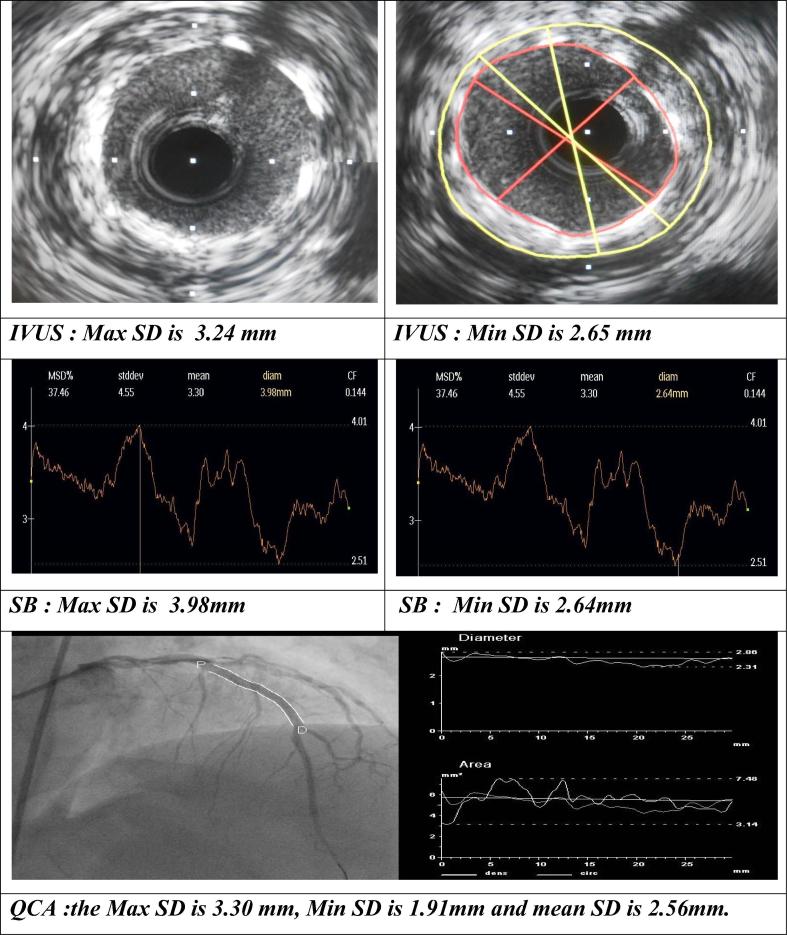
LAD midsegment stent diameters assessed by IVUS, SB and QCA.
Fig. 7.
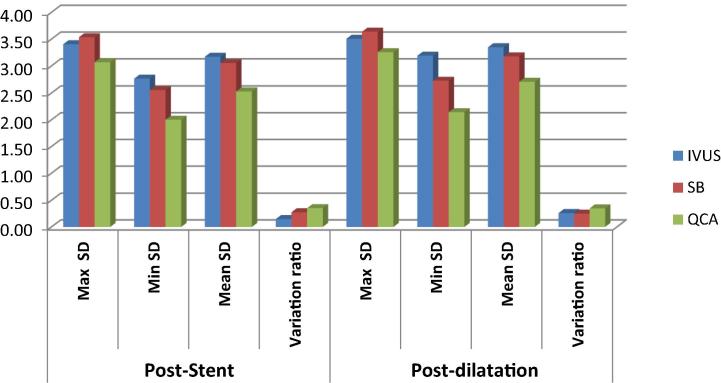
Poststenting and postdilatation stent diameters.
Intravascular ultrasound (IVUS) is a standard for detection of stent underexpansion.2, 3 However, IVUS is limited by professional and technical expertise, cost, procedural time, and the need for additional training of staff in catheterization laboratories, so it cannot be used extensively. Furthermore, there is a small risk of increased mechanical complications, particularly coronary spasm.6 Recently, optical coherence tomography (OCT) has also been used to assess vessel size and stent expansion, but the technique has similar limitations to IVUS.7.
StentBoost (SB) allows an improved angiographic visualization of the stent and of its relationship with the corresponding vessel lumen by enhancing the X-ray focus of the region where the stent is placed.4 It can detect the deployed stent very precisely, discriminate stent underexpansion, and define stent-vessel apposition (see Table 1, Table 2, Table 3, Table 4).
Table 1.
Demographic and angiographic data of the patients.
| Demographic characteristics | N (%) | |
|---|---|---|
| Age (years) | 51.83 ± 9.36 | |
| Sex | Male | 25 (83.3%) |
| Female | 5 (16.7%) | |
| Diabetes mellitus | 14 (46.7%) | |
| HTN | 19 (57.6%) | |
| Dyslipidaemia | 18 (60%) | |
| Smoking | 19 (63.3%) | |
| Family history of IHD | 12 (40%) | |
| Previous MI | 4 (13.3%) | |
| EF | 59.03 ± 5.93% | |
| Angiographic characteristics | ||
| Diseased vessel | LAD | 26 (78.8%) |
| CX | 3 (9.1%) | |
| RCA | 4 (12.1%) | |
| Lesion site | Proximal | 21 (63.6%) |
| Midsegment | 10 (30.3%) | |
| Distal | 2 (6.1%) | |
| Lesion class | A | 9 (27.3%) |
| B1 | 9 (27.3%) | |
| B2 | 6 (18.2%) | |
| C | 9 (27.3%) | |
| Direct stenting | 12 (36.4%) | |
| Pre dilatation | 21 (63.6%) | |
Table 2.
Stent diameters by IVUS, SB and QCA.
| Stent parameters | Measurements | |
|---|---|---|
| IVUS | Stent CSA | 8.17 ± 2.48 mm2 |
| Max SD | 3.45 ± 0.62 mm | |
| Min SD | 2.77 ± 0.53 mm | |
| Mean SD | 3.18 ± 0.47 mm | |
| Stent symmetry index | 0.24 ± 0.09 | |
| Stent boost | Max SD | 3.55 ± 0.47 mm |
| Min SD | 2.58 ± 0.56 mm | |
| Mean SD | 3.09 ± 0.58 mm | |
| Stent symmetry index | 0.34 ± 0.09 | |
| QCA | Max SD | 2.93 ± 0.61 mm |
| Min SD | 1.83 ± 0.57 mm | |
| Mean SD | 2.5 ± 0.48 mm | |
| Stent symmetry index | 0.14 ± 0.27 | |
| Acute gain | 0.94 ± 0.43 | |
Table 3.
Correlations between IVUS, SB and QCA.
| Stent diameters | IVUS | SB | P value |
|---|---|---|---|
| Max SD | 3.45 ± 0.62 | 3.55 ± 0.56 | 0.53 |
| Min SD | 2.77 ± 0.53 | 2.58 ± 0.56 | 0.07 |
| Mean SD | 3.18 ± 0.64 | 3.09 ± 0.58 | 0.54 |
| Stent symmetry index | 0.24 ± 0.09 | 0.34 ± 0.09 | 0.32 |
| IVUS | QCA | ||
| Max SD | 3.45 ± 0.62 | 2.97 ± 0.59 | 0.009 |
| Min SD | 2.77 ± 0.53 | 1.88 ± 0.60 | 0.001 |
| Mean SD | 3.18 ± 0.64 | 2.61 ± 0.50 | 0.001 |
| Stent symmetry index | 0.24 ± 0.09 | 0.14 ± 0.27 | 0.001 |
| SB | QCA | ||
| Max SD | 3.55 ± 0.56 | 2.97 ± 0.59 | 0.001 |
| Min SD | 2.58 ± 0.56 | 1.88 ± 0.60 | 0.001 |
| Mean SD | 3.09 ± 0.58 | 2.61 ± 0.50 | 0.001 |
| Stent symmetry index | 0.34 ± 0.09 | 0.14 ± 0.27 | 0.001 |
Table 4.
Poststenting and postdilatation stent parameters.
| Stent parameters | Poststenting | Postdilatation | P value | |
|---|---|---|---|---|
| IVUS | Stent CSA | 7.69 ± 3.06 | 9.03 ± 2.70 | 0.010 |
| Max SD | 3.25 ± 0.64 | 3.51 ± 0.56 | 0.017 | |
| Min SD | 2.86 ± 0.64 | 3.19 ± 0.51 | 0.008 | |
| Mean SD | 3.05 ± 0.64 | 3.35 ± 0.53 | 0.008 | |
| Stent symmetry index | 0.12 ± 0.04 | 0.09 ± 0.05 | 0.28 | |
| SB | Max SD | 3.46 ± 0.55 | 3.64 ± 0.42 | 0.25 |
| Min SD | 2.38 ± 0.33 | 2.73 ± 0.35 | 0.003 | |
| Mean SD | 2.87 ± 0.46 | 3.18 ± 0.43 | 0.04 | |
| Stent symmetry index | 0.31 ± 0.06 | 0.25 ± 0.04 | 0.16 | |
| QCA | Max SD | 3.17 ± 0.51 | 3.26 ± 0.65 | 0.64 |
| Min SD | 2 ± 0.52 | 2.14 ± 0.58 | 0.023 | |
| Mean SD | 2.61 ± 0.48 | 3.35 ± 0.53 | 0.001 | |
| Stent symmetry index | 0.38 ± 0.09 | 0.35 ± 0.08 | 0.35 | |
| Acute gain | 1.13 ± 0.64 | 1.27 ± 0.69 | 0.023 | |
1.1. Patients and methods
Thirty three elective stenting procedures were evaluated using IVUS, SB enhancement and QCA .We compared measurements of mean ± standard deviations of (Max SD, Min SD, Mean SD, stent symmetry index) using IVUS, SB and QCA after stent deployment and after postdilatation whenever necessary to optimize stent deployment. The Stent symmetry index was calculated [(maximum stent diameter minus minimum stent diameter) divided by maximum stent diameter].
Cardiac catheterization and PCI at culprit lesion was done using Digital Imaging and Communications in Medicine (DICOM)-compatible digital system (Philips CV20, 2011-Netherland) with imaging speed 15 frame per second (fps) .Quantitative coronary analysis of coronary lesions and classification of lesions according to modified ACC/AHA classification followed by IVUS assessment.
1.2. Quantitative Coronary Analysis (QCA)
All angiographic images were obtained with a digitalflat-panel cardiac imaging system (Allura Xper FD 20, Philips Medical Systems). Analysis was performed by validated and automated edge-detection software in all patients in two orthogonal views.8
1.3. Intravascular Ultrasound (IVUS) assessment
Imaging (iLab™, Boston Scientific Scimed Inc.,USA) was performed using a 40 MHz, 6 F compatible catheter (Atlantis SR Pro; Boston Scientific).
1.4. Stent boost enhancement
We used Stent boost (Philips Medical Systems) enhancing stent visualization angiographic technique. After stent deployment and balloon deflation, an enhanced stent image (ESI) is produced from a minimum of 20 cine frames over 3 s using the radiopaque markers of the delivery balloon as an anchor to align the stent across all frames. The StentOptimizer system automatically grabs the cine images to create a still image of the stent with enhanced edges and the associated region of interest.9
Inclusion criteria:
-
•
Stable or unstable angina.
-
•
Significant de novo lesions suitable for stent implementation.
-
•
Written informed consent.
Exclusion criteria:
-
•
Patients with haemodynamic instability.
-
•
Contraindication to aspirin or clopidogrel treatment.
1.5. Statistical analysis
Data was summarized using mean, standard deviation, median and inter quartile range for quantitative variables and frequency and percentage for qualitative ones using the Statistical Package of Social Science Software program, version 21 (SPSS). Pearson correlation coefficients were calculated to get the association between different quantitative variables. P values less than 0.05 were considered statistically significant, and Graphs were used to illustrate some information.
2. Results
2.1. Demographic characteristics
Our study included 30 patients (33 lesions) with a mean age of 51.83 ± 9.36 years, 25 males (83.3%) and 5 females (16.7%) 0.14 cases (46.7%) had diabetes mellitus, 19 cases (63.3%) had systemic hypertension, 18 cases (60%) had dyslipidaemia, 19 cases (63.3%) were smokers, 12 cases (40%) had positive family history of IHD, 4 cases (13.3%) had old myocardial infarction and the mean EF was 59.03 ± 5.93%.
2.2. Angiographic data
Thirty three coronary lesions were stented in in the 30 patients. LAD was the target vessel in 26 (78.8%) cases, while CX was target in 3 (9.1%) cases and RCA was culprit in 4 (12.1%) cases. 21 (63.6%) lesions were proximal, 10 (30.3%) lesions were midsegment and 2 (6.1%) lesions were distal. 9 (27.3%) of lesions were class A, 9 (27.3%) of lesions were class B1, 6 (18.2%) of lesions were class B2 and 9 (27.3%) of cases were class C. TIMI flow was III in all patients before and after PCI. direct stenting was done in 12 (36.4%) lesions while predilatation was done in 21 (63.6%) lesions.
2.3. Stent expansion assessment
Intravascular ultrasound assessment: the mean stent cross sectional area (CSA) was 8.17 ± 2.48 mm2, the mean maximum stent diameter (Max SD) was 3.45 ± 0.62 mm, the mean minimum stent diameter (Min SD) was 2.77 ± 0.53 mm, the mean stent diameter was 3.18 ± 0.47 mm and the stent symmetry index was 0.24 ± 0.09.
Stentboost enhancement assessment: the mean Max SD was 3.55 ± 0.47 mm, the mean Min SD was 2.58 ± 0.56 mm, the mean stent diameter was 3.09 ± 0.58 mm and the stent symmetry index was 0.34 ± 0.09.
Quantitative coronary analysis assessment: the mean Max SD was 2.93 ± 0.61 mm, the mean Min SD was 1.83 ± 0.57 mm, the mean stent diameter was 2.5 ± 0.48 mm, acute gain was 0.94 ± 0.43 and the stent symmetry index was 0.14 ± 0.27.
2.4. Correlations between IVUS, SB & QCA
2.4.1. Intravascular ultrasound &stentboost enhancement
SB was positively correlated with IVUS measurements of Max SD (p < .0001 & r 0.74), Min SD (p < .0001 & r 0.68) and mean SD (p < .0001 & r 0.771).
2.4.2. Intravascular ultrasound &quantitative coronary analysis
QCA was positively correlated with IVUS measurements of Max SD correlation (p < .0001 & r 0.69), Min SD (p < .0001 & r 0.63)and mean SD (p < .0001 & r 0.67).
2.4.3. Stentboost enhancement & quantitative coronary analysis
QCA was positively correlated with SB measurements of Max SD (p < .0001& r 0.61), Min SD (p .003 & r 0.49) and mean SD (p .001 & r 0.53).
2.5. Postdilatation and stent expansion
After satisfactory angiographic results in our study, 7 stents required postdilatation according to IVUS and SB enhancement data. The postdilatation stent diameters obtained by QCA, SB and IVUS were significantly higher than poststenting diameters.
3. Discussion
Stent underexpansion is a major risk factor for in-stent restenosis and acute in-stent thrombosis.1 StentBoost (SB) allows improved angiographic visualization of the stent and of its relationship with the corresponding vessel lumen by enhancing the X-ray focus of the region where the stent is placed.4
The aim of our study was to compare stent expansion by IVUS, SB enhancement and QCA and its value to guide dilatation post stent deployment.
According to our results, there was no statistically significant difference between IVUS & SB regarding maximal, minimal and mean stent diameters and there were strong positive correlations between both methods of assessment. Also there was no statistically significant differance between IVUS & SB regarding stent symmetry index without significant correlation.
From previous results, we found that SB enhancement is reliable for stent expansion assessment in comparison to IVUS and can replace it for post PCI assessment and decision making. StentBoost enhancement is superior than IVUS in identifying stent expansion in that it doesnot require insertion of additional catheter so avoiding rare but possible complications.
Intravascular ultrasound (IVUS) assessment allows circumferential stent assessment while SB enhancement is two dimensional and only diameters can be measured.
Our results were comparable to Fernando Cura et al.10, Sanidas et al.9, Yang et al.11, Mishell et al.12, Zhang et al.13 and Alghamdi et al.14 studies which concluded good correlation between IVUS and SB regarding stent diameters.
There were statistically significant differences between IVUS & QCA regarding maximal, minimal and mean stent diameters with strong positive correlations between both methods .Also there was statistically significant differance between IVUS & QCA regarding stent symmetry index without significant correlation.
From that results, we found that QCA is inaccurate for assessment of stent diameters and tends to underestimate diameters in comparison to IVUS parameters.
There were statistically significant differences between SB & QCA regarding maximal, minimal and mean stent diameters with strong positive correlations between both methods of assessment and there was statistically significant differance between SB & QCA regarding stent symmetry index without significant correlation.
Quantitative coronary analysis underestimate stent diameters but SB enhancement allowed improved stent visualization and thus more accurate assessment and larger diameters comparable to IVUS.
Our results were comparable to Cura et al.10, Sanidas et al.9, Yang et al.11, Mishell et al.12, Zhang et al.13 and Alghamdi et al.14 studies.
After satisfactory angiographic results in our study, 7 stents required postdilatation according to IVUS and SB enhancement data.
According to our results, the stent after postdilatation obtained by QCA, IVUS and SB were significantly larger than at poststenting, and the ratios (stent symmetry index) after postdilatation were smaller than poststenting.
Postdilatation improved stent expansion as measured by stent diameters and this will decrease risk of instent restenosis. There were comparable diameters measured by IVUS and SB after postdilatation but QCA data underestimated stent diameters after dilatation.
The postdilatation stent CSA was significantly higher than poststenting CSA measured by IVUS but the postdilatation acute gain was not statistically higher than poststenting gain measured by QCA.
According to our results, postdilatation improved stent expansion and we can conclude that improved stent visualization allowed detection of stent underexpansion and need for postdilatation. Our results were similar to Yang et al. study(258) that the minimum, maximum and mean diameters of postdilatation obtained by QCA, IVUS, SB were significantly larger than that of poststenting. That study concluded that there is an important advantage for SB in guiding the stent postdilatation.
4. Conclusions
StentBoost enhancement has superior correlations for stent expansion measured by IVUS when compared with QCA. StentBoost enhancement improved stent visualization and identification of stent underexpansion to guide stent postdilatation.
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Peer review under responsibility of Egyptian Society of Cardiology.
References
- 1.Fujii K., Mintz G.S., Kobayashi Y. Contribution of stent underexpansion to recurrence after sirolimus-eluting stent implantation for in-stent restenosis. Circulation. 2004;109:1085–1088. doi: 10.1161/01.CIR.0000121327.67756.19. [DOI] [PubMed] [Google Scholar]
- 2.de Feyter P.J., Kay P., Disco C., Serruys P.W. Reference chart derived from post-stent-implantation intravascular ultrasound predictors of 6-month expected restenosis on quantitative coronary angiography. Circulation. 1999;100:1777–1783. doi: 10.1161/01.cir.100.17.1777. [DOI] [PubMed] [Google Scholar]
- 3.Mintz G.S., Nissen S.E., Anderson W.D. ACC clinical expert consensus document on standards for the acquisition, measurement and reporting of intravascular ultrasound studies: a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents (Committee to Develop a Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS)) J Am Coll Cardiol. 2001;37:1478–1492. doi: 10.1016/s0735-1097(01)01175-5. [DOI] [PubMed] [Google Scholar]
- 4.Koolen J.J., van het Veer M., Hanekamp C.E. StentBoost image enhancement: first clinical experience. Medicamundi. 2005;49:1–6. [Google Scholar]
- 5.Serruys P.W., Ong A.T., van Herwerden L.A. Five-year outcomes after coronary stenting versus bypass surgery for the treatment of multivessel disease: the final analysis of the Arterial Revascularization Therapies Study (ARTS) randomized trial. J Am Coll Cardiol. 2005;46:575–581. doi: 10.1016/j.jacc.2004.12.082. [DOI] [PubMed] [Google Scholar]
- 6.Hausmann D., Erbel R., Alibelli-Chemarin M.J. The safety of intracoronary ultrasound. A multicenter survey of 2207 examinations. Circulation. 1995;91:623–630. doi: 10.1161/01.cir.91.3.623. [DOI] [PubMed] [Google Scholar]
- 7.Mehanna E.A., Attizzani G.F., Kyono H. Assessment of coronary stent by optical coherence tomography, methodology and definitions. Int J Cardiovasc Imaging. 2011;27:259–269. doi: 10.1007/s10554-010-9793-y. Epub 2011 Feb 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garrone P., Biondi-Zoccai G., Salvetti I. Quantitative coronary angiography in the current era: principles and applications. J Interv Cardiol. 2009;22:527–536. doi: 10.1111/j.1540-8183.2009.00491.x. [DOI] [PubMed] [Google Scholar]
- 9.Sanidas Elias A., Maehara Akiko, Barkama Ravit. Enhanced stent imaging improves the diagnosis of stent underexpansion and optimizes stent deployment. Catheterization Cardiovasc Intervent. 2013;81:438–445. doi: 10.1002/ccd.24353. [DOI] [PubMed] [Google Scholar]
- 10.Cura Fernando, Albertal Mariano, Candiello Alfonsina. StentBoost visualization for the evaluation of coronary stent expansion during percutaneous coronary interventions. Cardiol Ther. 2013;2:171–180. doi: 10.1007/s40119-013-0023-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Fei-fei, Zhang Li-wei, Huang Dang-sheng. A novel angiographic technique, StentBoost, in comparison with intravascular ultrasound to assess stent expansion. Chinese Med J. 2011;124:939–942. [PubMed] [Google Scholar]
- 12.Mishell Jacob M., Vakharia Kalpesh T., Ports Thomas A. Determination of adequate coronary stent expansion using StentBoost, a novel fluoroscopic image processing technique. Catheteriz Cardiovasc Intervent. 2007;69(1):84–93. doi: 10.1002/ccd.20901. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Jiao, Duan Yuan Yuan, Jin Zhi Geng. Stent boost subtract imaging for the assessment of optimal stent deployment in coronary ostial lesion intervention comparison with intravascular ultrasound. Int Heart J. 2015;56:37–42. doi: 10.1536/ihj.14-169. [DOI] [PubMed] [Google Scholar]
- 14.Alghamdi Ali, Al-khaldi Abdulaziz, Balgaith M. Stent boost versus intravascular ultrasound to determine stent expansion. J Saudi Heart Assoc. 2012;24(4):283–285. [Google Scholar]