Abstract
Electrospinning is one of the most effective approaches to fabricate tissue-engineered scaffolds composed of nano-to sub-microscale fibers that simulate a native extracellular matrix. However, one major concern about electrospun scaffolds for tissue repair and regeneration is that their small pores defined by densely compacted fibers markedly hinder cell infiltration and tissue ingrowth. To address this problem, researchers have developed and investigated various methods of manipulating scaffold structures to increase pore size or loosen the scaffold. These methods involve the use of physical treatments, such as salt leaching, gas foaming and custom-made collectors, and combined techniques to obtain electrospun scaffolds with loose fibrous structures and large pores. This article provides a summary of these motivating electrospinning techniques to enhance cell infiltration of electrospun scaffolds, which may inspire new electrospinning techniques and their new biomedical applications.
Keywords: Electrospinning, Scaffolds, Porosity control, Cell infiltration, Tissue regeneration
Graphical abstract
Highlights
-
•
Electrospinning is a popular and attractive technique to produce fibrous scaffolds for tissue regeneration.
-
•
One limitation for electrospun scaffolds is low cell infiltration.
-
•
This article summarizes innovative techniques to improve cell infiltration of electrospun scaffolds.
1. Introduction
First demonstrated in the 1930s by Anton Formhals, electrospinning has increasingly gained attention for various applications in the research community and industrial field [1]. Featured characteristics including simplicity and affordable cost, as well as controllable fiber diameter and arrangement of electrospinning technique make it a versatile approach to fabricating scaffolds with variable properties. One prominent feature of electrospun scaffolds is its ultrafine fibrous structure that reassembles the nanoscale of native extracellular matrix (ECM). They possess a large surface-to-volume ratio and are extremely conducive for cell attachment and growth. Therefore, they are widely used for tissue replacement and regeneration, including the myocardium [2], blood vessel [3], [4], heart valve [5], skin [6], bone [7], [8], cartilage [9], tendon [10], meniscus [11] and nerve [12].
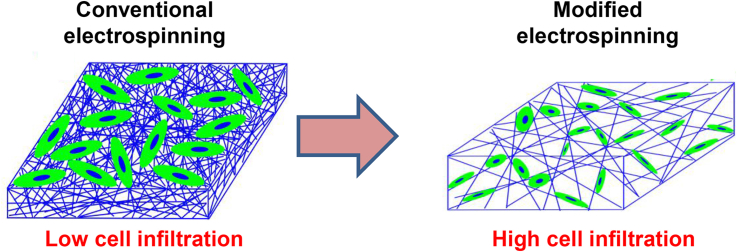
Electrospun scaffolds made from synthetic, natural, or combined materials have a nanofibrous structure that bears a close resemblance to native ECM. This structure provides the scaffolds with essential cues for cell growth and organization. Cell proliferation and ECM deposition on the electrospun scaffold have been well elaborated upon [13]. The conventional nanofibrous scaffolds (Fig. 1) merely mimic native ECMs in their fibrillary structures but not in their spatial characteristics. Specifically, during a conventional electrospinning process, generated fibers are densely compacted on a solid plate. The resulting electrospun scaffold is a planar substrate with a small interfiber distance much less than cell size. When cultured on conventional electrospun scaffolds, the cells experience a two-dimensional growth pattern with minimal penetration (Fig. 2) rather than a three-dimensional organization of cells embedded in native ECMs. Such poor cell infiltration into the scaffold due to the dense fibrous structure poses a significant challenge for tissue regeneration. Cell infiltration is essential for the formation of a three-dimensional (3D) cell-scaffold construct, subsequently promoting tissue ingrowth and facilitating integration between scaffold and host tissue post-implantation. Consequently, many significant techniques have been developed to improve cell infiltration for electrospun scaffolds [14], [15]. In this article, we review recent progress in promoting cell infiltration into the electrospun scaffolds by altering scaffold structure via a variety of techniques (Table 1).
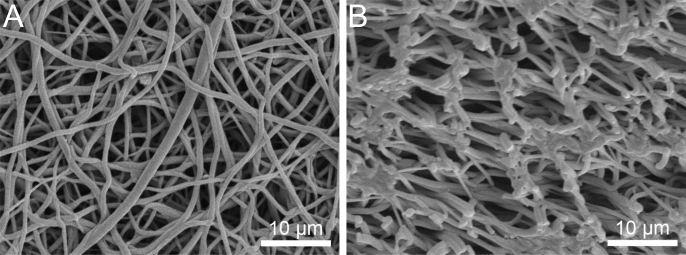
Fig. 1.
Representative SEM images of the surface (A) and cross-section (B) of an electrospun polyurethane scaffold fabricated by conventional electrospinning.
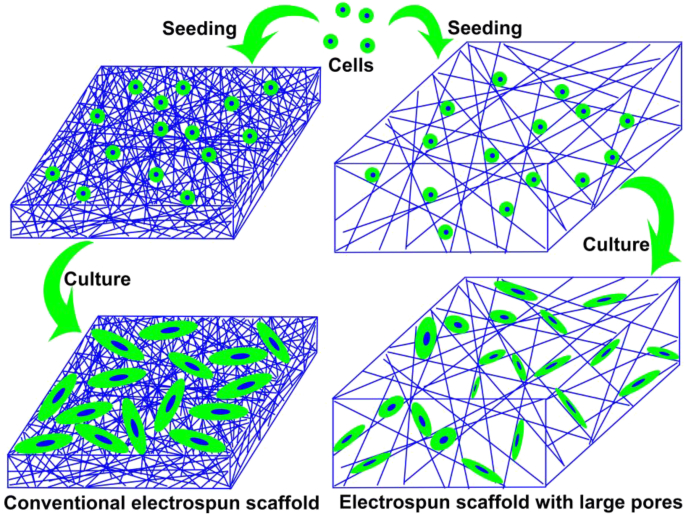
Fig. 2.
Schematic cell growth on the conventional electrospun scaffold and in the electrospun scaffold with large pores.
Table 1.
Summary of pore size and cell infiltrated depth of electrospun scaffolds.
| Technique | Material | Pore size | Cell type | Cell infiltrated depth | Reference |
|---|---|---|---|---|---|
| Micro/nano fibers | PCL | 20–45 μm | Rat MSC | ∼1.2 mm | [19] |
| Salt leaching | PCL | ∼200 μm | CFK2 cell | 4 mm | [22] |
| HA/collagen | 50–100 μm | Bovine chondrocyte | Not specified | [23] | |
| Cryogenic electrospinning | PLA | 10–500 μm | L929 fibroblast | 50 μm | [25] |
| Sacrificial fibers | PCL/PEO | Not specified | Bovine MSC | ∼800 μm | [28] |
| PLLA/PEO | 10–90 μm | MC3T3-E1 | ∼600 μm | [29] | |
| Electrospinning using a liquid bath collector | PLGA/PCL | Not specified | Rat BMSC | ∼200 μm | [34] |
| P(LLA-CL)/SF | 20–50 μm | L929 fibroblast | 100 μm | [36] | |
| P(LLA-CL)/collagen | ∼30 μm | PIEC | 300 μm | [37] | |
| P(LLA-CL)/collagen | ∼30 μm | TDSC | 1 mm | [39] | |
| Ultrasonication | PLLA | 6–14 μm | 3T3 fibroblast | ∼350 μm | [42] |
| Gas foaming | PCL/gelatin | ∼300 μm | Human MSC | ∼300 μm | [49] |
| PCL | ∼20 μm | 3T3 fibroblast | 1 cm | [50] | |
| Airflow perforated mandrel | PCL | 2–8 μm | Human dermal fibroblast | 186 μm | [52] |
| Electrospinning/electrospraying | PCL/collagen | Not specified | Human fetal osteoblast | ∼200 μm | [57] |
| PEUU | Not specified | Rat SMC | Full depth | [61] |
2. Techniques to enhance cell infiltration of electrospun scaffolds
2.1. Combination of nano and micro-fibers
Combining large microfibers with fine nanofibrous scaffolds can produce large pores with great pore interconnectivity. Balguid et al. illustrated that pore size strongly depended on fiber diameter, which ultimately determined the cell penetration behaviors of electrospun scaffolds [16]. The nanofibers exhibit advantages in improved cell adhesion and proliferation, while microfibers are advantageous in making pore size bigger and promoting cell infiltration [17], [18]. This leads to the methodology of combining nanofibers and microfibers to fabricate a scaffold that uses the inherent advantages of both electrospun fibers (Fig. 3A).
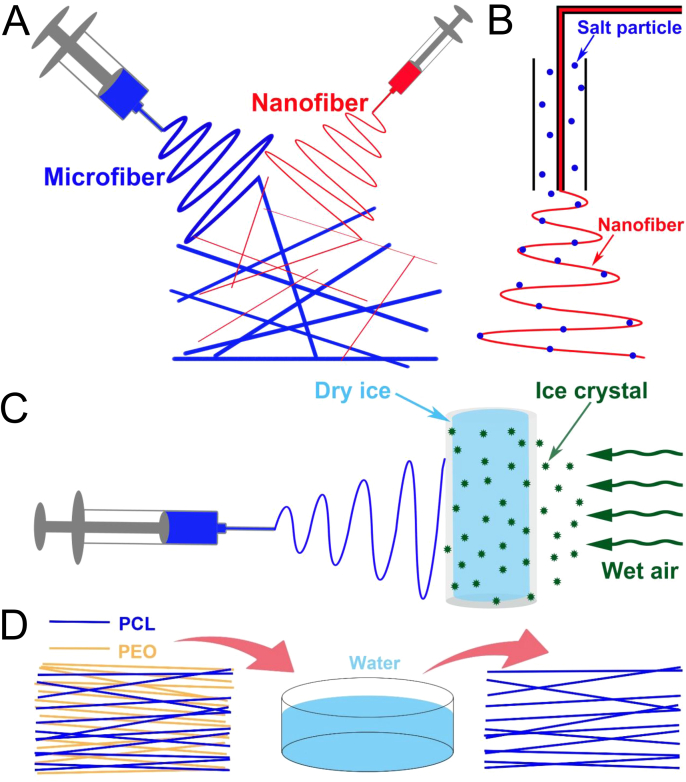
Fig. 3.
(A) Preparation of a micro- and nano-fiber hybrid scaffold by two-jet electrospinning [19]. (B) Introduction of salt particles into electrospun mesh in the surrounding sheath [22]. (C) Schematic setup for cryogenic electrospinning. A cylindrical aluminum drum containing dry ice is exposed in saturated atmosphere to simultaneously create ice crystals and collect the electrospun nanofibers [24]. (D) PEO nanofibers are used as a sacrificial component and leached out in a water bath to obtain electrospun PCL nanofibrous scaffolds with improved porosity [28].
Electrospun nano-/micro-fiber hybrid scaffolds can be prepared by two-stream electrospinning, where one stream creates nanofibers and the other generates microfibers. Pham et al. reported a poly(ɛ-caprolactone) (PCL) scaffold composed of 5 μm microfibers interspersed with 600 nm nanofibers supported completed cell infiltration throughout the whole scaffold in a bioreactor within 12 days [19]. Large pores defined by the microfibers allowed cells to infiltrate the scaffold freely, while the nanofibers facilitated cell spreading and improved cell growth inside the scaffold [20]. Furthermore, the presence of nanofibers in the nano-/micro-fiber hybrid scaffolds influenced stem cell differentiation [20], [21]. Levorson et al. demonstrated that fibrin nanofibers interspersed in a nano-/micro-fiber scaffold have a positive effect on the chondrogenic differentiation of human mesenchymal stem cells (MSCs) as increased glycosaminoglycan (GAG) production was found in two weeks of culture without the addition of growth factors [21].
2.2. Electrospinning with salt leaching
Salt leaching has been extensively used to prepare 3D porous scaffolds for tissue engineering applications. Salt particles are dispersed evenly in a polymer solution and leached out to create large pores with controllable pore size determined by particle size. Based on this principle, combining electrospinning with salt leaching leads to fibrous scaffolds with large pores. Nam et al. reported that the introduction of tiny salts (90–106 μm in diameter) into the Taylor Cone using a sheath surrounding the needle at intervals during electrospinning of PCL produced a uniform fiber network with a well-spread distribution of salt particles (Fig. 3B) [22]. Subsequently, salt leaching results in improved porosity and large pores with increased delamination within the PCL fibrous scaffold. After 3 weeks of culture, CFK2 cells (a cell line derived from fetal rat calvariae that has the phenotypic characteristics of chondrocytes) exhibited an extensive infiltrated depth of 4 mm along with up to 70% cell coverage within the delaminated scaffolds. Unlike adding salt particles into the Taylor Cone during the intervals of electrospinning, Kim et al. produced a homogeneous porous hyaluronic acid/collagen porous mesh by simultaneously depositing salt particles with nanofibers during electrospinning [23]. The resultant porous scaffold maintained structural integrity with acceptable dimensional shrinking after salt leaching. Bovine chondrocytes exhibited the roundness characteristic of typical chondrocyte phenotypes with extracellular matrix accumulation inside the scaffolds.
2.3. Cryogenic electrospinning
Using ice crystals as a porogen to induce large pores inside electrospun scaffolds was first published by Simonet et al. [24] and is also termed cryogenic electrospinning by Leong et al. [25], [26]. This approach involves the use of a low-temperature collecting system that allows the simultaneous formation of nanofibers and ice crystals, yielding an ice particle-embedded fibrous mesh (Fig. 3C). The ice particles are subsequently removed by freeze-drying to create pores inside the electrospun scaffolds. Therefore, the porosity and pore size of scaffolds are adjusted by varying the size and amount of the embedded ice crystals. Scaffold porosity increases with a greater amount of embedded ice crystals [24], and by alternating the humidity of the electrospinning environment to vary the size of ice crystals, the scaffold pore size can be adjusted from 10 to 500 μm [25]. The NIH 3T3 fibroblasts penetrated a 50 μm-thick porous scaffold under static culture condition within 7 days and showed a continuously increased number of cells during a period of 14 days, whereas no cell infiltration was found in conventional electrospun scaffolds. The ice crystal induced scaffold (400 μm thick) was then subcutaneously implanted into rat dorsum. Similar to the in vitro study, improved cell infiltration with macrophages and collagen-producing fibroblasts throughout the ice crystal induced scaffold at day 56, while poor cell infiltration was seen in the conventional electrospun scaffold [25]. Cryogenic electrospinning was also used for the chemoresistance of cancer cells by Bulysheva et al., where cryogenic electrospun silk fibroin (SF) scaffolds were fabricated to mimic cancer ECM [27]. HN12 cells derived from human head and neck squamous cell carcinoma were seeded with cryogenic electrospun SF scaffolds and then compared with an in vivo model of the same derivative human cancer to investigate cell-matrix interactions and drug resistance. Due to its highly porous structure, the cryogenic electrospun scaffold supported good cell infiltration, and the cells showed a profound protective effect on the scaffold compared to a conventional monolayer culture. It indicated that this approach is capable of replicating the in vivo conditions in a 3D culture model in terms of cell proliferation rate, differentiation, and infiltration throughout the scaffold.
2.4. Sacrificial fibers induced large pores
Washing out sacrificial fibers is also an effective approach to increasing pore size, which is similar to salt leaching (Fig. 3D). The typical procedure involves a target polymer and a water-soluble polymer that are concurrently electrospun using two independent spinnerets. Then the sacrificial fibers are removed by being dissolved in the water without structural disruption. Poly(ethylene oxide) (PEO) is one of the best candidates for the sacrificial fiber material due to its high water solubility. Baker et al. first introduced the combination of PCL and PEO fibers, where PEO fibers were sacrificed through dissolving in water to produce PCL scaffolds with large pores and reduced fiber entanglement [28]. The scaffold pore size and tensile strength could be tuned by varying the ratio of PCL/PEO. When seeded with bovine MSCs, the scaffolds showed better cell infiltration than conventional electrospun PCL scaffolds. Additionally, Whited et al. co-electrospun poly(L-lactide) (PLLA) with PEO to obtain porous scaffolds that supported MC3T3-E1 preosteoblast growth and osteogenic differentiation and facilitated cell infiltration into the scaffold with uniform cell distribution [29]. Recently, Klumpp et al. reported an aligned PCL/collagen-PEO (aPCL/Coll-PEO) scaffold which also possessed large pores created by removing PEO sacrificial fibers [30]. After 4 week implantation in rats, the aPCL/Coll-PEO scaffolds remained a porous structure with intensive cellular infiltration and tissue ingrowth, as well as 3D vascularity.
2.5. Electrospinning using a liquid bath collector
A technique using a liquid reservoir with a variety of solvents including water [31], methanol [32], tertiary-butyl alcohol [33], and ethanol [34] has gained increasing attention as a way to prepare electrospun scaffolds with large pores. A liquid reservoir increases the dispersion effect on fibers and allows significantly decreased fiber bonding, leading to larger pore size and improved porosity (Fig. 4A). This method is easily manipulated and effectively produces 3D porous scaffolds that hold promise for bone and cartilage tissue engineering [32], [34]. Yang et al. reported a cotton-like ploy(lactic-co-glycolic acid) (PLGA)/PCL scaffold using an ethanol bath to prepare electrospun nanofibrous scaffolds. Rat bone mesenchymal stem cells (BMSCs) infiltrated the scaffold and deposited an abundant cartilage-specific matrix under chondrogenic differentiation for 4 weeks. Subsequently, the cell-seeded scaffold was subcutaneously implanted into nude rats and exhibited an extensive new bone formation after 8 weeks [34].
Fig. 4.
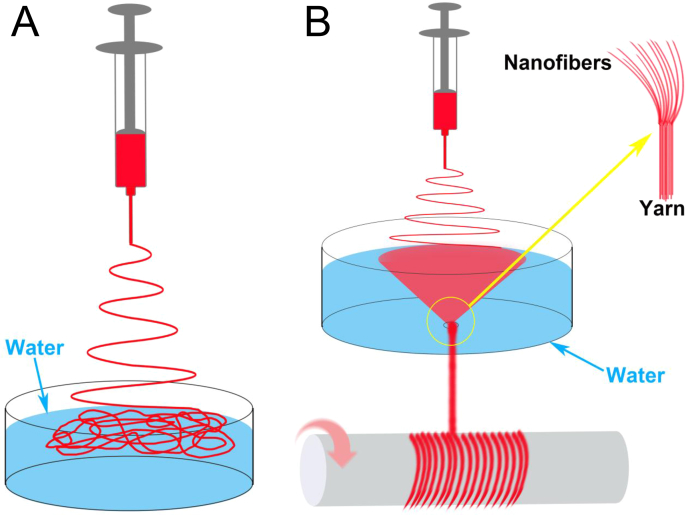
(A) Liquid bath is utilized to disperse electrospun nanofibers to create porous scaffolds [31]. (B) A water vortex is create in the liquid bath to deposit the nanofibers and twist them into aligned yarn that is collected by a rotating mandrel to prepare fibrous scaffolds [35].
Furthermore, with minor modifications, it can be adopted to fabricate a special fibrous structure termed “yarn” [35]. This approach involves collecting nanofibers with a dynamic liquid system created by a water vortex. The nanofibers deposited on the water's surface are pulled along by water vortex and twisted into a continuous yarn. The yarn is composed of a bundle of aligned nanofibers (Fig. 4B). Also, the use of a rotating mandrel to collect the yarns can obtain 3D nanoyarn scaffolds composed of aligned yarns [36]. These nanoyarn scaffolds have shown promise in a range of tissue engineering applications. Wu et al. reported that L929 fibroblasts infiltrated throughout a 100 μm-thick ploy(L-lactide-co-caprolactone) (P(LLA-CL))/SF nanoyarn scaffold under static culture for 7 days [36]. A later study showed pig iliac endothelial cells (PIECs) and MC3T3-E1 preosteoblasts penetrated throughout 300 μm-thick P(LLA-CL)/collagen nanoyarn scaffolds after 10 day static culture, and vascular-like structures were observed when the scaffold was seeded with PIECs [37]. Recent studies by Xu et al. highlighted the potential of P(LLA-CL)/collagen nanoyarn scaffolds for tendon tissue regeneration. Rabbit tendon cells and tendon-derived stem cells (TDSCs) exhibited extensive infiltration in the scaffolds and upregulated tendon-related gene expressions [38], [39]. P(LLA-CL)/collagen nanoyarns were recently combined with hyaluronate by lyophilization to obtain more complex 3D scaffolds by Zheng et al. [40] and Liu et al. [41] for cartilage tissue engineering. Those scaffolds supported cell infiltration and enhanced chondrogenic differentiation of rabbit BMSCs in vitro.
2.6. Ultrasonication
The conventional electrospun scaffolds can be loosened by post-electrospinning processing to increase pore size and porosity. For example, Lee et al. used ultrasonication to loosen densely packed fibers [42] (Fig. 5A). Ultrasonic manipulation represents a typical physical treatment that mechanically disperses tightly compacted fibers by the vibration of ultrasonication, thereby altering overall fiber density, pore size, porosity, and scaffold thickness [42], [43]. Although the increase in pore size and porosity is related to ultrasonic energy, it depends more on ultrasonic exposure time. The NIH 3T3 fibroblasts infiltrated up to ∼350 μm into the ultrasonication-treated PLLA scaffold under static culture for 7 days, whereas no evident cell infiltration was observed in the non-ultrasonication-treated electrospun PLLA scaffolds [42]. In addition, Gu et al. showed that ultrasonication-treated electrospun chitosan scaffolds supported a 1.4-fold higher proliferation rate of human dermal fibroblasts than that of non-ultrasonication treated electrospun chitosan scaffolds within 7 days [44]. A most recent study presented by Gu et al. also demonstrated the ultrasonication-treated electrospun chitosan/gelatin scaffold supported greater cell proliferation and infiltration of human dermal fibroblasts compared to non-ultrasonication-treated chitosan/gelatin scaffolds [45].
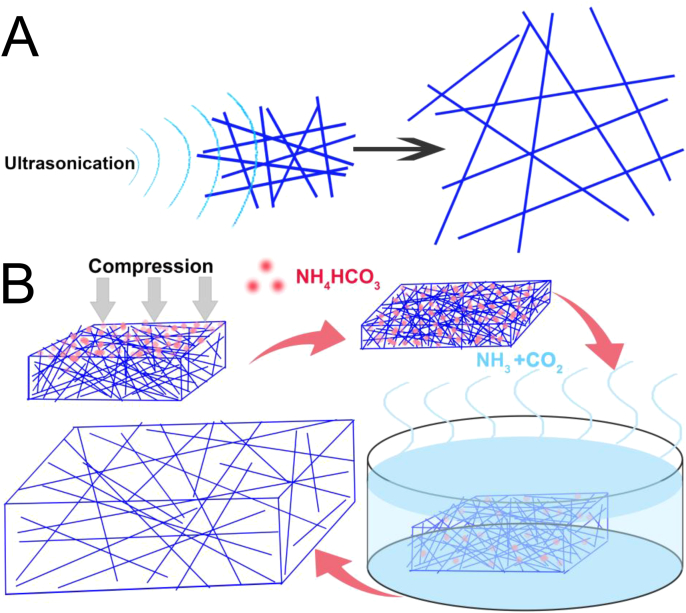
Fig. 5.
(A) Conventional electrospun scaffold is treated with sonication to loosen the densely compacted nanofibers [42]. (B) NH4HCO3 particles are mechanically kneaded into the conventional electrospun mesh and immersed in 90 °C water to generate gas bubbles to obtain large-pore fibrous scaffolds [47].
2.7. Electrospinning with gas foaming
Gas foaming has been widely used to fabricate 3D porous scaffolds, which is the introduction of gas (the most popular gas is carbon dioxide (CO2)) into a polymer solution and the rapid release of CO2 creates gas bubbles in the solution, which allows large pore formation [46]. The first report about employing gas foaming (Fig. 5B) to enlarge electrospun scaffold pore size was published by Lee et al., accompanying the use of salt leaching [47]. In this approach, ammonium bicarbonate (NH4HCO3)/sodium chloride (NaCl) particles were mechanically kneaded into electrospun PLLA fabric and compressively molded. Then the particle-loaded fabric was heated in water at 90 °C to decompose NH4HCO3 and generate ammonia and CO2, which enlarged scaffold pore size and porosity. Subsequently, the obtained fabric was immersed in 60 °C water to leach out residual NaCl to further improve the porosity and enlarge pore size. A similar technique reported by Kim et al. involving the use of a chemical blowing agent to loosen electrospun PCL nanofibers also attained highly porous scaffolds [48]. The gas foaming could be induced at a lower temperature (60 °C) by using sodium bicarbonate to minimize the loss of bioactivity of natural polymer as demonstrated by Hwang et al. [49]. A PCL/gelatin electrospun scaffold was fabricated using this method, and it supported human mesenchymal stem cell infiltration ∼300 μm into the scaffold. Another common gas foaming agent, sodium tetrahydridoborate (NaBH4), was effective in generating hydrogen gas to expand aligned electrospun PCL scaffolds, as reported by Jiang et al. [50]. It is notable that the NIH 3T3 fibroblasts penetrated throughout a 1 cm-thick gas foamed scaffold under static culture conditions within 7 days.
2.8. Custom-made collectors
Manipulation of the manner of electrospun fiber deposition is another effective way to loosen fibers. Blakeney et al. demonstrated a special electrospun collector composed of a spherical foam dish embedded with stainless steel probes [51]. This collector overcomes the inherent limitation of conventional solid flat collectors, allowing the electrospun nanofibers to be packed in a loose pattern, ultimately yielding a low-density, uncompressed, cotton ball-like PCL scaffold. When seeded with rat insulinoma INS-1 (823/13) cells, this scaffold not only supported a higher cell proliferation rate, but also promoted greater cell infiltration (up to 300 μm) compared with those cells seeded with a conventional electrospun PCL scaffold.
Electrospun fiber deposition could also be manipulated by airflow generated from a custom-made perforated mandrel as first illustrated by McClure et al. [52]. When connected with pressurized air, the perforated mandrel maintains a controllable airflow pressure that loosens electrospun fibers and allows the generation of less dense and more porous scaffolds. The pore size and porosity depend on the airflow velocity. This airflow-treated PCL scaffold showed similar compliance with the conventional electrospun PCL scaffold [52]. This method has been employed to electrospin several synthetic and natural polymers, including PCL [52], regenerative SF [53], polydioxanone (PDO) [54] and P(LLA-CL)/SF [55], yielding a number of porous scaffolds. Enhanced cell infiltration of various cells including human dermal fibroblasts [52], human breast epithelial cells [53], mouse bone marrow-derived macrophages [54] and human aortic smooth muscle cells [55] could be found in those airflow-treated scaffolds, implying a great potential of such scaffolds for a range of tissue engineering applications.
2.9. Electrospinning/electrospray
In addition to the above methods that increase scaffold pore size, improved cell infiltration can also be achieved by loosening fibers without significant change in pore size. Hashizume et al. reported a technique of “wet electrospinning,” in which electrospun poly(ester urethane)urea (PEUU) fibers were concurrently deposited with electrosprayed serum-based culture medium [56] (Fig. 6). The resulting wet electrospun scaffolds exhibit a relatively loose structure in that the fibers show a qualitatively higher degree of looping and more tortuosity than conventional electrospun scaffolds, and this structural feature was consistently observed throughout the wet electrospun scaffolds. When implanted in a rat model for abdominal wall replacement, the wet electrospun PEUU scaffold supported the extensive infiltration of smooth muscle cells with strong ECM elaboration.
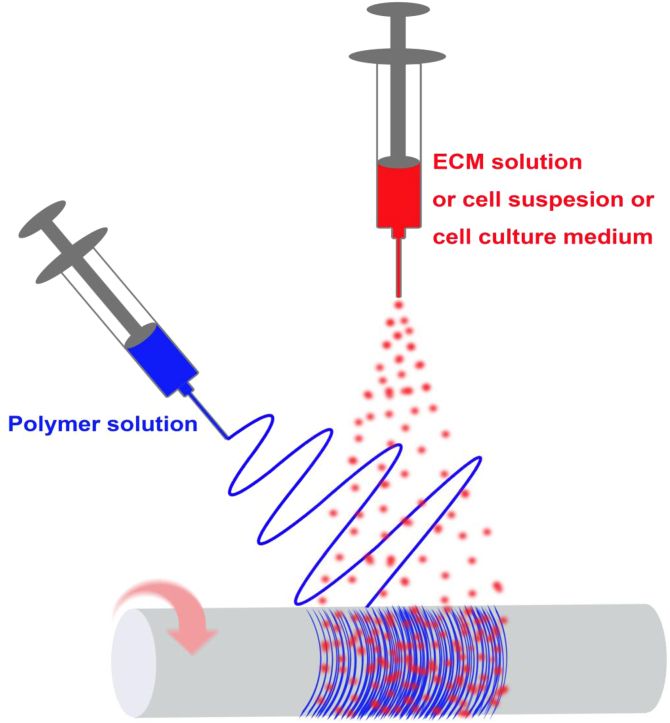
Fig. 6.
Concurrent electrospinning/electrospraying technique to prepare hybrid fibrous scaffold [59], [60], [61].
Another attractive technique involving the combination of electrospinning with electrospraying was introduced to fabricate a fiber/gel hybrid scaffold [57], [58], [59]. Methodologically, it combines the separately generated fibers and gel to reduce fiber bonding, which loosens the fibrous structure and allows significant cell infiltration and 3D cellularization. Hong et al. demonstrated the incorporation of ECM with a synthetic polymer using this approach, where electrospun PEUU fibers and electrospray decellularized dermal ECM (dECM) hydrogels were simultaneously collected using a rotating mandrel [59] (Fig. 6). The dECM hydrogel loosened the fibers that contribute to a relatively porous structure of the scaffold. The resulting PEUU fiber/dECM hydrogel composite scaffold supported cell infiltration throughout the whole scaffold in a rat full-thickness abdominal-wall defect model within 4 weeks. In contrast, minimal cell infiltration was observed in electrospun pure PEUU scaffolds [59]. A complex sandwich scaffold contains wet-electrospun PEUU fiber upper and lower layers to provide sufficient mechanical support, and a PEUU/dECM gel hybrid middle layer prepared by electrospinning/electrospraying to promote cell infiltration, which was reported by Takanari et al. [60]. When implanted in a full-thickness rat abdominal wall defect model, the scaffold maintained its thickness and exhibited extensive cell infiltration and vascular ingrowth with collagen deposition within 12 weeks.
Similar to the combination of electrospinning with electrospraying, a technique introduced by Stankus et al. involves the simultaneous deposition of electrospun fibers with electrosprayed cells that allows rapid creation of hybrid tissue engineered constructs with uniform cell distribution [61] (Fig. 6). The cellularized constructs sustained high cell survival rates under perfusion culture for 7 days. This technique is advantageous in the fabrication of tissue-engineered scaffolds with high cell densities that could facilitate cell-matrix interactions and improve scaffold mechanical properties for soft tissue replacement.
3. Conclusions and prospective
The increase in pore size and loosened fibrous structure is very effective in promoting cell infiltration, though the cell infiltrated depths into electrospun scaffolds vary from approach to approach and from in vitro study to in vivo study. One consideration regarding the methods to increase pore size or loosen the fiber is the significantly reduced mechanical properties compared with that of conventional electrospun scaffolds. Mechanical properties of electrospun fibrous scaffolds are intimately associated with their fiber density and junctions. Developing new robust biodegradable materials is the most effective way to strengthen the modified electrospun scaffolds, which may achieve a strong electrospun scaffold allowing cell ingrowth. Integrating other techniques or designing new collectors may be an alternative way to fabricate newly structural electrospun scaffolds with robust mechanical properties and great cell infiltration. Meanwhile, the scale-up of the electrospun scaffold is always a challenge for research and the industry. The scaling capability of a certain technique is largely dependent on its technical complexity. Conventional electrospinning technique exhibits high up-scaling potential in terms of production volume and reproducibility [62]. The complexity of these listed techniques (Table 2) markedly increases compared to conventional electrospinning alone, which further increases the difficulty of the scale-up. Some post-electrospun modifications, such as gas foaming and ultrasonication, may be good options for scale-up. Also, the upgraded collector, such as ethanol bath, may be suitable for scale-up. Multiple-streams with different functions, such as electrospinning/electrospray and electrospinning/salt leaching, significantly increase the complication and difficulty of the scale-up.
Table 2.
Pros and cons of the modified electrospinning techniques.
| Technique | Pros | Cons | References |
|---|---|---|---|
| Combination of nanofibers and microfibers | Controllable fiber diameter and pore size. | Small pores defined by the nanofibers in the scaffold still hinder cell infiltration. | [19], [20], [21] |
| Electrospinning with salt leaching | Controllable pore size. | Modifications of electrospinning setup need to disperse the salt particles into nanofibrous mats. | [22], [23] |
| Cryogenic electrospinning | Open 3D structure with super large pores. | Correct balance between crystal formation and fiber deposition is difficult to be achieved. Difficult to form a thick scaffold with homogeneous porous structure. |
[24], [25], [26], [27] |
| Sacrificial fibers to induce large pores | Adjustable porosity. Homogeneous porous structure. |
Difficult to increase scaffold pore size. | [28], [29], [30] |
| Electrospinning using a liquid bath collector | Dispersion effect of the liquid bath results in homogeneous pores with the scaffolds. | Difficult to scale up. | [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41] |
| Ultrasonication | Feasible process. | Difficult to obtain desirable shape of scaffold. | [42], [43], [44], [45] |
| Electrospinning with gas foaming | Homogeneous porous structure. | Chemical agent has a negative effect on electrospinning process. | [47], [48], [49], [50] |
| Electrospinning/electrospraying | Minimize the loss of bioactivity by separating natural component from highly volatile organic solution. Allow rapid formation of hybrid tissue engineered constructs with uniform cell distribution. |
Difficult to scale up. | [56], [57], [58], [59], [60], [61] |
The multi-technique-combination approach may be the key to achieving appropriate fibrous scaffolds with intensive cell infiltration. For example, the combination of the electrospinning/electrospraying [57], [58], [59] and the combination of electrospinning/liquid bath techniques [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41] are highly effective. It is notable that the separation of the electrospinning and electrospraying streams avoids direct contact of the electrosprayed bioactive materials with harsh organic solvents, avoiding a loss in bioactivity [59]. In addition, a quickly cellularized construct can be easily achieved by electrospraying cells into an electrospun nanofiber mesh [60]. Also, electrospinning can combine with other scaffold fabrication techniques to prepare a hybrid scaffold with biphase, such as an electrospun scaffold/porous scaffold combination, where the porous scaffold allows intensive cell ingrowth and an electrospun layer provides mechanical support [40]. These cases can inspire new electrospinning techniques through combination with other techniques to create new fibrous scaffolds with intensive cell infiltration.
In addition to the optimization of 3D electrospun scaffolds, improved cell infiltration can be attained by special cell seeding and dynamic cultivation techniques for in vitro study. Unlike in vivo study, in vitro cell cultivation lacks the inherently dynamic microenvironment. Consequently, initial cell seeding largely affects subsequent cell infiltration behaviors. Compared with the most commonly used method of seeding cells on the surface of scaffolds, centrifugal, rotational and vacuum seeding methods can significantly facilitate cell infiltration into porous scaffolds during the initial period [63], [64], [65]. Additionally, perfusion cell cultivation in bioreactors can enhance cell infiltration [66]. Furthermore, a strong understanding of how the cells dynamically interact with 3D nanostructures and new tissue formation to replace gradually degraded scaffolds may benefit the design of novel electrospun 3D scaffolds and yield improved cell infiltration.
In conclusion, these innovative approaches exhibited great promise in optimizing electrospun scaffolds to enhance cell infiltration, although challenges still exist. Future study should focus on the simplification of methods to make them more effective and controllable and on developing new techniques to allow industrial up-scaling. Ongoing efforts are required to improve cell infiltration of electrospun scaffolds further without sacrificing the necessary characteristics that are required for tissue repair and regeneration, which ultimately promote their clinical applications.
Acknowledgements
We greatly thank financial supports from the American Heart Association (Beginning Grant-in-Aid, #14BGIA20510066), and the National Scientific Foundation (CAREER, #1554835) in the United States of America.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.A. Formhals. Process and apparatus for preparing artificial threads. US Patent No. 1,975,504; (1934).
- 2.Guan J., Wang F., Li Z., Chen J., Guo X., Liao J. The stimulation of the cardiac differentiation of mesenchymal stem cells in tissue constructs that mimic myocardium structure and biomechanics. Biomaterials. 2011;32:5568–5580. doi: 10.1016/j.biomaterials.2011.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yin A., Zhang K., McClure M.J., Huang C., Wu J., Fang J. Electrospinning collagen/chitosan/poly(L-lactic acid-co-epsilon-caprolactone) to form a vascular graft: mechanical and biological characterization. J. Biomed. Mater. Res. Part A. 2013;101:1292–1301. doi: 10.1002/jbm.a.34434. [DOI] [PubMed] [Google Scholar]
- 4.Punnakitikashem P., Truong D., Menon J.U., Nguyen K.T., Hong Y. Electrospun biodegradable elastic polyurethane scaffolds with dipyridamole release for small diameter vascular grafts. Acta Biomater. 2014;10:4618–4628. doi: 10.1016/j.actbio.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masoumi N., Annabi N., Assmann A., Larson B.L., Hjortnaes J., Alemdar N. Tri-layered elastomeric scaffolds for engineering heart valve leaflets. Biomaterials. 2014;35:7774–7785. doi: 10.1016/j.biomaterials.2014.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin G., Prabhakaran M.P., Ramakrishna S. Stem cell differentiation to epidermal lineages on electrospun nanofibrous substrates for skin tissue engineering. Acta Biomater. 2011;7:3113–3122. doi: 10.1016/j.actbio.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Yoshimoto H., Shin Y.M., Terai H., Vacanti J.P. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials. 2003;24:2077–2082. doi: 10.1016/s0142-9612(02)00635-x. [DOI] [PubMed] [Google Scholar]
- 8.Jang J.-H., Castano O., Kim H.-W. Electrospun materials as potential platforms for bone tissue engineering. Adv. Drug Deliv. Rev. 2009;61:1065–1083. doi: 10.1016/j.addr.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Liao J., Guo X., Grande-Allen K.J., Kasper F.K., Mikos A.G. Bioactive polymer/extracellular matrix scaffolds fabricated with a flow perfusion bioreactor for cartilage tissue engineering. Biomaterials. 2010;31:8911–8920. doi: 10.1016/j.biomaterials.2010.07.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin Z., Chen X., Chen J.L., Shen W.L., Hieu Nguyen T.M., Gao L. The regulation of tendon stem cell differentiation by the alignment of nanofibers. Biomaterials. 2010;31:2163–2175. doi: 10.1016/j.biomaterials.2009.11.083. [DOI] [PubMed] [Google Scholar]
- 11.Baker B.M., Mauck R.L. The effect of nanofiber alignment on the maturation of engineered meniscus constructs. Biomaterials. 2007;28:1967–1977. doi: 10.1016/j.biomaterials.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang F., Murugan R., Wang S., Ramakrishna S. Electrospinning of nano/micro scale poly(l-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials. 2005;26:2603–2610. doi: 10.1016/j.biomaterials.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal S., Wendorff J.H., Greiner A. Progress in the field of electrospinning for tissue engineering applications. Adv. Mater. 2009;21:3343–3351. doi: 10.1002/adma.200803092. [DOI] [PubMed] [Google Scholar]
- 14.Zhong S., Zhang Y., Lim C.T. Fabrication of large pores in electrospun nanofibrous scaffolds for cellular infiltration: a review. Tissue Eng. Part B Rev. 2011;18:77–87. doi: 10.1089/ten.TEB.2011.0390. [DOI] [PubMed] [Google Scholar]
- 15.Rnjak-Kovacina J., Weiss A.S. Increasing the pore size of electrospun scaffolds. Tissue Eng. Part B Rev. 2011;17:365–372. doi: 10.1089/ten.teb.2011.0235. [DOI] [PubMed] [Google Scholar]
- 16.Balguid A., Mol A., van Marion M.H., Bank R.A., Bouten C.V.C., Baaijens F.P.T. Tailoring fiber diameter in electrospun poly(ɛ-Caprolactone) scaffolds for optimal cellular infiltration in cardiovascular tissue engineering. Tissue Eng. Part A. 2008;15:437–444. doi: 10.1089/ten.tea.2007.0294. [DOI] [PubMed] [Google Scholar]
- 17.Christopherson G.T., Song H., Mao H.-Q. The influence of fiber diameter of electrospun substrates on neural stem cell differentiation and proliferation. Biomaterials. 2009;30:556–564. doi: 10.1016/j.biomaterials.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Chen M., Patra P.K., Warner S.B., Bhowmick S. Role of fiber diameter in adhesion and proliferation of NIH 3T3 fibroblast on electrospun polycaprolactone scaffolds. Tissue Eng. 2007;13:579–587. doi: 10.1089/ten.2006.0205. [DOI] [PubMed] [Google Scholar]
- 19.Pham Q.P., Sharma U., Mikos A.G. Electrospun poly(ε-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: Characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules. 2006;7:2796–2805. doi: 10.1021/bm060680j. [DOI] [PubMed] [Google Scholar]
- 20.Kim B.S., Park K.E., Kim M.H., You H.K., Lee J., Park W.H. Effect of nanofiber content on bone regeneration of silk fibroin/poly(ε-caprolactone) nano/microfibrous composite scaffolds. Int. J. Nanomedicine. 2015;10:485–502. doi: 10.2147/IJN.S72730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levorson E.J., Raman Sreerekha P., Chennazhi K.P., Kasper F.K., Nair S.V., Mikos A.G. Fabrication and characterization of multiscale electrospun scaffolds for cartilage regeneration. Biomed. Mater. 2013;8:014103. doi: 10.1088/1748-6041/8/1/014103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nam J., Huang Y., Agarwal S., Lannutti J. Improved cellular infiltration in electrospun fiber via engineered porosity. Tissue Eng. 2007;13:2249–2257. doi: 10.1089/ten.2006.0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim T.G., Chung H.J., Park T.G. Macroporous and nanofibrous hyaluronic acid/collagen hybrid scaffold fabricated by concurrent electrospinning and deposition/leaching of salt particles. Acta Biomater. 2008;4:1611–1619. doi: 10.1016/j.actbio.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 24.Simonet M., Schneider O.D., Neuenschwander P., Stark W.J. Ultraporous 3D polymer meshes by low-temperature electrospinning: use of ice crystals as a removable void template. Polym. Eng. Sci. 2007;47:2020–2026. [Google Scholar]
- 25.Leong M.F., Rasheed M.Z., Lim T.C., Chian K.S. In vitro cell infiltration and in vivo cell infiltration and vascularization in a fibrous, highly porous poly(D,L-lactide) scaffold fabricated by cryogenic electrospinning technique. J. Biomed. Mater. Res. Part A. 2009;91A:231–240. doi: 10.1002/jbm.a.32208. [DOI] [PubMed] [Google Scholar]
- 26.Leong M.F., Chan W.Y., Chian K.S., Rasheed M.Z., Anderson J.M. Fabrication and in vitro and in vivo cell infiltration study of a bilayered cryogenic electrospun poly(D,L-lactide) scaffold. J. Biomed. Mater. Res. Part A. 2010;94A:1141–1149. doi: 10.1002/jbm.a.32795. [DOI] [PubMed] [Google Scholar]
- 27.Bulysheva A.A., Bowlin G.L., Petrova S.P., Yeudall W.A. Enhanced chemoresistance of squamous carcinoma cells grown in 3D cryogenic electrospun scaffolds. Biomed. Mater. 2013;8:055009. doi: 10.1088/1748-6041/8/5/055009. [DOI] [PubMed] [Google Scholar]
- 28.Baker B.M., Gee A.O., Metter R.B., Nathan A.S., Marklein R.A., Burdick J.A. The potential to improve cell infiltration in composite fiber-aligned electrospun scaffolds by the selective removal of sacrificial fibers. Biomaterials. 2008;29:2348–2358. doi: 10.1016/j.biomaterials.2008.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whited B.M., Whitney J.R., Hofmann M.C., Xu Y., Rylander M.N. Pre-osteoblast infiltration and differentiation in highly porous apatite-coated PLLA electrospun scaffolds. Biomaterials. 2011;32:2294–2304. doi: 10.1016/j.biomaterials.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Klumpp D., Rudisile M., Kuhnle R.I., Hess A., Bitto F.F., Arkudas A. Three-dimensional vascularization of electrospun PCL/collagen-blend nanofibrous scaffolds in vivo. J. Biomed. Mater. Res. Part A. 2012;100:2302–2311. doi: 10.1002/jbm.a.34172. [DOI] [PubMed] [Google Scholar]
- 31.Smit E., Bűttner U., Sanderson R.D. Continuous yarns from electrospun fibers. Polymer. 2005;46:2419–2423. [Google Scholar]
- 32.Ki C.S., Park S.Y., Kim H.J., Jung H.M., Woo K.M., Lee J.W. Development of 3-D nanofibrous fibroin scaffold with high porosity by electrospinning: implications for bone regeneration. Biotechnol. Lett. 2008;30:405–410. doi: 10.1007/s10529-007-9581-5. [DOI] [PubMed] [Google Scholar]
- 33.Yokoyama Y., Hattori S., Yoshikawa C., Yasuda Y., Koyama H., Takato T. Novel wet electrospinning system for fabrication of spongiform nanofiber 3-dimensional fabric. Mater. Lett. 2009;63:754–756. [Google Scholar]
- 34.Yang W., Yang F., Wang Y., Both S.K., Jansen J.A. In vivo bone generation via the endochondral pathway on three-dimensional electrospun fibers. Acta Biomater. 2013;9:4505–4512. doi: 10.1016/j.actbio.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Teo W.-E., Gopal R., Ramaseshan R., Fujihara K., Ramakrishna S. A dynamic liquid support system for continuous electrospun yarn fabrication. Polymer. 2007;48:3400–3405. [Google Scholar]
- 36.Wu J., Liu S., He L., Wang H., He C., Fan C. Electrospun nanoyarn scaffold and its application in tissue engineering. Mater. Lett. 2012;89:146–149. [Google Scholar]
- 37.Wu J., Huang C., Liu W., Yin A., Chen W., He C. Cell infiltration and vascularization in porous nanoyarn scaffolds prepared by dynamic liquid electrospinning. J. Biomed. Nanotechnol. 2014;10:603–614. doi: 10.1166/jbn.2014.1733. [DOI] [PubMed] [Google Scholar]
- 38.Xu Y., Wu J., Wang H., Li H., Di N., Song L. Fabrication of electrospun poly(L-lactide-co-epsilon-caprolactone)/collagen nanoyarn network as a novel, three-dimensional, macroporous, aligned scaffold for tendon tissue engineering. Tissue Eng. Part C Methods. 2013;19:925–936. doi: 10.1089/ten.tec.2012.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu Y., Dong S., Zhou Q., Mo X., Song L., Hou T. The effect of mechanical stimulation on the maturation of TDSCs-poly(L-lactide-co-e-caprolactone)/collagen scaffold constructs for tendon tissue engineering. Biomaterials. 2014;35:2760–2772. doi: 10.1016/j.biomaterials.2013.12.042. [DOI] [PubMed] [Google Scholar]
- 40.Zheng X., Wang W., Liu S., Wu J., Li F., Cao L. Enhancement of chondrogenic differentiation of rabbit mesenchymal stem cells by oriented nanofiber yarn-collagen type I/hyaluronate hybrid. Mater. Sci. Eng. C Mater. Biol. Appl. 2016;58:1071–1076. doi: 10.1016/j.msec.2015.07.066. [DOI] [PubMed] [Google Scholar]
- 41.Liu S., Wu J., Liu X., Chen D., Bowlin G.L., Cao L. Osteochondral regeneration using an oriented nanofiber yarn-collagen type I/hyaluronate hybrid/TCP biphasic scaffold. J. Biomed. Mater. Res. Part A. 2015;103:581–592. doi: 10.1002/jbm.a.35206. [DOI] [PubMed] [Google Scholar]
- 42.Lee J.B., Jeong S.I., Bae M.S., Yang D.H., Heo D.N., Kim C.H. Highly porous electrospun nanofibers enhanced by ultrasonication for improved cellular infiltration. Tissue Eng. Part A. 2011;17:2695–2702. doi: 10.1089/ten.TEA.2010.0709. [DOI] [PubMed] [Google Scholar]
- 43.Jeong S.I., Burns N.A., Bonino C.A., Kwon I.K., Khan S.A., Alsberg E. Improved cell infiltration of highly porous 3D nanofibrous scaffolds formed by combined fiber-fiber charge repulsions and ultra-sonication. J. Mater. Chem. B Mater. Biol. Med. 2014;2:8116–8122. doi: 10.1039/C4TB01487A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gu B.K., Park S.J., Kim M.S., Kang C.M., Kim J.-I., Kim C.-H. Fabrication of sonicated chitosan nanofiber mat with enlarged porosity for use as hemostatic materials. Carbohydr. Polym. 2013;97:65–73. doi: 10.1016/j.carbpol.2013.04.060. [DOI] [PubMed] [Google Scholar]
- 45.Gu B.K., Park S.J., Kim M.S., Lee Y.J., Kim J.-I., Kim C.-H. Gelatin blending and sonication of chitosan nanofiber mats produce synergistic effects on hemostatic functions. Int. J. Biol. Macromol. 2016;82:89–96. doi: 10.1016/j.ijbiomac.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 46.Liu X., Ma P.X. Polymeric scaffolds for bone tissue engineering. Ann. Biomed. Eng. 2004;32:477–486. doi: 10.1023/b:abme.0000017544.36001.8e. [DOI] [PubMed] [Google Scholar]
- 47.Lee Y.H., Lee J.H., An I.-G., Kim C., Lee D.S., Lee Y.K. Electrospun dual-porosity structure and biodegradation morphology of montmorillonite reinforced PLLA nanocomposite scaffolds. Biomaterials. 2005;26:3165–3172. doi: 10.1016/j.biomaterials.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 48.Kim G., Kim W. Highly porous 3D nanofiber scaffold using an electrospinning technique. J. Biomed. Mater. Res. Part B. 2007;81B:104–110. doi: 10.1002/jbm.b.30642. [DOI] [PubMed] [Google Scholar]
- 49.Hwang P.T.J., Murdock K., Alexander G.C., Salaam A.D., Ng J.I., Lim D.-J. Poly(ɛ-caprolactone)/gelatin composite electrospun scaffolds with porous crater-like structures for tissue engineering. J. Biomed. Mater. Res. Part A. 2016;104:1017–1029. doi: 10.1002/jbm.a.35614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang J., Carlson M.A., Teusink M.J., Wang H., MacEwan M.R., Xie J. Expanding two-dimensional electrospun nanofiber membranes in the third dimension by a modified gas-foaming technique. ACS Biomater. Sci. Eng. 2015;1:991–1001. doi: 10.1021/acsbiomaterials.5b00238. [DOI] [PubMed] [Google Scholar]
- 51.Blakeney B.A., Tambralli A., Anderson J.M., Andukuri A., Lim D.-J., Dean D.R. Cell infiltration and growth in a low density, uncompressed three-dimensional electrospun nanofibrous scaffold. Biomaterials. 2011;32:1583–1590. doi: 10.1016/j.biomaterials.2010.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McClure M.J., Wolfe P.S., Simpson D.G., Sell S.A., Bowlin G.L. The use of air-flow impedance to control fiber deposition patterns during electrospinning. Biomaterials. 2012;33:771–779. doi: 10.1016/j.biomaterials.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 53.Maghdouri-White Y., Elmore L.W., Bowlin G.L., Dréau D. Breast epithelial cell infiltration in enhanced electrospun silk scaffolds. J. Tissue Eng. Regen. Med. 2016;10:E121–E131. doi: 10.1002/term.1778. [DOI] [PubMed] [Google Scholar]
- 54.Garg K., Pullen N.A., Oskeritzian C.A., Ryan J.J., Bowlin G.L. Macrophage functional polarization (M1/M2) in response to varying fiber and pore dimensions of electrospun scaffolds. Biomaterials. 2013;34:4439–4451. doi: 10.1016/j.biomaterials.2013.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yin A., Li J., Bowlin G.L., Li D., Rodriguez I.A., Wang J. Fabrication of cell penetration enhanced poly (l-lactic acid-co-ɛ-caprolactone)/silk vascular scaffolds utilizing air-impedance electrospinning. Colloids Surf. B Biointerfaces. 2014;120:47–54. doi: 10.1016/j.colsurfb.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 56.Hashizume R., Fujimoto K.L., Hong Y., Amoroso N.J., Tobita K., Miki T. Morphological and mechanical characteristics of the reconstructed rat abdominal wall following use of a wet electrospun biodegradable polyurethane elastomer scaffold. Biomaterials. 2010;31:3253–3265. doi: 10.1016/j.biomaterials.2010.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ekaputra A.K., Prestwich G.D., Cool S.M., Hutmacher D.W. Combining electrospun scaffolds with electrosprayed hydrogels leads to three-dimensional cellularization of hybrid constructs. Biomacromolecules. 2008;9:2097–2103. doi: 10.1021/bm800565u. [DOI] [PubMed] [Google Scholar]
- 58.Ekaputra A.K., Prestwich G.D., Cool S.M., Hutmacher D.W. The three-dimensional vascularization of growth factor-releasing hybrid scaffold of poly (ɛ-caprolactone)/collagen fibers and hyaluronic acid hydrogel. Biomaterials. 2011;32:8108–8117. doi: 10.1016/j.biomaterials.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 59.Hong Y., Huber A., Takanari K., Amoroso N.J., Hashizume R., Badylak S.F. Mechanical properties and in vivo behavior of a biodegradable synthetic polymer microfiber–extracellular matrix hydrogel biohybrid scaffold. Biomaterials. 2011;32:3387–3394. doi: 10.1016/j.biomaterials.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takanari K., Hong Y., Hashizume R., Huber A., Amoroso N.J., D'Amore A. Abdominal wall reconstruction by a regionally distinct biocomposite of extracellular matrix digest and a biodegradable elastomer. J. Tissue Eng. Regen. Med. 2013;7 doi: 10.1002/term.1834. [DOI] [PubMed] [Google Scholar]
- 61.Stankus J.J., Guan J., Fujimoto K., Wagner W.R. Microintegrating smooth muscle cells into a biodegradable, elastomeric fiber matrix. Biomaterials. 2006;27:735–744. doi: 10.1016/j.biomaterials.2005.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Persano L., Camposeo A., Tekmen C., Pisignano D. Industrial upscaling of electrospinning and applications of polymer nanofibers: a review. Macromol. Mater. Eng. 2013;298:504–520. [Google Scholar]
- 63.Villalona G.A., Udelsman B., Duncan D.R., McGillicuddy E., Sawh-Martinez R.F., Hibino N. Cell-seeding techniques in vascular tissue engineering. Tissue Eng. Part B Rev. 2010;16:341–350. doi: 10.1089/ten.teb.2009.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fu W.J., Xu Y.D., Wang Z.X., Li G., Shi J.G., Cui F.Z. New ureteral scaffold constructed with composite poly(L-lactic acid)-collagen and urothelial cells by new centrifugal seeding system. J. Biomed. Mater. Res. Part A. 2012;100:1725–1733. doi: 10.1002/jbm.a.34134. [DOI] [PubMed] [Google Scholar]
- 65.Soletti L., Nieponice A., Guan J., Stankus J.J., Wagner W.R., Vorp D.A. A seeding device for tissue engineered tubular structures. Biomaterials. 2006;27:4863–4870. doi: 10.1016/j.biomaterials.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 66.Nerurkar N.L., Sen S., Baker B.M., Elliott D.M., Mauck R.L. Dynamic culture enhances stem cell infiltration and modulates extracellular matrix production on aligned electrospun nanofibrous scaffolds. Acta Biomater. 2011;7:485–491. doi: 10.1016/j.actbio.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]