Abstract
Background
Chinese herbal injections (CHIs) are prepared by extracting and purifying effective substances from herbs (or decoction pieces) using modern scientific techniques and methods. CHIs combined with aspirin + anticoagulants + dehydrant + neuroprotectant (AADN) are believed to be effective for the treatment of acute cerebral infarction (ACI). However, no randomized controlled trial (RCT) has been performed to directly compare the efficacies of different regimens of CHIs. Therefore, we performed a systematic review and network meta-analysis (NMA) to compare the efficacies of different regimens of CHIs for ACI.
Methods
We conducted an overall and systematic retrieval from literature databases of RCTs focused on the use of CHIs to treat ACI up to June 2016. We used the Cochrane Handbook version 5.1.0 and CONSORT statement to assess the risk of bias. The data were analyzed using STATA 13.0 and WinBUGS 1.4.3 software.
Results
Overall, 64 studies with 6225 participants involving 15 CHIs were included in the NMA. In terms of the markedly effective rate, Danhong (DH) + AADN had the highest likelihood of being the best treatment. In terms of the improvement of neurological impairment, Shuxuening (SXN) + AADN had the highest likelihood of being the best treatment. Considering two outcomes, injections of SXN, Yinxingdamo (YXDM), DH, Shuxuetong (SXT), HongHuaHuangSeSu (HHHSS), DengZhanXiXin (DZXX) and Shenxiong glucose (SX) plus AADN were the optimum treatment regimens for ACI, especially SXN + AADN and YXDM + AADN.
Conclusions
Based on the NMA, SXN, YXDM, DH, SXT, HHHSS, DZXX and SX plus AADN showed the highest probability of being the best treatment regimens. Due to the limitations of the present study, our findings should be verified by well-designed RCTs.
Electronic supplementary material
The online version of this article (10.1186/s12906-018-2178-9) contains supplementary material, which is available to authorized users.
Keywords: Network meta-analysis, Acute cerebral infarction, Chinese herbal injection
Background
Acute cerebral infarction (ACI) is one of the most common cerebral vascular diseases, also referred to as ischemic stroke, which is result from ischemia, hypoxia and cerebral blood circulation [1–3]. ACI has the characteristics of high disability and recurrence [4–6]. Besides, it is a major disease leading to serious damage of central nervous system or death [4, 7]. It was estimated that ACI cause 6.2 million mortalities annually worldwide [8]. In traditional Chinese medicine (TCM) theories, ACI refers to “apoplexy”, majorly due to blood stasis syndrome [3]. Therefore, promoting blood flow is of primary importance. It has been proven that TCM is an effective complementary intervention for stroke, especially in the treatment of ischemic stroke [9–13].
Currently, therapies for ACI include thrombolytics, antithrombotics, anticoagulants, and neuroprotectants [14]; this was the Grade-I recommendation in the guidance of diagnosis and treatment of acute ischemic stroke in China 2010, which fully considered the national conditions and clinical experience. Among them, thrombolytic has a short therapeutic time window. Thus, many patients easily miss the effective time window of thrombolysis. ACI patients who are not eligible for thrombolysis therapy should be given oral aspirin, which was approved by the US Food and Drug Administration (FDA), at a dose ranging from 150 to 300 mg/d as soon as possible (level of evidence: A) [15, 16]. And patients with brain edema could use a mannitol intravenous drip. Although more high-quality clinical trials are needed to further demonstrate the efficacy and safety of neuroprotective agents, a number of RCTs have suggested that edaravone and cerebroprotein hydrolysate improve functional outcomes and the safety of patients with ACI [17–20]. Additionally, the therapeutic principle of invigorating blood circulation for removing blood stasis of TCM holds a significant position for ACI. Chinese herbal injections (CHIs) have the characteristics of rapid efficacy and high bioavailability [21–23].Presently, 37 injections are often used in the treatment of cerebral infarction, such as Xueshuantong injection, Shuxuening injection, Mailuoning injection, and Danshenchuanxiongqin injection [24–27]. Clinical data [28–32] from systematic reviews of RCTs have demonstrated the beneficial effects of CHIs for inhibiting platelet aggregation, improving blood microcirculation and nerve function, enhancing the tolerance of ischemic tissue to hypoxia, and protecting against ischemic reperfusion injury.
Hence, this study systematically evaluated the clinical effectiveness of CHIs combined with an aspirin + anticoagulants + dehydrant + neuroprotectant (AADN) regimen in ACI patients that conformed to the standardized treatment of ischemic cerebrovascular disease: integration, individualization and sequencing. However, there is no direct head-to-head evidence revealing the best CHIs for ACI treatment. Determining the superiority of a treatment based on a pairwise comparison meta-analysis is difficult. A network meta-analysis (NMA), which is an extension of a traditional meta-analysis, synthesizes the available evidence to enable simultaneous comparisons of different treatment options that lack direct head-to-head evaluations [33–35]. Therefore, the present study performed a NMA to compare the clinical efficacy of 37 CHIs combined with the AADN regimen to reveal the best CHIs for ACI, aiming to provide more sights for selection of ACI.
Methods
Eligibility criteria
Studies meeting the following criteria were included: (1) Clinical randomized controlled trials (RCTs) using CHIs + AADN to treat ACI regardless of blinding. (2) Cerebrovascular disease was diagnosed according to the standards revised by the Fourth National Conference on Cerebrovascular Disease by the Chinese Medical Association in 1995 [36]. The acute phase of ACI generally refers to 2 weeks after the onset of disease. Thus, this NMA enrolled patients with the course of disease within 2 weeks. No cerebral hemorrhage was detected using cranial computed tomography (CT) or magnetic resonance imaging (MRI). There were no limits on age, gender, race or disease severity. (3) Eligible comparisons were CHIs + AADN regimens versus the AADN regimen alone and CHIs + AADN regimens versus other CHIs + AADN regimens. There was no limitation on the dosages or treatment courses. (4) Outcome measures included the markedly effective rate, improvement of neurological impairment, activities of daily living function, and death from all causes within the treatment and during the entire follow-up period. The following formula was used: the markedly effective rate (%) = (number of recovered patients + number of patients with significant progress) / total number × 100%. The efficacy criteria were predominantly based on the reduction of the neurological deficit score and could be divided into four grades: recovery, significant progress, progress, and no change or deterioration. Recovery, significant progress, progress, and no change or deterioration were determined when the neurological deficit score decreased from 91% to 100%, between 46% and 90%, between 18% and 45%, and < 17%, respectively. The improvement of neurological impairment is expressed as the mean ± standard deviation.
The following studies were excluded: (1) studies that did not refer to the acute phase; (2) studies that did not meet the curative effect valuation standard; (3) studies involving patients who had a severe cognitive disorder, hemorrhagic tendency, or serious complications, such as atrial fibrillation, severe heart failure, severe liver and kidney diseases, undergoing surgery, acupuncture or other physical therapy; (4) data that were incorrect, incomplete or unavailable; (5) reviews or meta-analyses, experimental research, retrospective studies, case reports, and conference abstracts.
Data sources and search strategy
A systematic literature search was performed using the following databases from inception to June 2016: PubMed, Cochrane Library, Embase, China National Knowledge Infrastructure Database (CNKI), Wanfang Database, and Chinese Biomedical Literature Database (CBM). The medical subject headings (MeSH) and free text words were used. No language or other restrictions were imposed. Furthermore, we hand searched the reference lists of all retrieved studies. The specific Chinese and English search terms for each CHIs are shown in Additional file 1: Table S1 and the detailed search strategy is shown in Additional file 2.
Literature selection and data extraction
Two reviewers independently read the titles and abstracts of the literature to exclude literature that was obviously not relevant as well as reviews and pharmacological experiments. We retrieved the full text of the articles to determine whether they were eligible.
The data of interest from each included RCT were collected using a standard data abstraction form created in Microsoft Excel 2013 (Microsoft Corp, Redmond, WA, USA). The main components of the extracted data were as follows: (1) General information: author names and publication data; (2) Patient information: median age, number of patients, gender, and acute phase; (3) Intervention: names, dosages, and treatment; (4) Outcomes: the markedly effective rate, improvement of neurological deficit score, adverse drug reactions/adverse drug events (ADRs/ADEs), activities of daily living function, and death from all causes within the treatment and during the entire follow-up period.
Quality assessment
The methodological quality of each included study was evaluated using the Cochrane Risk of Bias tool [37] and the CONSORT statement [38]. The items included randomization, blinding, dropout, eligibility criteria for participants, adverse events, and statistical methods. The judgments for each entry involved were divided into 3 grades: “high”, “unclear”, and “low”. A quality assessment was performed by two independent reviews, and disagreements were resolved by consensus.
Statistical analysis
We performed a pairwise meta-analysis using STATA 13.0 software (Stata Corporation, College Station, TX, USA). The pooled odds ratios (ORs) were calculated for dichotomous data and standardized mean differences (SMDs) were calculated for continuous variables, both with 95% confidence interval (95% CI). The Chi-squared test was used to evaluate the heterogeneity between studies, and I2 was used to show the extent of heterogeneity. When P was ≥0.1 and I2 was ≤50%, no statistical heterogeneity was suggested and the Mantel-Haenszel fixed-effects model was used for the meta-analysis. When P < 0.1 and I2 was > 50%, we explored the sources of heterogeneity using a subgroup analysis and meta-regression. When there was no clinical heterogeneity, the Mantel-Haenszel random-effects model was used to perform the meta-analysis [37].
A Bayesian NMA was conducted using WinBUGS 1.4.3 software (MRC Biostatistics Unit, Cambridge, UK). The random-effects model with vague priors for multi-arm trials was used [39]. The model parameters were estimated using a Markov chain Monte Carlo method called Gibbs sampling. Convergence was found to be adequate after running 1000 samples. These samples were discarded as “burn-in,” and posterior summaries were based on 100,000 subsequent simulations. The results are reported as the OR and SMD with 95% CI. To evaluate the inconsistency between direct and indirect effect estimates for the same comparison, we evaluated each closed loop in the network. In a closed loop, we employed the inconsistency factor (IF) to evaluate heterogeneity among the included studies. If the 95% CIs of the IF values were truncated at zero, it indicated that the 2 sources were in agreement [39]. To rank the treatments, we used the surface under the cumulative ranking probabilities (SUCRA); a SUCRA value of 100% is assigned to the best treatment and 0% for the worst treatment [39]. A comparison-adjusted funnel plot was used to assess the presence of small-study effect [40]. Egger’s test was used to assess the symmetry of the funnel plot [41].
To account for both the markedly effective rate and neurological deficits, we used multivariate methods to determine the dependency between outcomes. Clustering methods and 2-dimensional plots were used to produce clusters of treatments [42]. Using the clusterank command, clustered ranking plots can be obtained using the STATA program. The markedly effective rate and neurological deficits became the data variable containing the SUCRA scores for all treatments in this network. The different colors correspond to the estimated clusters and were utilized for grouping the treatments according to their similarity for both outcomes.
Results
Literature search and characteristics of the included studies
Figure 1 shows the PRISMA flow diagram. A total of 13,764 articles were identified from electronic databases. After the exclusion of duplications, reviews, and obviously irrelevant studies by reading titles and abstracts, 3493 papers were downloaded for additional review. A total of 3429 RCTs were excluded for the following reasons: non-RCTs, non-acute phase, not meeting intervention and the curative effect valuation standard, incorrect data, no treatment time, and no outcomes of interest. Hence, 64 studies and 15 CHIs were included in the NMA. All studies were published in Chinese from 2006 to 2015.
Fig. 1.
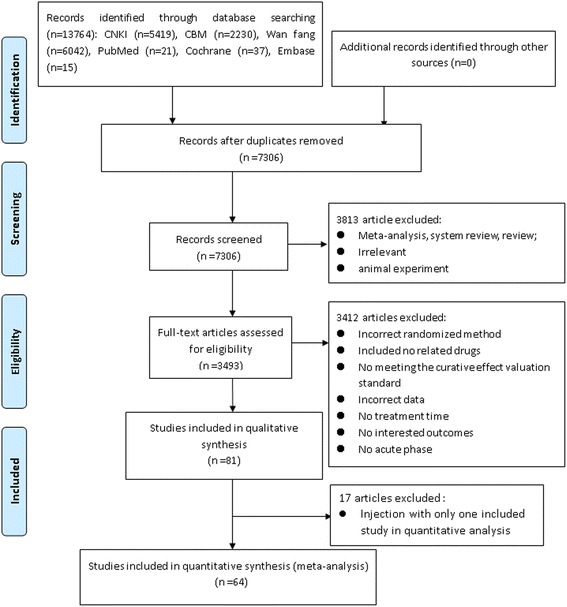
Flow diagram of the study search
Characteristics of the included studies
The 64 RCTs [28, 43–105] included 6225 participants, with sample sizes varying from 26 to 300 participants. All RCTs were conducted among Chinese populations in China. All participants were evaluated using cranial CT or NMRI. The rang of participants was approximately 35 to 87 years. There were more male patients (59.4%) than females. This study included 16 treatments for ACI: AADN, Shuxuening injection(SXN) + AADN, Shuxuetong injection (SXT) + AADN, Shenxiong injection (SX) + AADN, Mailuoning injection (MLN) + AADN, Honghuahuangsesu injection (HHHSS) + AADN, Fufangdanshen injection (FFDS) + AADN, Dengzhanhuasu injection (DZHS) + AADN, Dengzhanxixin injection (DZXX) + AADN, Danshenchuanxiongqin injection (DSCXQ) + AADN, Danshen injection (DS) + AADN, Danhong injection (DH) + AADN, Yinxingdamo injection (YXDM) + AADN, Ligustrazine injection (LI) + AADN, Xuesaitong injection (XST2) + AADN, and Xueshuantong injection (XST1) + AADN; to concisely express the results of this research, we used the abbreviations of the TCM injections to replace the treatments. The treatment abbreviations are shown in Table 1. The acute phase was no more than 30 days, with 62.5% of the cases having an acute phase of less than 72 h. The duration of treatment for both the experimental and control groups was no more than 30 days. Figure 2 shows a network graph comparing fifteen CHIs. There were 120 pairwise comparisons including 40 direct comparisons. Table 2 provides a summary of the included studies. Additional file 3 showed the more details of included CHIs.
Table 1.
Treatment abbreviations
| Full name | Abbreviations |
|---|---|
| Aspirin + Anticoagulants + Dehydrant + Neuroprotectant | AADN |
| Ligustrazine injection | LI |
| Xueshuantong injection | XST1 |
| Xuesaitong injection | XST2 |
| Shuxuening injection | SXN |
| Dengzhanxixin injection | DZXX |
| Dengzhanhuasu injection | DZHS |
| Shuxuetong injection | SXT |
| Danhong injection | DH |
| Fufangdanshen injection | FFDS |
| Yinxingdamo injection | YXDM |
| Mailuoning injection | MLN |
| Honghuahuangsesu injection | HHHSS |
| Shenxiong glucose injection | SX |
| Danshen Chuanxiongqin injection | DSCXQ |
| Danshen injection | DS |
Fig. 2.
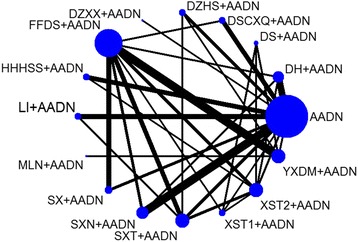
Network of the included comparisons. Note: Nodes are proportional to the number of patients included in the corresponding treatments, and edges are weighted according to the number of studies included in the respective comparisons
Table 2.
Characteristics of the studies included in this meta-analysis
| Study | Acute phase | Sex (M/F) | Age | Experimental group | Control group | Course | Outcomes | ADRs/ADEs | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | T1 | Dosage | N | T2 | Dosage | |||||||
| Yu YC 2014 | 72 h | 38/30 | 62.5 (44–76) | 34 | XST1 | 500 mg | 34 | AADN | – | 14d | (1) | None |
| Yu YM 2009 | 72 h | 71/45 | 62.5 (40–72) | 58 | DH | 20 ml | 58 | FFDS | 20 ml | 15d | (1)(2) | Unclear |
| Yu Y 2015 | 72 h | 35/21 | 54.3 (38–71) | 28 | XST1 | 500 mg | 28 | FFDS | 20 ml | 14d | (1)(2) | 6 |
| Zhang ZJ 2008 | 74 h | 41/30 | 64.3 | 35 | SX | 100 ml | 36 | FFDS | 20 ml | 14d | (1) | None |
| Zheng XD 2007 | 72 h | 39/21 | 66.4 | 30 | YXDM | 20 mL | 30 | FFDS | 30 ml | 14d | (1) | None |
| Zhou SJ 2011 | 1 M | 46/34 | 62 (42–75) | 40 | SX | 100 ml | 40 | AADN | – | 14d | (1)(2) | None |
| Zhou SF 2009 | 48 h | 52/42 | 65.6 (62–86) | 48 | SX | 200 ml | 46 | FFDS | 20 mL | 21d | (1)(2) | 10 |
| Zhou SH 2013 | 72 h | 50/40 | 62 | 45 | SXN | 20 ml | 45 | AADN | – | 14d | (1)(4) | None |
| Xu XY 2011 | 12 h | 40/20 | 55.9 (45–73) | 30 | YXDM | 20 ml | 30 | DS | 20 ml | 21d | (1)(2)(3) | Unclear |
| Xie S 2011 | 24 h | 42/30 | 61.4 (53–78) | 36 | SXT | 6 ml | 36 | FFDS | 20 ml | 14d | (1) | None |
| Xie YG 2010 | 48 h | 39/31 | 61.6 (50–76) | 35 | SXT | 6 ml | 35 | DZHS | 50 mg | 14d | (1)(2) | None |
| Xu LL 2011 | 72 h | 48/34 | 63.0 (55–81) | 42 | LI | 120 mg | 42 | AADN | – | 14d | (1)(2) | Unclear |
| Xu XQ 2012 | 72 h | 64/44 | 57.9 | 54 | YXDM | 20 ml | 54 | FFDS | 20 ml | 14d | (1)(2) | 1 |
| Yang HJ 2007 | 24 h | 50/34 | 53.6 (39–75) | 48 | DSCXQ | 10 ml | 36 | FFDS | 10 ml | 30d | (1)(2) | 1 |
| Yang YF 2012 | 48 h | – | 68 | 112 | DH | 30 ml | 101 | AADN | – | 21d | (1)(2) | Unclear |
| Yao QY 2010 | 72 h | 64/40 | 70.8 (54–82) | 56 | SXT | 6 ml | 48 | LI | 12 ml | 14d | (1)(2) | 2 |
| Yao J 2010 | 72 h | 37/27 | 60.5 (49–76) | 32 | YXDM | 20 ml | 32 | DZHS | 50 mg | 14d | (1)(2) | None |
| Xie RP 2010 | 72 h | 52/28 | 42–80 | 40 | SXN | 20 ml | 40 | FFDS | 20 mL | 15d | (1) | None |
| Tan SY 2016 | 72 h | 49/37 | 39–82 | 43 | DH | 30 ml | 43 | AADN | – | 14d | (1)(2) | None |
| Wang WP 2015 | 72 h | 49/41 | 68.2 (60–78) | 45 | SXN | 20 mL | 45 | FFDS | 20 mL | 14d | (1)(2) | Unclear |
| Ren HM 2009 | 72 h | 38/26 | 40–78 | 32 | SX | 100 ml | 32 | AADN | – | 14d | (1)(2) | None |
| Sun HJ 2013 | 72 h | 42/36 | 60.9 (40–70) | 39 | LI | 120 mg | 39 | AADN | – | 14d | (1)(2) | Unclear |
| Tang JP 2013 | 72 h | 47/45 | 51.8 (43–73) | 46 | HHHSS | 100 mg | 46 | AADN | – | 14d | (1)(2) | None |
| Tang FY 2009 | 72 h | 39/33 | 64.0 (50–78) | 36 | DZHS | 50 mg | 36 | AADN | – | 14d | (1)(2) | None |
| Tang XJ 2013 | 72 h | 45/33 | 58 | 39 | YXDM | 20 mg | 39 | AADN | – | 14d | (1)(2) | None |
| Wang L 2010 | 48 h | 26/22 | 65.6 (62–86) | 40 | SX | 200 mL | 40 | FFDS | 20 mL | 21d | (1)(2) | Unclear |
| Wang YL 2013 | 168 h | 33/23 | 32–88 | 28 | SXT | 6 ml | 28 | DZHS | 40 ml | 14d | (2) | Unclear |
| Lan Y 2015 | 72 h | 41/39 | 54–72 | 40 | DSCXQ | 10 ml | 40 | AADN | – | 14d | (1)(2) | 3 |
| Li M 2014 | 24 h | 63/17 | 48–76 | 40 | DH | 30 ml | 40 | AADN | – | 14d | (2) | Unclear |
| Liu YP 2010 | 72 h | 45/35 | 51–71 | 40 | DH | 40 ml | 40 | AADN | – | 15d | (1) | 1 |
| Ma J 2010 | 24 h | 29/31 | 59.1 (50–68) | 30 | DZHS | 20 mg | 30 | AADN | – | 14d | (1)(2) | Unclear |
| Ma J 2015 | 72 h | 49/41 | 65.5 (45–79) | 45 | DH | 20 ml | 45 | AADN | – | 28d | (1)(4) | Unclear |
| Chen H 2015 | 24 h | 74/60 | 59.4 (46–87) | 67 | SXT | 6 ml | 67 | AADN | – | 14d | (1)(2) | 5 |
| Luan T 2014 | 48 h | 52/43 | 57.5 | 50 | XST2 | 0.4 g | 45 | DS | 20 ml | 15d | (1)(4) | 7 |
| Huang ML 2012 | 72 h | 158/142 | 62.6 (42–71) | 150 | XST1 | 500 mg | 150 | DS | 25 ml | 14d | (1) | 1 |
| Li X 2015 | 72 h | 129/71 | 62.9 | 100 | HHHSS | 0.1 g | 100 | AADN | – | 14d | (1)(2) | Unclear |
| Ma ZL 2011 | 72 h | 74/46 | 62.8 (45–84) | 60 | HHHSS | 0.1 g | 60 | AADN | – | 14d | (1)(2) | None |
| Zhang Y 2012 | 48 h | 52/28 | 57.5 (35–80) | 40 | DSCXQ | 100 mg | 40 | AADN | – | 14d | (1)(2) | 1 |
| Liu M 2014 | 48 h | 79/57 | 60.2 (40–76) | 68 | DSCXQ | 10 mL | 68 | AADN | – | 14d | (1)(2) | 2 |
| Chen YC 2011 | 72 h | 44/24 | 57.9 | 34 | YXDM | 20 ml | 34 | FFDS | 20 ml | 14d | (1)(2) | 1 |
| Yang RF 2013 | 48 h | 52/48 | 60.8 (30–83) | 50 | SXT | 6 g | 50 | AADN | – | 14d | (1) | Unclear |
| Lin YF 2008 | 72 h | 39/21 | 66.5 | 30 | YXDM | 20 ml | 30 | FFDS | 20 ml | 14d | (1)(3) | None |
| Peng T 2006 | 48 h | 51/48 | 68 | 49 | SXT | 7 ml | 50 | FFDS | 20 ml | 14d | (1)(3) | 1 |
| Li XH 2011 | 168 h | – | 63.5(40–78) | 120 | SXN | 20 ml | 120 | AADN | – | 14d | (1)(2) | Unclear |
| Liu L 2015 | 6-72 h | 74/46 | 61.4 (39–76) | 60 | DH | 20 ml | 60 | SXN | 20 ml | 14d | (1) | None |
| Zhang YH 2010 | 6-72 h | 62/34 | 58.9 (39–81) | 48 | DH | 30 ml | 48 | XST2 | 400 mg | 14d | (1)(2) | 5 |
| Fan J 2006 | 72 h | 56/32 | 64 (41–82) | 44 | YXDM | 20 ml | 44 | FFDS | 20 ml | 14d | (2) | Unclear |
| Liu HY 2014 | 96 h | 67/57 | 61.9 (38–83) | 64 | XST2 | 0.4 g | 60 | FFDS | 30 ml | 15d | (1) | None |
| Li FQ 2010 | 72 h | 74/46 | 64 | 60 | YXDM | 30 ml | 60 | FFDS | 40 ml | 15d | (1) | Unclear |
| Lian CL 2013 | 360 h | 49/43 | 62 (43–77) | 46 | LI | 120 mg | 46 | AADN | – | 30d | (1) | None |
| Chen S 2006 | 72 h | 81/53 | 67.9 | 70 | XST2 | 800 mg | 64 | XST1 | 10 ml | 14d | (1)(2) | None |
| Cao LZ 2012 | 72 h | 71/9 | 59.3 | 40 | XST2 | 500 mg | 40 | FFDS | 1.0 g | 14d | (1)(2) | Unclear |
| Liu Y 2009 | 72 h | 79/43 | 65.1 (43–73) | 62 | SXT | 6 ml | 60 | XST2 | 8 ml | 14d | (1) | 1 |
| Luo XD 2011 | 72 h | 35/25 | 61.8 | 30 | XST2 | 0.4 g | 30 | AADN | – | 14d | (1)(2) | 3 |
| Liao MJ 2014 | 6-72 h | 43/17 | 62.5 (36–80) | 30 | HHHSS | 100 ml | 30 | XST2 | 400 mg | 14d | (1) | Unclear |
| Shi JL 2010 | 72 h | 51/33 | 62 | 42 | YXDM | 20 ml | 42 | LI | 200 mg | 30d | (2) | Unclear |
| Ni H 2010 | 24 h | 61/55 | 62.7 (54–74) | 59 | SXN | 20 ml | 57 | AADN | – | 14d | (1) | Unclear |
| Li CP 2007 | 8-72 h | 106/54 | 63.8 (42–85) | 80 | SXN | 10-20 ml | 80 | AADN | – | 14d | (1) | Unclear |
| Zhou ZP 2011 | 24 h | 48/33 | 62.2 | 42 | DZXX | 30 ml | 39 | AADN | – | 15d | (1) | Unclear |
| Chen C 2015 | 72 h | 37/31 | 67.5 | 34 | SXT | 6 ml | 34 | XST2 | 400 mg | 14d | (1)(2) | 14 |
| Chen JY 2012 | 72 h | 73/35 | 67 (40–76) | 54 | SXT | 6 ml | 54 | FFDS | 20 ml | 14d | (1)(2) | Unclear |
| Luo QY 2007 | 72 h | 54/36 | 63.6 (37–81) | 45 | SXN | 6 ml | 45 | AADN | 20 ml | 14d | (1) | None |
| Chen J 2007 | 72 h | 45/35 | 61 | 40 | YXDM | 20 ml | 40 | MLN | 20 ml | 15d | (1)(2)(3) | Unclear |
| Zhang XL 2005 | 6d | 37/13 | 35–80 | 26 | MLN | 20 ml | 24 | FFDS | 20 m l | 21d | (2) | None |
M male, F female, AVG average, E experimental group, C control group, W week, D day, AADN aspirin + anticoagulants + dehydrant + neuroprotectant, DH Danhong injection + AADN, DS Danshen injection + AADN, DSCXQ Danshenchuangxiongqin injection + AADN, DZHS Dengzhanhuasu injection + AADN, DZXX Dengzhanxixin injection + AADN, FFDS Fufangdanshen injection + AADN, HHHSS Honghuahuangsesu injection + AADN, SX Shenxiong glucose injection + AADN, SXT Shuxuetong injection + AADN, XST1 Xueshuantong injection + AADN, XST2 Xuesetong injection + AADN, LI Ligustrazine injection + AADN, YXDM Yinxingdamo injection + AADN, SXN Shuxuening injection + AADN, MLN Mailuoning injection + AADN, ADRs adverse drug reactions, ADEs adverse drug events; (1): markedly effective rate; (2): neurological impairment; (3): death; (4): activities of daily living function; N: sample size; T1: therapy of experiment; T2: Therapy of control; N:Number of studies; −: No report
Quality of the included studies
We used the Cochrane Handbook version 5.1.0 and CONSORT statement to conduct a quality evaluation of the included studies. All studies mentioned the use of random distribution, while ten studies [44, 64, 68, 73, 75, 82, 95–97, 101] described a satisfactory method of randomization including random number tables or the envelope method. Two studies [60, 85] reported information about blinding. All studies provided information on patients who were lost to follow-up or dropped out. All studies reported the eligibility criteria for participants and statistical methods. Approximately 74.6% of the studies provided information about adverse events. Details on risk of bias are shown in Additional file 4: Figure S1.
Pairwise meta-analysis
Pairwise meta-analysis of the markedly effective rate
Fifty-nine RCTs reported markedly effective rates; in these RCTs, 5864 patients were involved and 34 regimens were included. Table 3 summarizes the results of the pairwise meta-analysis regarding the markedly effective rates. There was no significant heterogeneity in the pooled analysis of all included studies (P > 0.1; I 2 < 50%); the results of the heterogeneity test are shown in Table 2. The direct comparison showed that DH and SXN were more beneficial in improving the markedly effective rate than AADN (AADN versus DH: OR = 0.61, 95% CI = 0.45–0.84; versus SXN: OR = 0.57, 95% CI = 0.44–0.73). SX and XST2 were more beneficial than FFDS (FFDS versus SX: OR = 0.61, 95% CI = 0.38–0.98; versus XST2: OR = 0.54, 95% CI = 0.34–0.86). Other treatment comparisons failed to reach statistical significance (the 95% CI included 1). The detailed results are shown in Fig. 3.
Table 3.
A summary of the meta-analysis for the markedly effective rate
| AADN | 0.61(0.45,0.84) P = 0.96 i2 = 0% | 0.75(0.48,1.16) P = 0.97 l2 = 0% | 0.72(0.42,1.23) P = 0.82 I2 = 0% | 0.56(0.26,1.17) | – | 0.74(0.54,1.01) P = 0.91 I2 = 0% | 0.67(0.40,1.14) P = 0.92 I2 = 0% | 0.90(0.61,1.32) P = 0.71 I2 = 0% | 0.93(0.46, 1.90) | 0.72(0.33, 1.59) | 0.81(0.54, 1.21) P = 0.79 I2 = 0% | 0.61(0.30,1.26) | 0.57(0.44,0.73) P = 0.19 I2 = 35.4% | – | |
| 3.89 (2.26, 6.26) | DH | – | – | – | – | 1.53 (0.84,2.80) |
– | – | – | – | 1.73 (0.89, 3.34) |
– | – | 1.22 (0.69,2.16) |
– |
| 0.8 (0.29, 1.8) | 0.22 (0.07,0.52) |
DS | – | – | – | – | – | – | – | 0.79 (0.53,1.19) |
0.75 (0.41, 1.36) |
– | 0.64 (0.28,1.47) |
– | – |
| 2.14 (1.02, 3.99) | 0.59 (0.23,1.23) |
3.3 (0.91,8.58) |
DSCXQ | – | – | 1.70 (0.81,3.59) |
– | – | – | – | – | – | – | – | – |
| 2.22 (0.95, 4.47) | 0.61 (0.22,1.35) |
3.38 (0.92,8.96) |
1.16 (0.37,2.82) |
DZHS | – | – | – | – | 0.70 (0.32,1.48) |
– | – | – | 0.75 (0.33,1.72) |
– | |
| 5.36 (1.06,16.68) | 1.47 (0.26,4.87) |
8.32
(1.12,30.55) |
2.82 (0.44,9.73) |
2.82 (0.42,9.88) |
DZXX | – | – | – | – | – | – | – | – | – | – |
| 0.94 (0.57, 1.46) | 0.25 (0.13,0.44) |
1.41 (0.51,3.11) |
0.49 (0.21,0.96) |
0.48 (0.19,1.01) |
0.29
(0.05,0.93) |
FFDS | – | 0.61(0.38, 0.98) P = 0.89 I2 = 0% | 0.74(0.51,1.07)P = 0.86 I2 = 0% | 0.76(0.33, 1.76) | 0.54(0.34,0.86)P = 0.70 I2 = 0% | – | 0.75(0.53,1.06)P = 0.98 I2 = 0% | 0.81(0.50,1.31)P = 0.65 I2 = 0% | – |
| 3.34 (1.66, 6.14) | 0.91 (0.37,1.91) |
5.14
(1.48,13.28) |
1.75 (0.62,4) |
1.75 (0.56,4.22) |
1.02 (0.16,3.51) |
3.76
(1.61,7.65) |
HHHSS | – | – | – | 1.85 (0.79, 4.29) |
– | – | – | – |
| 2.9 (1.36, 5.46) | 0.79 (0.32,1.65) |
4.43
(1.29,11.37) |
1.52 (0.53,3.47) |
1.51 (0.49,3.6) |
0.89 (0.14,3.03) |
3.18
(1.56,5.83) |
0.97 (0.34,2.21) |
SX | – | – | – | – | – | – | – |
| 3.27 (1.86, 5.35) | 0.89 (0.42,1.66) |
4.96
(1.68,11.54) |
1.71 (0.68,3.58) |
1.68 (0.67,3.51) |
1 (0.17,3.27) |
3.6
(2.03,5.97) |
1.09 (0.44,2.23) |
1.25 (0.52,2.55) |
SXT | – | 1.38(0.86, 2.22) P = 0.86 I2 = 0% | 1.47 (0.79, 2.78) |
– | – | – |
| 1.27 (0.53, 2.6) | 0.34 (0.13,0.76) |
1.78 (0.7,3.81) |
0.66 (0.21,1.62) |
0.66 (0.2,1.63) |
0.39 (0.06,1.38) |
1.39 (0.59,2.84) |
0.42 (0.14,1) |
0.49 (0.16,1.16) |
0.41 (0.16,0.87) |
XST1 | 0.65 (0.37, 1.15) |
– | – | – | – |
| 2.24 (1.23, 3.77) | 0.61 (0.29,1.12) |
3.31
(1.25,7.23) |
1.17 (0.46,2.51) |
1.16 (0.43,2.56) |
0.68 (0.12,2.28) |
2.47
(1.36,4.14) |
0.74 (0.31,1.5) |
0.86 (0.35,1.77) |
0.71 (0.38,1.25) |
1.99 (0.85,3.94) |
XST2 | – | – | – | – |
| 1.64 (0.78, 3.09) | 0.45 (0.18,0.96) |
2.54 (0.7,6.6) |
0.86 (0.29,2.01) |
0.86 (0.27,2.06) |
0.5 (0.08,1.73) |
1.85 (0.76,3.8) |
0.55 (0.19,1.25) |
0.64 (0.22,1.48) |
0.53 (0.22,1.07) |
1.52 (0.47,3.66) |
0.79 (0.31,1.68) |
LI | – | – | – |
| 2.99 (1.53, 5.34) | 0.81 (0.36,1.6) |
4.43
(1.57,10.1) |
1.56 (0.59,3.4) |
1.52 (0.59,3.24) |
0.91 (0.15,3.1) |
3.24
(1.85,5.34) |
1 (0.37,2.17) |
1.14 (0.46,2.36) |
0.96 (0.45,1.82) |
2.69
(1.01,5.84) |
1.41 (0.65,2.69) |
2.05 (0.74,4.58) |
YXDM | – | 1.32 (0.67,2.61) |
| 3.3 (2, 5.14) | 0.9 (0.46,1.59) |
5.09
(1.64,12.18) |
1.73 (0.71,3.61) |
1.73 (0.64,3.79) |
1.01 (0.18,3.3) |
3.69
(1.97,6.31) |
1.1 (0.45,2.25) |
1.28 (0.53,2.61) |
1.08 (0.52,1.98) |
3.04
(1.12,6.65) |
1.59 (0.75,2.97) |
2.27 (0.91,4.75) |
1.21 (0.54,2.33) |
SXN | – |
| 1.33 (0.2, 4.65) | 0.36 (0.05,1.3) |
1.98 (0.24,7.46) |
0.69 (0.09,2.61) |
0.68 (0.09,2.47) |
0.41 (0.03,1.84) |
1.45 (0.23,4.95) |
0.44 (0.06,1.65) |
0.51 (0.07,1.86) |
0.43 (0.06,1.52) |
1.2 (0.15,4.48) |
0.63 (0.09,2.24) |
0.91 (0.11,3.45) |
0.45 (0.08,1.44) |
0.42 (0.06,1.51) |
MLN |
The upper right corner is the Meta-analysis results. The bottom left corner is the network Meta-analysis results. Results are the odds ratios (OR) and related 95% credibility interval (95% CI) in the row-defining treatment compared with the column -defining treatment. OR higher than 1 favor the row-defining treatment, and vice versa. Significant effects are printed in bold. AADN aspirin + anticoagulants + dehydrant + neuroprotectant, DH Danhong injection + AADN, DS Danshen injection + AADN, DSCXQ Danshenchuangxiongqin injection + AADN, DZHS Dengzhanhuasu injection + AADN, DZXX Dengzhanxixin injection + AADN, FFDS Fufangdanshen injection + AADN, HHHSS Honghuahuangsesu injection + AADN, SX Shenxiong glucose injection + AADN, SXT Shuxuetong injection + AADN, XST1 Xueshuantong injection + AADN, XST2 Xuesetong injection + AADN, LI Ligustrazine injection + AADN, YXDM Yinxingdamo injection + AADN, SXN Shuxuening injection + AADN, MLN Mailuoning injection + AAD
Fig. 3.
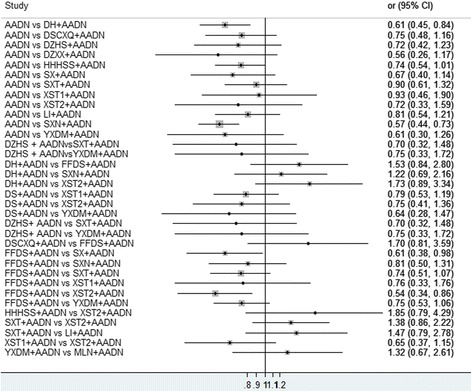
Forest graph of Meta-analysis on the markedly effective rate
Pairwise meta-analysis of the improvement of neurological impairment
Forty-one RCTs reported an improvement of neurological impairment; in these RCTs, 3828 patients were involved and 29 regimens were included. When P was ≥0.1 and I2 was ≤50%, the Mantel-Haenszel fixed-effects model was used for the meta-analysis and vice versa. The results of the heterogeneity test are shown in Table 4. Table 4 summarizes the results of the pairwise meta-analysis regarding the improvement of neurological impairment. The direct comparison showed that DH, DZHS, HHHSS, SX, SXT, XST2, LI, YXDM, and SXN were more effective than AADN alone in the reduction of the neurological impairment score (AADN versus DH: SMD = 0.54, 95% CI = 0.33–0.75; versus DZHS: SMD = 1.01, 95% CI = 0.04–1.98; versus HHHSS: SMD = 0.64, 95% CI = 0.44–0.84; versus SX: SMD = 0.77, 95% CI = 0.43–1.11; versus SXT: SMD = 0.79, 95% CI = 0.44–1.14; versus XST2: SMD = 0.53, 95% CI = 0.01–1.04; versus LI: SMD = 0.83, 95% CI = 0.51–1.16; versus YXDM: SMD = 1.03, 95% CI = 0.56–1.50; versus SXN: SMD = 0.41, 95% CI = 0.15–0.66). DH was more effective than FFDS (SMD = − 1.01, 95% CI = − 1.40 to − 0.62) and XST2 (SMD = − 0.61, 95% CI = − 1.02 to − 0.20). DSCXQ was more effective than FFDS (SMD = − 0.82, 95% CI = − 1.27 to − 0.37). YXDM and SXT were more effective than DZHS (DZHS versus YXDM: SMD = 0.75, 95% CI = 0.24–1.26; versus SXT: SMD = 0.84, 95% CI = 0.35–1.33). SX, XST1, XST2, and SXN were more effective than FFDS (FFDS versus SX: SMD = 0.79, 95% CI = 0.45–1.13; versus XST1: SMD = 1.15, 95% CI = 0.58–1.71; versus XST2: SMD = 0.95, 95% CI = 0.49–1.41; versus SXN: SMD = 2.41, 95% CI = 1.85–2.97). SXT was more effective than LI (SMD = − 0.79, 95% CI = − 1.19 to − 0.39). YXDM was more effective than LI (LI versus YXDM: SMD = 0.96, 95% CI = 0.51–1.42). MLN was more effective than YXDM (YXDM versus MLN: SMD = 0.51, 95% CI = 0.06–0.95). Other treatment comparisons failed to reach statistical significance (the 95% CI included 0). The detailed results are shown in Fig. 4.
Table 4.
A summary of the meta-analysis for the improvement of neurological impairment
| AADN | 0.54(0.33, 0.75) I2 = 13.4% P = 0.32 | – | 0.51(− 0.02, 1.03) I2 = 79.8% P = 0.007 | 1.01(0.04, 1.98) I2 = 85.2% P = 0.009 | – | – | 0.64(0.44, 0.84) I2 = 87.2% P = 0.000 |
0.77(0.43, 1.11)
I2 = 0% P = 0.60 |
0.79(0.44,1.14) | – | 0.53(0.01, 1.04) |
0.83
(0.51, 1.16) I2 = 80.4% P = 0.02 |
1.03(0.56, 1.50) | 0.41(0.15, 0.66) | – |
| − 0.71 (− 1.28,-0.13) | DH | – | – | – | – | − 1.01 (− 1.40,-0.62) | – | – | – | – |
− 0.61
(− 1.02,-0.20) |
– | – | – | |
| − 0.83 (− 2.21,0.56) | −0.12 (− 1.58,1.3) | DS | – | – | – | – | – | – | – | – | – | – | 0.31 (− 0.20, 0.81) | – | – |
| − 0.52 (− 1.15,0.1) | 0.19 (− 0.64,1.01) | 0.31 (− 1.18,1.8) | DSCXQ | – | – | − 0.82 (− 1.27,-0.37) | – | – | – | – | – | – | – | – | – |
| −0.59 (− 1.26,0.07) | 0.12 (− 0.74,0.96) | 0.24 (− 1.23,1.69) | −0.07 (− 0.96,0.8) | DZHS | – | – | – | – | 0.84 (0.35, 1.33) | – | – | – | 0.75 (0.24, 1.26) | – | – |
| −0.78 (− 2.19,0.63) | −0.07 (− 1.57,1.41) | 0.05 (− 1.88,1.97) | −0.26 (− 1.78,1.2) | −0.19 (− 1.68,1.2) | DZXX | – | – | – | 0.03 (− 0.49, 0.55) | – | – | – | – | – | – |
| 0.22 (− 0.3,0.73) | 0.92 (0.26,1.58) | 1.05 (− 0.32,2.4) | 0.74 (0.01,1.47) | 0.81 (0.05,1.58) | 1.00 (− 0.43,2.43) | FFDS | – | 0.79 (0.45,1.13) I2 = 0% P = 1 | 0.07 (−0.03, 0.45) | 1.15 (0.58, 1.71) | 0.95 (0.49, 1.41) | – | 1.31 (−0.05,2.66) I2 = 95.7% P = 0 | 2.41 (1.85, 2.97) | 0.44 (−0.12, 1.01) |
| −0.78 (− 1.47,-0.09) | − 0.07 (− 0.97,0.83) | 0.05 (− 1.49,1.58) | −0.26 (− 1.19,0.6) | −0.19 (− 1.16,0.7) | 0 (− 1.57,1.57) | − 1 (− 1.86,-0.13) | HHHSS | – | – | – | – | – | – | – | – |
| − 0.67 (− 1.34,0) | 0.04 (− 0.8,0.87) | 0.16 (− 1.34,1.64) | −0.14 (− 1.03,0.7) | −0.08 (− 0.99,0.8) | 0.11 (− 1.41,1.64) | −0.88 (− 1.55,-0.21) | 0.11 (− 0.85,1.08) | SX | – | – | – | – | – | – | – |
| − 0.81 (− 1.44,− 0.18) | -0.1 (− 0.89,0.69) | 0.02 (− 1.44,1.47) | −0.29 (− 1.14,0.5) | −0.22 (− 0.99,0.5) | −0.03 (− 1.29,1.23) | −1.03 (− 1.7,-0.36) | −0.03 (− 0.97,0.91) | −0.14 (− 1,0.72) | SXT | – | − 0.20 (− 0.68,0.28) | −0.79 (− 1.19,-0.39) | – | – | – |
| − 0.6 (− 1.61,0.39) | 0.11 (− 0.97,1.16) | 0.23 (−1.41,1.85) | −0.08 (− 1.22,1.0) | −0.01 (− 1.16,1.1) | 0.18 (− 1.48,1.83) | −0.82 (− 1.76,0.12) | 0.18 (−1.05,1.39) | 0.06 (− 1.06,1.18) | 0.21 (− 0.87,1.28) | XST1 | 0.24 (− 0.10,0.58) | – | – | – | – |
| −0.56 (− 1.22,0.09) | 0.14 (− 0.59,0.88) | 0.27 (− 1.22,1.75) | −0.04 (− 0.92,0.8) | 0.03 (− 0.85,0.9) | 0.22 (−1.25,1.69) | −0.78 (− 1.44,-0.12) | 0.22 (− 0.74,1.17) | 0.1 (− 0.77,0.97) | 0.25 (− 0.5,1) | 0.04 (− 0.88,0.97) | XST2 | – | – | – | – |
| −0.47 (− 1.13,0.19) | 0.24 (− 0.61,1.08) | 0.36 (−1.09,1.82) | 0.05 (− 0.84,0.9) | 0.12 (− 0.74,0.9) | 0.31 (− 1.16,1.79) | −0.69 (− 1.43,0.07) | 0.31 (− 0.65,1.27) | 0.2 (− 0.71,1.1) | 0.34 (− 0.42,1.1) | 0.13 (−1.01,1.28) | 0.09 (− 0.78,0.96) | LI | 0.96 (0.51, 1.42) | – | – |
| −1.14 (− 1.74,-0.54) | −0.43 (− 1.19,0.34) | −0.31 (− 1.57,0.94) | −0.61 (− 1.43,0.2) | −0.55 (− 1.3,0.22) | −0.36 (− 1.82,1.12) | −1.35 (− 1.89,-0.81) | −0.36 (− 1.27,0.56) | −0.47 (− 1.28,0.33) | −0.33 (− 1.08,0.42) | −0.53 (− 1.59,0.52) | −0.57 (− 1.36,0.21) | −0.67 (− 1.42,0.08) | YXDM | – | 0.51 (0.06,0.95) |
| −1.25 (− 2.14,-0.37) | − 0.54 (− 1.56,0.47) | −0.41 (− 2.01,1.18) | −0.72 (− 1.79,0.3) | −0.65 (− 1.73,0.4) | −0.46 (− 2.09,1.17) | − 1.46 (− 2.36,-0.58) | −0.47 (− 1.6,0.65) | −0.58 (− 1.63,0.48) | −0.43 (− 1.48,0.59) | −0.64 (− 1.9,0.62) | −0.68 (− 1.73,0.36) | −0.77 (− 1.86,0.29) | −0.11 (− 1.1,0.88) | SXN | – |
| − 0.44 (− 1.45,0.57) | 0.27 (− 0.84,1.37) | 0.39 (− 1.16,1.94) | 0.09 (− 1.06,1.2) | 0.16 (− 0.98,1.2) | 0.35 (− 1.32,2.02) | −0.65 (− 1.58,0.27) | 0.34 (− 0.89,1.57) | 0.23 (− 0.9,1.36) | 0.37 (− 0.73,1.48) | 0.17 (− 1.14,1.48) | 0.13 (− 0.99,1.24) | 0.03 (− 1.1,1.17) | 0.7 (− 0.22,1.62) | 0.81 (− 0.45,2.08) | MLN |
The upper right corner is the Meta-analysis results. The bottom left corner is the network Meta-analysis results. Results are the SMD and related 95% credibility interval (95% CI) in the row-defining treatment compared with the column -defining treatment. SMD higher than 0 favor the column-defining treatment, and vice versa. Significant effects are printed in bold. AADN aspirin + anticoagulants + dehydrant + neuroprotectant, DH Danhong injection + AADN, DS Danshen injection + AADN, DSCXQ Danshenchuangxiongqin injection + AADN, DZHS Dengzhanhuasu injection + AADN, DZXX Dengzhanxixin injection + AADN, FFDS Fufangdanshen injection + AADN, HHHSS Honghuahuangsesu injection + AADN, SX Shenxiong glucose injection + AADN, SXT Shuxuetong injection + AADN, XST1 Xueshuantong injection + AADN, XST2 Xuesetong injection + AADN, LI Ligustrazine injection + AADN, YXDM Yinxingdamo injection + AADN, SXN Shuxuening injection + AADN, MLN Mailuoning injection + AADN
Fig. 4.
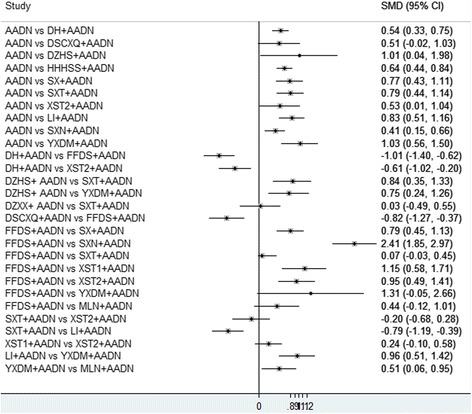
Forest graph of Meta-analysis on the neurological impairment
Pairwise meta-analysis of the death
Most studies did not report any deaths during the treatment period or during follow up after the treatment ended in all trials. Four studies [50, 81, 82, 102] reported no death during the treatment period. This result may mean that CHIs plus AADN is an effective approach in the treatment of ACI or short follow-up time.
Pairwise meta-analysis of the activities of daily living function
Three studies [46, 67, 71] assessed the activities of daily living function using the Barthel Index. Due to the limited quantity of the included studies, the Mantel-Haenszel random-effects model was used. There was a significant difference between the treatment group and the control group (SX versus AADN: SMD = 0.83, 95% CI = 0.41–1.25; DSCXQ versus AADN: SMD = 0.73, 95% CI = 0.28–1.18; DH versus AADN: SMD = 1.69, 95% CI = 1.21–2.17).
Results of the Bayesian network meta-analysis
In the original analysis, most studies did not mention the activities of daily living function or death from all causes within the treatment period or during the entire follow-up period. Therefore, the present study did not compare death or the activities of daily living function among different treatments; we only performed a NMA to compare the markedly effective rate and the improvement of neurological impairment among the different regimens of CHIs for ACI.
Bayesian network meta-analysis of the markedly effective rate
According to the network of comparisons (Table 3), DH, DSCXQ, DZXX, HHHSS, SX, SXT, XST2, YXDM, and SXN improved the markedly effective rate more significantly than AADN alone (DH: OR = 3.89, 95% CI = 2.26–6.26; DSCXQ: OR = 2.14, 95% CI = 1.02–3.99; DZXX: OR = 5.36, 95% CI = 1.06–16.68; HHHSS: OR = 3.34, 95% CI = 1.66–6.14; SX: OR = 2.90, 95% CI = 1.36–5.46; SXT: OR = 3.27, 95% CI = 1.86–5.35; XST2: OR = 2.24, 95% CI = 1.23–3.77; YXDM: OR = 2.99, 95% CI = 1.53–5.34; SXN: OR = 3.3, 95% CI = 2–5.14). Moreover, DZXX, HHHSS, SX, SXT, XST2, YXDM, and SXN were better than DS (DZXX: OR = 8.32, 95% CI = 1.12–30.55; HHHSS: OR = 5.14, 95% CI = 1.48–13.28; SX: OR = 4.43, 95% CI = 1.29–11.37; SXT: OR = 4.96, 95% CI = 1.68–11.54; XST2: OR = 3.31, 95% CI = 1.25–7.23; YXDM: OR = 4.43, 95% CI = 1.57–10.1; SXN: OR = 5.09, 95% CI = 1.64–12.18). Additionally, HHHSS, SX, SXT, XST2, YXDM, and SXN were more effective than FFDS (HHHSS: OR = 3.76, 95% CI = 1.61–7.65; SX: OR = 3.18, 95% CI = 1.56–5.83; SXT: OR = 3.60, 95% CI = 2.03–5.97; XST2: OR = 2.47, 95% CI = 1.36–4.14; YXDM: OR = 3.24, 95% CI = 1.85–5.34; SXN: OR = 3.69, 95% CI = 1.97–6.31). YXDM and SXN were more effective than XST1 (YXDM: OR = 2.69, 95% CI = 1.01–5.84; SXN: OR = 3.04, 95% CI = 1.12–6.65).
Additional file 5: Figure S2 shows the inconsistency plot used to identify heterogeneity among studies in the closed loop of this NMA. Eleven triangular loops and 25 quadratic loops were present in the NMA; 83% of IF values with 95% CIs were truncated at zero, suggesting no significant inconsistency.
Rank probability
Figure 5 shows the cumulative probabilities (SUCRA results) of CHIs that were the most effective when combined with AADN. DH had the highest likelihood of being the best treatment for the markedly effective rate (SUCRA-85.2%), followed by DZXX (SUCRA-80.4%), SXN (SUCRA-76.3%), SXT (SUCRA-75.9%), HHHSS (SUCRA-74.4%), YXDM (SUCRA-69.2%), SX (SUCRA-66.3%), XST2 (SUCRA-51.9%), DZHS (SUCRA-50.3%), DSCXQ (SUCRA-49.2%), LI (SUCRA-36.0%), XST1 (SUCRA-24.4%), MLN (SUCRA-22.9%), FFDS (SUCRA-12.9%), and DS (SUCRA-8.2%).
Fig. 5.
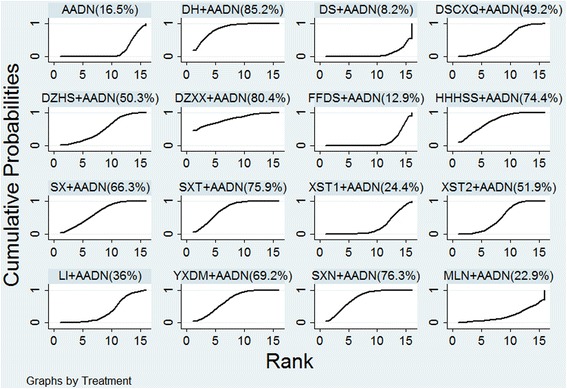
SUCRA for the markedly effective rate
Assessment of publication bias
The comparison-adjusted funnel plots (Additional file 6: Figure S3) for the markedly effective rate were asymmetric near the zero line. The result from Egger’s test was P = 0.047. Therefore, this study may have a small sample effect and publication bias.
Bayesian network meta-analysis of the improvement of neurological impairment
According to the network of comparisons (Table 4), DH, HHHSS, SXT, YXDM, and SXN improved neurological impairment more significantly than AADN alone (DH: SMD = − 0.71, 95% CI = − 1.28 to − 0.13; HHHSS: SMD = − 0.78, 95% CI = − 1.47 to − 0.09; SXT: SMD = − 0.81, 95% CI = − 1.44 to − 0.18; YXDM: SMD = − 1.14, 95% CI = − 1.74 to − 0.54; SXN: SMD = − 1.25, 95% CI = − 2.14 to − 0.37). Moreover, DH, DSCXQ, DZHS, HHHSS, SX, SXT, XST2, YXDM, and SXN were more effective than FFDS (FFDS versus DH: SMD = 0.92, 95% CI = 0.26–1.58; versus DSCXQ: SMD = 0.74, 95% CI = 0.01–1.47; versus DZHS: SMD = 0.81, 95% CI = 0.05–1.58; HHHSS: SMD = − 1, 95% CI = − 1.86 to − 0.13; SX: SMD = − 0.88, 95% CI = − 1.55 to − 0.21; SXT: SMD = − 1.03, 95% CI = − 1.7 to − 0.36; XST2: SMD = − 0.78, 95% CI = − 1.44 to − 0.12; YXDM: SMD = − 1.35, 95% CI = − 1.89 to − 0.81; SXN: SMD = − 1.46, 95% CI = − 2.36 to − 0.58). There was no statistical significance in other treatment comparisons.
As shown in Additional file 7: Figure S4, 9 triangular loops and seventeen quadratic loops were present in the NMA; 81% of IF values with 95% CIs were truncated at zero, suggesting no significant inconsistency.
Rank probability
The cumulative probability analysis (SUCRA results) showed that SXN + AADN had the highest likelihood of improving the neurological impairment scores (SUCRA-84.7%), followed by YXDM (SUCRA-84.4%), SXT (SUCRA-63.8%), HHHSS (SUCRA-60.7%), DS (SUCRA-60.6%), DZXX (SUCRA-58.1%), DH (SUCRA-56.3%), SX (SUCRA-53.2%), XST1 (SUCRA-49.0%), DZHS (SUCRA-47.7%), XST2 (SUCRA-45.5%), DSCXQ (SUCRA-43.1%), LI (SUCRA-39.2%), MLN (SUCRA-38.9%), and FFDS (SUCRA-3.9%). The results are shown in Fig. 6.
Fig. 6.
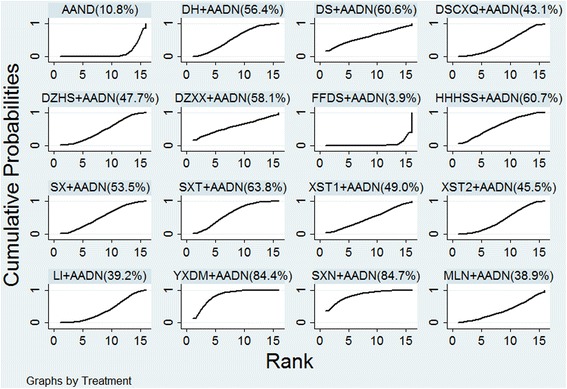
SUCRA for the improvement of neurological impairment
Simultaneous ranking of the interventions for two outcomes
Clustered ranking plots of the network for the markedly effective rate and the improvement of neurological impairment score are shown in Fig. 7. Each color represents a group of treatments that belong to the same cluster. Treatments lying in the upper right corner are more effective than the other treatments. The upper right corner in Fig. 7 shows that SXN, YXDM, DH, SXT, HHHSS, DZXX and SX produce significantly better outcomes in ACI patients.
Fig. 7.
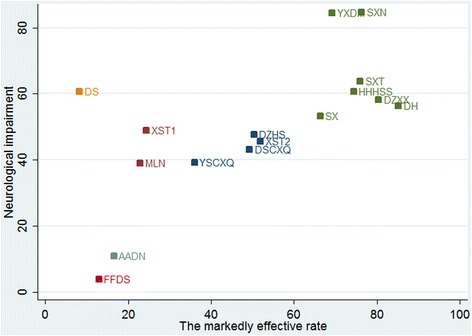
Clustered ranking plot of the networks
Discussion
Considerable evidence exists regarding the clinical effectiveness of CHIs in ACI patients. Some CHIs have been widely used to strengthen clinical effectiveness, reduce neurological impairments and improve the patient’s quality of life. However, the majority of these findings have not been analyzed in head-to-head comparisons. Clinicians must decide among several therapeutic options for ACI patients. To address the absence of comparative data, we conducted an NMA to comprehensively estimate the effectiveness of different CHIs combined with AADN for ACI.
This NMA consisted of 64 RCTs that included 6225 participants; fifteen CHIs were identified in the treatment of ACI, including injections of SXN, SXT, SX, MLN, HHHSS, FFDS, DZHS, DZXX, DSCXQ, DS, DH, YXDM, LI, XST1, and XST2. In terms of the improvement of the markedly effective rate, DH had the highest likelihood of being the best treatment in terms of the markedly effective rate. On account of improvement of neurological impairment, SXN had the highest probability of being the best treatment.
The clustered ranking according to two outcomes revealed that the markedly effective rate and the improvement of the neurological impairment cluster were best for SXN, YXDM, DH, SXT, HHHSS, DZXX and SX. SXN and YXDM are shown at the top right corner. Previous meta-analyses [106–108] found that SXN and YXDM as adjuvant treatments for ACI were beneficial compared to AADN alone. SXN and YXDM are Ginkgo biloba extracts (GBEs), both of which are extracted from Ginkgo biloba leaves. Ginkgo biloba leaves, the TCM for activating blood circulation, mainly contain ginkgo flavonoids, ginkgolides, and bilobalide and have been used as a therapeutic agent for managing cerebrovascular and neurological disorders [109, 110]. GBE exhibits a wide variety of biological activities, including anti-inflammation and antioxidant effects [111, 112]. ACI is the process whereby artery stenosis or blockage causes brain tissue hypoxic ischemia, resulting in brain dysfunction [6]. There is considerable evidence suggesting the active repair and recovery mechanisms following stroke, and neurogenesis is one of them [113]. GBE not only has antioxidant, anti-atherogenesis and angiogenic properties but can also strengthen repair and regeneration mechanisms and prevent cell death, protect the brain from further damage and improve neurological deficits following stroke [113–115]. The neuroprotective mechanism has been attributed to the heme oxygenase 1 (HO1)/Wnt canonical pathway as well as neuritogenic and angiogenic effects [113, 116]. HO1, a key component of the EGb 761 neuroprotective signaling pathway, activates the signaling pathway mechanisms of angiogenesis, cell survival and neuroplasticity, and neurogenesis [113]. Thus, GBE could enhance the post-stroke regeneration process to improve treatments for stroke recovery. Further research is desirable to shed more light on the mechanism underlying the effects of GBE on ACI.
A NMA was used to compare the effectiveness of different CHIs to identify the best CHIs for ACI. This study is the first indirect comparison using a network approach to compare the effectiveness of CHIs, which is valuable for clinicians selecting CHIs for ACI treatment. However, some limitations existed in this NMA.
First, all trials reported random distribution, while ten studies described the randomization methods including random number tables or the envelope method. Information about allocation concealment and blinding was not clear in the majority of trials and may have therefore affected the reliability of the results. Second, the systematic review included only published studies in the database, with no relevant gray literature, which likely caused a selection bias in the literature. Third, the study aimed to use a NMA to evaluate the clinical effectiveness of 37 CHIs combined with an AADN regimen; however, only 15 CHIs were included in the NMA. Thus, more rigorously designed RCTs focused on the 22 additional CHIs are needed to confirm the effectiveness of CHIs for ACI. Fourth, due to the original research limitation, we failed to evaluate the long-term effect of CHIs. Additionally, with the limited data extracted from the original research, we failed to evaluate the ability of CHIs to improve the activities of daily living function and reduce mortality. Fifth, our results might have limited generalizability because all of the included RCTs were conducted in China among Chinese populations. Therefore, it is uncertain whether the effect may change when CHIs are used in populations of other ethnicities and in different geographical locations. In addition, though acute phases were limited, the severities of patient were various. This point may influence the results. Sixth, a NMA compares multiple treatments by incorporating direct and indirect evidence into a general statistical framework. One issue with the validity of a NMA is the inconsistency between direct and indirect evidence. Hence, to improve the reliability of our results, we used a random-effects model within a Bayesian framework. Although, head-to-head trials provide the highest level of evidence when comparing interventions, the quantity of data for some CHI direct trials was small, such as DH versus FFDS, SXN, and XSTT. Large RCTs are needed to specifically compare CHIs with one another.
Conclusions
In summary, our evidence suggested that DH injection plus AADN was the optimum treatment regimen for patients with ACI to improve the markedly effective rate. SXN injection plus AADN was the optimum treatment regimen for ACI to improve the neurological impairment score. Considering both the markedly effective rate and the improvement of neurological impairment, SXN, YXDM, DH, SXT, HHHSS, DZXX and SX plus AADN were the optimum treatment regimens for ACI, especially SXN + AADN and YXDM + AADN. In terms of limitations, highest levels of evidence need to support our conclusions.
Additional files
Table S1. List of search terms. (DOC 20 kb)
Search strategy. (DOC 18 kb)
More details about the product information of CHIs. (DOC 42 kb)
Figure S1. Risk of bias summary. Note: Green: low risk of bias; Yellow: unclear risk of bias; Red: high risk of bias. (JPEG 1934 kb)
Figure S2. Inconsistency test for the markedly effective rate. (JPEG 953 kb)
Figure S3. Comparison adjusted funnel plot. (JPEG 533 kb)
Figure S4. Inconsistency test for improvement of neurological impairment. (JPEG 693 kb)
Acknowledgments
Funding
National Natural Science Foundation of China (Nos. 81473547 and 81673829).
Availability of data and materials
All data generated or analyzed during this study are included in the published article.
Abbreviations
- AADN
Aspirin + anticoagulants + dehydrant + neuroprotectant
- ACI
Acute cerebral infarction
- CBM
Chinese biomedical literature database
- CHIs
Chinese herbal injections
- CNKI
China national knowledge infrastructure database
- CI
Credible interval
- CT
Computed tomography
- DH
Danhong injection
- DS
Danshen injection
- DSCXQ
Danshenchuanxiongqin injection
- DZHS
Dengzhanhuasu injection
- DZXX
Dengzhanxixi injection
- FFDS
Fufangdanshen injection
- GBEs
Ginkgo biloba extracts
- HHHSS
Honghuahuangsesu injection
- IF
Inconsistency factor
- LI
Ligustrazine injection
- MeSH
Medical subject heading
- MLN
Mailuoning injection
- MRI
Magnetic resonance imaging
- NMA
Network meta-analysis
- ORs
Odds ratios
- RCTs
Randomized controlled trials
- SMDs
Standardized mean differences
- SUCRA
Surface under the cumulative ranking probabilities
- SX
Shenxiong injection
- SXN
Shuxuening injection
- SXT
Shuxuetong injection
- TCM
traditional Chinese medicine
- XST1
Xueshuantong injection
- XST2
Xuesaitong injection
- YXDM
Yinxingdamo injection
Authors’ contributions
Conception and design of the network meta-analysis: SL, JRW. Performance of the network meta-analysis: SL, DZ, and KHW. Quality assessment of the network meta-analysis: JRW, DZ, and BZ. Analysis of study data: XMZ, DT, and XJD. Writing of the paper: SL, YYC, and XKL. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
Ethical approval was not necessary in the current meta-analysis because our meta-analysis only gathered RCTs from a literature search; hence, this procedure did not require any personal data and did not harm any patient.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12906-018-2178-9) contains supplementary material, which is available to authorized users.
Contributor Information
Shi Liu, Email: 1048823645@qq.com.
Jia-Rui Wu, Email: exogamy@163.com.
Dan Zhang, Email: 2426394372@qq.com.
Kai-Huan Wang, Email: wkhlinda@163.com.
Bing Zhang, Email: zhangbing6@263.net.
Xiao-Meng Zhang, Email: zhangxm0320@163.com.
Di Tan, Email: tandi1993@qq.com.
Xiao-Jiao Duan, Email: duanxiaojiaodgh@163.com.
Ying-Ying Cui, Email: 867422664@qq.com.
Xin-Kui Liu, Email: 1020172827@qq.com.
References
- 1.Luo JN. New progress on treating acute cerebral infarction. Pract J card Cereb Pneumal. Vasc Dis. 2010;18:1546–1547. [Google Scholar]
- 2.Hui Z, Sha DJ, Wang SL, et al. Panaxatriol saponins promotes angiogenesis and enhances cerebral perfusion after ischemic stroke in rats. BMC Complement Altern Med. 2017;17:70. doi: 10.1186/s12906-017-1579-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang K, Zhang D, Wu J, et al. A comparative study of Danhong injection and Salvia miltiorrhiza injection in the treatment of cerebral infarction: a systematic review and meta-analysis. Med. 2017;96:e7079. doi: 10.1097/MD.0000000000007079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inoue T, Kobayashi M, Uetsuka Y, Uchiyama S. Pharmacoeconomic analysis of cilostazol for the secondary prevention of cerebral infarction. Circ J. 2006;70:453–458. doi: 10.1253/circj.70.453. [DOI] [PubMed] [Google Scholar]
- 5.Sun W, Ou Q, Zhang Z, et al. Chinese acute ischemic stroke treatment outcome registry (CASTOR): protocol for a prospective registry study on patterns of real-world treatment of acute ischemic stroke in China. BMC Complement Altern Med. 2017;17:357. doi: 10.1186/s12906-017-1863-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei J, Yang W, Yin S, et al. The quality of reporting of randomized controlled trials of electroacupuncture for stroke. BMC Complement Altern Med. 2016;16:512. doi: 10.1186/s12906-016-1497-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang KH, Wu JR, Liu S, Zhang D, Duan XJ, Zhang B. Meta-analysis on randomized controlled trials of xingnaojing injection for treating acute cerebral infarction. Chin J Pharmacoepidemiol. 2017;15:471–6. [Google Scholar]
- 8.You YN, Cho MR, Kim JH, et al. Assessing the quality of reports about randomized controlled trials of scalp acupuncture combined with another treatment for stroke. BMC Complement Altern Med. 2017;17:452. doi: 10.1186/s12906-017-1950-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun K, Fan J, Han J. Ameliorating effects of traditional Chinese medicine preparation, Chinese materia medica and active compounds on ischemia/reperfusion-induced cerebral microcirculatory disturbances and neuron damage. Acta Pharm Sin B. 2015;5:8–24. doi: 10.1016/j.apsb.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao W, Liu W. Wu T, Zhong D, Liu G. Dengzhanhua preparations for acute cerebral infarction. Cochrane Database Syst Rev. 2008;63:CD005568. doi: 10.1002/14651858.CD005568.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liao P, Wang L, Guo L, Zeng R, Huang J, Zhang M. Danhong injection (a traditional Chinese patent medicine) for acute myocardial infarction: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2015;2015:646530. doi: 10.1155/2015/646530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie CL, Wang WW, Xue XD, Zhang SF, Gan J, Liu ZG. A systematic review and meta-analysis of Ginsenoside-Rg1 (G-Rg1) in experimental ischemic stroke. Sci Rep. 2015;5:7790. doi: 10.1038/srep07790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li B, Wang Y, Lu J, Liu J, Yuan Y, Yu Y, et al. Evaluating the effects of Danhong injection in treatment of acute ischemic stroke: study protocol for a multicenter randomized controlled trial. Trials. 2015;16:561. doi: 10.1186/s13063-015-1076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu YC, Wu SZ, Hou Q. Clinical study on Xueshuantong combined with aspirinon in treatment of senium acute cerebral infarction. Drugs & Clinic. 2014;7:782–785. [Google Scholar]
- 15.CAST (Chinese Acute Stroke Trial) Collaborative Group CAST: randomised placebo-controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. Lancet. 1997;349:1641–1649. doi: 10.1016/S0140-6736(97)04010-5. [DOI] [PubMed] [Google Scholar]
- 16.The International Stroke Trial (IST) A randomized trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. International stroke trial collaborative group. Lancet. 1997;349:1569–1581. doi: 10.1016/S0140-6736(97)04011-7. [DOI] [PubMed] [Google Scholar]
- 17.Edaravone Acute Infarction Study Group Effect of a novel free radical scavenger, edaravone (MCI-186), on acute brain infarction. Randomized, placebo-controlled, double-blind study at multicenters. Cerebrovasc Dis. 2003;15:222–229. doi: 10.1159/000069318. [DOI] [PubMed] [Google Scholar]
- 18.Gu XL, Ding XS, Di Q. Clinical study on edaravone injection in treatment of acute cerebral infarction. Chinese J New Drugs Clin Remedies. 2005;24:113–116. [Google Scholar]
- 19.Zhang M, Xu LJ, Deng LY. Efficacy and safety of Edaravone injection in the treatment of acute cerebral infarction: a randomized, double blind, multicenter study. Chinese J New Drugs Clin Remedies. 2007;26:105–108. [Google Scholar]
- 20.Davalos A, Castillo J, Alvarez-Sabin J, Secades JJ, Mercadal J, López S, et al. Oral citicoline in acute ischemic stroke: an individual patient data pooling analysis of clinical trials. Stroke. 2002;33:2850–2857. doi: 10.1161/01.STR.0000038691.03334.71. [DOI] [PubMed] [Google Scholar]
- 21.National Pharmacopoeia Committee . Chinese pharmacopoeia, part 1, Appendix 13. Beijing, China: Chemical Industry Press; 2005. [Google Scholar]
- 22.Xie YY, Xiao X, Luo J-M, Fu C, Wang Q-W, Wang Y-M, et al. Integrating qualitative and quantitative characterization of traditional Chinese medicine injection by high-performance liquid chromatography with diode array detection and tandem mass spectrometry. J Sep Sci. 2014;37:1438–1447. doi: 10.1002/jssc.201400129. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Liu F, Li PH. Analysis of the adverse reaction of traditional Chinese medicine injections activating blood stasis. China Pharmacy. 2011;22:646–648. [Google Scholar]
- 24.Tan D, Wu JR, Liu S, Zhang B. Meta-analysis of efficacy of shuxuening injection in the treatment of cerebral infarction. J Pharmacoepidemiol. 2016;8:492–498. [Google Scholar]
- 25.Wang X, Wu JR, Zhang D, Wang KH, Zhang B. Meta-analysis of efficacy of xueshuantong injection in the treatment of acute cerebral infarction. J Pharmacoepidemiol. 2016;10:616–622. [Google Scholar]
- 26.Wang KH, Wu JR, Zhang D, Liu S, Zhang XM, Zhang B. Meta-analysis of efficacy of mailuoning injection in the treatment of acute cerebral infarction. J Pharmacoepidemiol. 2016;9:544–548. [Google Scholar]
- 27.Duan XJ, Wu JR, Liu S, Zhang D, Fang J, Zhang B. Meta-analysis of efficacy of danshenchuanxiongqin injection in the treatment of acute cerebral infarction. J Pharmacoepidemiol. 2017;1:27–32. [Google Scholar]
- 28.Liu X-T, Ren P-W, Peng L, Kang D-Y, Zhang T-L, Wen S, et al. Effectiveness and safety of ShenXiong glucose injection for acute ischemic stroke: a systematic review and GRADE approach. BMC Complement Altern Med. 2016;16:68. doi: 10.1186/s12906-016-1038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu J, Zhang XM, Zhang B. Qingkailing injection for the treatment of acute stroke: a systematic review and meta-analysis. J Tradit Chin Med. 2014;34:131–139. doi: 10.1016/S0254-6272(14)60066-2. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Wu J, Zhang B. Xuesaitong injection as one adjuvant treatment of acute cerebral infarction: a systematic review and meta-analysis. BMC Complement Altern Med. 2015;15:36. doi: 10.1186/s12906-015-0560-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng W, Yang J, Wang Y, Wang W, Xu J, Wang L, et al. Systematic review and meta-analysis of randomized controlled trials of Xingnaojing treatment for stroke. Evid Based Complement Altern Med. 2014;2014:210851. doi: 10.1155/2014/210851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu B, Liu M, Liu H, Li W, Tan S, Zhang S, et al. Meta-analysis of traditional Chinese patent medicine for ischemic stroke. Stroke. 2007;38:1973–1979. doi: 10.1161/STROKEAHA.106.473165. [DOI] [PubMed] [Google Scholar]
- 33.Jinatongthai P, Kongwatcharapong J, Foo CY, Phrommintikul A, et al. Comparative efficacy and safety of reperfusion therapy with fibrinolytic agents in patients with ST-segment elevation myocardial infarction: a systematic review and network meta-analysis. Lancet. 2017;390:747–759. doi: 10.1016/S0140-6736(17)31441-1. [DOI] [PubMed] [Google Scholar]
- 34.Sun F, Chai SB, Li LS, et al. Effects of glucagon-like peptide-1 receptor agonists on weight loss in patients with type 2 diabetes: a systematic review and network meta-analysis. J Diabetes Res. 2015;2015:157201. doi: 10.1155/2015/157201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23:3105–3124. doi: 10.1002/sim.1875. [DOI] [PubMed] [Google Scholar]
- 36.The fourth session of the National Cerebrovascular Conference. The norm of clinical neurologic deficit score. Chin J Neurol. 1996;29:381–3.
- 37.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [EB/OL]. The Cochrane collaboration (2011). http://training.cochrane.org/handbook. Accessed 16 May 2013.
- 38.Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol. 2010;63:e1–37. doi: 10.1016/j.jclinepi.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Salanti G, Ades AE, Ioannidis JPA. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64:163–171. doi: 10.1016/j.jclinepi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 40.Riley RD, Higgins JPT, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. 2011;342:d549. doi: 10.1136/bmj.d549. [DOI] [PubMed] [Google Scholar]
- 41.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chaimani A, Higgins JPT, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One. 2013;8:e76654. doi: 10.1371/journal.pone.0076654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu YM, Ran ZJ. Clinical study on 58 cases acute cerebral infarction treated by Danhong injection. Pract J Card Cereb Pneum Vasc Dis. 2009;17:477–478. [Google Scholar]
- 44.Yu Y. Clinical study on Xueshuantong in treatment of acute cerebral infarction. Chinese Foreign Med Res. 2015;13:32–33. [Google Scholar]
- 45.Zhang ZJ, Cao CY. Clinical study on Shenxiong glucose injection in treatment of acute cerebral infarction and its effect on plasma endothelin. J Guangdong Med Coll. 2008;26:408–409. [Google Scholar]
- 46.Zheng XD. Curative effect with dipyridamole plus aspirin therapy in acute cerebral infarction. J Mod Clin Med. 2007;33:171–173. [Google Scholar]
- 47.Zhou SJ. Study on Shenxiong glucose injection in treatment of acute cerebral infarction. Mod. Prev Med. 2011;38:2656–2659. [Google Scholar]
- 48.Zhou SF, Huo Y. Study on Ginkgo dipyridolum injection combined with aspirinon in treatment of acute cerebral infarction. J Mudanjiang Med Univ. 2009;20:56–58. [Google Scholar]
- 49.Zhou SH. Effect of Shu Xue Ning injection on serum IL - 6 and its efficacy in patients with acute cerebral infarction. Zhejiang J Tradit Chin Med. 2013;48:923. [Google Scholar]
- 50.Xu XY. Study on Ginkgo dipyridolum injection in treatment of acute cerebral infarction. In: Shandong province, the third integrated traditional Chinese medicine and western medicine academic symposium. Jinan; 2011. p. 268–270.
- 51.Xie S, Cao C. Effect of Shuxuening injection in patients with acute cerebral infarction. J Med Theory Pract. 2011;24:1891–1892. [Google Scholar]
- 52.Xie YG, Zhou JH. Observation of effect of Suxuetong injection on acute cerebral infarction. China Trop Med. 2010;10:352–392. [Google Scholar]
- 53.Xu LL, Meng QY, Tian L, Liu XH, Liu T. Curative effection of tetramethylpyrazine on acute cerebral infarction. J Pract Tradit Chinese Intern Med. 2011;25:57–58. [Google Scholar]
- 54.Xu XQ. The observation of aplication of Ginkgo leaf extract and diphyridamole injection in the treatment of acute cerebral infarction. Chinese J Exp Tradit Med Formulae. 2012;18:211–213. [Google Scholar]
- 55.Yang HJ. Effect of Xuetong injection in patients with acute cerebral infarction. J Pract Med Tech. 2007;14:2157. [Google Scholar]
- 56.Yang YF. Study on Danhong injection combined with aspirinon in treatment of acute cerebral infarction. J Psychol Doctor. 2012;3:30. [Google Scholar]
- 57.Yao QY, Gong SJ. Effect of Shuxuetong injection in patients with acute cerebral infarction. Mod J Integr Tradit Chinese Western Med. 2010;19:3389–3390. [Google Scholar]
- 58.Yao J, Lu XR. The observation of aplication of Ginkgo leaf extract and diphyridamole injection in the treatment of acute cerebral infarction. Clin Med. 2010;30:44–45. [Google Scholar]
- 59.Xie RP. Clinical study on Shuxuening in treatment of acute cerebral infarction. Proc Clin Med. 2010;19:758–759. [Google Scholar]
- 60.Tan SY, Tan ZL. Clinical study of Danhong injection in the treatment of cerebral infarction caused by branch atheromatous disease. J Shenyang Med Coll. 2016;18:18–20. [Google Scholar]
- 61.Wang WP. Study on Ginkgo dipyridolum injection combined with Fufangdanshen injection in treatment of acute cerebral infarction. Shenzhen J Integr Tradit Chinese and Western Med. 2015;25:21–22. [Google Scholar]
- 62.Ren HM. The observation of aplication of Shenxiong injection in the treatment of acute cerebral infarction. China Healthcare Innov. 2009;4:27. [Google Scholar]
- 63.Sun HJ, Zheng MH, Liang TS. Effects of Chuangxiongqin injection on serum sCD40L and the level of hs-CRP in patients with angina pectoris. J Shanghai Univ Tradit Chin Med. 2013;27:39–41. [Google Scholar]
- 64.Tang JP, Jiang P, Yang XY. Clinical efficacy and safety of safflower yellow pigment injection in the treatment of acute cerebral infarction. J Mod Med Health. 2013;29:2524–2525. [Google Scholar]
- 65.Tang FY, Zhang GW. Clinical study on 36 cases acute cerebral infarction treated by breviscapine injection. Chinese J Pract Med. 2009;36:51–52. [Google Scholar]
- 66.Tang XJ. Study on Ginkgo damole injection combined with aspirinon in treatment of acute cerebral infarction. Hebei Med J. 2013;35:1188–1189. [Google Scholar]
- 67.Wang L. Clinical study on 48 cases acute cerebral infarction treated by Shenxiong glucose injection. China Foreign Med Treat. 2010;29:116. [Google Scholar]
- 68.Wang YL, Zou XH, Dang LH. Expression of TNF-a and IL-6 in patients with acute cerebral infarction after clinical intervention of Shuxuetong injection and clinical significance. J Yunnan UnivTradit Chin Med. 2013;36:70–72. [Google Scholar]
- 69.Lan Y, Xiao JX, Zheng TY, ZX L. Effect of salvia ligustrazin injection on lysophospholipids acid, P selection and its efficacy in patients with acute cerebral infarction. Mod J Integr Tradit Chinese Western Med. 2015;24:840–842. [Google Scholar]
- 70.Li M, Li Q, Zhang M, Zhang XL, Hu Q, Pang Y. Effect of Dan Hong injection on serum S100B protein and neuron specific alcohol in patients with acute cerebral infarction. Herald Med. 2014;33:1596–1599. [Google Scholar]
- 71.Liu YP, Wang ZG, Wu H. Effect of Dan Hong injection on C-reactive protein and its efficacy in patients with acute cerebral infarction. Pract J Card Cereb Pneum Vasc Dis. 2010;18:1433–1434. [Google Scholar]
- 72.Ma J, Yu NW, Ren ZW, Chen M, Wu HH. Effect of breviscapine on serum NSE,Ang-2 and IL-6 levels and its efficacy in patients with acute cerebral infarction. Chinese J Biochem Pharm. 2010;35:110–112. [Google Scholar]
- 73.Ma J, Yu NW, Wu HH, Chen M, Ren ZW, Zhao DD. Effect of Danhong injection on nerve function and hemorheology for patients with acute ischemic stroke. Chinese J Exp Tradit Med Formulae. 2015;21:204–207. [Google Scholar]
- 74.Chen H. Clinical study on 67 cases acute cerebral infarction treated by Shuxuetong injection. Asia-Pacific Tradit Med. 2015;11:137–138. [Google Scholar]
- 75.Luan T. The clinical effects of Xuesaitong injection in the treatment of acute cerebral infarction. Guide China Med. 2014;12:42–43. [Google Scholar]
- 76.Huang ML. Clinical study on 150 cases acute cerebral infarction treated by Xueshuangtong injection. Mod Diagn Treat. 2012;23:2254–2255. [Google Scholar]
- 77.Li X, Zheng WW, Gao YB. Effect of carthamin yellow injection on NT-proBNP and TXB_2 for patients with acute ischemic stroke. Chinese J Integr Med Cardio/Cerebrovascular Dis. 2015;13:1900–1902. [Google Scholar]
- 78.Ma ZL, Wu BX, Cheng DM, Zhang XL, Hu Y. Clinic observation of the curative effect of safflor yellow in the treatment of acute cerebral infarction. Chinese J Pract Nerv Dis. 2011;14:30–31. [Google Scholar]
- 79.Zhang Y, Hou J, Hu Y, Mu ZB, HY M. Study on Danshen Chuanxiongqin injection combined with edaravone in treatment of acute cerebral infarction. Chinese J Integr Med Cardio/Cerebrovascular Dis. 2012;10:168–169. [Google Scholar]
- 80.Liu M. Study on Danshen Chuanxiongqin injection combined with edaravone in treatment of acute cerebral infarction. Mod J Integr Tradit Chinese Western Med. 2014;23:2105–2107. [Google Scholar]
- 81.Chen YC. Clinic observation of the curative effect of Ginkgo damole injection in the treatment of acute cerebral infarction. Chinese Foreign Med Res. 2011;9:45–46. [Google Scholar]
- 82.Yang RF. Clinical research of edaravone combined with Shuxuetong in treating acute cerebral infarction. China J Chinese Med. 2013;28:1228–1229. [Google Scholar]
- 83.Lin YF, Wang ML, Huang ZQ, Yan P, Su Y. Clinical research of combined Ginkgo damole injection with aspirin in treating acute cerebral infarction. Proc Clin Med. 2008;17:761–762. [Google Scholar]
- 84.Peng T, Pang YL. Clinic observation of the curative effect of Shuxuetong injection in the treatment of acute cerebral infarction. Pract J Card Cereb Pneum Vasc Dis. 2006;14:388. [Google Scholar]
- 85.Li XH. Clinic observation of the curative effect of Shuxuening injection in the treatment of acute cerebral infarction. Asia-Pacific Tradit Med. 2011;7:129–130. [Google Scholar]
- 86.Liu L, Yang XS, Chen W, Liu ZT. Clinic observation of the curative effect of Danhong injection in the treatment of acute cerebral infarction. Clin J Tradit Chin Med. 2014;26:166–167. [Google Scholar]
- 87.Zhang YH, Liu WJ, Ding S. The clinical research of Danhong combinded with low molecular heparin calcium to treat the acute cerebral infarction. Pract J Card Cereb Pneum Vasc Dis. 2010;18:3–5. [Google Scholar]
- 88.Fan J. Clinic observation of the curative effect of Ginkgo damole injection in the treatment of acute cerebral infarction. China Pharm. 2006;15:54. [Google Scholar]
- 89.Liu HY, Liu YM. Clinical study on 64 cases acute cerebral infarction treated by Xuesaitong injection. Jilin Med J. 2014;2014:4963–4964. [Google Scholar]
- 90.Li FQ. Clinical study on 64 cases acute cerebral infarction treated by Ginkgo damole injection. Chinese J Ethnomed Ethnopharmacy. 2010;19:106. [Google Scholar]
- 91.Lian CL. Clinical observation on 46 cases of acute cerebral infarction treated with combination of traditional Chinese and western medicine. J Pract Tradit Chin Med. 2013;29:456. [Google Scholar]
- 92.Chen S, Jiang KW, Lu HZ. Clinic observation of the curative effect of Xuesaitong injection in the treatment of acute cerebral infarction. J Pract Med. 2006;22:1405–1406. [Google Scholar]
- 93.Cao L. Clinic observation of the curative effect of Xueshuangtong injection in the treatment of acute cerebral infarction. Chinese J Ethnomed Ethnopharmacy. 2012;21:71. [Google Scholar]
- 94.Liu Y. Effective observation on acute cerebral infarction treated by Shuxuetong injection. Chinese J Med Guide. 2009;11:1157–1158. [Google Scholar]
- 95.Luo XD, Wang P, Zeng XR. Xuesaitong injection plus routine therapy for acute cerebral infarction and the influence on plasma C-reactive protein. Pract J Clin Med. 2011;8:96–98. [Google Scholar]
- 96.Liao MJ, Chen ZB. Clinical study on 30 cases acute cerebral infarction treated by safflower yellow injection. Med Inf. 2014;27:109–110. [Google Scholar]
- 97.Shi JL, Cheng JL. The clinical research of Ginkgo-damole combinded with atorvastatin calcium to treat the acute cerebral infarction. Chinese J Pract Nerv Dis. 2010;13:52–53. [Google Scholar]
- 98.Ni H. Clinical study on 59 cases of Shuxuening combined with sodium ozagrel to treat the acute cerebral infarction. Jilin Med J. 2010;30:3078–3079. [Google Scholar]
- 99.Li CP. Clinical study on 80 cases acute cerebral infarction treated by Shuxuening injection. J Shanxi Coll Tradit Chin Med. 2007;8:37–38. [Google Scholar]
- 100.Zhou ZP, Yang GS, Wang AY. Effect of erigeron injection on MMP- 9 in patient with acute cerebral infarction. Mod Prev Med. 2011;38:2660–2661. [Google Scholar]
- 101.Chen C. Efficacy and safety of aspirin plus clopidogrel and Shuxuetong in treatment of patients with acute cerebral infarction. Eval Anal Drug-Use Hosp China. 2015;15:808–810. [Google Scholar]
- 102.Chen JY, Wang DC, Luo SW. Clinical study on 54 cases of Shuxuetong plus aspirin and clopidogrel to treat the acute cerebral infarction. China Pharmaceuticals. 2012;21:53–54. [Google Scholar]
- 103.Luo QY. Clinic observation of the curative effect of Ginkgo bilboa injection in the treatment of acute cerebral infarction. Youjiang Med J. 2007;35:133–134. [Google Scholar]
- 104.Chen J, Li SJ. Clinical observation of Ginkgo-dipyidamolum injection in treatment of acute cerebral infarction. Neural Inj Funct Reconstr. 2007;7:291–293. [Google Scholar]
- 105.Zhang XL, Zhang LL, Heng XL, Ma Y, Pang W. Effect of Mailuoning injection on blood rheology for patients with acute ischemic stroke. Nerv Dis Mental Health. 2015;5:216–217. [Google Scholar]
- 106.Ni HQ, Wu L, Li JF, Chen J. Meta-analysis of comparative study on Ginkgo dipyridolum injection in the treatment of acute cerebral infarction. China Pharm. 2008;19:1655–1658. [Google Scholar]
- 107.Xi BC, Zhang C, Sun LL. Meta-analysis of comparative study on Shuxuening injection in the treatment of acute cerebral infarction. Pharm Care Res. 2012;12:354–357. doi: 10.5428/pcar20120512. [DOI] [Google Scholar]
- 108.Zheng WK, Zhang L, Shang HC. Systematic review on Shuxuening injection in treating acute cerebral infarction. Chinese Licensed Pharmacist. 2012;9:33–41. [Google Scholar]
- 109.Rodríguez M, Ringstad L, Schäfer P, Just S, Hofer HW, Malmsten M, et al. Reduction of atherosclerotic nanoplaque formation and size by Ginkgo biloba (EGb 761) in cardiovascular high-risk patients. Atherosclerosis. 2007;192:438–444. doi: 10.1016/j.atherosclerosis.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 110.Luo Y. Alzheimer's disease, the nematode Caenorhabditis elegans, and Ginkgo biloba leaf extract. Life Sci. 2006;78:2066–2072. doi: 10.1016/j.lfs.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 111.Park Y-M, Won J-H, Yun K-J, Ryu J-H, Han Y-N, Choi S-K, et al. Preventive effect of Ginkgo biloba extract (GBB) on the lipopolysaccharide-induced expressions of inducible nitric oxide synthase and cyclooxygenase-2 via suppression of nuclear factor-κB in RAW 264.7 cells. Biol Pharm Bull. 2006;29:985–990. doi: 10.1248/bpb.29.985. [DOI] [PubMed] [Google Scholar]
- 112.Biddlestone L, Corbett AD, Dolan S. Oral administration of Ginkgo biloba extract, EGb-761 inhibits thermal hyperalgesia in rodent models of inflammatory and post-surgical pain. Br J Pharmacol. 2007;151:285–291. doi: 10.1038/sj.bjp.0707220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nada SE, Tulsulkar J, Shah ZA. Heme oxygenase 1-mediated neurogenesis is enhanced by Ginkgo biloba (EGb 761®) after permanent ischemic stroke in mice. Mol Neurobiol. 2014;49:945–956. doi: 10.1007/s12035-013-8572-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tulsulkar J, Shah ZA. Ginkgo biloba prevents transient global ischemia-induced delayed hippocampal neuronal death through antioxidant and anti-inflammatory mechanism. Neurochem Int. 2013;62:189–197. doi: 10.1016/j.neuint.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen J-S, Huang P-H, Wang C-H, Lin F-Y, Tsai H-Y, Wu T-C, et al. Nrf-2 mediated heme oxygenase-1 expression, an antioxidant-independent mechanism, contributes to anti-atherogenesis and vascular protective effects of Ginkgo biloba extract. Atherosclerosis. 2011;214:301–309. doi: 10.1016/j.atherosclerosis.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 116.Shah ZA, Nada SE, Doré S. Heme oxygenase 1, beneficial role in permanent ischemic stroke and in Ginkgo biloba (EGb 761) neuroprotection. Neuroscience. 2011;180:248–255. doi: 10.1016/j.neuroscience.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. List of search terms. (DOC 20 kb)
Search strategy. (DOC 18 kb)
More details about the product information of CHIs. (DOC 42 kb)
Figure S1. Risk of bias summary. Note: Green: low risk of bias; Yellow: unclear risk of bias; Red: high risk of bias. (JPEG 1934 kb)
Figure S2. Inconsistency test for the markedly effective rate. (JPEG 953 kb)
Figure S3. Comparison adjusted funnel plot. (JPEG 533 kb)
Figure S4. Inconsistency test for improvement of neurological impairment. (JPEG 693 kb)
Data Availability Statement
All data generated or analyzed during this study are included in the published article.


