Highlights
-
•
The founds may result in an important advance in neurological rehabilitation.
-
•
A single-point cane may help improving walking of slow and intermediate walkers after stroke.
-
•
If walking is enhanced, the benefits may be carried over to participation.
Keywords: Clinical trial, Stroke, Gait, Cane, Rehabilitation
Abstract
Background
Canes are usually prescribed for individuals with stroke with the purpose of improving walking and increasing safety. However, there is no consensus regarding the clinical effects of these aids on walking and participation.
Objective
This study will examine the efficacy of the provision of a cane to improve walking and increase participation after stroke.
Methods
This is a two-arm, prospectively registered, randomized trial with concealed allocation, blinded measurers, and intention-to-treat analysis. Fifty individuals with chronic stroke, categorized as slow or intermediate walkers (walking speeds ≤0.8 m/s), will participate. The experimental group will receive a single-point cane and instructions to use the cane anytime they need to walk. The control group will receive a placebo intervention, consisting of self-stretching exercises of the lower limb muscles and instructions to not use assistive devices. The primary outcome will be comfortable walking speed. Secondary outcomes will include walking step length, walking cadence, walking capacity, walking confidence, and participation. Outcomes will be collected by a researcher blinded to group allocation at baseline (Week 0), after intervention (Week 4), and one month beyond intervention (Week 8).
Conclusion
The provision of a single-point cane may help improving walking of slow and intermediate walkers after stroke. If walking is enhanced, the benefits may be carried over to participation, and individuals may experience greater free-living physical activity at home and in the community.
Introduction and rationale
Stroke is the leading cause of adult disability worldwide.1 Amongst the limitations in daily living activities, the ability to walk is reported by patients as the most important activity to recover after a stroke.2, 3 In addition, higher walking ability is related to greater independence and social participation; both performance and capacity of walking have been shown to predict participation.4 Thus, recovery of walking after stroke is one of the most important goals in neurological rehabilitation.5
Assistive devices, such as canes and crutches, are usually prescribed for individuals after stroke with the purpose of improving walking and increasing safety.6 Previous studies have examined the effects of assistive devices on walking parameters in individuals with stroke.7, 8, 9, 10, 11 The results suggested that assistive devices increase step length8 and comfortable and maximum walking speeds,7, 11 decrease cadence,7 and improve walking symmetry.9 No significant changes in maximum joint angles7 or trunk movements10 have been found. A narrative review12 summarized the effects of using a cane on walking in people with stroke. Although 19 experimental studies were included, methodological shortcomings, such as the absence of randomized trials and the predominance of cross-sectional studies with small samples (n < 20 participants), prevent the drawing of convincing conclusions regarding the effects of using a cane on walking. In addition, many of these studies included participants, who had been habitually using a cane, so that the magnitude of the benefits may have been overestimated.
More recently, Nascimento et al.13 conducted an experimental study to investigate the effects of the provision of a single-point cane in a heterogeneous group of community-dwelling people with stroke, who were naïve to the use of assistive devices for walking. Overall, the provision of a cane did not improve walking speed or cadence, and produced a small benefit in step length. However, sub-group analyses demonstrated clinically meaningful increases in walking speed, step length, and cadence for individuals classified as slow and intermediate walkers, i.e., walking speeds ≤0.8 m/s. These results reinforce the need to target interventions to those who will most benefit and avoid the risk of not implementing worthwhile interventions.13, 14
It has also been suggested that the provision of a cane can improve walking confidence.15 Even though walking ability is an important predictor of participation in people with stroke,4 there were not found any studies on the benefits of using a cane on community participation. The most logical time to prescribe walking aids to people with stroke is after their independent walking has stabilized, since, at this stage, there would be no likelihood of interfering with the development of independent walking. A randomized trial to investigate the effects of the provision of a cane to ambulatory individuals with chronic stroke, naïve to the use of assistive devices, on walking and participation after stroke is, therefore, warranted. The specific research questions are:
-
1.
Does the provision of a cane improve walking (speed, step length, cadence, capacity, confidence) in ambulatory individuals with chronic stroke?
-
2.
Are the benefits carried over to participation?
Methods
Design
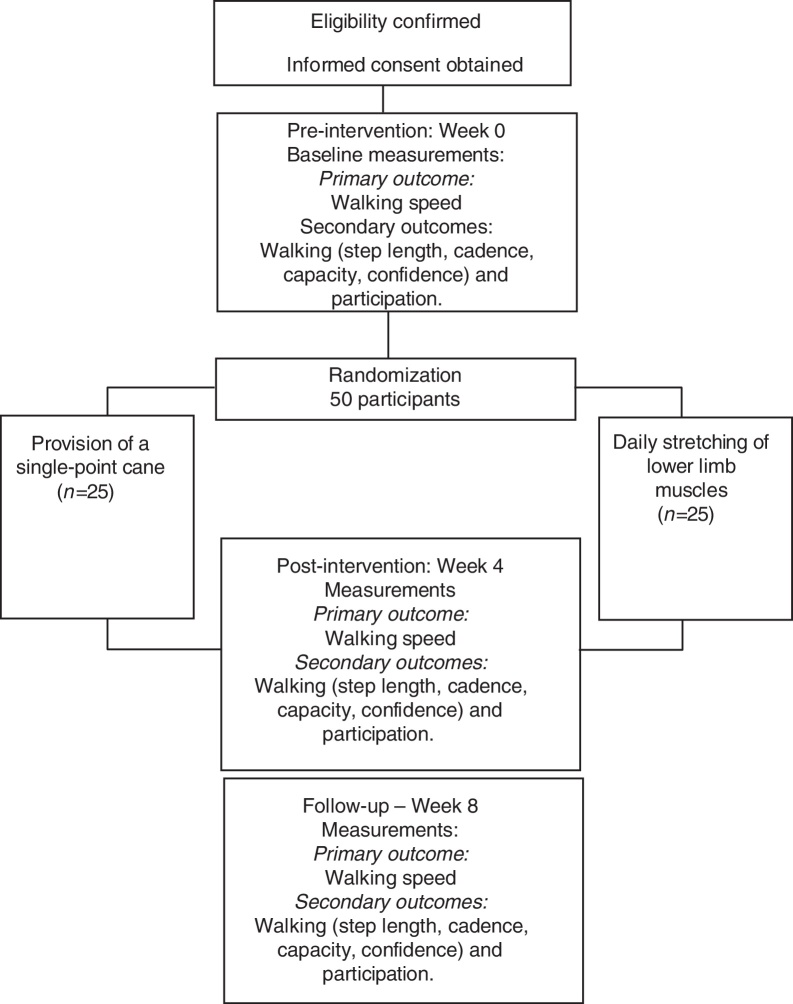
A prospective, randomized controlled trial with concealed allocation, blinded measurers, and intention-to-treat analysis will be carried-out (Fig. 1). Community-dwelling people with chronic stroke will be recruited from the general community, by means of advertisements and by screening public rehabilitation services and lists of previous research projects. Participants will be randomly allocated into either experimental group (i.e., provision of a cane) or control group (i.e., placebo intervention). Outcome measures will be collected by trained researchers at baseline (Week 0), at the end of the intervention (Week 4), and one month beyond the intervention (Week 8). Analyses of inclusion criteria, getting the informed consent, data collection, and statistical analyses will be carried-out by researchers, who will be blinded to group allocation. All the participants will be evaluated and receive all the information regarding the interventions in a research laboratory. The study obtained ethical approval from the Research Ethical Committee (CAAE: 65765817.3.0000.5149) of the Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil. The trial was prospectively registered at the www.ClinicalTrials.gov (NCT03150979).
Figure 1.
Design of the trial.
Participants and therapists – inclusion and exclusion criteria
Participants will be individuals with stroke, who will be eligible, if they:
-
•
are >20 years of age;
-
•
are between 6 months and 5 years after their last episode of stroke;
-
•
have hemiparesis, i.e., weakness of the knee flexor/extensor and/or hip flexor muscles16;
-
•
are able to walk at least 14 m, independently;
-
•
walk at speeds ≤ 0.8 m/s;
-
•
are naïve to the use of assistive devices for walking; and
-
•
provide written consent.
They will be excluded, if they have:
-
•
cognitive deficits, which will be screened by the Mini-Mental State Examination. The cut-off scores are 26 for people with high levels of education, 18 for people with elementary and middle levels, and 13 for illiterate people17;
-
•
other non-stroke related conditions.
Therapists, who will deliver the intervention, will be eligible if they have more than two years of clinical experience in the area of neurological rehabilitation.
Randomization
Randomization will be computer-generated, by a researcher not involved in participant recruitment, and stratified according to the baseline walking speeds: slow (<0.4 m/s) and intermediate (0.4–0.8 m/s) walkers, to ensure an even spread between the groups. The allocation of the participants will be concealed in sequentially numbered, and sealed in opaque envelopes, prepared prior to the study by a research assistant, who will not be involved in the study. After the baseline measures have been collected, participants will be randomly assigned to the experimental or control groups by the treating therapist, after revealing the content of the sealed opaque envelopes. All outcomes will be measured by blinded assessors. All enrolled participants will receive a code, in order to protect confidentiality before, during, and after the trial.
Intervention
The experimental group will receive a single-point cane, with ergonomic handgrip, which will be individually adjusted to the height of the ulnar process of the participants’ non-paretic upper limb, while they are standing with their elbows in extension.7 A physical therapist will provide instructions and training on how to walk with the cane. Individuals will be instructed to hold the cane with their non-paretic hand, to allow for the maintenance of reciprocal walking patterns, taking the first step by moving forward the paretic limb and cane together.7 Participants will practice for about 15 min or until they feel comfortable to walk with the cane. At that time, they will receive the cane and instructions to use it for walking, according to the following sentence: “Feel free to use the cane anytime you need to walk”. Once a week, the participants will be contacted by a physical therapist, to ensure that they are comfortable in using the cane and clarify any problems. In the middle of the intervention period (Week 2), a home visit is planned and the treating therapist will make adjustments on the cane and solve any doubts, if necessary.
The control group will receive a placebo intervention. A physical therapist will provide instructions and training on how to perform self-stretching exercises of the lower limb muscles. Participants will receive a booklet, containing written and visual descriptions of the stretching exercises and recommendations for daily practice. A recommendation for not using assistive devices when walking will be provided, as follows: “avoid using assistive devices for walking, such as canes, stickers, and walkers; however, if you decide to use them, write it down”. The control group will also receive the phone calls/home visits and undertake the same testing protocol as the experimental group. This will avoid bias related to the amount of attention. If training proves to be effective, the control group will be offered the intervention.
The intervention will be undertaken in the participant's home, which will reduce costs and may result in good compliance.18, 19 On the other hand, this means that the training is not directly supervised. To record compliance, participants will receive the Life-Space Diary,20 in which the information regarding the use and non-use of the cane for walking in various environments will be registered. The Life-Space diary is a sheet of A4 paper, with five concentric zones listed in the first column (bedroom, rest of the dwelling, grounds surrounding the dwelling, the “block” in which the dwelling is located, and the area across the traffic-bearing street), and with 31 other columns each representing a day in the month. The diary is ruled and divided into boxes. Participants will be instructed to place a tick in each box every evening, representing the zones to where they had moved during that day and to indicate whether they used or not a cane for walking. Additional rows are provided to indicate other places where the participant might go.20 When required, a caregiver will be instructed to help them. To encourage the participants to comply with the protocol, both groups will be asked to sign a symbolic contract of commitment to the proposed protocol.19
Primary outcome
The primary outcome is comfortable walking speed, measured by the 10-m Walk Test, and reported in m/s. The participants will be instructed to walk at their “comfortable speed” along a 14-m hallway, and the time to cover the central 10 m will be recorded with a digital stopwatch and converted to speed.21 Walking speed will be measured with and without the cane.
Secondary outcomes
Secondary outcomes are walking step length, walking cadence, walking capacity, walking confidence, and participation.
Walking step length and cadence will be measured using the 10-m Walk Test. Step length will be calculated by dividing the covered distance, i.e., 10 m, by the number of steps to cover the distance, and reported in meters. Walking cadence will be calculated by dividing the number of steps by the time to cover the distance, i.e., 10 m, and reported in steps/min.14
Walking capacity will be measured using the 6-min Walk Test, and reported as the covered distance (m). By using a standardized protocol,22 participants will be instructed to cover the maximum distance, as possible, taking rests as needed.
Walking confidence will be measured using the Modified Gait Efficacy Scale, and reported as scores ranging from 10 to 100. This scale is a 10-item measure that addresses the perception of the level of confidence in walking during challenging circumstances. The items include walking on a level surface and on grass, stepping over an obstacle, stepping up and down a curb, ascending and descending stairs (with and without a handrail), and walking over a long distance.23 The items are individually scored on a 10-point Likert scale, with 1 indicating “no confidence”, and 10 indicating “complete confidence”.23
Participation will be measured using the Brazilian version of the Stroke Impact Scale 3.0. The scale covers eight domains and the participation domain will be used. Scores range from zero to 100 and higher scores indicate higher levels of participation.24
Data monitoring body
An independent researcher, who will be blind to the group allocations, will monitor any adverse effects and perform database management and statistical analyses. The treating therapists will be responsible for monitoring of doses and compliance.
Sample size estimation
Fifty participants will be recruited, with walking speed as the primary outcome. The sample size has been calculated, to reliably detect a between-group difference of 0.20 m/s in walking speed, with 80% power, at a two-tailed significance level of 0.05. In a previous trial13 with a similar sample of community-dwelling people after stroke, the mean walking speed of the slow and intermediate walkers was 0.46 m/s (SD 0.24 m/s), using the same measurement procedure as the present protocol. The least number of participants needed to detect a 0.20 m/s difference between two independent groups is 23 per group, i.e., 46 participants in total. Based on the assumption that about 10% of participants may dropout during the study, a target of 50 participants in total has been set.
Statistical analyses
Data collection will yield six variables, which reflect walking and participation: walking speed (m/s), walking step length (m), walking cadence (steps/min), walking capacity (m), walking confidence (Gait Efficacy Scale score), and participation (Stroke Impact Scale 3.0 participation sub-scale score). There are two factors (group*time), with repeated measures on the time factor. Two-way analyses of variance with repeated measures at all time-points for all outcomes will be reported to evaluate the statistical significance of the between-group differences. The mean between-group differences, along with 95% confidence intervals, will be reported for all outcomes. The effect of the intervention will be calculated based on intention-to-treat analyses.
Study organization and funding
This trial will be conducted according to relevant ethical frameworks and has received approval from the institutional ethical review board. It is funded by the following Brazilian national funding agencies: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG).
Discussion
This trial will examine the efficacy of the provision of a cane on walking and participation in individuals with chronic stroke. Although previous studies7, 8, 9, 10, 11, 12, 13 have investigated the effect of using a cane for walking after stroke, methodological shortcomings (e.g., design and small sample sizes) and differences regarding sample characteristics (e.g., time since stroke) and characteristics of interventions (e.g., time to implement intervention, type of cane) prevent drawing clear conclusions, which could help clinicians in their decision-making process. In addition, no previous trials have investigated whether benefits in walking carry over to participation. In response to this challenge, a single-blind randomized trial will be conducted. High internal validity is expected, due to randomization, concealed allocation, blinding of assessors, intention-to-treat analysis,25 and appropriate sample size.
The most logical time to prescribe walking aids to people with stroke is after their independent walking has stabilized, since, at this stage, there would be no likelihood of interfering with the development of independent walking. In addition, the question as to whether a cane can improve walking is best answered during the chronic stages, so that it is not confounded by recovery during active rehabilitation at the acute stages.13 Therefore, this trial will only include participants at the chronic stages after stroke. Furthermore, the experimental intervention will be a single-point cane with ergonomic handgrip (Mercur®), which is preferred by patients, requires less oxygen use at a given speed,26, 27 and is relatively inexpensive (costs about US$17).
This trial has some limitations. Participants and therapists cannot be blind, which is unpractical during the delivery of complex interventions. In addition, the experimental and control interventions consist of home exercises with no direct supervision, and, therefore, depend on the participant's motivation. Strategies to encourage participants to comply with the protocol, such as contracts, phone calls, and weekly visits, are planned.19
In conclusion, the results of this trial may result in an important advance in neurological rehabilitation. First, a low-cost, simple intervention may help improving walking of slow and intermediate walkers after stroke. Second, if walking is enhanced, the benefits may be carried over to participation, and individuals may experience greater free-living physical activity at home and in the community,3, 28, 29 decreased disability and increased social interactions,30, 31 and increased ability to engage in work and leisure activities, the ultimate goal for both patients and rehabilitation professionals.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq (grant number 304434/2014-0) and Fundação de Amparo à Pesquisa de Minas Gerais-FAPEMIG (PPM-00082-16). Data will be stored at the Universidade Federal de Minas Gerais, Brazil.
Footnotes
Trial registration: Clinical Trials, NCT03150979. Registered on May 11th, 2017 (https://clinicaltrials.gov/ct2/show/NCT03150979).
References
- 1.Roger V.L., Go A.S., Lloyd-Jones D.M. Heart diseases and stroke statistic update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caty G.D., Arnould C., Stoquart G.G., Thonnard J.L., Lejeune T.M. ABILOCO: a Rasch-built 13-item questionnaire to assess locomotion ability in stroke patients. Arch Phys Med Rehabil. 2008;89:284–290. doi: 10.1016/j.apmr.2007.08.155. [DOI] [PubMed] [Google Scholar]
- 3.Alzahrani M., Dean C., Ada L. Relationship between walking performance and types of community-based activities in people with stroke: an observational study. Braz J Phys Ther. 2011;15:45–51. doi: 10.1590/s1413-35552011005000002. [DOI] [PubMed] [Google Scholar]
- 4.Faria-Fortini I., Basílio M.L., Scianni A.A., Faria C.D.C.M., Teixeira-Salmela L.F. Performance and capacity-based measures of locomotion, compared to impairment-based measures, best predicted participation in individuals with hemiparesis due to stroke. Disabil Rehabil. 2017;11:1–8. doi: 10.1080/09638288.2017.1312570. [DOI] [PubMed] [Google Scholar]
- 5.Skilbeck C.E., Wade D.T., Hewer R.L., Wood V.A. Recovery after stroke. J Neurol Neurosurg Psychiatry. 1983;46:5–8. doi: 10.1136/jnnp.46.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bateni H., Maki B.E. Assistive devices for balance and mobility: benefits, demands, and adverse consequences. Arch Phys Med Rehabil. 2005;86:134–145. doi: 10.1016/j.apmr.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 7.Polese J.C., Teixeira-Salmela L.F., Nascimento L.R. The effects of walking sticks on gait kinematics and kinetics with chronic stroke survivors. Clin Biomech. 2012;27(2):131–137. doi: 10.1016/j.clinbiomech.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Kuan T., Tsou J., Su F. Hemiplegic gait of stroke patients: the effect of using a cane. Arch Phys Med Rehabil. 1999;80:777–784. doi: 10.1016/s0003-9993(99)90227-7. [DOI] [PubMed] [Google Scholar]
- 9.Beauchamp M.K., Skrela M., Southmayd D. Immediate effects of cane use on gait symmetry in individuals with subacute stroke. Physioter Can. 2009;61:154–160. doi: 10.3138/physio.61.3.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tyson S. Trunk kinematics in hemiplegic gait and effect of walking aids. Clin Rehabil. 1999;13:295–300. doi: 10.1191/026921599666307333. [DOI] [PubMed] [Google Scholar]
- 11.Jeong Y.G., Jeong Y.J., Kim T. A randomised comparison of energy consumption when using different canes, inpatients after stroke. Clin Rehabil. 2015;29(2):129–134. doi: 10.1177/0269215514543932. [DOI] [PubMed] [Google Scholar]
- 12.Pinheiro H.A. The effects of a cane in rehabilitation of hemiplegic patients. Rev Neurocienc. 2011;19(2):358–364. [Google Scholar]
- 13.Nascimento L.R., Ada L., Teixeira-Salmela L.F. The provision of a cane provides greater benefit to community-dwelling people after stroke with a baseline walking speed between 0.4 and 0.8 m/s: an experimental study. Physiotherapy. 2016;102(4):351–356. doi: 10.1016/j.physio.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Dean C.M., Ada L., Lindley R.I. Treadmill training provides greater benefit to the subgroup of community-dwelling people after stroke who walk faster than 0.4 m/s: a randomised trial. J Physiother. 2014;60(2):97–101. doi: 10.1016/j.jphys.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Polese J.C., Nascimento L.R., Faria C.D. Perception of patients with chronic hemiplegia regarding the use of assistive walking devices. Rev Panam Salud Publica. 2011;30(3):204–208. doi: 10.1590/s1020-49892011000900003. [DOI] [PubMed] [Google Scholar]
- 16.Faria C.D.C.M., Teixeira-Salmela L.F., Nadeau S. Predicting levels of basic functional mobility, as assessed by the Timed “Up and Go” test, for individuals with stroke: discriminant analyses. Disabil Rehabil. 2013;35:146–152. doi: 10.3109/09638288.2012.690497. [DOI] [PubMed] [Google Scholar]
- 17.Bertolucci P., Brucki S., Campacci S. The Mini-mental state examination in an outpatient population: influence of literacy. Arq Neuropsiquiatr. 1994;52:1–7. [PubMed] [Google Scholar]
- 18.Siemonsma P., Döpp C., Alpay L., Tak E., Meeteren N., Chorus A. Determinants influencing the implementation of home-based stroke rehabilitation: a systematic review. Disabil Rehabil. 2014;36(24):2019–2030. doi: 10.3109/09638288.2014.885091. [DOI] [PubMed] [Google Scholar]
- 19.De Menezes K.K.P., Nascimento L.R., Polese J.C., Ada L., Teixeira-Salmela L.F. Effect of high-intensity home-based respiratory muscle training on strength of respiratory muscles following a stroke: a protocol for a randomized controlled trial. BJPT. 2017;21(5):372–377. doi: 10.1016/j.bjpt.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.May D., Nayak U.S., Isaacs B. The life-space diary: a measure of mobility in old people at home. Int Rehabil Med. 1985;7(4):182–186. doi: 10.3109/03790798509165993. [DOI] [PubMed] [Google Scholar]
- 21.Nascimento L.R., Caetano L.C., Freitas D.C., Morais T.M., Polese J.C., Teixeira-Salmela L.F. Different instructions during the ten-meter walking test determined significant increases in maximum gait speed in individuals with chronic hemiparesis. BJPT. 2012;16(2):122–127. doi: 10.1590/s1413-35552012005000008. [DOI] [PubMed] [Google Scholar]
- 22.American Thoracic Society/European Respiratory Society ATS/ERS statement on respiratory muscle testing. Am J Respir Care Med. 2002;166:518–624. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- 23.Newell A.M., VanSwearingen J.M., Hile E., Brach J.S. The Modified Gait Efficacy Scale: establishing the psychometric properties in older adults. Phys Ther. 2012;92(2):318–328. doi: 10.2522/ptj.20110053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carod-Artal F.J., Coral L.F., Trizotto D.S., Moreira C.M. The stroke impact scale 3.0: evaluation of acceptability, reliability, and validity of the Brazilian version. Stroke. 2008;39(9):2477–2484. doi: 10.1161/STROKEAHA.107.513671. [DOI] [PubMed] [Google Scholar]
- 25.Slack M.K., Draugalis J.R. Establishing the internal and external validity of experimental studies. Am J Health Syst Pharm. 2001;58(22):2173–2181. [PubMed] [Google Scholar]
- 26.Allet L., Leeman B., Guyen E. Effect of different walking aids on walking capacity of patients with post-stroke hemiparesis. Arch Phys Med Rehabil. 2009;90:1408–1413. doi: 10.1016/j.apmr.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 27.Jeong Y., Jeong Y.J., Kim T. A randomised comparison of energy consumption when using different canes, inpatients after stroke. Clin Rehabil. 2015;29(2):129–134. doi: 10.1177/0269215514543932. [DOI] [PubMed] [Google Scholar]
- 28.Fulk G.D., Reynolds C., Mondal S., Deutsch J.E. Predicting home and community walking activity in people with stroke. Arch Phys Med Rehabil. 2010;91(10):1582–1586. doi: 10.1016/j.apmr.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Michaelsen S.M., Ovando A.C., Romaguera F., Ada L. Effect of backward walking treadmill training on walking capacity after stroke: a randomised clinical trial. Int J Stroke. 2014;9(4):529–532. doi: 10.1111/ijs.12255. [DOI] [PubMed] [Google Scholar]
- 30.Kim M., Cho K., Lee W. Community walking training program improves walking function and social participation in chronic stroke patients. Tohoku J Exp Med. 2014;234(4):281–286. doi: 10.1620/tjem.234.281. [DOI] [PubMed] [Google Scholar]
- 31.Tilson J.K., Sullivan K.J., Cen S.Y., Locomotor Experience Applied Post Stroke (LEAPS) Investigative Team Meaningful gait speed improvement during the first 60 days poststroke: minimal clinically important difference. Phys Ther. 2010;90(2):196–208. doi: 10.2522/ptj.20090079. [DOI] [PMC free article] [PubMed] [Google Scholar]