Abstract
Back ground
Drug resistant tuberculosis is alarmingly on the rise especially in developing countries. Skeletal tuberculosis accounts up to 10% of all extra pulmonary tuberculosis. World Health Organisation (WHO) has not formulated guidelines for the management of Multi-drug resistant skeletal tuberculosis.
Results
A retrospective analysis of patients treated for musculoskeletal tuberculosis was done, to study drug resistance patterns. The outcome was assessed both clinically and radiologically.
898 patients were treated for skeletal tuberculosis during the period of 2006–2013 (96 months). 478 (53.2%) patients were treated for tubercular spondylitis and 420 (46.8%) for extra–spinal skeletal tuberculosis. Ninety two patients (10.2%) had documented resistance to the anti-tubercular drugs. There were 42 mono resistant tuberculosis cases (4.7%), 13 poly resistant cases (1.4%), 33 multi-drug resistant cases (MDR TB) (3.7%) and 4 (0.4%) extremely drug resistant tuberculosis cases (XDR). All the patients were treated medically as per drug susceptibility patterns and protocols. Surgery was performed when indicated in 59 (66%) cases. 85% completed their course of treatment and were successfully healed as per pre-set clinical, biochemical and radiological criteria. The remaining were lost to follow up. One patient died as a result of post op respiratory infection.
Conclusions
The prevalence of Multi-drug resistant tuberculosis patients in our centre was 3.7% and that of Extremely drug resistant tuberculosis cases was 0.4%. A Multi-disciplinary approach with drug susceptibility tests, sensitive drugs, and surgery if required is essential. Health education is essential to improve awareness among health care professionals about the danger of drug resistance in tuberculosis.
1. Introduction
Tuberculosis has emerged as one of the leading health problems in developing countries to an extent that World Health Organization (WHO) has declared it ‘a global health emergency.1 In spite of the development of newer drugs to combat this disease, drug resistance has emerged as a major concern.1 4.1% of new TB patients have multi drug resistant tuberculosis as per the latest update in 2017.3 Drug resistance is more common in the developing countries. India had reported 2.8% of new multi-drug resistant cases.3 There is limited literature on the incidence and treatment of drug resistant skeletal tuberculosis.2, 4, 5 In this paper, we aim to study the clinical characteristics, drug resistance patterns and outcome − both clinical and radiological − of drug resistant skeletal tuberculosis at our tertiary care centre.
2. Materials and methods
We conducted a retrospective analysis on culture isolates from our high volume mycobacterial laboratory during the period 2006 −2013(96 months) after getting clearance from the Institutional Review Board. Patients with culture proven drug resistant skeletal tuberculosis, with a minimum follow-up of 18 months were included in the study. The mycobacterial culture was done on both solid culture- (Lowenstein Jensen media) and Middle brook 7H9 liquid media by automated mycobacterial growth indicator tube (MGIT) system as per the standard operating procedures prescribed by the manufacturer. Drug susceptibility tests for 1st line (Isoniazid, Streptomycin, Rifampicin, Ethambutol) and 2nd line(Ciprofloxacin/Ofloxacin, Capreomycin, Kanamycin and Ethionamide) drugs were done using 1% proportion methods as per standard operation methods. All drug susceptibility tests (DST) were performed by the mycobacteriology section at our Clinical Microbiology Department − which is externally accredited by the Revised National Tuberculosis Control Program and the Central Tuberculosis Division, Ministry of Health, Government of India.
Drug resistance patterns were classified according to WHO definition of drug resistant tuberculosis. Drug resistance was defined as ‘Mono resistance’ when there is resistance to one first line anti–TB drug only, ‘Poly resistance’ when the resistance is more than one first line anti–TB drug, other than both Isoniazid and Rifampicin. ‘Multi-drug resistance’ (MDR) was defined as resistance to both Isoniazid and Rifampicin (the first line drugs) and ‘Extremely drug resistance’ (XDR) was defined as resistance to the above first line drugs along with resistance to at least one fluoroquinolone and at least one injectable agent.6 The term ‘primary drug resistance’ was used for cases that had not received any treatment previously, and ‘secondary drug resistance’ for those who received some form of primary treatment for tuberculosis.
Patients with skeletal tuberculosis were analysed in two subgroups − spinal tuberculosis and extra-spinal tuberculosis. The demography, resistance patterns, site of involvement, management (conservative or surgical) and the outcome −clinical, biochemical and radiological parameters were assessed. Positive outcome was confirmed clinically by reduction in pain and returning to occupation using McNab’s criteria,7 and bio-chemically with normalization of ESR and CRP. Radiological healing was confirmed by sclerosis of bone and fusion between the vertebra (Sentinel’s sign)8 and re-mineralization of the vertebra.9
3. Results
Of the 4693 tissue samples sent by orthopaedic surgeons for mycobacterial culture during the period 2006–2013 (96 months). 898 were positive for mycobacterium tuberculosis. Of these 478(53.2%) patients were treated for spinal tuberculosis and 420(46.8%) for extra–spinal skeletal tuberculosis. Of the 898 patients, 92 patients (10.2%) had documented resistance to the anti-tubercular drugs. There were 42 mono resistant tuberculosis cases (4.7%), 13 poly resistant cases (1.4%), 33 multi-drug resistant cases (MDR TB) (3.7%) and 4 (0.4%) extremely drug resistant tuberculosis cases(XDR) [Table 1]. None of the patients with drug resistance were positive for HIV. Thirty one (55%) patients with resistant spinal tuberculosis had surgical interventions and 28 patients (78%) with resistant extra-spinal tuberculosis had surgery. 76 patients (85%) completed their treatment and were healed as per the set criteria and 13 patients were lost to follow up.
Table 1.
Demographic details.
| Susceptible TB | Mono- resistant TB | Poly resistant TB | MDR TB | XDR TB | |
|---|---|---|---|---|---|
| Musculoskeletal Tuberculosis (n = 898) | |||||
| Total No. Of Patients | 806 | 42 (4.7%) | 13(1.4%) | 33(3.7%) | 4 (0.4%) |
| Spinal Tuberculosis (n = 478) [53.2%] | |||||
| Total No. Of Patients | 422 | 24 | 7 | 21 | 4 |
| Mean Age | 43.2 | 42 | 42 | 32 | 22 |
| M: F ratio | 233:189 | 14:10 | 5:2 | 10:11 | 3:1 |
| Extra Spinal Tuberculosis (n = 420) [46.8%] | |||||
| Total No. Of Patients n | 384 | 18 | 6 | 12 | 0 |
| Mean Age | 42 | 45.6 | 42 | 32 | 0 |
| M: F ratio | 263:121 | 16:2 | 4:2 | 6:6 | 0 |
Legend − Demographic details of patients with tuberculosis. (TB: Tuberculosis; MDR: Multidrug resistant; XDR: Extremely drug resistant)
3.1. Mono and Poly Resistant Tuberculosis
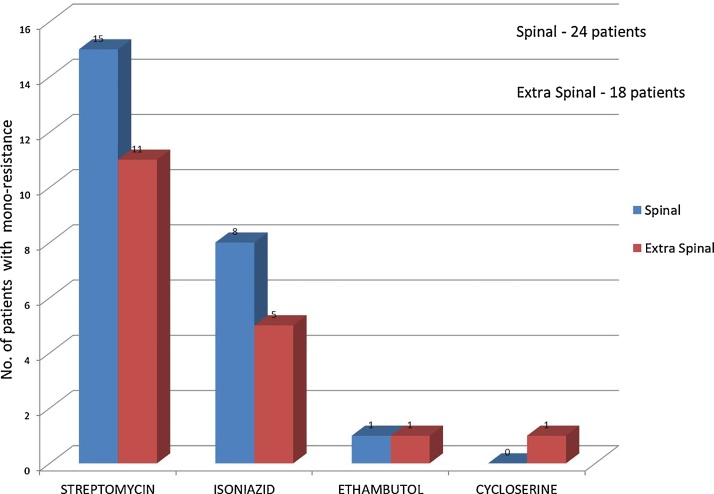
Of the 55 cases of mono and poly resistant tuberculosis, 31 had tuberculosis of the spine and the rest were extra-spinal. Of the 31patients, 24 had mono resistance (streptomycin being the most common) [Fig. 1] and 7 had poly resistance [Table 2]. Of the 24 patients with extra-spinal drug resistant tuberculosis, 18 had mono-resistance [Fig. 1]. Six had poly resistance (4 had resistance to two drugs and 2 had drug resistance to more than 2 drugs) [Table 2]. Of the 31 patients with spinal tuberculosis, 9(29%) required surgery, while the others were treated only with medications. Of the 9 patients who had surgery for spinal tuberculosis, 7 went on to heal, while 2 were lost to follow up. Of the 22 patients who were treated with medication only, 4 were lost to follow up and 18 were healed of the disease. Of the 24 patients with extra spinal tuberculosis, 16(66%) required surgery, while the rest were treated conservatively. Among these 16 patients who had surgery, 14 completed their treatment and were cleared of their disease, while 2 were lost to follow up. Only one of the 8 patients treated conservatively with medications was lost to follow up. The remaining patients completed their course of treatment and went to heal as per the set criteria.
Fig. 1.
Graph showing patients with mono-resistance pattern of tuberculosis in Spinal and Extra spinal Cases.
Table 2.
Pattern of Poly resistance (Total Number = 13).
| No. of drugs | Drug Resistance | Spinal Tuberculosis (n = 7) | Extra spinal tuberculosis (n = 6) |
|---|---|---|---|
| 2 | INH & Streptomycin | 4 | 4 |
| INH & Ethambutol | 2 | ||
| Ethambutol & Streptomycin | 1 | ||
| 3 | INH, Streptomycin & Ethambutol. | 1 | |
| 5 | INH, Streptomycin, | 1 | |
| Cycloserine, Ethambutol, | |||
| Capreomycin. |
3.2. Multi-drug Resistant (MDR) Tuberculosis
Of the 33 cases of MDR tuberculosis, 21(64%) had infection of the spine and 12 (36%) were extra −spinal in location.
The mean age of the 21 patients with MDR tuberculosis of the spine was 32years. There was no sex predilection [Table 1] and the resistance was predominantly of the secondary type (80%). The Thoracolumbar spine was the most commonly affected site of involvement [Table 3]. Fifteen patients had surgical intervention for either progressive neurological deterioration (10/15), mechanical instability10 (13/15) or deformity (8/15). 13 patients underwent extended posterior circumferential decompression (EPCD) surgical drainage,11 debridement and anterior column reconstruction with cage filled with autogenous cancellous bone graft and 2 patients underwent surgical drainage and decompression without instrumentation. Another three patients had pig tail drainage for psoas abscess [Table 3]. Of the 18 patients who had surgical intervention, 17 completed their treatment and were healed. One patient expired due to pulmonary infection in the post-operative period following an extended posterior circumferential decompression and stabilisation for a thoracic spine (D2–D3) tubercular spondylitis. Of the 3 patients who had conservative treatment, one patient lost to follow up, while the remaining two went to heal as per the set criteria.
Table 3.
Multi − Drug Resistant Tuberculosis.
| Spinal Group (n = 21) | Extra-spinal Group(n = 12) | |
|---|---|---|
| Prevalence | 4.4% | 2.8% |
| Site of Involvement | Thoracic − 5 | Knee − 5 |
| Thoraco −Lumbar − 11 | Hip − 2 | |
| Lumbar − 5 | Foot − 2 | |
| Hand − 2 | ||
| Ribs − 1 | ||
| Interventions | Surgical decompression | Knee Joint |
| With instrumented fusion − 13 | Arthrodesis − 2 | |
| Surgical decompression | Synovectomy − 3 | |
| without instrumentation − 2 | Hip Joint | |
| U/s guided | Excision Arthroplasty − 1 | |
| Pig tail drainage − 3 | Debridement − 1 | |
| Foot | ||
| Debridement − 2 | ||
| Hand | ||
| Debridement of | ||
| Wrist − 1 | ||
| 3rd MCP joint | ||
| Synovectomy − 1 | ||
| Ribs | ||
| Debridement − 1 |
In the extra-spinal group, the mean age of the patients with MDR TB was 32.3 (16 −65) years. 67% of the patients were secondary MDR tuberculosis. There was no sex predilection and none were HIV positive. The knee joint was the most commonly affected joint. Of the 5 patients with knee involvement, 3 had synovectomy and biopsy of the knee and 2 patients underwent arthrodesis. The treatment for the other patients with extra-spinal MDR tuberculosis is described in Table 3. All patients required some surgery as part of the work up for a histological diagnosis. Among the 12 Osteo-articular MDR patients, 10 had completed their treatment and were healed of the disease, while 2 patients were lost to follow up.
All MDR patients were treated with Pyrazinamide, a Fluoroquinolone, a 2nd line injectable agent (for a period of 6 months) and one bacteriostatic drug for a period of 24 months as per WHO recommendations for pulmonary MDR tuberculosis. 12, 13
3.3. Extremely drug Resistant (XDR) Tuberculosis
The Prevalence of XDR tuberculosis was 0.4% - all in patients with spinal tuberculosis. Of the 4 patients who had XDR spinal tuberculosis, 3 patients underwent surgical EPCD procedure and one underwent pig tail insertion for psoas abscess. The three patients who underwent surgery completed treatment successfully, while the patient who had the pig tail insertion was lost to follow up. There were no patients with XDR tuberculosis in the extra-spinal group.
4. Discussion
Tuberculosis has emerged as a major cause of mortality and morbidity in the recent years, particularly in developing countries like India. Also there has been an alarming increase in both MDR TB and XDR TB worldwide. It is a serious disease associated with administration of long duration of significantly toxic second line drugs and is associated with higher morbidity and mortality rates. In 2016, 6.3 million new (incident) TB cases were reported. 47% of these cases were in India, China and the Russian Federation. According to the WHO 2017 report, India had the largest estimated burden of tuberculosis (2·79 million cases) and Rifampicin-resistant or Multi-drug resistant tuberculosis (1,47,000 cases) in the world in 2016.3 The prevalence of MDR TB was reported to be 4.1% (primary) new cases and 19% in previously treated cases (secondary MDR). What is more worrying is the emergence of XDR TB and TDR TB (totally drug resistant TB). In 2009, Velayati et al14 from Iran reported the first case of TDR TB and in 2012, Udwadia et al15 from India reported the first case of TDR TB in India.
Though tuberculosis commonly affects the respiratory system, other organ systems like the central nervous system, abdomen, head and neck and musculo-skeletal system are also often affected. In large studies, extra-pulmonary TB accounts for 15–20% of all cases of tuberculosis and of this, 10% accounts for skeletal tuberculosis.16 50% of Musculo-skeletal tuberculosis has been reported to be in the spine17– as has been borne out in our study (53.2%). The other common sites are the hip, knee, pelvis and small bones of the hand and foot.
There are only a few case reports and studies that have looked at the problems associated with Osteo-articular MDR tuberculosis. Li et al2 from China retrospectively analysed 35 cases of Drug resistant spine TB. Of these, 23 were resistant to a single drug and the remaining 12 were MDR TB. 32 were treated surgically and 3 underwent percutaneous drainage. The indications for surgery were severe deformity, presence of neurological deficit, and spinal instability. At the final follow up, 33 had been successfully treated without major complications, and 2 were still undergoing treatment.
Pawar et al4 from India reported on 25 cases of MDR TB spine, of which 7 were children. The average treatment duration was 24 months and almost 50% of the patients had drug related complications. Only four patients required surgical management for neurological deterioration and mechanical instability. Among the 25 patients, 19 of them achieved “healed status” and 6 were undergoing treatment.
Mohan et al18 in his study involving 111 drug resistant Osteo-articular tuberculosis cases reported that the drug sensitivity testing revealed 87 (78.3%) cases of multi-drug resistance (resistance to both isoniazid and rifampicin) and 3(2.7%) cases of XDR-TB spine. Of the individual drugs, widespread resistance was present to both isoniazid (92.7%) and rifampicin (81.9%), as well as to streptomycin (69.3%).
In our series, we found the prevalence for MDR spinal tuberculosis to be 4.4% (21/478) and that for extra-spinal MDR tuberculosis to be 2.8% (12/420). Of the 21 patients with MDR tuberculosis of the spine 80% had secondary resistance. 15 patients required surgical drainage and decompression with or without instrumentation and graft. Of the 21 patients with MDR tuberculosis of the spine, 81% (17) completed their treatment and were healed.
As drug therapy schedule for MDR Osteo-articular drugs tuberculosis have not been formulated. WHO has laid down guidelines for the treatment of multi-drug resistant pulmonary tuberculosis. These guidelines have been followed for the Osteo-articular MDR in our study. 78% of the 33 patients with MDR tuberculosis responded to the guidelines and have been cured of the disease.
Surgical indications for MDR tuberculosis did not differ from the indications for the treatment of conventional tubercular spondylitis such as pain, mechanical instability, progressive neurological deficit and deformity in similar lines to the indications described by Agarwal et al.19 In the case of extra- spinal osteo-articular tuberculosis, most patients required a surgical intervention as part of the work up for a histological diagnosis.
The large sample size, availability of Xpert PCR for early diagnosis and a high volume Microbiology department were the strengths of the study. However, being a tertiary care referral centre in South India, the prevalence of drug resistance reported in the study centre cannot necessarily be extrapolated to the whole country.
It must be recognised that drug resistant tuberculosis is emerging as a problem with increased secondary drug resistance patterns. Factors that contribute to an increase in MDR TB cases include poor compliance to drug therapy, delay in diagnosis, inappropriate and inadequate doses and regimens prescribed by the treating physicians, cost of medications, lack of direct observed therapy, lack of awareness of importance of continued therapy, poor drug supplies, misuse of drugs for non-tubercular cases and poorly-managed National Tuberculosis Control Programmes.20, 21, 22
5. Conclusions
The prevalence of a 10.2% resistance to anti-tuberculous drugs is of extreme concern. More worrying is the persistence of Multi-drug resistant tuberculosis (3.7%) and of Extremely drug resistant tuberculosis cases (0.4%). A Multidisciplinary approach with drug susceptibility tests, sensitive drugs and surgery if required is essential. It is essential to improve awareness among health care professionals about the danger of drug resistance in tuberculosis and there should be more emphasis on training clinicians on the rational use of appropriate anti-tuberculous drugs.
Conflicts of interest
All authors have none to declare.
Funding sources
No disclosures.
Prior publication
NIL.
Pharmacological or industrial support
NIL.
Contributor Information
J. Arockiaraj, Email: svjustin@cmcvellore.ac.in.
G.S. Balaji, Email: Gopisankar.g@jipmer.edu.in.
V.M. Cherian, Email: vmcherian@cmcvellore.ac.in.
Jepegnanam T.S., Email: jepegnanamt@cmcvellore.ac.in.
B.P. Thomas, Email: binu@cmcvellore.ac.in.
Joy S. Michael, Email: joymichael@cmcvellore.ac.in.
P.M. Poonnoose, Email: reenamg@cmcvellore.ac.in.
References
- 1.Kochi A. The global tuberculosis situation and the new control strategy of the World Health Organization. Tubercle. 1991;71:1–6. doi: 10.1016/0041-3879(91)90017-m. [DOI] [PubMed] [Google Scholar]
- 2.Li Litao, Zhang Zehua, Luo Fei. Management of drug-resistant spinal tuberculosis with a combination of surgery and individualised chemotherapy: a retrospective analysis of thirty-five patients. Int Orthopaedics (SICOT) 2012;36:277–283. doi: 10.1007/s00264-011-1398-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO; Geneva: 2016. Global tuberculosis report 2017.www.who.int/tb/publications/global_report/en [Google Scholar]
- 4.Pawar U.M., Kundnani V., Agashe V. Multidrug-resistant tuberculosis of the spine–is it the beginning of the end? A study of twenty-five culture proven multidrug-resistant tuberculosis spine patients. Spine (Phila Pa 1976) 2009;34(October (22)):E806–E810. doi: 10.1097/BRS.0b013e3181af7797. [DOI] [PubMed] [Google Scholar]
- 5.Balaji V., Daley P., Anand A.A. Risk factors for MDR and XDR-TB in a tertiary referral hospital in India. PLoS One. 2010;5(March (3)):e9527. doi: 10.1371/journal.pone.0009527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Extensively drug-resistant tuberculosis (XDR-TB): recommendations for prevention and control. Wkly Epidemiol Rec. 2006;81:430–432. [PubMed] [Google Scholar]
- 7.Macnab I. Negative disc exploration: an analysis of the causes of nerve-root involvement in sixty-eight patients. J Bone Joint Surg Am. 1971;53:891–903. [PubMed] [Google Scholar]
- 8.McAfee P.C. Interbody fusion cages in reconstructive operations on the spine. J Bone Joint Surg Am. 1999;81:859–880. doi: 10.2106/00004623-199906000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Jain A.K., Dhammi I.K., Modi P. Tuberculosis spine: therapeutically refractory disease. Indian J Orthop. 2012 Mar;46(2):171–178. doi: 10.4103/0019-5413.93685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sundararaj G.D., Babu N., Amritanand R. Treatment of haematogenous pyogenic vertebral osteomyelitis by single-stage anterior debridement, grafting of the defect and posterior instrumentation. J Bone Joint Surg Br. 2007;89:1201–1205. doi: 10.1302/0301-620X.89B9.18776. [DOI] [PubMed] [Google Scholar]
- 11.Rathinavelu B., Arockiaraj J., Krishnan V. The extended posterior circumferential decompression technique in the management of tubercular spondylitis with and without paraplegia. Asian Spine J. 2014 Dec;8(6):711–719. doi: 10.4184/asj.2014.8.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization; Geneva: 2008. Guidelines for the programmatic management of drug-resistant tuberculosis Emergency update 2008. [PubMed] [Google Scholar]
- 13.World Health Organization; Geneva: 2011. Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis. Update 2011. [ISBN-13: 978–92-4–150158-3. [PubMed] [Google Scholar]
- 14.Velayati A.A., Masjedi M.R., Farnia P. Emergence of new forms of totally drug-resistant tuberculosis bacilli: super extensively drug-resistant tuberculosis or totally drug-resistant strains in iran. Chest. 2009;136(August (2)):420–425. doi: 10.1378/chest.08-2427. [DOI] [PubMed] [Google Scholar]
- 15.Udwadia Z.F. Totally drug-resistant tuberculosis in India: who let the djinn out? Respirology. 2012 Jul;17(5):741–742. doi: 10.1111/j.1440-1843.2012.02192.x. [DOI] [PubMed] [Google Scholar]
- 16.Denkinger C.M., Schumacher S., Boehme C.C. Xpert MTB/RIF assay for the diagnosis of extrapulmonary tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2014;44:435–446. doi: 10.1183/09031936.00007814. [DOI] [PubMed] [Google Scholar]
- 17.Tuli S.M. Severe kyphotic deformity in tuberculosis of the spine. Int Orthopaedics (SICOT) 1995;19:327–331. doi: 10.1007/BF00181121. [DOI] [PubMed] [Google Scholar]
- 18.Mohan K., Rawall S., Pawar U.M. Drug resistance patterns in 111 cases of drug-resistant tuberculosis spine. Eur Spine J. 2013;22(Suppl. 4):647–652. doi: 10.1007/s00586-012-2154-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agrawal V., Patgaonkar P.R., Nagariya S.P. Tuberculosis of spine. J Craniovertebr Junction Spine. 2010;1:74–85. doi: 10.4103/0974-8237.77671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Espinal M.A., Laserson K., Camacho M. Determinants of drug-resistant tuberculosis: analysis of 11 countries. Int J Tuberculosis Lung Dis. 2001;5:887–893. [PubMed] [Google Scholar]
- 21.Farmer P., Bayona J., Becerra M. Multidrug resistant tuberculosis and the need for biosocial perspectives. Int J Tuberculosis Lung Dis. 2001;5:885–886. [PubMed] [Google Scholar]
- 22.Zaman K., Rahim Z., Yunus M. Drug resistance of Mycobacterium tuberculosis in selected urban and rural areas in Bangladesh. Scand J Infect Dis. 2005;37:21–26. doi: 10.1080/00365540410026095. [DOI] [PubMed] [Google Scholar]