Abstract
Objective:
Leptin plays a pathophysiologic role in the pathogenesis of aortic dysfunction and peripheral arterial stiffness (PAS). Our aim was to evaluate the risk factors for developing PAS and the association of leptin and PAS in patients with type 2 diabetes mellitus (DM).
Materials and Methods:
Fasting blood samples were obtained for biochemical data and leptin determinations from 105 patients with type 2 DM. In this study, we applied an automatic pulse wave analyzer (VaSera VS-1000) to measure the brachial-ankle pulse wave velocity (baPWV); a baPWV value >14.0 m/s on either side was considered high PAS.
Results:
Seventy-five patients (71.4%) had high PAS and they included a higher percentage of patients with hypertension (P < 0.001), older age (P < 0.001), and a higher body fat mass (P = 0.043), systolic blood pressure (P < 0.001), diastolic blood pressure (P = 0.016), serum blood urea nitrogen (P = 0.003), and leptin level (P < 0.001), and lower height (P = 0.027) and glomerular filtration rate (P < 0.001) compared with type 2 DM patients with low PAS. After adjusting for factors significantly associated with PAS in these patients by multivariate logistic regression analysis, age (β = 0.470, adjusted R2 change = 0.279; P < 0.001), logarithmically transformed leptin (log-leptin, β = 0.259, adjusted R2 change = 0.085; P = 0.001), and hypertension (β = 0.197, adjusted R2 change = 0.031; P = 0.011) were significant independent predictors of PAS in type 2 DM patients.
Conclusion:
The serum leptin level could be a predictor of PAS in type 2 DM patients.
KEYWORDS: Brachial-ankle pulse wave velocity, Leptin, Peripheral arterial stiffness, Type 2 diabetes mellitus
INTRODUCTION
The arterial system undergoes structural and functional changes with age, characterized by endothelial dysfunction, wall thickening, reduced distensibility, and arterial stiffening [1]. Arterial stiffness represents vascular damage and has been identified as an independent risk factor for the development of cardiovascular mortality [2]. Arterial stiffness can be measured by pulse wave velocity (PWV). This method is noninvasive and reproducible and is the simplest way to measure arterial stiffness [3]. Brachial-ankle PWV (baPWV) is one measure arterial stiffness using brachial to ankle arterial wave analyses and has been used to assess peripheral arterial stiffness (PAS) compared with carotid-femoral PWV which is used to assess central arterial stiffness using carotid-to-femoral arterial waves [3,4]. A meta-analysis of 12 cohort studies confirmed that baPWV is an independent predictor for cardiovascular disease onset, and an increase of 1 m/s was associated with a 12% increase in the risk of cardiovascular events [5]. baPWV is also a useful early marker for the detection of vascular dysfunction in patients with type 2 diabetes mellitus (DM) [6].
Leptin, a 16-kDa adipokine hormone peptide product encoded by the obesity (ob) gene, regulates energy balance by inhibiting hunger [7]. Leptin exerts many potentially atherogenic effects such as induction of endothelial dysfunction and stimulation of the inflammatory reaction, oxidative stress, platelet aggregation, and vascular smooth muscle hypertrophy [8]. Circulating leptin is considered a risk factor for coronary heart disease [9], and a predictor of central arterial stiffness in patients with coronary artery disease [10], hypertension [11], and kidney transplantation [12]. Moreover, the serum leptin level is also a predictor of PAS in kidney transplantation patients [13]. baPWV was a predictor of all-cause mortality in diabetic patients after nontraumatic lower extremity amputation [14]. The aim of the present study was to determine the risk factors for PAS and the relationship between the serum leptin concentration and PAS in type 2 DM patients.
MATERIALS AND METHODS
Participants
We enrolled 105 patients with type 2 DM from a medical center in Hualien, Taiwan from November 2014 to March 2015. This study was conducted in accordance with the Declaration of Helsinki and was approved by the Protection of the Human Subjects Institutional Review Board of Tzu Chi University and Hospital (IRB103-136-A). Informed written consent was obtained from all participants before their enrolment in this study. The study participants were recruited in the outpatient metabolism and endocrinology clinic at Buddhist Tzu Chi General Hospital, Hualien, Taiwan. Patients were excluded if they had an acute infection, limb amputation, acute myocardial infarction, heart failure, or malignancy at the time of blood sampling, or if they refused to provide informed consent for the study.
Anthropometric analysis
Body weight of the participant was measured in light clothing and without shoes to the nearest 0.5 kg, and body height was measured to the nearest 0.5 cm. Waist circumference was measured using a tape measure around the waist from the point between the lowest ribs and the hip bones with the hands on the hips. Body mass index (BMI) was calculated as the weight in kilograms divided by the height in meters squared. Bioimpedance measurements of fat mass were performed at the bedside according to the standard tetrapolar whole-body (hand-foot) technique, using a single-frequency (50 kHz) analyzer (Biodynamic-450, Biodynamics Corporation, Seattle, USA) [10,11,12,13,15,16]. All measurements were carried out by the same operator.
Biochemical investigations
Fasting blood samples (approximately 5 mL) were immediately centrifuged at 3000 × g for 10 min. Serum levels of albumin, blood urea nitrogen (BUN), creatinine (Cre), fasting glucose, glycated hemoglobin, total cholesterol, triglycerides (TG), high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol were measured using an autoanalyzer (Siemens Advia 1800, Siemens Healthcare GmbH, Henkestr, Germany) [10,11,12,13,15,16]. Serum leptin (SPI-BIO, Montigny le Bretonneux, France) concentrations were determined using a commercially available enzyme immunoassay [10,11,12,13,15]. The estimated glomerular filtration rate (GFR) was calculated using the Modification of Diet in Renal Disease equation in this study.
Measurements of blood pressure and brachial-ankle pulse wave velocity
After blood sampling, patients rested in the supine position for 10 min. Blood pressure was determined by an automatic upper arm oscillometric device, and systolic blood pressure (SBP) and diastolic blood pressure (DBP) were checked three times in the right brachial artery. Hypertension was defined as SBP ≥140 mmHg, and/or DBP ≥90 mmHg, or if the patient had received any antihypertensive medication in the past 2 weeks. Then, four pneumatic cuffs connected to both plethysmographic and oscillometric sensors were wrapped around both upper arms and ankles for the assessment of the baPWV by a volume-plethysmographic apparatus (VaSera VS-1000, Fukuda Denshi Co. Ltd., Tokyo, Japan) [13,16]. To monitor the electrocardiogram and heart sounds, a microphone was placed on the left side of the sternum, and electrocardiogram electrodes were placed on both wrists. Phonocardiography, electrography, and arterial pressure waveforms of the brachial and ankle arteries were measured, and the left-baPWV and right-baPWV were automatically calculated by the analyzer. The baPWV was calculated as the length of an arterial segment between the brachium and ankle divided by the time interval between the wavefront of the brachial waveform and that of the ankle waveform. Based on the recursive partitioning analysis in the Kyushu Prevention Study of Atherosclerosis, a baPWV of 14 m/s was considered a statistically adequate cutoff point for coronary artery events and cerebrovascular events in diabetic populations [17]. Therefore, in the present study, patients were in the high PAS group if their left or right baPWV values were >14.0 m/s [13,16,18].
Statistical analysis
Data were tested for normal distribution using the Kolmogorov–Smirnov test. Data were expressed as means ± standard deviation (SD) for normally distributed data and comparisons between patients were performed using the Student's independent t-test (two-tailed). Data were expressed as medians and interquartile ranges for nonnormally distributed data (TG, fasting glucose, BUN, Cre, and leptin) and comparisons between patients were performed using the Mann–Whitney U-test. Data expressed as the number and percentage of patients were analyzed by the Chi-square test. TG, fasting glucose, BUN, Cre, and leptin were not normally distributed and underwent base 10 logarithmic transformations to achieve normality. Variables that were significantly associated with PAS in type 2 DM patients were tested for independence in multivariate forward stepwise regression analysis (adopted factors: age, hypertension, height, body fat mass, SBP, DBP, log-BUN, GFR, and log-leptin). Data were analyzed using SPSS for Windows (version 19.0; SPSS Inc., Chicago, IL, USA). The P < 0.05 was considered statistically significant.
RESULTS
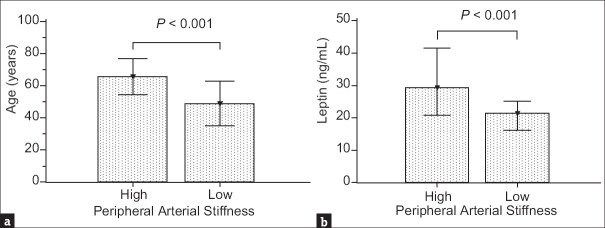
The clinical characteristics and biochemical data of our 105 type 2 DM patients are presented in Table 1. Seventy-five patients (71.4%) were in the high PAS group with a left or right baPWV value >14.0 m/s, and the others were classified as the low PAS group. Compared with the low PAS group, type 2 DM patients in the high PAS group were older age (P < 0.001) and had higher body fat mass (P = 0.043), SBP (P < 0.001), DBP (P = 0.016), BUN (P = 0.003), and leptin levels (P < 0.001), and lower height (P = 0.027) and GFR (P < 0.001). Comparisons of age and leptin levels in the high and low PAS groups in patients with type 2 DM are presented in Figure 1.
Table 1.
Clinical variables of 105 diabetic patients with high and low peripheral arterial stiffness
| Characteristics | All patients (n=105) | Low PAS group (n=30) | High PAS group (n=75) | P |
|---|---|---|---|---|
| Age (years) | 60.91±14.11 | 49.03±13.95 | 65.67±11.11 | <0.001* |
| Height (cm) | 161.16±8.48 | 164.03±8.09 | 160.01±8.40 | 0.027* |
| Body weight (kg) | 71.67±14.32 | 73.71±16.29 | 70.85±13.49 | 0.357 |
| BMI (kg/m2) | 27.42±3.96 | 27.21±4.80 | 27.51±3.61 | 0.732 |
| Body fat mass (%) | 33.06±7.10 | 30.85±7.46 | 33.95±6.81 | 0.043* |
| Waist circumference (cm) | 91.44±9.95 | 88.47±11.81 | 92.63±8.92 | 0.053 |
| SBP (mmHg) | 142.25±20.53 | 130.80±16.35 | 146.83±20.33 | <0.001* |
| DBP (mmHg) | 83.02±11.92 | 78.63±9.87 | 84.77±12.27 | 0.016* |
| Left-baPWV (m/s) | 16.31±3.39 | 12.82±0.74 | 17.70±3.00 | <0.001* |
| Right-baPWV (m/s) | 16.13±3.17 | 12.64±0.83 | 17.53±2.63 | <0.001* |
| Albumin (mg/dL) | 4.27±0.27 | 4.34±0.31 | 4.25±0.25 | 0.088 |
| Total cholesterol (mg/dL) | 161.79±31.27 | 158.87±33.27 | 162.96±30.58 | 0.547 |
| Triglycerides (mg/dL) | 114.00 (89.50-162.00) | 107.50 (87.50-149.00) | 119.00 (90.00-171.00) | 0.314 |
| HDL-C (mg/dL) | 47.10±13.17 | 47.30±13.66 | 47.01±13.06 | 0.920 |
| LDL-C (mg/dL) | 100.24±26.34 | 101.97±24.20 | 99.55±27.28 | 0.673 |
| BUN (mg/dL) | 16.00 (12.00-18.00) | 13.00 (12.00-16.25) | 17.00 (14.00-21.00) | 0.003* |
| Creatinine (mg/dL) | 0.80 (0.70-1.00) | 0.80 (0.60-0.90) | 0.90 (0.70-1.00) | 0.119 |
| GFR (mL/min) | 89.09±29.06 | 104.64±33.81 | 82.88±24.52 | <0.001* |
| Fasting glucose (mg/dL) | 139.00 (121.50-172.50) | 130.00 (122.50-163.75) | 142.00 (121.00-175.00) | 0.419 |
| Glycated hemoglobin (%) | 7.99±1.78 | 7.93±1.88 | 8.02±1.75 | 0.823 |
| Leptin (ng/mL) | 25.06 (20.06-34.89) | 21.45 (16.00-25.38) | 29.31 (20.79-41.86) | <0.001* |
*P < 0.05 was considered statistically significant. Values for continuous variables are given as means±SD and were tested by Student's t-test; variables not normally distributed are given as medians and interquartile range and were tested by the Mann–Whitney U-test. PAS: Peripheral arterial stiffness, HDL-C: High-density lipoprotein cholesterol, LDL-C: Low-density lipoprotein cholesterol, baPWV: Brachial-ankle pulse wave velocity, SD: Standard deviation, BMI: Body mass index, GFR: Glomerular filtration rate, DBP: Diastolic blood pressure, SBP: Systolic blood pressure, BUN: Blood urea nitrogen
Figure 1.
Comparison of (a) age (mean ± standard deviation) and (b) leptin levels (median and interquartile range) in the high and low peripheral arterial stiffness groups in patients with type 2 diabetes mellitus
The data for gender, hypertension, and drugs use are presented in Table 2. Among these patients, 59 had hypertension (56.2%). Type 2 DM patients with hypertension (P < 0.001) were prone to develop high PAS. No statistically significant differences in PASs were found in patients using statins, fibrates, or antidiabetic drugs.
Table 2.
Distribution of diabetic patients with high and low peripheral arterial stiffness in subgroup analysis
| Characteristics | Low PAS group (%) | High PAS group (%) | P |
|---|---|---|---|
| Gender | |||
| Male | 17 (56.7) | 34 (45.3) | 0.294 |
| Female | 13 (43.3) | 41 (54.7) | |
| Hypertension | |||
| No | 22 (73.3) | 24 (32.0) | <0.001* |
| Yes | 8 (26.7) | 51 (68.0) | |
| Statins | |||
| No | 16 (53.3) | 38 (50.7) | 0.805 |
| Yes | 14 (46.7) | 37 (49.3) | |
| Fibrates | |||
| No | 29 (96.7) | 71 (94.7) | 0.664 |
| Yes | 1 (3.3) | 4 (5.3) | |
| Metformin | |||
| No | 17 (56.7) | 30 (40.0) | 0.121 |
| Yes | 13 (43.3) | 45 (60.0) | |
| Sulfonylureas | |||
| No | 13 (43.3) | 36 (48.0) | 0.665 |
| Yes | 17 (56.7) | 39 (52.0) | |
| DDP-4 inhibitors | |||
| No | 9 (30.0) | 34 (45.3) | 0.149 |
| Yes | 21 (70.0) | 41 (54.7) | |
| Thiazolidinediones | |||
| No | 30 (100.0) | 74 (98.7) | 0.525 |
| Yes | 0 | 1 (1.3) | |
| Insulin | |||
| No | 26 (86.7) | 54 (72.0) | 0.111 |
| Yes | 4 (13.3) | 21 (28.0) |
*P < 0.05 was considered statistically significant. Data are expressed as n (%) of patients. Analysis was done using the Chi-square test. PAS: Peripheral arterial stiffness, DDP-4: Dipeptidyl peptidase 4
Multivariable forward stepwise linear regression analysis was used to delineate the significant variables associated with PAS in type 2 DM patients, and the results are presented in Table 3. We found that age (β = 0.470, adjusted R2 change = 0.279; P < 0.001), logarithmically transformed leptin (log-leptin, β =0.259, adjusted R2 change = 0.085; P = 0.001), and hypertension (β = 0.197, adjusted R2 change = 0.031; P = 0.011) were independent predictors of PAS in type 2 DM patients.
Table 3.
Multivariable stepwise linear regression analysis of peripheral artery stiffness in 105 diabetic patients
| Items | β | Adjusted R2 | Adjusted R2 change | P |
|---|---|---|---|---|
| Age (years) | 0.470 | 0.279 | 0.279 | <0.001* |
| Log-leptin (ng/mL) | 0.259 | 0.364 | 0.085 | 0.001* |
| Hypertension | 0.197 | 0.395 | 0.031 | 0.011* |
Data for BUN and leptin levels showed skewed distributions and therefore were log-transformed before analysis. *P<0.05 was considered statistically significant in multivariable stepwise linear regression analysis (adopted factors: Age, hypertension, height, body fat mass, SBP, DBP, log-BUN, GFR, and log-leptin). BUN: Blood urea nitrogen, GFR: Glomerular filtration rate, DBP: Diastolic blood pressure, SBP: Systolic blood pressure
DISCUSSION
The results of our study showed a high prevalence of PAS in patients with type 2 DM. The fasting serum leptin level, hypertension, and older age were independent predictors of PAS among the type 2 DM patients.
Arterial stiffness is related to atherosclerosis and cardiovascular disease and has been considered a strong independent predictor of coronary events and cardiovascular mortality [2]. Katakami et al. noted the cumulative incidence rates of cardiovascular events (coronary heart disease, cerebrovascular events, peripheral artery disease, and heart failure) were significantly higher in patients with high baPWV values (≥1550 cm/s) than those with low baPWV values (<1550 cm/s), and a multivariate analysis in 1040 type 2 DM patients revealed that baPWV was still an independent predictor for cardiovascular events even after adjusting for conventional cardiovascular risk factors [19]. In a Mendelian randomization analysis, type 2 DM was causally associated with arterial stiffening using the measurement of baPWV in 11,385 participants from a well-defined community study in Shanghai from 2011 to 2013 [20]. Diabetes may enhance arterial stiffness through pathological changes in the vascular bed, such as reduced nitric oxide bioavailability, increased formation of advanced glycation endproducts, and collagen cross-linking in the arterial wall [21]. In this study, 71.4% of type 2 DM patients were in the high PAS group.
Age is an important determinant of PWV and induces structural and functional abnormalities such as arterial wall hypertrophy and degeneration or disorganization of the medial layer [22]. SBP was significantly associated with baPWV in patients with type 2 diabetes [23]. The frequencies of hypertension, older age, and SBP were significantly higher in type 2 DM patients with higher baPWV values compared with those with lower baPWV values [19]. Recent study showed that each SD increase in fat percentage in the trunk was associated with a 1.03 m/s increase in baPWV [24]. Increased visceral adiposity with normal weight (BMI <25 kg/m2 and visceral fat area ≥100 cm2) remained independently associated with baPWV levels in multivariate linear regression analysis in type 2 DM patients [25]. Our results found that hypertension, older age, body fat mass, SBP, and DBP were higher in the high PAS group than the low PAS among the type 2 DM patients. After adjustment for a variety of confounders in multivariable forward stepwise linear regression analysis, age and hypertension remained independent predictors of PAS among the type 2 DM patients.
High PWV values are significantly and independently associated with reduced GFR in normal individuals [26]. Arterial stiffness increases circumferential and shear stresses in the arterial lumen and results in endothelial dysfunction and microvascular ischemia leading to kidney injury [27]. Other possible mechanisms include chronic inflammation, oxidative stress, and activation of the renin-angiotensin system which reduce GFR [28]. Sedaghat et al. noted each SD higher PWV was associated with a 7% higher risk of incident chronic renal disease [29]. Our study revealed that the GFR was lower in the high PAS group than the low PAS among the type 2 DM patients.
Leptin has peripheral actions to stimulate vascular inflammation, oxidative stress, and vascular smooth muscle hypertrophy which may exert actions on endothelial function, vascular homeostasis, and atherogenesis [30]. Hyperleptinemia associated inversely with vasodilatation in resistance arteries by endothelial-dependent vasodilatation and endothelial-independent vasodilatation and associated with arterial stiffness and hypertension in a cross-sectional study which included 1016 participants aged 70 years [31]. Furthermore, the serum leptin level is also a predictor for PAS in kidney transplantation patients [13]. Our study also found that the leptin level was higher in the high PAS group than the low PAS among the type 2 DM patients. After multivariate stepwise linear regression analysis, log-leptin remained an independent predictor of PAS among the type 2 DM patients.
There were some limitations in this current study. First, this was an observational, single-center study with a small number of participants with type 2 DM. Second, participants in this study were not differentiated by race, limiting the conclusions of our findings in other ethnicities. Third, although we collected data on antidiabetic and anti-lipid drugs, we did not collect data on medications such as antihypertensives which could have potentially affected serum leptin levels or baPWV values. Another limitation is that the blood pressure was measured with the patient in a supine position, which may have affected the reading. Usually, blood pressure is significantly lower in the sitting than the supine position [32]. Whether a patient's position affects baPWV values is unclear. Further long-term prospective studies are needed to follow-up on the trend between serum leptin and PAS in type 2 DM patients.
CONCLUSION
The present study revealed a high prevalence of PAS in type 2 DM patients. Hyperleptinemia positively correlated with PAS in type 2 DM patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Harvey A, Montezano AC, Lopes RA, Rios F, Touyz RM. Vascular fibrosis in aging and hypertension: Molecular mechanisms and clinical implications. Can J Cardiol. 2016;32:659–68. doi: 10.1016/j.cjca.2016.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Veerasamy M, Ford GA, Neely D, Bagnall A, MacGowan G, Das R, et al. Association of aging, arterial stiffness, and cardiovascular disease: A review. Cardiol Rev. 2014;22:223–32. doi: 10.1097/CRD.0000000000000009. [DOI] [PubMed] [Google Scholar]
- 3.Tomiyama H, Yamashina A. Non-invasive vascular function tests: Their pathophysiological background and clinical application. Circ J. 2010;74:24–33. doi: 10.1253/circj.cj-09-0534. [DOI] [PubMed] [Google Scholar]
- 4.Munakata M. Brachial-ankle pulse wave velocity: Background, method, and clinical evidence. Pulse (Basel) 2016;3:195–204. doi: 10.1159/000443740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vlachopoulos C, Aznaouridis K, Terentes-Printzios D, Ioakeimidis N, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with brachial-ankle elasticity index: A systematic review and meta-analysis. Hypertension. 2012;60:556–62. doi: 10.1161/HYPERTENSIONAHA.112.194779. [DOI] [PubMed] [Google Scholar]
- 6.Yapei Y, Xiaoyan R, Sha Z, Li P, Xiao M, Shuangfeng C, et al. Clinical significance of arterial stiffness and thickness biomarkers in type 2 diabetes mellitus: An up-to-date meta-analysis. Med Sci Monit. 2015;21:2467–75. doi: 10.12659/MSM.894693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adamczak M, Wiecek A. The adipose tissue as an endocrine organ. Semin Nephrol. 2013;33:2–13. doi: 10.1016/j.semnephrol.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Beltowski J. Leptin and atherosclerosis. Atherosclerosis. 2006;189:47–60. doi: 10.1016/j.atherosclerosis.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Sattar N, Wannamethee G, Sarwar N, Chernova J, Lawlor DA, Kelly A, et al. Leptin and coronary heart disease: Prospective study and systematic review. J Am Coll Cardiol. 2009;53:167–75. doi: 10.1016/j.jacc.2008.09.035. [DOI] [PubMed] [Google Scholar]
- 10.Tsai JP, Wang JH, Chen ML, Yang CF, Chen YC, Hsu BG, et al. Association of serum leptin levels with central arterial stiffness in coronary artery disease patients. BMC Cardiovasc Disord. 2016;16:80. doi: 10.1186/s12872-016-0268-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsai JP, Hsu BG, Lee CJ, Hsieh YH, Chen YC, Wang JH, et al. Serum leptin is a predictor for central arterial stiffness in hypertensive patients. Nephrology (Carlton) 2017;22:783–9. doi: 10.1111/nep.12859. [DOI] [PubMed] [Google Scholar]
- 12.Tsai JP, Lee MC, Chen YC, Ho GJ, Shih MH, Hsu BG, et al. Hyperleptinemia is a risk factor for the development of central arterial stiffness in kidney transplant patients. Transplant Proc. 2015;47:1825–30. doi: 10.1016/j.transproceed.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Lee MC, Chen YC, Ho GJ, Shih MH, Chou KC, Hsu BG, et al. Serum leptin levels positively correlate with peripheral arterial stiffness in kidney transplantation patients. Transplant Proc. 2014;46:353–8. doi: 10.1016/j.transproceed.2013.11.145. [DOI] [PubMed] [Google Scholar]
- 14.Ikura K, Hanai K, Oka S, Watanabe M, Oda Y, Hamada M, et al. Brachial-ankle pulse wave velocity, but not ankle-brachial index, predicts all-cause mortality in patients with diabetes after lower extremity amputation. J Diabetes Investig. 2017;8:250–3. doi: 10.1111/jdi.12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen MC, Hsu BG, Lee CJ, Wang JH. Hyperleptinemia positively correlates with cardiometabolic syndrome in hypertensive patients. Int J Clin Exp Pathol. 2016;9:12959–67. [Google Scholar]
- 16.Hsu BG, Liou HH, Lee CJ, Chen YC, Ho GJ, Lee MC, et al. Serum sclerostin as an independent marker of peripheral arterial stiffness in renal transplantation recipients: A cross-sectional study. Medicine (Baltimore) 2016;95:e3300. doi: 10.1097/MD.0000000000003300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maeda Y, Inoguchi T, Etoh E, Kodama Y, Sasaki S, Sonoda N, et al. Brachial-ankle pulse wave velocity predicts all-cause mortality and cardiovascular events in patients with diabetes: The Kyushu Prevention Study of Atherosclerosis. Diabetes Care. 2014;37:2383–90. doi: 10.2337/dc13-1886. [DOI] [PubMed] [Google Scholar]
- 18.Kato A, Ishida J, Endo Y, Takita T, Furuhashi M, Maruyama Y, et al. Association of abdominal visceral adiposity and thigh sarcopenia with changes of arteriosclerosis in haemodialysis patients. Nephrol Dial Transplant. 2011;26:1967–76. doi: 10.1093/ndt/gfq652. [DOI] [PubMed] [Google Scholar]
- 19.Katakami N, Osonoi T, Takahara M, Saitou M, Matsuoka TA, Yamasaki Y, et al. Clinical utility of brachial-ankle pulse wave velocity in the prediction of cardiovascular events in diabetic patients. Cardiovasc Diabetol. 2014;13:128. doi: 10.1186/s12933-014-0128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu M, Huang Y, Xie L, Peng K, Ding L, Lin L, et al. Diabetes and risk of arterial stiffness: A mendelian randomization analysis. Diabetes. 2016;65:1731–40. doi: 10.2337/db15-1533. [DOI] [PubMed] [Google Scholar]
- 21.Prenner SB, Chirinos JA. Arterial stiffness in diabetes mellitus. Atherosclerosis. 2015;238:370–9. doi: 10.1016/j.atherosclerosis.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 22.Camici GG, Savarese G, Akhmedov A, Lüscher TF. Molecular mechanism of endothelial and vascular aging: Implications for cardiovascular disease. Eur Heart J. 2015;36:3392–403. doi: 10.1093/eurheartj/ehv587. [DOI] [PubMed] [Google Scholar]
- 23.Bouchi R, Ohara N, Asakawa M, Nakano Y, Takeuchi T, Murakami M, et al. Is visceral adiposity a modifier for the impact of blood pressure on arterial stiffness and albuminuria in patients with type 2 diabetes? Cardiovasc Diabetol. 2016;15:10. doi: 10.1186/s12933-016-0335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee M, Choh AC, Demerath EW, Towne B, Siervogel RM, Czerwinski SA, et al. Associations between trunk, leg and total body adiposity with arterial stiffness. Am J Hypertens. 2012;25:1131–7. doi: 10.1038/ajh.2012.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouchi R, Minami I, Ohara N, Nakano Y, Nishitani R, Murakami M, et al. Impact of increased visceral adiposity with normal weight on the progression of arterial stiffness in Japanese patients with type 2 diabetes. BMJ Open Diabetes Res Care. 2015;3:e000081. doi: 10.1136/bmjdrc-2015-000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Safar ME, Plante GE, Mimran A. Arterial stiffness, pulse pressure, and the kidney. Am J Hypertens. 2015;28:561–9. doi: 10.1093/ajh/hpu206. [DOI] [PubMed] [Google Scholar]
- 27.Safar ME, London GM, Plante GE. Arterial stiffness and kidney function. Hypertension. 2004;43:163–8. doi: 10.1161/01.HYP.0000114571.75762.b0. [DOI] [PubMed] [Google Scholar]
- 28.Upadhyay A, Hwang SJ, Mitchell GF, Vasan RS, Vita JA, Stantchev PI, et al. Arterial stiffness in mild-to-moderate CKD. J Am Soc Nephrol. 2009;20:2044–53. doi: 10.1681/ASN.2009010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sedaghat S, Mattace-Raso FU, Hoorn EJ, Uitterlinden AG, Hofman A, Ikram MA, et al. Arterial stiffness and decline in kidney function. Clin J Am Soc Nephrol. 2015;10:2190–7. doi: 10.2215/CJN.03000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koh KK, Park SM, Quon MJ. Leptin and cardiovascular disease: Response to therapeutic interventions. Circulation. 2008;117:3238–49. doi: 10.1161/CIRCULATIONAHA.107.741645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonzalez M, Lind L, Söderberg S. Leptin and endothelial function in the elderly: The prospective investigation of the vasculature in uppsala seniors (PIVUS) study. Atherosclerosis. 2013;228:485–90. doi: 10.1016/j.atherosclerosis.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 32.Alexis O. Providing best practice in manual blood pressure measurement. Br J Nurs. 2009;18:410–5. doi: 10.12968/bjon.2009.18.7.41654. [DOI] [PubMed] [Google Scholar]