Abstract
Living cells orient the cytoskeleton polarity and directional migration in response to spatial gradients of multiple types of cues. The resulting tactic behaviors are critical for the proper cell localization in the context of complex single-cell and tissue behaviors. In this perspective, we highlight the recent discovery of, to our knowledge, a new -taxis phenomenon, the topotaxis, which mediates directional cell migration in response to the gradients of such topographic features as the density of extracellular matrix fibers. The direction of topotactic migration critically depends on the effective stiffness of the cortical cytoskeleton, which is controlled by the balance between two parallel signaling pathways activated by the extracellular matrix input. Topotaxis can account for such striking cell behaviors as the opposite directionality of migration of benign and metastatic cancer cells and certain aspects of the wound-healing process. We anticipate that, in conjunction with other tactic phenomena, topotaxis can provide critical information for understanding and design of tissue structure and function.
Main Text
Normal and cancerous cells can sense graded density of topographic matrix features as a cue to orient their polarity and migration
One key characteristic of life is the ability of cells and organisms to actively respond to changes in their environment. For many cells, these changes include active migration, frequently guided by diverse environmental cues. Living cells can detect gradients of distinct chemical, mechanical, electrical and other kinds of signals. The corresponding “-taxis” phenomena include the ability to recognize gradients of soluble (chemotaxis) (1) and immobilized chemoattractants and chemorepellents (haptotaxis) (2, 3, 4), gradients of rigidity of the adhesive substratum (durotaxis) (5), and gradients of electrical field intensity (galvanotaxis) (6). This striking capability to undergo biased migration betrays the complexity of native cell micro-environments both within living tissues and, for single-cell organisms, in their natural habitats.
In living tissues, both during their development and during their homeostatic maintenance, cells are commonly embedded within intricate networks of extracellular matrix (ECM). The fibers of the matrix present the cells with structurally and chemically complex contact interfaces. Cells use a range of specialized receptors (most notably a family of integrin hetero-dimers) to attach to and be stimulated by the surrounding ECM fibers (7, 8, 9, 10). This interaction can also lead to matrix remodeling by the cells, with the ECM fibers being deformed, re-oriented, degraded, and synthesized by the cells, particularly fibroblasts and other cells specialized for this function (11, 12). These mutual effects might lead to complex interactions, critical to tissue morphogenesis and maintenance, with many details still only poorly understood. They can lead, in particular, to alignment of ECM fibers before the initial invasive dissemination in metastatic cancers of the breast and likely many other tissues (13, 14, 15, 16). The aligned matrix fibers are also a feature of many normal tissues, providing a highly anisotropic environment for the cells at the interface with the matrix (17, 18). Cells respond to this specific oriented matrix organization by polarizing their cytoskeleton and moving along the oriented fibers, a phenomenon known as “contact guidance” (19).
Recently, we identified, to our knowledge, a new cellular “-taxis” phenomenon that occurs while cells are engaging in complex interactions with the surrounding or underlying structured ECM (20, 21, 22). Using engineered surfaces mimicking the size, biochemistry, and orientation of highly organized ECM fibers (17), we discovered that when the density of these fibers was varied, different cell types were capable of sensing this graded cue and undergoing gradual migration up or down this gradient (Fig. 1). This migration across the arrays of fibers was commonly coupled to the contact guidance along the fibers, representing complex directional cell responses. This phenomenon was also observed in more complex two-dimensional structures, with fibers woven together into criss-cross patterns of different density, mimicking such ECM structures as basement membranes (Fig. 1 D) (20, 21). A potentially related phenomenon was reported for another type of graded topographic features (micro-craters) that might also correspond to certain matrix structures (23). Strikingly, we found that the same fiber-density gradient could be interpreted by cells as a cue pointing in opposite directions, with cells navigating either up or down the same gradient, depending on their genetic status (e.g., melanoma cells of different degrees of aggressiveness) or average fiber density (skin fibroblasts). This finding suggested that the interpretation of the gradients of topographic cues can depend on complex intracellular control mechanisms affecting the molecular “circuits” determining the directionality of cell polarity.
Figure 1.
Cells can interface with complex local extracellular matrix organization, leading to a change in their polarity and migration patterns. (A) An electron micrograph of rat dermis illustrating local variability in the collagen-rich extracellular matrix density and organization. (B) Modeling of the matrix structure and graded density by engineering of cell adhesive substrata with nanoscale graded texture (electron micrographs of arrays of partially interrupted nanothreads composed on nanoposts are shown (graded post density array; GPDA)). (C) Scanning electron microscope image of a melanoma cell cultured on the surface shown in (B). The arrow indicates the direction of topotaxis, from more dense to less dense areas (22); (D) Scanning electron microscope image of a fibroblast cell interfaced with a more complex microscale texture of threads modeling intertwined matrix fibers. The arrow displays topotactic guidance from less dense to more dense areas (21).
Topotaxis depends on the effective cortical cell stiffness defining the degree of compliance of cell membrane at the matrix interface
Can topotactic guidance be explained solely by invoking other tactic mechanisms, e.g., those controlling durotaxis or haptotaxis? The ability of the cells to navigate both up and down the gradients of topographic features immediately suggests that the answer is “no,” as both durotaxis and haptotaxis only account for the movement of cells up the density of the rigidity of the substratum or the surface density of a specific biochemical matrix component. What then are the mechanisms guiding cells in gradients of topographic features? We found that the explanation lies in the ability of the cells to interface with the complex and intricate topography of the surrounding matrix features. Previously, it has been suggested that cells have limited capacity to “envelop” microfabricated pillars and other topographic features, leading to the effect of “cells sitting on a bed of micro-needles”, which allows measurement of microscale forces exerted by the cells and also presents the cells with variable effective surface rigidity inputs (24, 25, 26). However, the ability of the cell’s surface to deform sufficiently to comply with the complex topography of the adhesive substratum can vary, depending on, e.g., the spacing between the nano- and microscale features that the cells can explore and the deformability of the cell’s plasma membrane and the underlying cortical cytoskeleton. Previously, we found, for example, that this differential deformability can explain how nanoscale changes in the spacing of model ECM fibers can affect the macroscopic characteristics of collective cell behavior, such as action-potential propagation in cardiac myocytes on the scale of centimeters, extending the effect of the matrix organization over at least six orders of magnitude in space (27). It is possible, therefore, that, depending on the effective cortical stiffness and the characteristics of the local topographic landscape, cells can differentially penetrate into the spaces between the topographic features, leading to a different overall degree of contact with the ECM, which can determine cell polarization and movement.
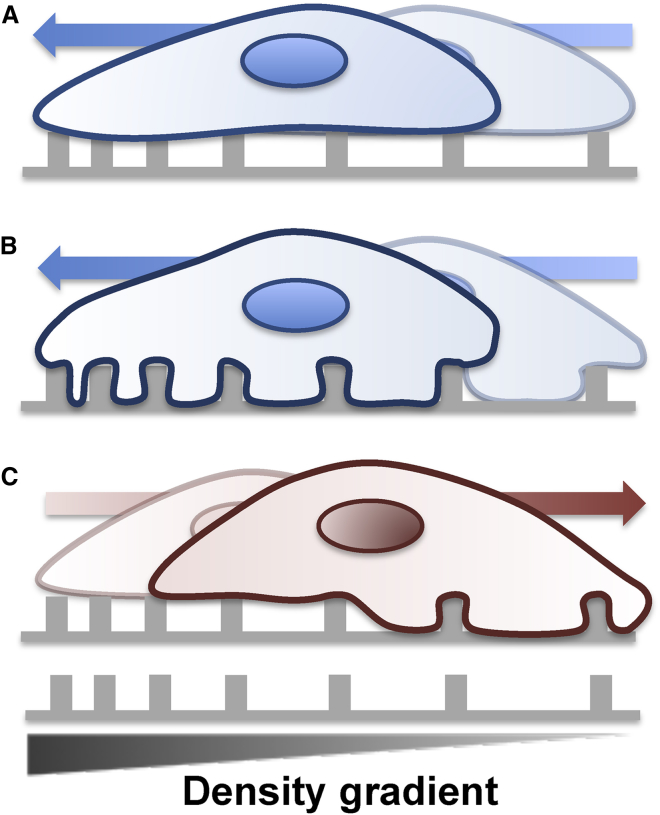
If the topographic features are distributed in a spatially graded fashion, there may be three distinct cases of cell-substratum topography interfacing. First, the cell cortex may be effectively so stiff that it would not allow the cell to penetrate into the spacing between topographic features at all, limiting cell contact to the most proximate surfaces (e.g., tops of the fiber or stubs (pillars), see Fig. 2). Second, the cell cortex may be so soft that it would allow a complete penetration of the cell protrusions into the spaces between the matrix features, as well as effective enveloping of the fibers, substantially increasing the area of the cell-matrix interface. Finally, and most interestingly, the degree of penetration into, and envelopment of, the topographic substratum features can vary across the cell length, being maximal in the sparser areas of the topography and minimal in the denser areas. In the former two cases, the resultant cell behavior can be guided by and accounted for by the haptotaxis and/or durotaxis mechanisms, without the need to invoke new types of -taxis phenomena. The cells would move toward the increasing density of the topographic features, e.g., the matrix fibers. However, in the case of differential penetration, the cells can maximize the matrix contact at the points of higher penetration, i.e., in the sparser rather than denser area of the gradient, with the resulting movement being reversed, i.e., in the direction away from denser and toward sparser matrix areas. This effect essentially depends on the three-dimensional (3D) topographic organization of the fibers and how it is interpreted by the cells, and thus cannot be solely interpreted by referring to haptotaxis or durotaxis (which have been mostly studied in 2D). As discussed in more detail below, ECM input can in turn affect the effective cell stiffness, resulting in an interesting feedback effect that can either facilitate or prevent cell compliance with the nanotopographic features. Overall, this model, which was experimentally confirmed, as discussed below, underscores the importance of taking the material properties of the cells and the surrounding matrix into account when exploring cell behavior.
Figure 2.
Three scenarios of a cell interfacing with an adhesive substratum containing spatially graded topographic features and the corresponding topotaxis directionality. (A) Stiffer cells show limited interfiber penetration and thus limited extracellular matrix contact. They are guided toward higher-matrix-input areas, i.e., topotaxis is toward denser fiber zones. (B) Softer cells can show a high degree of compliance with the complex matrix organization, penetrating into the interfiber spaces and substantially increasing the contact with and input from the extracellular matrix. This contact increases with increasing fiber density. Topotaxis is toward denser fiber zones. (C) At intermediate cell stiffness, there can be a differential degree of interfiber penetration leading to maximization of cell-matrix contact in the sparser, but not the denser, matrix zones. Topotaxis is toward sparser fiber zones.
Topotaxis directionality is defined by the interplay of two signaling pathways activated by ECM and impinging on control of cell stiffness
Do the cells indeed exhibit differential penetration into and between the substratum features? And if so, what regulatory mechanisms control this ability and thus, ultimately, the topotactic cell guidance? In our analysis, we indeed observed differential penetration of various types of cells into the spaces separating matrix features, to the degree defined by the cell stiffness and the local topographic features, in a way fully consistent with the model above (22). This effect was observed on the scale of individual cells over large ranges of fiber density, with fiber sizes and inter-fiber distances similar to those of collagen fibers in vivo (1–10 μm of pore diameters, i.e., distances between fibers) (28), demonstrating that the part of a cell exposed to the sparser region penetrates into the interfiber space better than the part of the cell on the relatively denser side of the model matrix. Furthermore, we found that the cell stiffness, and thus the cortical deformability and the degree of topographic compliance, strongly depended on the genetic makeup of melanoma cells and their degree of aggressiveness. We furthermore found that cortical stiffness and deformability were controlled by the opposite effects of two signaling pathways: the phosphatidylinositide-3-kinase (PI3K)-dependent increase in cortical “softness,” deformability, and protrusive activity counterbalanced by the Rho-associated-protein-kinase (ROCK)-dependent increase in the membrane stiffness (Fig. 3). Interestingly, both these pathways were activated by cell-ECM contact through regulation of integrin receptors, implying that the control was exercised through a feed-forward signaling-loop regulation. The outcome of activation of this signaling network depends on the balance between these two parallel pathways. If, in a given cell, the ROCK signaling dominates over the PI3K signaling pathway for the same ECM input (as was found, e.g., in the relatively nonaggressive melanoma cells), the membrane and underlying cortical cytoskeleton are stiffer, limiting the ability of a cell to conform to the complex surrounding ECM structure. Furthermore, the potential for increased cell-ECM interaction through cell penetration between the ECM fibers (or artificial adhesive substratum features mimicking them) in sparser areas would be counteracted by the negative feedback of increasing ECM-mediated ROCK signaling and thus cell stiffness. As a result, there will be a very limited cell-ECM interfacing, regardless of the local fiber density, leading to topotactic movement up the density gradient. This was indeed experimentally observed. On the other hand, the relative dominance of PI3K signaling, due to mutations in this pathway observed in many aggressive cancers, including metastatic melanoma, can lead to a more compliant cell membrane-cortex and greater local interfacing with the ECM features. This in turn can result in a positive rather than negative feedback, with an increasing cell-ECM contact leading to further effective cortical softening through higher PI3K signaling. The end result would be increased cellular compliance and higher propensity for differential penetration between topotactic features and thus topotaxis from denser to sparser matrix area. This was indeed found to be the case for aggressive melanoma cells, within a wide range of matrix density values similar to those observed in vivo, creating the striking effect whereby aggressive and less aggressive melanoma cells displayed opposite topotactic guidance on the same topographic gradient. This effect immediately suggested that topographic gradients can be used to quickly sort out aggressive and less aggressive cancer cells, a phenomenon that would be useful in diagnostic and prognostic testing.
Figure 3.
The effective cell stiffness, and thus the direction of topotaxis, are governed by the interplay between two parallel signaling pathways diverging from the same input and converging on the same target, both activated by the biochemical input from the extracellular matrix: PI3K and ROCK (see text for details). The PI3K pathway decreases, and the ROCK pathway increases, the effective cell stiffness by regulating the cortical cytoskeleton. The relative balance of activity of these pathways determines the direction of topotaxis and can be affected by cell identity, genetic mutations, or application of pharmacological agents. The balancing interplay between these two pathways is partially affected by signaling cross talk.
If the biochemical control circuit presented above indeed underlies the topotactic guidance, one should be able to reverse the direction of topotaxis by simple pharmacological and/or genetic manipulations of either PI3K or ROCK signaling pathways. We indeed found this to be the case, as, in a series of experiments, we could turn back the direction of topotactic cell migration by reversing the dominance between PI3K and ROCK signaling pathways. This effect was particularly striking when the directionality of the aggressive melanoma cells was switched so that they behaved as benign cells, suggesting, as discussed more fully below, opportunities for therapeutic interventions in this and other aggressive cancers.
Of interest, PI3K and the associated Rac1 small GTPase signaling were previously implicated in controlling cell polarity through specification of the cell leading edge. On the other hand, ROCK signaling and the associated RhoA small GTPase activity were implicated in cell trailing edge, likely through limiting the protrusive activity and promoting cortical contractility (29, 30, 31). Both these pathways were implicated in mesenchymal (Rac1) and amoeboid (RhoA) 3D migration (32, 33). Thus, the signaling networks controlling topographic cell compliance and topotaxis directly feed into the known migration mechanisms accounting for the observed cell polarization and migration.
Implications of topotactic migration for wound healing, aggressive cancer spread, and other processes
Cells in multicellular organisms are commonly embedded in the ECM, which can display various degrees of complexity and organization. 3D cell migration through the intricate meshwork of this matrix displays complex modes defined in part by the degrees of the matrix density and the extent of its alignment (32, 34, 35). Topotactic cell guidance suggests that the effective cell stiffness may define the extent of cell interfacing with the surrounding matrix fibers, which in turn can determine the direction of cell migration. How can this mechanism influence the normal physiological, as well as pathological, processes?
One interesting example of topotactic guidance is observed in skin fibroblasts (20). These cells take an active part in wound healing by mediating the synthesis and organization of the damaged ECM and thus paving the road for subsequent reepithelization. Currently, it is not understood how fibroblasts clear from the wound site before the influx of epithelial cells, with suggested mechanisms including cell death triggered through unknown mechanisms. We found that fibroblasts can be topotactically guided by the local matrix density, displaying bidirectional guidance away from both very high and very low density areas toward the location of optimal intermediate density. This behavior is well explained by the theory of topotaxis briefly presented above, as shown in Fig. 2. The topotactic guidance thus can help in both removing fibroblasts from the areas of newly synthesized high-density matrix and recruiting them to areas of active repair with lower matrix densities. This in turn would create a self-organized “matrix repair wave” of fibroblast localization that would naturally shift as the matrix is repaired and would ultimately force fibroblasts to clear from the repair site, without invoking other previously hypothesized mechanisms, such as cell death.
Another important context in which topotaxis may have clear physiological and pathophysiological roles is in the control of cell localization within a tissue and any change in this localization after either environmentally mediated alterations of the matrix or genetic mutations controlling the effective cortical cell stiffness. In our analysis of topotactic migration in aggressive melanoma, we found that a simple alteration of expression or function of phosphatase and tensin homolog (PTEN), a protein whose gene has the infamous distinction of being the second-most mutated gene across all known cancers (36, 37). However, the significance of this mutation, and, in particular, its putative role in the transition to a more invasive spread, as observed in melanoma, is often not clear. Our analysis showed that the loss of PTEN can dramatically change the PI3K signaling and thus the balance of signaling pathways controlling cell stiffness, making cells effectively softer. This in turn leads to a switch in topotactic polarity and migration allowing the cells to migrate toward sparser matrix areas, which can in turn promote more directional, faster spread. Furthermore, lower cortical tension can make cells more deformable and allow them to more easily “squeeze” through porous matrix areas. This behavior is in contrast to the behavior of less aggressive, more benign cells expressing fictional PTEN, in which topotactic migration promotes cell movement into denser matrix areas, thus disfavoring aggressive spread. Similar mechanisms can ensure appropriate or pathogenic localization patterns of other cells within complex tissues.
These examples highlight the importance of examining such biophysical properties of cells and tissues as the effective cell stiffness and the local density and organization of the extracellular matrix. One can easily envision that if, as is often the case, the matrix organization can be altered in aging or disease, cell localization and function can be significantly perturbed. Indeed, gradual disorganization of the matrix in aging can lead not only to visible wrinkling of the skin, but also to differential contexts for cell navigation, influencing the propensity and degree of potential cell spread from the sites of otherwise benign tumor growth (38, 39). A flip side of this process can occur during tissue fibrosis, resulting in inappropriately dense matrix areas, which could lead to reorganization of the associated tissues through mechanisms including topotactic migration. These and other settings are common enough to warrant additional deep analysis of cell-matrix interactions and investigation of the role of topotaxis in mediating the ensuing tissue reorganization.
Conclusions
Topotaxis is a phenomenon whose role in cell behavior emphasizes the importance of an integrative view, combining the genetic, biochemical, and physical aspects of cell regulation. This integrative view can also help in reexamining the role of other tactic processes, particularly haptotaxis and durotaxis. Despite the perceived importance of these cell guidance mechanisms, they historically have been analyzed mostly on flat surfaces commonly used for cell culture. However, the reality of in vivo cell migration is often vastly different, with cells navigating through complex 3D matrices, with cell-matrix interactions subject to variable interactions. A unified matrix-mediated cell guidance model thus has to include this added complexity and reinterpret the relative roles of distinct -tactic phenomena in different local contexts. We propose that this synthesis can occur through analysis of the underlying signaling networks, which are likely to be overlapping and lead to cell polarity control, and through more accurate modeling of the cell microenvironment. This synthesis will likely reveal the essential features of cell guidance in complex microenvironments in ways that are much more relevant and consequential for our understanding of tissue function. We anticipate that topotaxis will be invoked in many other contexts, including developmental processes, and will serve as an important consideration in diagnostic analysis of cell biopsies and in tissue engineering efforts.
Acknowledgments
We thank Dr. Hong-Nam Kim at the Korea Institute of Science and Technology for the scanning electron microscope image in Fig. 1 A.
J.P. is a recipient of a Samsung scholarship. This work was also supported by National Institutes of Health grants U54 CA209992 and U01 CA155758 to A.L. and R01 GM109463 and R43 CA221659 to D.-H.K. This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA015704 (to D.-H.K.).
Editor: Brian Salzberg.
References
- 1.Devreotes P., Janetopoulos C. Eukaryotic chemotaxis: distinctions between directional sensing and polarization. J. Biol. Chem. 2003;278:20445–20448. doi: 10.1074/jbc.R300010200. [DOI] [PubMed] [Google Scholar]
- 2.Chelberg M.K., Tsilibary E.C., McCarthy J.B. Type IV collagen-mediated melanoma cell adhesion and migration: involvement of multiple, distinct domains of the collagen molecule. Cancer Res. 1989;49:4796–4802. [PubMed] [Google Scholar]
- 3.Isenberg B.C., Dimilla P.A., Wong J.Y. Vascular smooth muscle cell durotaxis depends on substrate stiffness gradient strength. Biophys. J. 2009;97:1313–1322. doi: 10.1016/j.bpj.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCarthy J.B., Furcht L.T. Laminin and fibronectin promote the haptotactic migration of B16 mouse melanoma cells in vitro. J. Cell Biol. 1984;98:1474–1480. doi: 10.1083/jcb.98.4.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lo C.M., Wang H.B., Wang Y.L. Cell movement is guided by the rigidity of the substrate. Biophys. J. 2000;79:144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mycielska M.E., Djamgoz M.B. Cellular mechanisms of direct-current electric field effects: galvanotaxis and metastatic disease. J. Cell Sci. 2004;117:1631–1639. doi: 10.1242/jcs.01125. [DOI] [PubMed] [Google Scholar]
- 7.Boudreau N.J., Jones P.L. Extracellular matrix and integrin signalling: the shape of things to come. Biochem. J. 1999;339:481–488. [PMC free article] [PubMed] [Google Scholar]
- 8.Damsky C.H., Werb Z. Signal transduction by integrin receptors for extracellular matrix: cooperative processing of extracellular information. Curr. Opin. Cell Biol. 1992;4:772–781. doi: 10.1016/0955-0674(92)90100-q. [DOI] [PubMed] [Google Scholar]
- 9.Hynes R.O. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Juliano R.L., Haskill S. Signal transduction from the extracellular matrix. J. Cell Biol. 1993;120:577–585. doi: 10.1083/jcb.120.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shieh A.C., Rozansky H.A., Swartz M.A. Tumor cell invasion is promoted by interstitial flow-induced matrix priming by stromal fibroblasts. Cancer Res. 2011;71:790–800. doi: 10.1158/0008-5472.CAN-10-1513. [DOI] [PubMed] [Google Scholar]
- 12.Zamir E., Katz M., Geiger B. Dynamics and segregation of cell-matrix adhesions in cultured fibroblasts. Nat. Cell Biol. 2000;2:191–196. doi: 10.1038/35008607. [DOI] [PubMed] [Google Scholar]
- 13.Conklin M.W., Eickhoff J.C., Keely P.J. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am. J. Pathol. 2011;178:1221–1232. doi: 10.1016/j.ajpath.2010.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nam K.H., Kim P., Kim D.H. Multiscale cues drive collective cell migration. Sci. Rep. 2016;6:29749. doi: 10.1038/srep29749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Provenzano P.P., Eliceiri K.W., Keely P.J. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4:38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang K., Corsa C.A., Longmore G.D. The collagen receptor discoidin domain receptor 2 stabilizes SNAIL1 to facilitate breast cancer metastasis. Nat. Cell Biol. 2013;15:677–687. doi: 10.1038/ncb2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim D.H., Provenzano P.P., Levchenko A. Matrix nanotopography as a regulator of cell function. J. Cell Biol. 2012;197:351–360. doi: 10.1083/jcb.201108062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liliensiek S.J., Nealey P., Murphy C.J. Characterization of endothelial basement membrane nanotopography in rhesus macaque as a guide for vessel tissue engineering. Tissue Eng. Part A. 2009;15:2643–2651. doi: 10.1089/ten.tea.2008.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teixeira A.I., Abrams G.A., Nealey P.F. Epithelial contact guidance on well-defined micro- and nanostructured substrates. J. Cell Sci. 2003;116:1881–1892. doi: 10.1242/jcs.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim D.H., Han K., Levchenko A. Mechanosensitivity of fibroblast cell shape and movement to anisotropic substratum topography gradients. Biomaterials. 2009;30:5433–5444. doi: 10.1016/j.biomaterials.2009.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim D.H., Seo C.H., Suh K.Y. Guided cell migration on microtextured substrates with variable local density and anisotropy. Adv. Funct. Mater. 2009;19:1579–1586. doi: 10.1002/adfm.200990041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park J., Kim D.H., Levchenko A. Directed migration of cancer cells guided by the graded texture of the underlying matrix. Nat. Mater. 2016;15:792–801. doi: 10.1038/nmat4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeon H., Koo S., Healy K.E. Directing cell migration and organization via nanocrater-patterned cell-repellent interfaces. Nat. Mater. 2015;14:918–923. doi: 10.1038/nmat4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saez A., Buguin A., Ladoux B. Is the mechanical activity of epithelial cells controlled by deformations or forces? Biophys. J. 2005;89:L52–L54. doi: 10.1529/biophysj.105.071217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan J.L., Tien J., Chen C.S. Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proc. Natl. Acad. Sci. USA. 2003;100:1484–1489. doi: 10.1073/pnas.0235407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang M.T., Fu J., Chen C.S. Assaying stem cell mechanobiology on microfabricated elastomeric substrates with geometrically modulated rigidity. Nat. Protoc. 2011;6:187–213. doi: 10.1038/nprot.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim D.H., Lipke E.A., Levchenko A. Nanoscale cues regulate the structure and function of macroscopic cardiac tissue constructs. Proc. Natl. Acad. Sci. USA. 2010;107:565–570. doi: 10.1073/pnas.0906504107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolf K., Te Lindert M., Friedl P. Physical limits of cell migration: control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J. Cell Biol. 2013;201:1069–1084. doi: 10.1083/jcb.201210152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Machacek M., Hodgson L., Danuser G. Coordination of Rho GTPase activities during cell protrusion. Nature. 2009;461:99–103. doi: 10.1038/nature08242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Srinivasan S., Wang F., Bourne H.R. Rac and Cdc42 play distinct roles in regulating PI(3,4,5)P3 and polarity during neutrophil chemotaxis. J. Cell Biol. 2003;160:375–385. doi: 10.1083/jcb.200208179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu J., Wang F., Bourne H.R. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell. 2003;114:201–214. doi: 10.1016/s0092-8674(03)00555-5. [DOI] [PubMed] [Google Scholar]
- 32.Friedl P., Wolf K. Plasticity of cell migration: a multiscale tuning model. J. Cell Biol. 2010;188:11–19. doi: 10.1083/jcb.200909003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanz-Moreno V., Gadea G., Marshall C.J. Rac activation and inactivation control plasticity of tumor cell movement. Cell. 2008;135:510–523. doi: 10.1016/j.cell.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 34.Doyle A.D., Wang F.W., Yamada K.M. One-dimensional topography underlies three-dimensional fibrillar cell migration. J. Cell Biol. 2009;184:481–490. doi: 10.1083/jcb.200810041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Even-Ram S., Yamada K.M. Cell migration in 3D matrix. Curr. Opin. Cell Biol. 2005;17:524–532. doi: 10.1016/j.ceb.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 36.Chalhoub N., Baker S.J. PTEN and the PI3-kinase pathway in cancer. Annu. Rev. Pathol. 2009;4:127–150. doi: 10.1146/annurev.pathol.4.110807.092311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu H., Goel V., Haluska F.G. PTEN signaling pathways in melanoma. Oncogene. 2003;22:3113–3122. doi: 10.1038/sj.onc.1206451. [DOI] [PubMed] [Google Scholar]
- 38.Labat-Robert J., Robert L. Aging of the extracellular matrix and its pathology. Exp. Gerontol. 1988;23:5–18. doi: 10.1016/0531-5565(88)90015-0. [DOI] [PubMed] [Google Scholar]
- 39.Robert L. Cell-matrix interactions in cancer spreading--effect of aging. Semin. Cancer Biol. 2002;12:157–163. doi: 10.1016/S1044-579X(02)00019-6. [DOI] [PubMed] [Google Scholar]