Abstract
Background
Hand-foot-mouth disease (HFMD) is a common infectious disease in China and occurs mostly in infants and children. Beijing is a densely populated megacity, in which HFMD has been increasing in the last decade. The aim of this study was to quantify spatio-temporal characteristics of HFMD and the relationship between meteorological factors and HFMD incidence in Beijing, China.
Methods
Daily counts of HFMD cases from January 2010 to December 2012 were obtained from the Beijing Center for Disease Prevention and Control (CDC). Seasonal trend decomposition with Loess smoothing was used to explore seasonal patterns and temporal trends of HFMD. Bayesian spatiotemporal Poisson regression models were used to quantify spatiotemporal patterns of HFMD incidence and associations with meteorological factors.
Results
There were 114,777 HFMD cases reported to Beijing CDC from 1 January 2010 to 31 December 2012 and the raw incidence was 568.6 per 100,000 people. May to July was the peak period of HFMD incidence each year. Low-incidence townships were clustered in central, northeast and southwest regions of Beijing. Mean temperature, relative humidity, wind velocity and sunshine hours were all positively associated with HFMD. The effect of wind velocity was significant with a RR of 3.30 (95%CI: 2.37, 4.60) per meter per second increase, as was sunshine hours with a RR of 1.20 (95%CI: 1.02, 1.40) per 1 hour increase.
Conclusions
The distribution of HFMD in Beijing was spatiotemporally heterogeneous, and was associated with meteorological factors. Meteorological monitoring could be incorporated into prediction and surveillance of HFMD in Beijing.
Electronic supplementary material
The online version of this article (10.1186/s12879-018-3071-3) contains supplementary material, which is available to authorized users.
Keywords: Hand-foot-mouth disease, Spatio-temporal analysis, Bayesian approach, Meteorological factors
Background
Hand, foot and mouth disease (HFMD) is a common infectious disease that occurs mostly in infants and children, but can also occur in adolescents and occasionally in adults. HFMD is mainly caused by coxsackievirus A16 (Cox A16), which usually results in a mild self-limiting disease with few complications, and enterovirus 71 (EV71), which has been associated with serious complications and may be fatal. [1, 2] Outbreaks of HFMD have occurred in the Western Pacific Region in recent years, and China is one of the Asian countries with the highest number of reported cases [2, 3]. An EV71 vaccine was first generated and approved in China in 2016. However, several recent HFMD outbreaks have been caused by other pathogens, making it important to develop multivalent vaccines to control HFMD epidemics [4].
HFMD has been listed as a notifiable Class C infectious disease in the national communicable disease surveillance and reporting system of China since May 2008. Compared with 2008, the number of reported cases in 2010 was approximately four times higher [5]. In January 2017, there were 77,412 cases of HFMD including 5 deaths reported in mainland China [6]. The increasing burden of HFMD has become a serious public health problem in China, causing government concern about the risk factors of this disease and greater interest in approaches to effectively prevent and control HFMD.
In China, the epidemiological characteristics, risk factors and spatiotemporal patterns of HFMD have been studied at a national scale [3, 7–10]. Considering the extensive area and diverse demographic, economic and climatic characteristics of China, the distribution and risk factors likely vary in different regions. Previous studies in Guangzhou, Wuhan, Zhengzhou, Shanxi and Hong Kong have explored relationships between meteorological variables and HFMD using time-series analysis, through which the seasonal and temporal characteristics of HFMD have been characterized. Temperature and relative humidity were found to be associated with incidence of this disease [11–16]. In Beijing, Xu et al. [17] found a non-linear association between temperature and HFMD incidence.
In addition to temporal dynamics, HFMD epidemics demonstrate spatial patterns, which have been characterized in different areas [18–22]. However, few spatio-temporal studies have explored the relationship between HFMD incidence and meteorological factors and simultaneously quantified spatial and temporal patterns. Bayesian approaches could be utilized both to quantify spatio-temporal variation and identify how meteorological factors affect HFMD incidence [23, 24]. Such studies could help establish the utility of meteorological monitoring as an adjunct for the surveillance and control of HFMD.
This study aims to quantify seasonal patterns, temporal trends and the spatio-temporal distribution of HFMD incidence at a township level in Beijing, and investigate the relationship between meteorological factors and HFMD incidence in this city.
Methods
Study area
Beijing, the capital city of China, is located in the northern tip of the roughly triangular North China Plain, at a latitude of 39″26′ to 41″03’ N. The area of Beijing is 16,410 km2 with 14 urban administrative districts and two rural counties, comprised of 304 townships. The population is 19.6 million, with townships ranging from 2000 to 359,400 people (The Sixth National Population Census, Beijing, 2010). Beijing has a rather dry, monsoon-influenced continental climate with hot, humid summers and cold, dry winters. As townships are the smallest administrative units for monitoring of infectious diseases, they were used as the geographical unit for this spatiotemporal analysis.
HFMD data
Data on HFMD cases that were reported from January 2010 to December 2012 were obtained from the Beijing Center for Disease Prevention and Control (CDC). The data were collected from the China Information System for Disease Control and Prevention (CISDCP) with age, gender, occupation, address and dates of onset and diagnosis. HFMD cases were diagnosed based on the symptoms of fever, vesicular lesions on hands, feet, mouth and occasionally the buttocks, which are defined by the National Guidelines published by Chinese Ministry of Health in 2009 [25]. Severe cases are HFMD cases associated with meningitis, encephalitis, and severe complications, including neurological, cardiovascular and respiratory problems. All HFMD cases are required to be reported to CISDCP within 24 h after diagnosis.
Meteorological and population data
Meteorological factors investigated included mean temperature, maximum temperature, minimum temperature, relative humidity, atmospheric pressure, precipitation, sunshine hours and wind velocity. Daily data on these variables were collected from the China Meteorological Data Sharing Service System (http://data.cma.cn/). A weather monitoring station is located in Daxing District (N39°48′, E116°28′) in southeast Beijing, from which the data are usually used to represent the meteorological conditions of the whole of Beijing city [17]. Thus, the meteorological data were temporally but not spatially variable. We obtained population data from the Beijing Area Statistics Yearbook from 2005 to 2015, which were used to calculate linear monthly growth rates at the township level and to estimate monthly population counts between January 2010 and December 2012.
Statistical analysis
Seasonal trend decomposition with Loess smoothing was used to explore seasonal patterns and temporal trends of HFMD, using the statistical software R version 3.3.2 (R Development Core Team, 2016).
Pairwise Spearman correlation analysis was conducted to detect correlations between the meteorological factors. For pairs of variables with a correlation coefficient > |0.7|, only one member of the pair was included in multivariable models (the member with the lowest p-value in a bivariate regression model).
The standardized morbidity ratios (SMRs) of each town were calculated by dividing the observed number of cases by the expected number, which was calculated as the product of overall incidence of the city and the average population for each township during the study period.
In this study we used a Bayesian conditional auto regressive (CAR) model approach, with parameter estimation done by Markov chain Monte Carlo (MCMC) methods. This approach allows estimation of spatial variability of disease risk and the effect of covariates [26, 27], overcoming the issue of spatial autocorrelation violating the assumption of independence [23, 28]. In recent years, CAR models have been used to analyze the spatial distribution of malaria, filariasis, and schistosomiasis [26, 29, 30], but there have been fewer applications to other infectious diseases.
Using the Bayesian framework, Poisson regression models were used to quantify spatiotemporal variation of HFMD incidence and associations with selected meteorological factors. The models were implemented using the WinBUGS software, version 1.4.3 (Medical Research Council, Biostatistics Unit, Cambridge, United Kingdom). Three Bayesian models (non-spatial, spatial and spatiotemporal) were constructed. The regression models assumed that the observed HFMD cases followed a Poisson distribution with a mean (μ):
where Yij is the observed number of cases in town i, month j; Eij is the expected number of cases in town i, month j; and θij is the relative risk (SMR) in town i, month j.
The non-spatial model was defined as:
The spatial model was defined as:
The spatio-temporal model was defined as:
where α is the intercept; βk are the k regression coefficients and Xkij are the covariates (different meteorological factors); vi is an unstructured random effect with mean zero and variance ; ui is a spatially structured random effect with mean zero and variance ; gj is the autoregressive time effect with mean zero and variance ; di are the spatially smoothed town-level temporal trend coefficients; and ω is the provincial average temporal trend coefficient.
For the intercept (α) flat prior distributions was applied. For all the coefficients (β) and the provincial average temporal trend coefficient (ω), normal prior probability distributions were used and assumed with a mean = 0 and a precision (inverse of variance) = 0.0001. The spatial structuring in structured random effect (ui) and spatially smoothed town-level temporal trend coefficients (di) was modelled using a CAR prior structure, which is defined by a simple adjacency weights matrix to determine the spatial relationships between townships. If two towns were adjacent, the weight = 1 and if they were not adjacent, the weight = 0. The weights matrix was generated in ArcGIS (version 10.3, ESRI, Redlands, CA). The priors for the precision of vi, ui, gj and di were specified using non-informative gamma distributions with a shape parameter = 0.5 and a scale parameter = 0.0005.
The model with the lowest deviance information criterion (DIC) was chosen as the best-fitting model. The model was run for 300,000 iterations after an initial burn-in of 10,000 iterations, and was assessed for convergence. After model convergence, samples from the posterior distributions of each random variable were stored and analyzed to provide summary estimates.
Results
Seasonal patterns and temporal trends of HFMD
There were 114,777 HFMD cases reported to Beijing CDC from 1 January 2010 to 31 December 2012, of which the sex ratio was 1.5 (male/female). There were 1244 (1.08%) severe cases and 27 (0.02%) deaths. Five thousand eight hundred and ninety-one cases were laboratory confirmed with 2516 infected with Cox A16 (42.71%) and 2406 infected with EV17 (40.84%). Most cases were distributed in children aged 1 to 4 years old (78.88%). Of all the children who were reported to have HFMD, 51.29% were cared for in homes, with an incidence of 292.7 per 100,000 people from 2010 to 2012, and 42.63% were cared for in nurseries, with an incidence of 243.3 per 100,000 people from 2010 to 2012 (Table 1). Table 2 shows the Spearman correlation of meteorological factors. Mean temperature, relative humidity, precipitation, wind velocity and sunshine hours were included in multivariable models for pairs of these variables with correlation coefficients less than |0.7|.
Table 1.
Summary characteristics of HFMD cases in Beijing, 2010–2012
| 2010 | 2011 | 2012 | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | Male | Female | Total | Male | Female | Total | Male | Female | Total | |
| 0- | 1606 | 972 | 2578 | 1081 | 610 | 1691 | 1055 | 722 | 1777 | 6046 |
| 1- | 5819 | 3770 | 9589 | 3823 | 2383 | 6206 | 4409 | 3070 | 7479 | 23,274 |
| 2- | 5851 | 3888 | 9739 | 3882 | 2710 | 6592 | 4460 | 2884 | 7344 | 23,675 |
| 3- | 6505 | 4187 | 10,692 | 4310 | 2906 | 7216 | 5423 | 3426 | 8849 | 26,757 |
| 4- | 3674 | 2408 | 6082 | 2775 | 1849 | 4624 | 3684 | 2445 | 6129 | 16,835 |
| 5- | 1918 | 1332 | 3250 | 1279 | 834 | 2113 | 1967 | 1297 | 3264 | 8627 |
| 6–14 | 1745 | 1166 | 2911 | 1168 | 861 | 2029 | 1869 | 1335 | 3204 | 8144 |
| ≥15 | 253 | 313 | 566 | 155 | 216 | 371 | 212 | 270 | 482 | 1419 |
| Occupation | ||||||||||
| Scattered children | 14,246 | 9226 | 23,472 | 9415 | 6147 | 15,562 | 11,938 | 7902 | 19,840 | 58,874 |
| Nursery children | 11,688 | 7642 | 19,330 | 8128 | 5383 | 13,511 | 9725 | 6365 | 16,090 | 48,931 |
| Others | 1437 | 1168 | 2605 | 930 | 839 | 1769 | 1412 | 1182 | 2594 | 6968 |
| Pathogen | ||||||||||
| Cox A16 | 371 | 255 | 626 | 361 | 246 | 607 | 787 | 496 | 1283 | 2516 |
| EV17 | 583 | 405 | 988 | 351 | 243 | 594 | 488 | 336 | 824 | 2406 |
| Other | 281 | 166 | 447 | 122 | 81 | 203 | 193 | 126 | 319 | 969 |
| Number of severe cases | 378 | 232 | 610 | 175 | 103 | 278 | 215 | 141 | 356 | 1244 |
| Number of deaths | 14 | 4 | 18 | 4 | 1 | 5 | 3 | 1 | 4 | 27 |
| Total | 27,371 | 18,036 | 45,407 | 18,473 | 12,369 | 30,842 | 23,079 | 15,449 | 38,528 | 114,777 |
Table 2.
Pairwise Spearman correlation of meteorological factors
| Meant | Maxt | Mint | rh | ap | Rain | ws | |
|---|---|---|---|---|---|---|---|
| Maxt | 0.987* | ||||||
| Mint | 0.981* | 0.947* | |||||
| rh | 0.389* | 0.334* | 0.474* | ||||
| ap | −0.869* | −0.865* | −0.851* | −0.375* | |||
| Rain | 0.181* | 0.142* | 0.259* | 0.471* | −0.248* | ||
| ws | −0.009 | −0.015 | −0.003 | −0.448* | −0.02 | 0.033 | |
| sun | 0.165* | 0.248* | 0.032 | −0.571* | −0.072* | −0.339* | 0.273* |
Meant mean temperature, maxt maximum temperature, mint minimum temperature, rh relative humidity, ap atmospheric pressure, rain precipitation, ws wind velocity, sun sunshine hours
*P < 0.05
Figure 1 shows the time-series decompositions of HFMD cases. There was an evident seasonal pattern with a peak occurring between May and July of each year. After the seasonal pattern was removed, the inter-annual pattern was characterized by peaks at the start and towards the end of the study period.
Fig. 1.
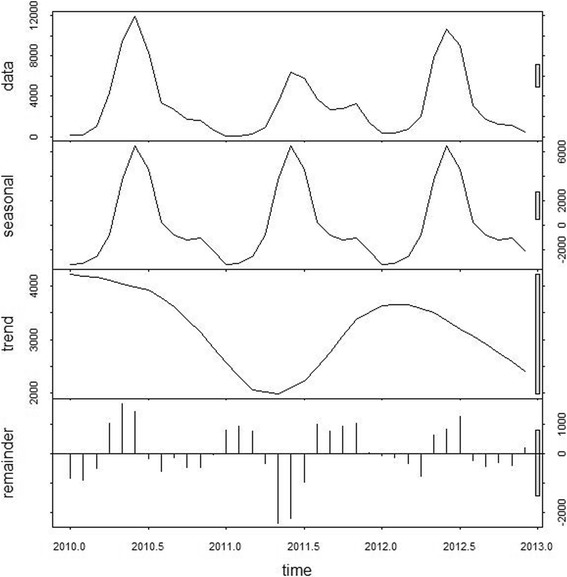
Decomposed HFMD time-series in Beijing, 2010–2012
Figure 2 shows the spatial distribution of cumulative incidence of HFMD at the township level during the study period. The raw incidence was 231.5 cases per 100,000 people in 2010, 152.8 cases per 100,000 in 2011 and 186.2 cases per 100,000 people in 2012, with an average annual incidence of 190.2 for these 3 years. Most cases were reported in central and southern regions (Additional file 2: Figure S1).
Fig. 2.
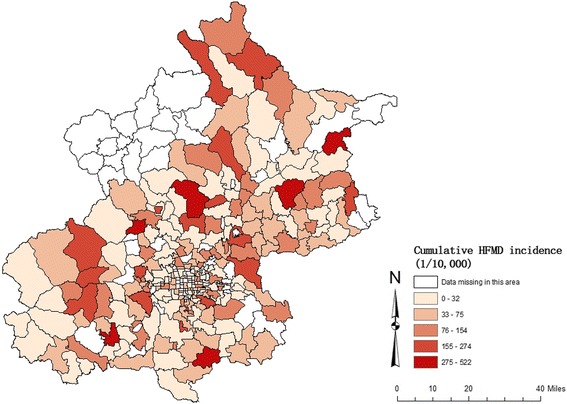
Spatial distribution of cumulative HFMD incidence in Beijing, 2010–2012
Temporal variability of meteorological variables
The monthly time-series of the meteorological factors is illustrated in Fig. 3, with summary statistics provided in Table 4. Mean temperature had a seasonal pattern with peaks in July and August (summer in Beijing). Relative humidity and precipitation had similar patterns, peaking just after temperature (late summer). Wind velocity did not show a regular pattern and sunshine hours were longest in spring and autumn.
Fig. 3.
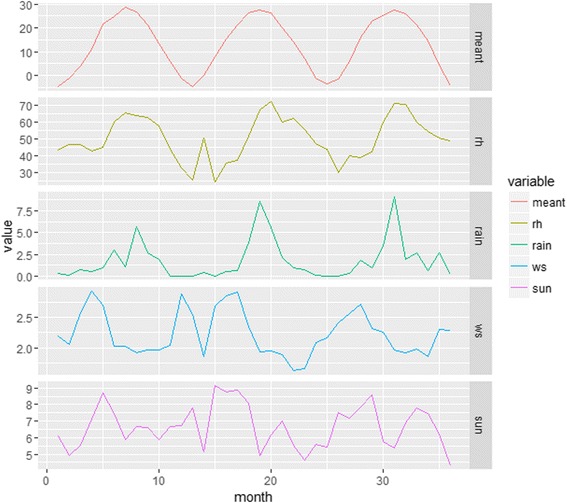
Monthly time-series for the meteorological factors in Beijing, 2010–2012. (meant, mean temperature (°C); rh, relative humidity (%); rain, precipitation (mm); ws, wind velocity (m/s); sun, sunshine (hour))
Table 4.
Bayesian Poisson regression model of the association between meteorological factors and HFMD in Beijing, 2010–2012
| Variable | Mean | Range | Coefficient (95%CI) | Relative Risk (95%CI) |
|---|---|---|---|---|
| Mean temperature (°C) | 12.94 | −4.78-28.61 | 0.06(0.03,0.09) | 1.06(1.03,1.10) |
| Relative humidity (%) | 50.35 | 24.77–71.90 | 0.05(0.01,0.08) | 1.05(1.01,1.08) |
| Precipitation (mm) | 1.79 | 0–9.16 | 0.03(−0.06,0.12) | 1.03(0.94,1.12) |
| Wind velocity (m/s) | 2.23 | 1.62–2.93 | 1.19(0.86,1.53) | 3.30(2.37,4.60) |
| Sunshine (hour) | 6.67 | 4.32–9.14 | 0.18(0.02,0.34) | 1.20(1.02,1.40) |
| Overall Intercept | −9.53(−11.37,-7.68) |
Statistical models
A high level of correlation was found between mean temperature and both maximum temperature (r = 0.987) and minimum temperature (r = 0.981). Mean temperature was included in the subsequent model as a temperature index. Air pressure was also highly correlated with mean temperature (r = − 0.869) and was excluded. Thus five meteorological factors (mean temperature, relative humidity, precipitation, sunshine hours and wind velocity) were chosen for inclusion in the regression models for further analysis. Table 3 summarizes the DIC values for the three models. The spatio-temporal model had the lowest DIC, indicating it was the best performing model. The spatio-temporal model was therefore selected as the final model, with meteorological factors included as covariates.
Table 3.
The Deviance Information Criterion values for Bayesian models of HFMD incidence
| Model | DIC | |
|---|---|---|
| Without covariates | With covariates | |
| Non-spatial model | 55,654.9 | – |
| Spatial model | 55,579.0 | – |
| Spatial-temporal model | 52,352.4 | 52,254.8 |
Table 4 presented the association between meteorological factors and HFMD incidence, which indicated that mean temperature, relative humidity, wind velocity and sunshine hours were all positively associated with HFMD risk, whereas precipitation was not significantly associated with HFMD risk. The effect of wind velocity, sunshine hours, mean temperature and relative humidity was significant with RRs of 3.30 (95%CI: 2.37, 4.60), 1.20 (95%CI: 1.02, 1.40), 1.06 (95%CI: 1.03, 1.10) and 1.05 (95%CI: 1.01, 1.08) per unit increase, respectively.
Figure 4 shows maps of the township-level mean for each of the variance components of the model (A – unstructured spatial random effect; B – structured spatial random effect; and C – spatially smoothed temporal trend). Clear clusters of low relative risk were present in central, northeast and southwest Beijing, with clusters of high relative risk evident in the north and southeast. The spatially smoothed temporal trend did not show a very clear pattern, with small clusters of higher and lower than average trends interspersed throughout Beijing.
Fig. 4.
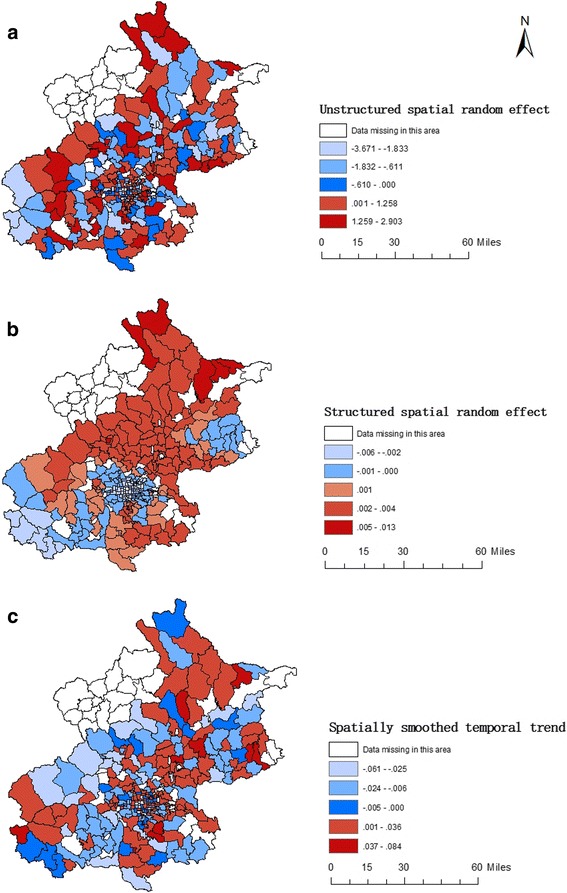
Unstructured, structured spatial random effect and spatially smoothed temporal trend for HFMD in Beijing, 2010–2012
Discussion
There were 114,777 HFMD cases from 2010 to 2012, with an average incidence of 190.2 cases per 100,000 people, which was higher than some other provinces in China like Sichuan (43.7/100,000, 2008–2013) [31], Shandong (104.4/100,000, 2008–2012) [23], Jiangsu (126.3/100,000, 2009–2013) [24], and Guangdong (167.8/100,000, 2008–2011) [32], but was lower than Guangxi (298.3/100,000, 2008–2013) [21]. The total incidence in males was higher than that in females, which was consistent with previous studies [23, 24], suggesting the susceptibility of males. Most cases were concentrated in children from 1 to 4 years old, which were the main target groups for the surveillance and control of HFMD in Beijing.
From 2010 to 2012, there was an oscillating inter-annual pattern with peaks at the start and towards the end of the study period in HFMD incidence. The peaks of HFMD cases were in May to July every year, which corresponds to early summer in Beijing. There was another lower peak occurring in the autumn of 2011. Previous studies in Singapore and Malaysia have shown seasonal outbreaks of HFMD between March and May [22], whereas in Japan, outbreaks typically occur during the summer months [33]. In China, single seasonal peaks have been shown to appear between April and August in Shandong province, as well as Henan province, both also located in North China [23]. Double peaks have been observed in some southern districts of China, usually in the warm months of spring and autumn [34, 35].
The areas of Beijing with high HFMD incidence were distributed in districts circumjacent to central Beijing like Mentougou, Fangshan, Changping, Daxing, Tongzhou and north Huairou district (Fig. 2). The spatial random effect (Fig. 4) represented the residual spatial clustering after accounting for the meteorological variables [36]. Socio-economic levels, medical and health facility access, surveillance and control capacities of HFMD in different districts might be potential factors influencing this residual variation, which are more amenable to control than meteorological factors. Thus, clusters of high residual risk should be the focus of greater attention in the prevention and control of HFMD with measures like reinforcing community health education and improving health care levels of nurseries.
Mean temperature, relative humidity, wind velocity and sunshine hours were positively associated with HFMD incidence. Temperature and relative humidity were found to be associated with HFMD in previous studies [14, 33, 37]. There are two main ways that meteorological factors can influence HFMD: by affecting the external environment to change the biological activity, propagation and transmission of pathogen; and by impacting on human behavior [38–40]. During warmer months, communal physical activity among children and adolescents increases, which may promote the risk of contact transmission of HFMD [41–43]. A study in Shandong province [44] revealed a strong association between HFMD incidence and wind speed as shown in our study. They suggested that wind can promote air pollutants like particulate matter carrying enterovirus and thus accelerate the spread of HFMD [38]. As a major atmospheric pollutant, fine particulate matter (PM2.5, defined as particle less than 2.5 mm aerodynamic diameter) has a small size and a relatively large surface area, which makes it easy to absorb viruses in the air. Especially considering the high levels of PM2.5 pollution in Beijing [45, 46], it is plausible that high wind velocity is a risk factor for the spread of HFMD. On the other hand, children spend more time with indoor activities in confined spaces during windy periods, which would increase the chances of EV transmission, a possible indirect explanation supporting the association of increased transmission with windy conditions. Longer sunshine hours was also found to be a risk factor in our study, which was different from studies in other places [23, 24]. Sunlight could promote virus replication or inactivate human virus to some extent [47–49]. It is also possible that children engage more in outside activities on sunny days, thus increasing contact among children. Precipitation was not significantly associated with HFMD incidence in this study, which was consistent with the study conducted in Shandong Province [23]. A study in Hefei [50] of China found that extreme precipitation (90th percentile of precipitation as the analytical cut-off point) was significantly associated with childhood HFMD. Beijing is a temperate city with lower mean monthly precipitation (54.9 mm) than Hefei (130.8 mm), which has a subtropical monsoon humid climate The threshold effect of precipitation on HFMD might be the reason for inconsistent conclusions [50].
There were previous studies conducted in Beijing investigating spatio-temporal patterns of HFMD and the effects of meteorological factors on this disease. Additional file 1: Table S1 showed the detailed comparison of these studies with ours. Wang et al. [18] used spatial filtering combined with scan statistics methods to detect HFMD clusters in Beijing from 2008 to 2012, finding that the most likely space-time cluster was located in the southwest of Beijing. Dong et al. [38] used geographically weighted regression model to explore the seasonal influence of weather factors on incidents of HFMD from 2008 to 2011 in Beijing. Our study considered spatial and temporal variability of all the variables in Bayesian CAR model, which could quantify spatio-temporal variation and identify the effects of meteorological factors at the same time. Compared with the study conducted by Dong et al., we found that sunshine hours was also positively associated with HFMD incidence. The effects of mean temperature and wind velocity were consistent while relative humidity and precipitation showed different effect estimates. The threshold effect of precipitation on HFMD incidence might be the reason for the discordant conclusions.
Demographic and socio-economic characteristics which include child population density, Gross Domestic Product per capita, number of health agencies, proportion of children in nursery, and proportion of children in primary school might be confounders affecting the incidence of HFMD. Additional file 3: Figure S2, Additional file 4: Figure S3, Additional file 5: Figure S4, Additional file 6: Figure S5, Additional file 7: Figure S6 were the spatial distributions of these factors, which did not show obvious association with HFMD clusters. However, data of the demographic and socio-economic characteristics that we obtained from Beijing Area Statistics Yearbook are in district level for now and further statistical analysis considering these factors in higher resolution should be conducted if available.
In terms of study limitations, the meteorological data were obtained from one monitoring center, which was assumed to represent the meteorological conditions of the whole city. It would be preferable to incorporate spatial variation in the meteorological variables. However, Beijing has a relatively small area, and we do not expect as much spatial variability between townships as temporal variability across months and years. Secondly, the time-series was short (3 years) and to quantify seasonal and interannual variability and associated factors it would be better to analyze data from a much longer time period. Finally, this was an ecological study and it is important to note that measured associations were only valid at the level of the township and cannot be applied to individuals, or different levels of spatial aggregation [23].
Conclusions
In this study, we detected the spatial and temporal distribution of HFMD and its association with meteorological factors by using Bayesian spatiotemporal regression models in Beijing, China. The incidence of HFMD cases had a seasonal pattern in Beijing and the peaks were often in warm months. Certain districts with high HFMD incidence should be the focus of greater attention. Meteorological factors like temperature, relative humidity, wind velocity and sunshine hours might be the driving factors for the spatiotemporal dynamics of HFMD, and could be considered as targets for meteorological monitoring of HFMD risk.
Additional files
Table S1. Comparison of studies conducted in Beijing investigating spatio-temporal patterns of HFMD and the effects of meteorological factors. (DOCX 16 kb)
Figure S1. Spatial distribution of cumulative HFMD cases in Beijing, 2010–2012. (JPEG 289 kb)
Figure S2. Spatial distribution of average child population density in Beijing, 2010–2012. (JPEG 128 kb)
Figure S3. Spatial distribution of Gross Domestic Product per capita (billion) in Beijing, 2012. (JPEG 134 kb)
Figure S4. Spatial distribution of health agency counts (per square kilometers) in Beijing, 2012. (JPEG 141 kb)
Figure S5. Spatial distribution of proportion of children in nursery in Beijing, 2012. (JPEG 137 kb)
Figure S6. Spatial distribution of proportion of children in primary school in Beijing, 2012. (JPEG 123 kb)
Acknowledgments
The authors thank the Beijing Center for Disease Control and Prevention and China Meteorological Data Sharing System for providing the data.
Funding
None.
Availability of data and materials
The dataset and materials used for this study can be available only upon the approval by the Beijing Center for Disease Control and Prevention.
Abbreviations
- CAR
Bayesian conditional auto regressive
- CDC
Center for Disease Prevention and Control
- CISDCP
China Information System for Disease Control and Prevention
- Cox A16
Coxsackievirus A16
- DIC
Deviance information criterion
- EV71
Enterovirus 71
- HFMD
Hand-foot-mouth disease
- MCMC
Markov chain Monte Carlo
- SMRs
Standardized morbidity ratios
Authors’ contributions
ACAC conceived and designed the study; LT undertook statistical analysis and drafted the manuscript; MX and FL helped supervise the data quality; LJ contributed data collection and database conduction; XP directed its implementation. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12879-018-3071-3) contains supplementary material, which is available to authorized users.
Contributor Information
Lin Tian, Email: tianlin@bjmu.edu.cn.
Fengchao Liang, Email: liangfengchao126@126.com.
Meimei Xu, Email: xumeimei2005@163.com.
Lei Jia, Email: lailajia@126.com.
Xiaochuan Pan, Email: xcpan@bjmu.edu.cn.
Archie C. A. Clements, Email: director.rsph@anu.edu.au
References
- 1.Aswathyraj S, et al. Hand, foot and mouth disease (HFMD): emerging epidemiology and the need for a vaccine strategy. Med Microbiol Immunol. 2016;205(5):397–407. doi: 10.1007/s00430-016-0465-y. [DOI] [PubMed] [Google Scholar]
- 2.WHO . Hand, foot and mouth disease fact sheets. 2012. [Google Scholar]
- 3.Xiao X, et al. The exposure-response relationship between temperature and childhood hand, foot and mouth disease: a multicity study from mainland China. Environ Int. 2017;100:102–109. doi: 10.1016/j.envint.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 4.Mao QY, et al. EV71 vaccine, a new tool to control outbreaks of hand, foot and mouth disease (HFMD) Expert Rev Vaccines. 2016;15(5):599–606. doi: 10.1586/14760584.2016.1138862. [DOI] [PubMed] [Google Scholar]
- 5.WHO . A guide to clinical management and public health response for hand, foot and mouth disease. 2011. [Google Scholar]
- 6.WHO . Hand, foot and mouth disease surveillance summary in the western Pacific region. 2017. [Google Scholar]
- 7.Zhuang ZC, et al. Epidemiological research on hand, foot, and mouth disease in mainland China. Viruses. 2015;7(12):6400–6411. doi: 10.3390/v7122947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang C, et al. Spatiotemporal cluster patterns of hand, foot, and mouth disease at the county level in mainland China, 2008-2012. PLoS One. 2016;11(1):e0147532. doi: 10.1371/journal.pone.0147532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liao J, et al. Short-term effects of climatic variables on hand, foot, and mouth disease in mainland China, 2008-2013: a multilevel spatial Poisson regression model accounting for Overdispersion. PLoS One. 2016;11(1):e0147054. doi: 10.1371/journal.pone.0147054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang C, et al. Different effects of meteorological factors on hand, foot and mouth disease in various climates: a spatial panel data model analysis. BMC Infect Dis. 2016;16:233. doi: 10.1186/s12879-016-1560-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song Y, et al. Time series analyses of hand, foot and mouth disease integrating weather variables. PLoS One. 2015;10(3):e0117296. doi: 10.1371/journal.pone.0117296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei J, et al. The effect of meteorological variables on the transmission of hand, foot and mouth disease in four major cities of Shanxi province, China: a time series data analysis (2009-2013) PLoS Negl Trop Dis. 2015;9(3):e0003572. doi: 10.1371/journal.pntd.0003572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang Y, et al. Effect of meteorological variables on the incidence of hand, foot, and mouth disease in children: a time-series analysis in Guangzhou, China. BMC Infect Dis. 2013;13:134. doi: 10.1186/1471-2334-13-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang P, Goggins WB, Chan EY. Hand, foot and mouth disease in Hong Kong: a time-series analysis on its relationship with weather. PLoS One. 2016;11(8):e0161006. doi: 10.1371/journal.pone.0161006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng H, et al. Time series analysis of hand-foot-mouth disease hospitalization in Zhengzhou: establishment of forecasting models using climate variables as predictors. PLoS One. 2014;9(1):e87916. doi: 10.1371/journal.pone.0087916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen B, et al. Time series analysis of reported cases of hand, foot, and mouth disease from 2010 to 2013 in Wuhan, China. BMC Infect Dis. 2015;15:495. doi: 10.1186/s12879-015-1233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu M, et al. Non-linear association between exposure to ambient temperature and Children's hand-foot-and-mouth disease in Beijing, China. PLoS One. 2015;10(5):e0126171. doi: 10.1371/journal.pone.0126171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, et al. Epidemiological analysis, detection, and comparison of space-time patterns of Beijing hand-foot-mouth disease (2008-2012) PLoS One. 2014;9(3):e92745. doi: 10.1371/journal.pone.0092745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao F, Huang P. Epidemiological characteristics and temporal-spatial clustering analysis of hand, foot and mouth disease in Nanchang city 2008-2012. Infect Dis (Lond) 2015;47(1):33–38. doi: 10.3109/00365548.2014.964762. [DOI] [PubMed] [Google Scholar]
- 20.Mao YJ, et al. Epidemiological features and spatio-temporal clusters of hand-foot-mouth disease at town level in Fuyang, Anhui Province, China (2008-2013) Epidemiol Infect. 2016;144(15):3184–3197. doi: 10.1017/S0950268816001710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie YH, et al. Spatio-temporal clustering of hand, foot, and mouth disease at the county level in Guangxi, China. PLoS One. 2014;9(2):e88065. doi: 10.1371/journal.pone.0088065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sham NM, et al. Temporal and spatial mapping of hand, foot and mouth disease in Sarawak, Malaysia. Geospat Health. 2014;8(2):503–507. doi: 10.4081/gh.2014.39. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, et al. Spatio-temporal analysis of the relationship between climate and hand, foot, and mouth disease in Shandong province, China, 2008-2012. BMC Infect Dis. 2015;15:146. doi: 10.1186/s12879-015-0901-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu W, et al. Spatiotemporal dynamics of hand-foot-mouth disease and its relationship with meteorological factors in Jiangsu Province, China. PLoS One. 2015;10(6):e0131311. doi: 10.1371/journal.pone.0131311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Health, C.M.o . National guidelines for HFMD prevention and control. 2009. [Google Scholar]
- 26.Mabaso ML, et al. Spatio-temporal analysis of the role of climate in inter-annual variation of malaria incidence in Zimbabwe. Int J Health Geogr. 2006;5:20. doi: 10.1186/1476-072X-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Escaramis G, Carrasco JL, Ascaso C. Detection of significant disease risks using a spatial conditional autoregressive model. Biometrics. 2008;64(4):1043–1053. doi: 10.1111/j.1541-0420.2007.00981.x. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez-Prieto V, et al. A Bayesian approach to study the risk variables for tuberculosis occurrence in domestic and wild ungulates in south Central Spain. BMC Vet Res. 2012;8:148. doi: 10.1186/1746-6148-8-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang GJ, et al. A Bayesian-based approach for spatio-temporal modeling of county level prevalence of Schistosoma japonicum infection in Jiangsu province, China. Int J Parasitol. 2005;35(2):155–162. doi: 10.1016/j.ijpara.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Clements AC, et al. Bayesian spatial analysis and disease mapping: tools to enhance planning and implementation of a schistosomiasis control programme in Tanzania. Tropical Med Int Health. 2006;11(4):490–503. doi: 10.1111/j.1365-3156.2006.01594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu L, et al. Spatio-temporal clustering of hand, foot and mouth disease at the county level in Sichuan province, China, 2008-2013. Epidemiol Infect. 2015;143(4):831–838. doi: 10.1017/S0950268814001587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deng T, et al. Spatial-temporal clusters and risk factors of hand, foot, and mouth disease at the district level in Guangdong Province, China. PLoS One. 2013;8(2):e56943. doi: 10.1371/journal.pone.0056943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Onozuka D, Hashizume M. The influence of temperature and humidity on the incidence of hand, foot, and mouth disease in Japan. Sci Total Environ. 2011;410-411:119–125. doi: 10.1016/j.scitotenv.2011.09.055. [DOI] [PubMed] [Google Scholar]
- 34.Zou XN, et al. Etiologic and epidemiologic analysis of hand, foot, and mouth disease in Guangzhou city: a review of 4,753 cases. Braz J Infect Dis. 2012;16(5):457–465. doi: 10.1016/j.bjid.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Ma E, et al. Changing epidemiology of hand, foot, and mouth disease in Hong Kong, 2001-2009. Jpn J Infect Dis. 2010;63(6):422–6. [PubMed] [Google Scholar]
- 36.Clements AC, et al. Space-time variation of malaria incidence in Yunnan province, China. Malar J. 2009;8:180. doi: 10.1186/1475-2875-8-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen HX, et al. Temporal and spatial analysis of hand, foot, and mouth disease in relation to climate factors: a study in the Mekong Delta region, Vietnam. Sci Total Environ. 2017;581-582:766–772. doi: 10.1016/j.scitotenv.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 38.Dong W, et al. The effects of weather factors on hand, foot and mouth disease in Beijing. Sci Rep. 2016;6:19247. doi: 10.1038/srep19247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Humpel N, Owen N, Leslie E. Environmental factors associated with adults’ participation in physical activity: a review. Am J Prev Med. 2002;22(3):188–199. doi: 10.1016/S0749-3797(01)00426-3. [DOI] [PubMed] [Google Scholar]
- 40.Frumkin H. Environmental health: from global to local. 2010. [Google Scholar]
- 41.Suminski RR, et al. Meteorological conditions are associated with physical activities performed in open-air settings. Int J Biometeorol. 2008;52(3):189–197. doi: 10.1007/s00484-007-0110-y. [DOI] [PubMed] [Google Scholar]
- 42.Ooi EE, et al. Seroepidemiology of human enterovirus 71, Singapore. Emerg Infect Dis. 2002;8(9):995–997. doi: 10.3201/eid0809.010397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Belanger M, et al. Influence of weather conditions and season on physical activity in adolescents. Ann Epidemiol. 2009;19(3):180–186. doi: 10.1016/j.annepidem.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 44.Liao Y, et al. A study of spatiotemporal delay in hand, foot and mouth disease in response to weather variations based on SVD: a case study in Shandong Province, China. BMC Public Health. 2015;15:71. doi: 10.1186/s12889-015-1446-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu M, et al. Spatiotemporal analysis of particulate air pollution and ischemic heart disease mortality in Beijing, China. Environ Health. 2014;13:109. doi: 10.1186/1476-069X-13-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeng Q, et al. Characteristics of the exposure-response relationship of particulate matter and mortality: a time series analysis of 7 cities in China. J Occup Environ Med. 2015;57(10):e93–100. doi: 10.1097/JOM.0000000000000541. [DOI] [PubMed] [Google Scholar]
- 47.Wang B, et al. MEK1-ERKs signal cascade is required for the replication of Enterovirus 71 (EV71) Antivir Res. 2012;93(1):110–117. doi: 10.1016/j.antiviral.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 48.Silverman AI, et al. Sunlight inactivation of human viruses and bacteriophages in coastal waters containing natural photosensitizers. Environ Sci Technol. 2013;47(4):1870–1878. doi: 10.1021/es3036913. [DOI] [PubMed] [Google Scholar]
- 49.Carratala A, et al. Solar disinfection of viruses in polyethylene Terephthalate bottles. Appl Environ Microbiol. 2015;82(1):279–288. doi: 10.1128/AEM.02897-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng J, et al. Associations between extreme precipitation and childhood hand, foot and mouth disease in urban and rural areas in Hefei, China. Sci Total Environ. 2014;497-498:484–490. doi: 10.1016/j.scitotenv.2014.08.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparison of studies conducted in Beijing investigating spatio-temporal patterns of HFMD and the effects of meteorological factors. (DOCX 16 kb)
Figure S1. Spatial distribution of cumulative HFMD cases in Beijing, 2010–2012. (JPEG 289 kb)
Figure S2. Spatial distribution of average child population density in Beijing, 2010–2012. (JPEG 128 kb)
Figure S3. Spatial distribution of Gross Domestic Product per capita (billion) in Beijing, 2012. (JPEG 134 kb)
Figure S4. Spatial distribution of health agency counts (per square kilometers) in Beijing, 2012. (JPEG 141 kb)
Figure S5. Spatial distribution of proportion of children in nursery in Beijing, 2012. (JPEG 137 kb)
Figure S6. Spatial distribution of proportion of children in primary school in Beijing, 2012. (JPEG 123 kb)
Data Availability Statement
The dataset and materials used for this study can be available only upon the approval by the Beijing Center for Disease Control and Prevention.


